Recent Advances in Conductive Composite Hydrogels for Electronic Skin Applications
Abstract
1. Introduction
2. Materials for Preparing Hydrogels
2.1. Synthetic Polymers
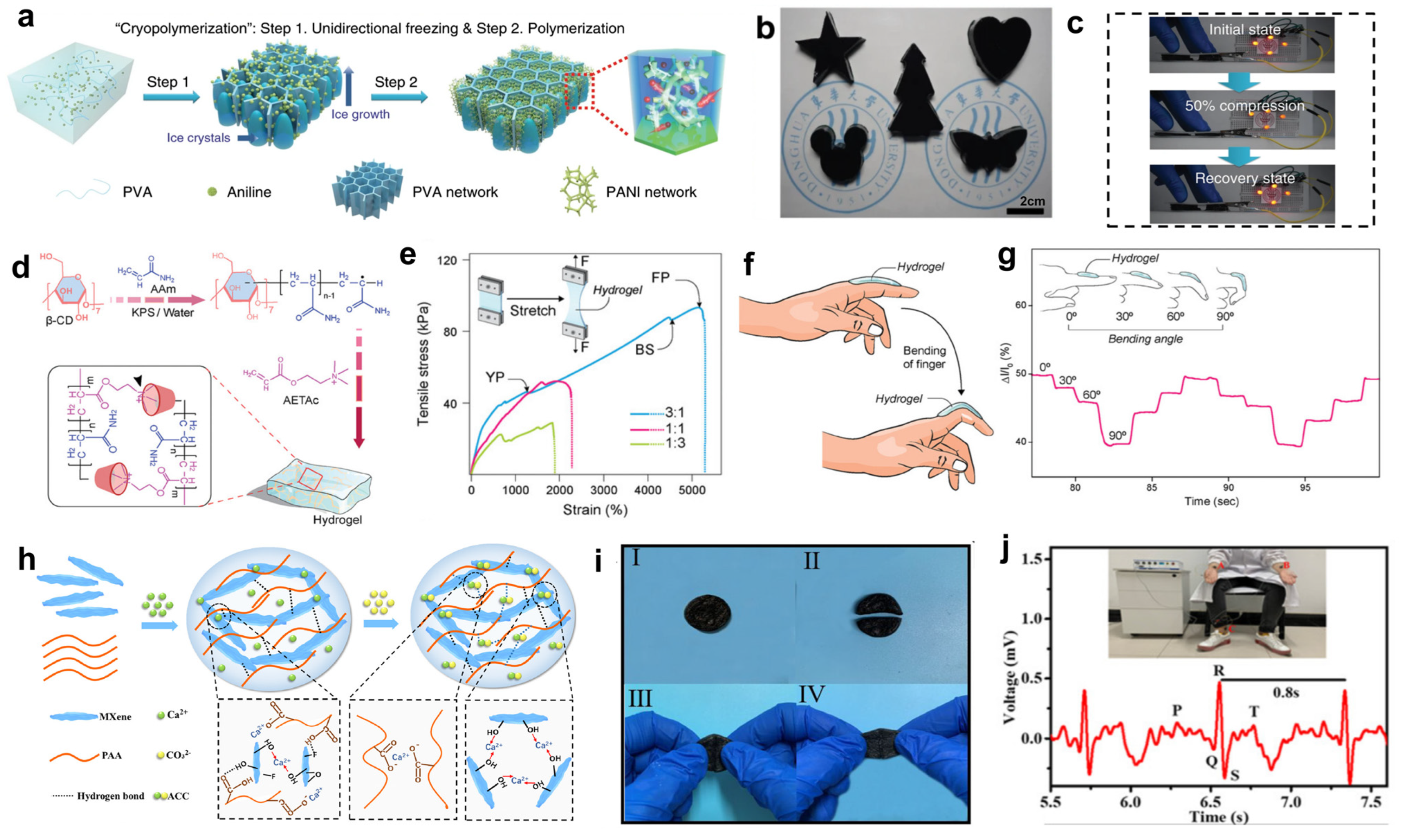
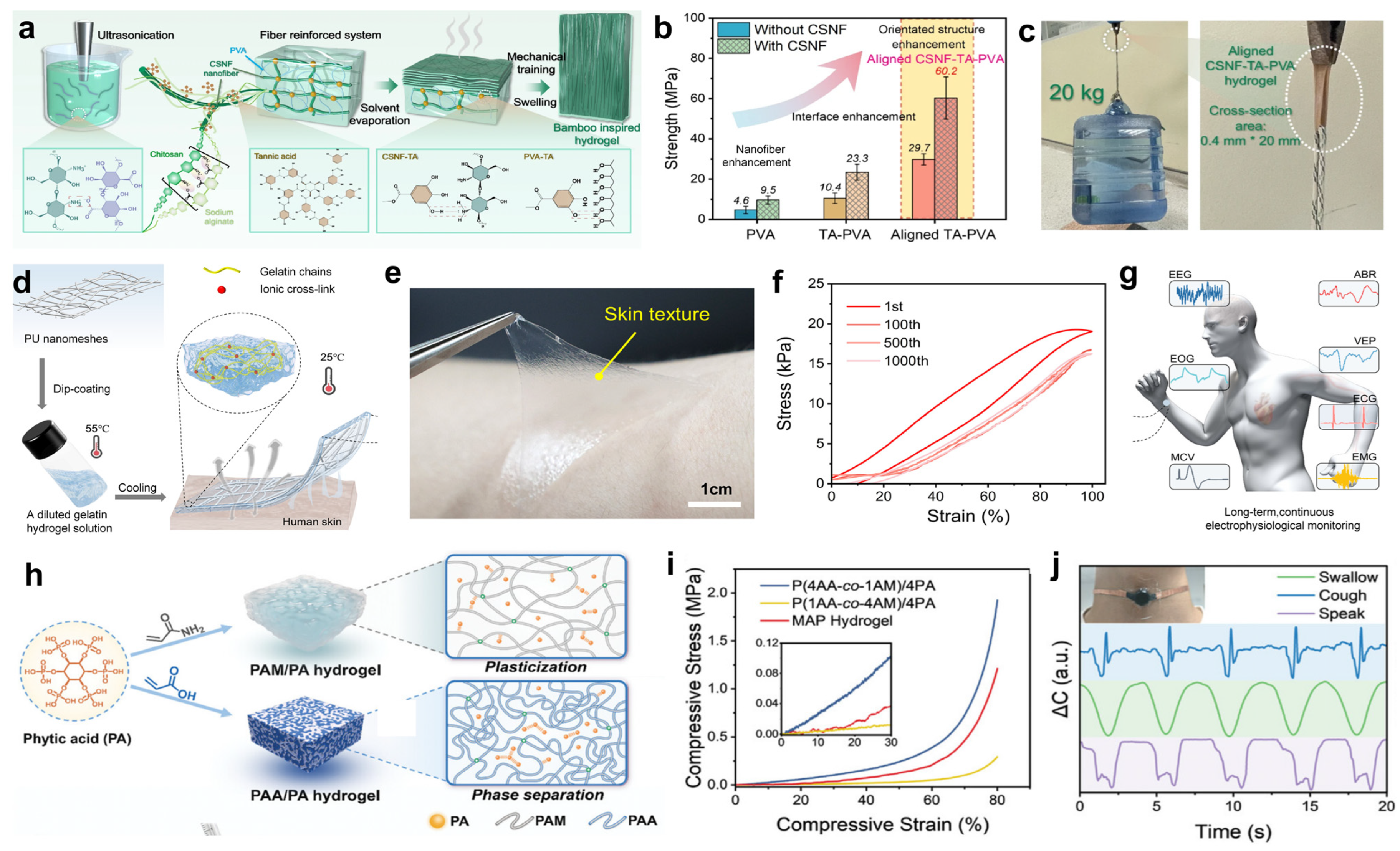
2.2. Natural Polymer Materials
3. Conductive Filler Materials
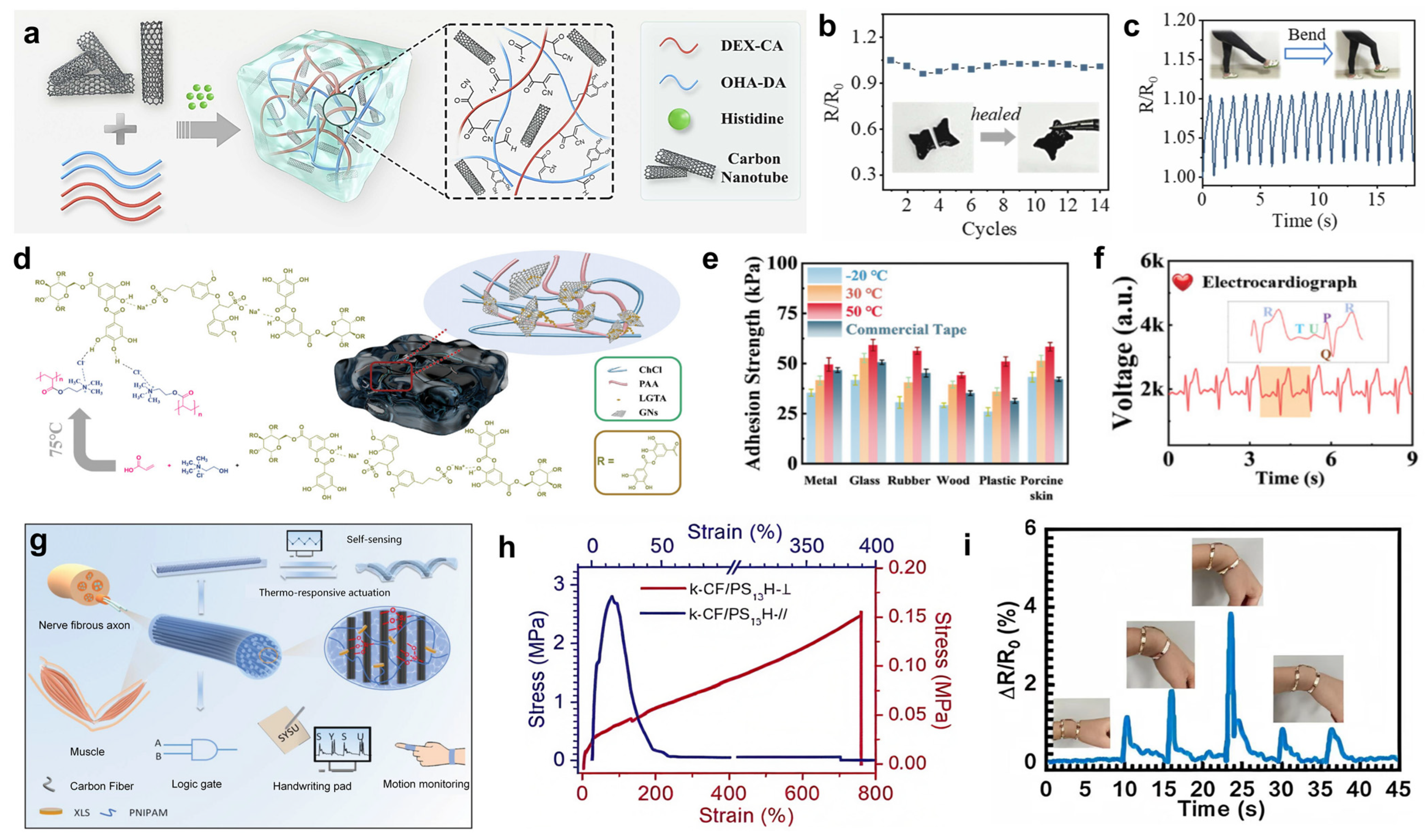
3.1. Carbon Materials
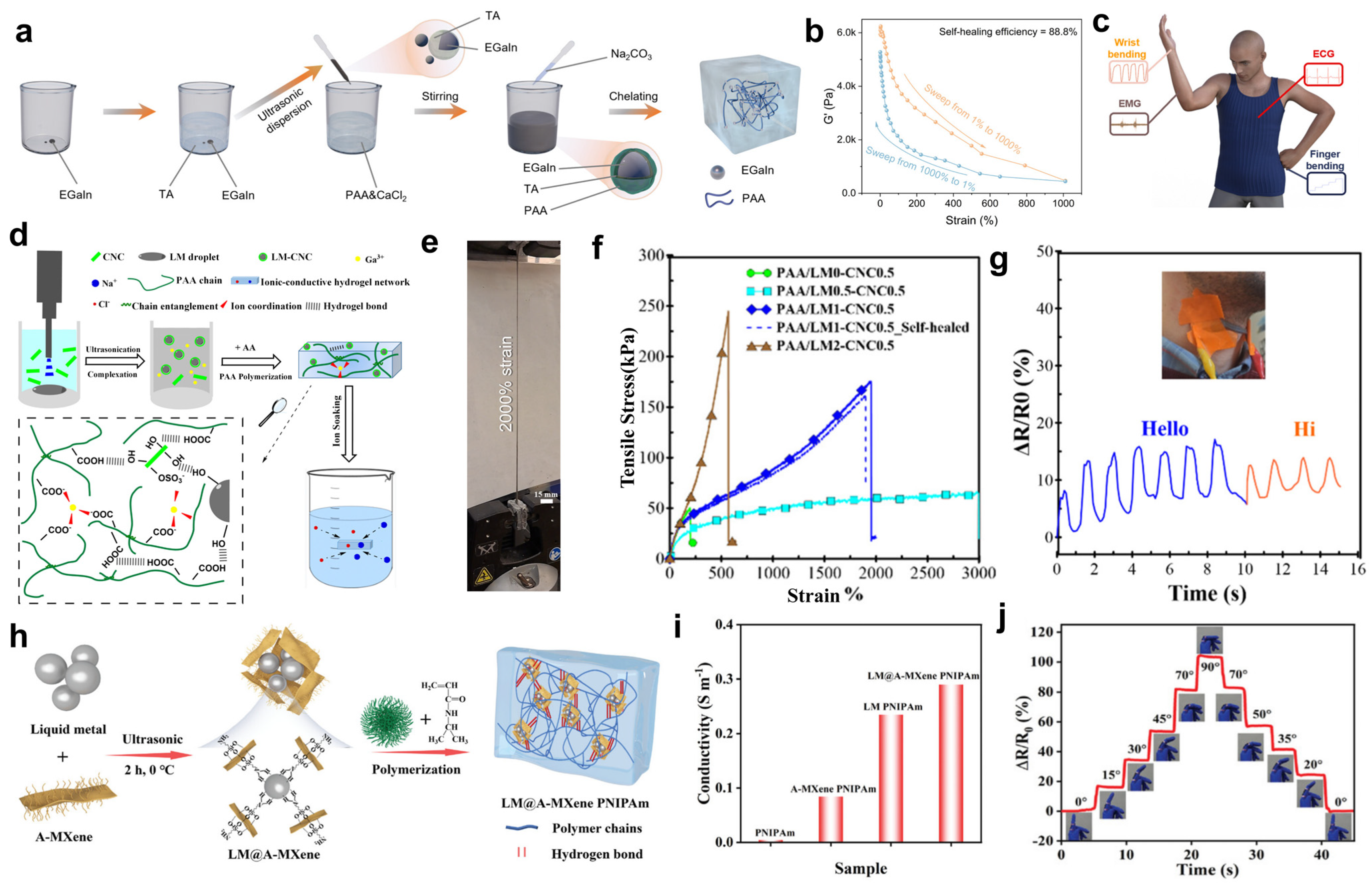
3.2. Liquid Metals
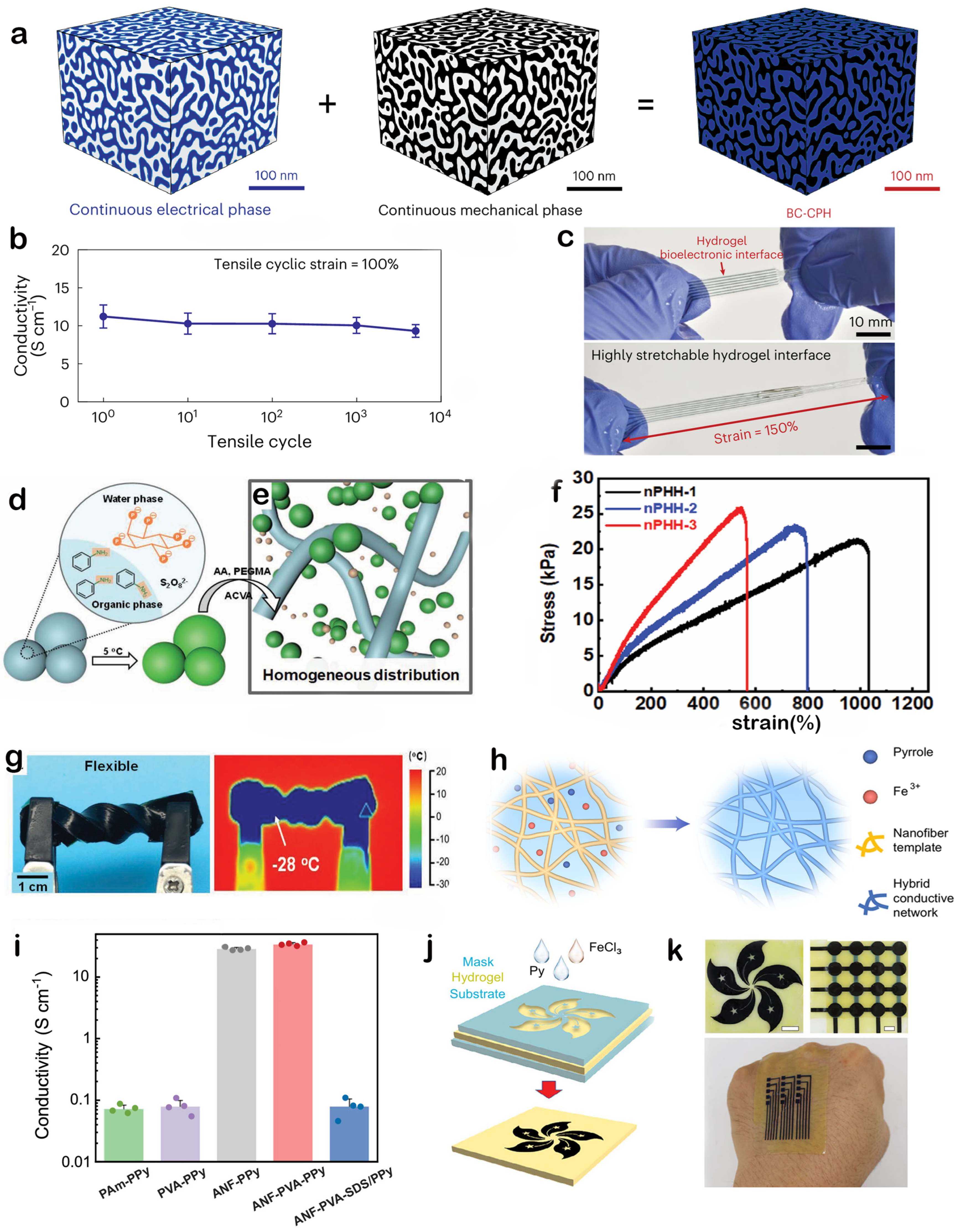
3.3. Conductive Polymers
4. Wearable E-Skin Applications
4.1. Electrophysiological Signals Monitoring
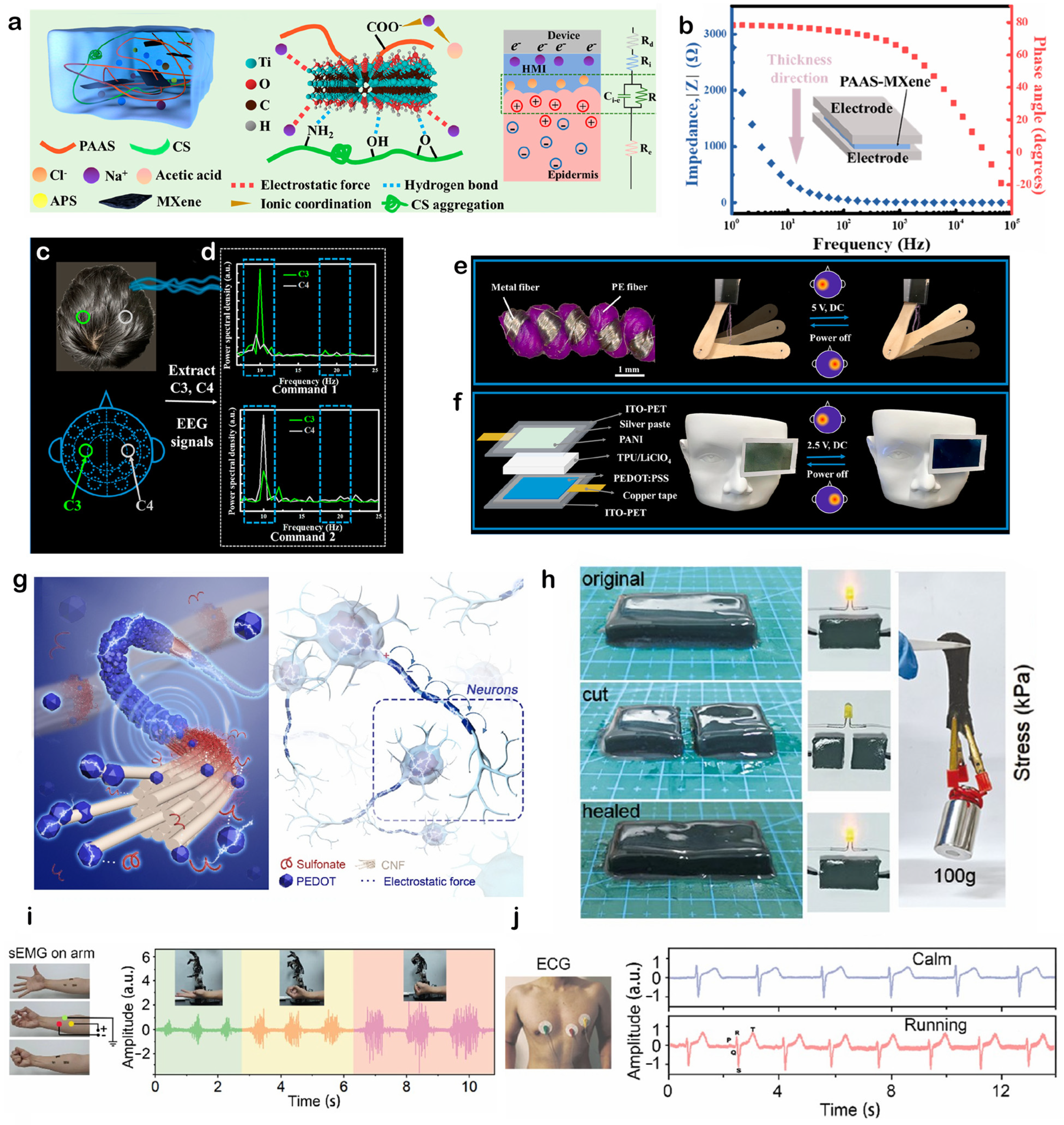
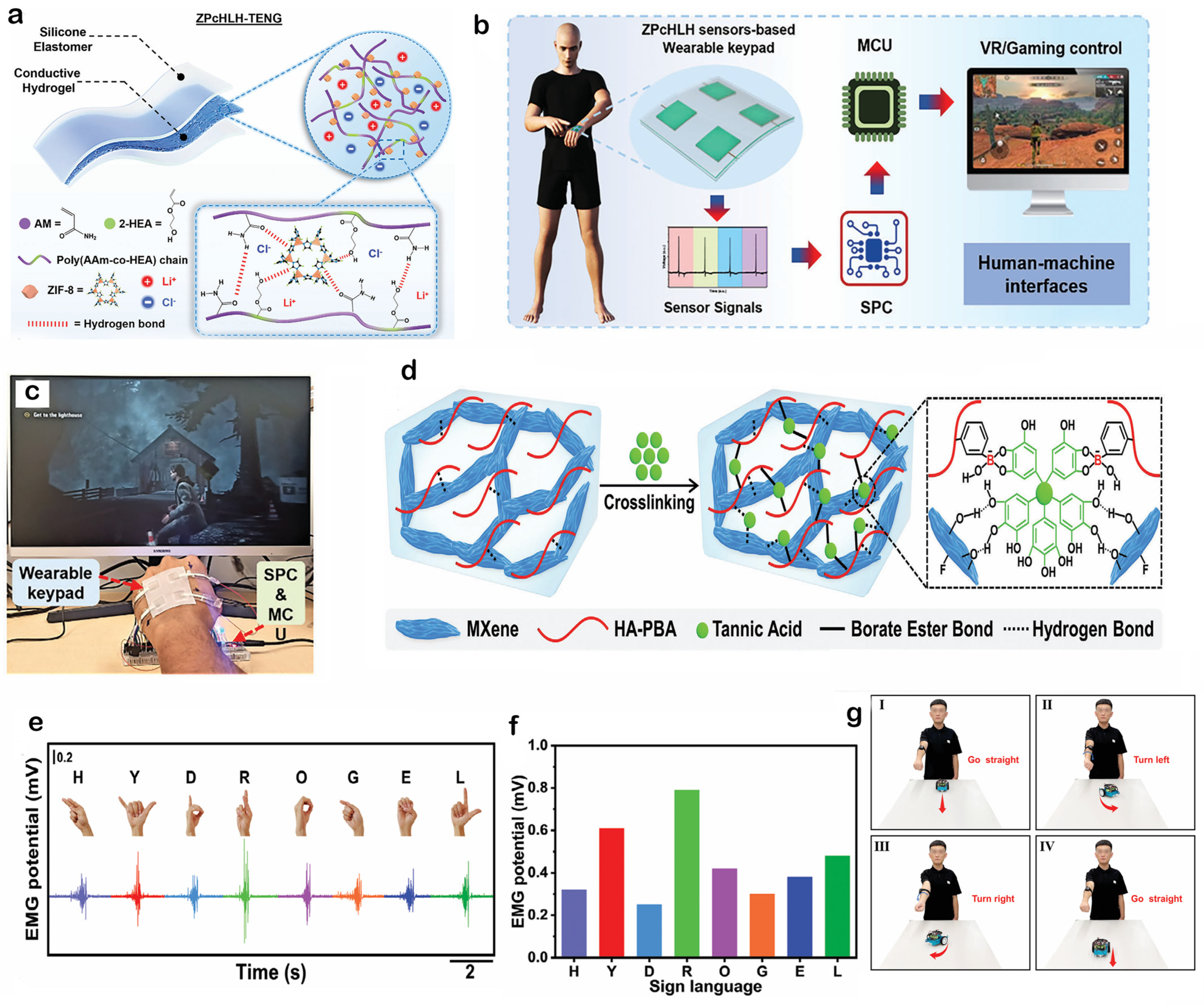
4.2. Human–Machine Interaction
4.3. Body Motion Monitoring

5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mu, G.; Zhang, Y.; Yan, Z.; Yu, Q.; Wang, Q. Recent advancements in wearable sensors: Integration with machine learning for human–machine interaction. RSC Adv. 2025, 15, 7844–7854. [Google Scholar] [CrossRef]
- Kumari, P.; Mathew, L.; Syal, P. Increasing trend of wearables and multimodal interface for human activity monitoring: A review. Biosens. Bioelectron. 2017, 90, 298–307. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, K.; Xu, H.; Li, T.; Jin, Q.; Cui, D. Recent developments in sensors for wearable device applications. Anal. Bioanal. Chem. 2021, 413, 6037–6057. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Huang, F.; Zhang, X.; Song, P.; Zheng, H.; Chevali, V.; Wang, H.; Xu, Z. Flexible pressure sensors via engineering microstructures for wearable human-machine interaction and health monitoring applications. iScience 2022, 25, 104148. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Cheng, Y.; Yue, Y.; Chen, Y.; Gao, H.; Li, L.; Cai, B.; Liu, W.; Wang, Z.; Guo, H.; et al. High-performance flexible pressure sensor with a self-healing function for tactile feedback. Adv. Sci. 2022, 9, 2200507. [Google Scholar] [CrossRef]
- Sun, W.; Guo, Z.; Yang, Z.; Wu, Y.; Lan, W.; Liao, Y.; Wu, X.; Liu, Y. A review of recent advances in vital signals monitoring of sports and health via flexible wearable sensors. Sensors 2022, 22, 7784. [Google Scholar] [CrossRef] [PubMed]
- Trung, T.Q.; Lee, N.E. Recent progress on stretchable electronic devices with intrinsically stretchable components. Adv. Mater. 2017, 29, 1603167. [Google Scholar] [CrossRef]
- Alam, F.; Ashfaq Ahmed, M.; Jalal, A.H.; Siddiquee, I.; Adury, R.Z.; Hossain, G.M.; Pala, N. Recent progress and challenges of implantable biodegradable biosensors. Micromachines 2024, 15, 475. [Google Scholar] [CrossRef]
- He, R.; Liu, H.; Niu, Y.; Zhang, H.; Genin, G.M.; Xu, F. Flexible miniaturized sensor technologies for long-term physiological monitoring. NPJ Flex. Electron. 2022, 6, 20. [Google Scholar] [CrossRef]
- Yuk, H.; Zhang, T.; Lin, S.; Parada, G.A.; Zhao, X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 2016, 15, 190–196. [Google Scholar] [CrossRef]
- Faisal, A.I.; Majumder, S.; Mondal, T.; Cowan, D.; Naseh, S.; Deen, M.J. Monitoring methods of human body joints: State-of-the-art and research challenges. Sensors 2019, 19, 2629. [Google Scholar] [CrossRef]
- Huang, X.; Xue, Y.; Ren, S.; Wang, F. Sensor-based wearable systems for monitoring human motion and posture: A review. Sensors 2023, 23, 9047. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and applications in biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Zhu, T.; Ni, Y.; Biesold, G.M.; Cheng, Y.; Ge, M.; Li, H.; Huang, J.; Lin, Z.; Lai, Y. Recent advances in conductive hydrogels: Classifications, properties, and applications. Chem. Soc. Rev. 2023, 52, 473–509. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xiao, G.; Wang, Y.; Chen, X.; Duan, G.; Wu, Y.; Gong, X.; Wang, H. Design and fabrication of conductive polymer hydrogels and their applications in flexible supercapacitors. J. Mater. Chem. A 2020, 8, 23059–23095. [Google Scholar] [CrossRef]
- Lei, K.; Chen, M.; Guo, P.; Fang, J.; Zhang, J.; Liu, X.; Wang, W.; Li, Y.; Hu, Z.; Ma, Y.; et al. Environmentally adaptive polymer hydrogels: Maintaining wet-soft features in extreme conditions. Adv. Funct. Mater. 2023, 33, 2303511. [Google Scholar] [CrossRef]
- Ouyang, Y.; Huang, G.; Cui, J.; Zhu, H.; Yan, G.; Mei, Y. Advances and challenges of hydrogel materials for robotic and sensing applications. Chem. Mater. 2022, 34, 9307–9328. [Google Scholar] [CrossRef]
- Sun, X.; Yao, F.; Li, J. Nanocomposite hydrogel-based strain and pressure sensors: A review. J. Mater. Chem. A 2020, 8, 18605–18623. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, H.; Huang, Y.; Wei, Y.; Chen, J. Naturally sourced hydrogels: Emerging fundamental materials for next-generation healthcare sensing. Chem. Soc. Rev. 2023, 52, 2992–3034. [Google Scholar] [CrossRef]
- Liu, K.; Wei, S.; Song, L.; Liu, H.; Wang, T. Conductive hydrogels—A novel material: Recent advances and future perspectives. J. Agric. Food Chem. 2020, 68, 7269–7280. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Ye, Z.; Liu, W.; Liu, X.; Wang, X. Conductive hydrogels for bioelectronics: Molecular structures, design principles, and operation mechanisms. J. Mater. Chem. C 2023, 11, 10785–10808. [Google Scholar] [CrossRef]
- Kim, S.; Shin, Y.; Han, J.; Kim, H.J.; Sunwoo, S.-H. Introductory review of soft implantable bioelectronics using conductive and functional hydrogels and hydrogel nanocomposites. Gels 2024, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, M.; Chong, C.-M.; Lin, D.; Chen, S.; Zhen, Y.; Ding, H.; Zhong, H.-J. Recent advances in the 3D printing of conductive hydrogels for sensor applications: A review. Polymers 2024, 16, 2131. [Google Scholar] [CrossRef]
- Tang, H.; Li, Y.; Liao, S.; Liu, H.; Qiao, Y.; Zhou, J. Multifunctional conductive hydrogel interface for bioelectronic recording and stimulation. Adv. Healthc. Mater. 2024, 13, 2400562. [Google Scholar] [CrossRef]
- Hu, C.; Wang, L.; Liu, S.; Sheng, X.; Yin, L. Recent development of implantable chemical sensors utilizing flexible and biodegradable materials for biomedical applications. ACS Nano 2024, 18, 3969–3995. [Google Scholar] [CrossRef]
- Liu, D.; Huyan, C.; Wang, Z.; Guo, Z.; Zhang, X.; Torun, H.; Mulvihill, D.; Xu, B.B.; Chen, F. Conductive polymer based hydrogels and their application in wearable sensors: A review. Mater. Horiz. 2023, 10, 2800–2823. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.D.; Elias, A.L.; Chung, H.-J. Flexible electronics under strain: A review of mechanical characterization and durability enhancement strategies. J. Mater. Sci. 2016, 51, 2771–2805. [Google Scholar] [CrossRef]
- Sun, X.; Qin, Z.; Ye, L.; Zhang, H.; Yu, Q.; Wu, X.; Li, J.; Yao, F. Carbon nanotubes reinforced hydrogel as flexible strain sensor with high stretchability and mechanically toughness. Chem. Eng. J. 2020, 382, 122832. [Google Scholar] [CrossRef]
- He, Q.; Cheng, Y.; Deng, Y.; Wen, F.; Lai, Y.; Li, H. Conductive hydrogel for flexible bioelectronic device: Current progress and future perspective. Adv. Funct. Mater. 2024, 34, 2308974. [Google Scholar] [CrossRef]
- Peng, S.; Yu, Y.; Wu, S.; Wang, C.-H. Conductive polymer nanocomposites for stretchable electronics: Material selection, design, and applications. ACS Appl. Mater. Interfaces 2021, 13, 43831–43854. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.; Kwon, S.H.; Ibrahim, M.; Dang, A.; Dong, L. Flexible Sensor-Based Human–Machine Interfaces with AI Integration for Medical Robotics. Adv. Robot. Res. 2025, 202500027. [Google Scholar] [CrossRef]
- Lee, E.S.; Lee, M.Y.; Kim, D.H.; Koo, J.H. Recent Advances in Hydrogel-Based Soft Bioelectronics and its Convergence with Machine Learning. Adv. Eng. Mater. 2024, 26, 2401432. [Google Scholar]
- Zhang, Z.; Yang, J.; Wang, H.; Wang, C.; Gu, Y.; Xu, Y.; Lee, S.; Yokota, T.; Haick, H.; Someya, T.; et al. A 10-micrometer-thick nanomesh-reinforced gas-permeable hydrogel skin sensor for long-term electrophysiological monitoring. Sci. Adv. 2024, 10, eadj5389. [Google Scholar] [CrossRef]
- Li, X.; He, L.; Li, Y.; Chao, M.; Li, M.; Wan, P.; Zhang, L. Healable, degradable, and conductive MXene nanocomposite hydrogel for multifunctional epidermal sensors. ACS Nano 2021, 15, 7765–7773. [Google Scholar] [CrossRef]
- Rahman, M.T.; Rahman, M.S.; Kumar, H.; Kim, K.; Kim, S. Metal-Organic Framework Reinforced Highly Stretchable and Durable Conductive Hydrogel-Based Triboelectric Nanogenerator for Biomotion Sensing and Wearable Human-Machine Interfaces. Adv. Funct. Mater. 2023, 33, 2303471. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.; Xu, Z.; Li, Z.; Zhang, L.; Wan, P. Flexible Conformally Bioadhesive MXene Hydrogel Electronics for Machine Learning-Facilitated Human-Interactive Sensing. Adv. Mater. 2024, 36, e2401035. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, C.; Zhang, B.; Wan, Z.; Wang, S.; Zhang, K.; Yang, L.; Wei, R. A Highly Sensitive, Conductive, and Flexible Hydrogel Sponge as a Discriminable Multimodal Sensor for Deep-Learning-Assisted Gesture Language Recognition. Adv. Funct. Mater. 2024, 35, 2416453. [Google Scholar] [CrossRef]
- Zarei, M.; Lee, G.; Lee, S.G.; Cho, K. Advances in biodegradable electronic skin: Material progress and recent applications in sensing, robotics, and human–machine interfaces. Adv. Mater. 2023, 35, 2203193. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lv, Z.; Batool, S.; Li, M.Z.; Zhao, P.; Guo, L.; Wang, Y.; Zhou, Y.; Han, S.T. Biocompatible material-based flexible biosensors: From materials design to wearable/implantable devices and integrated sensing systems. Small 2023, 19, 2207879. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Q.; Guo, M.; Zhi, X.; Xia, Y.; Shi, G.; Wang, X. Ultrastretchable freezing-tolerant organohydrogels for health monitoring and triboelectric nanogenerator-based gesture recognition. Nano Energy 2024, 131, 110261. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, J.; Pan, G.; Lin, Y.; Chen, D.; Lu, J.; Li, J.; Huang, J.; Li, Z.; Lin, X.; et al. Multifunctional Wide-Temperature Threshold Conductive Eutectic Hydrogel Sensor for Shoulder Pain Monitoring and Photothermal Therapy. Adv. Funct. Mater. 2025, 2509779. [Google Scholar] [CrossRef]
- Omidian, H.; Chowdhury, S.D. High-performing conductive hydrogels for wearable applications. Gels 2023, 9, 549. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Fu, Q.; Meng, L.; Hao, S.; Dai, R.; Yang, J. Recent progress in natural biopolymers conductive hydrogels for flexible wearable sensors and energy devices: Materials, structures, and performance. ACS Appl. Bio Mater. 2020, 4, 85–121. [Google Scholar] [CrossRef]
- Cheng, H.; Devi, R.K.; Huang, K.-Y.; Ganesan, M.; Ravi, S.K.; Lin, C.C. Highly Biocompatible Antibacterial Hydrogel for Wearable Sensing of Macro and Microscale Human Body Motions. Small 2024, 20, 2401201. [Google Scholar] [CrossRef]
- Luo, Y.; Abidian, M.R.; Ahn, J.-H.; Akinwande, D.; Andrews, A.M.; Antonietti, M.; Bao, Z.; Berggren, M.; Berkey, C.A.; Bettinger, C.J.; et al. Technology roadmap for flexible sensors. ACS Nano 2023, 17, 5211–5295. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Zhang, T. Flexible sensing electronics for wearable/attachable health monitoring. Small 2017, 13, 1602790. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Carlos, C.; Zhou, H.; Sui, J.; Wang, Y.; Silva-Pedraza, Z.; Yang, F.; Dong, Y.; Zhang, Z.; Hacker, T.A.; et al. Stretchable piezoelectric biocrystal thin films. Nat. Commun. 2023, 14, 6562. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Lu, H.; Wang, Y.; Xu, J.; Zhu, J.; Zhang, C.; Liu, T. Cryopolymerization enables anisotropic polyaniline hybrid hydrogels with superelasticity and highly deformation-tolerant electrochemical energy storage. Nat. Commun. 2020, 11, 62. [Google Scholar] [CrossRef]
- Lu, P.; Xu, J.; Liu, S.; Fu, L.; Wu, S.; Liu, Z.; Hou, T.; Liu, H.; Huang, D. Facile synthesis of ultratough conductive gels with swelling and freezing resistance for flexible sensor applications. Sci. Rep. 2025, 15, 7335. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, Z.; Zhao, W.; He, M.; Guo, N.; Weng, L.; Lin, Z.; Taleb, M.F.A.; Ibrahim, M.M.; Singh, M.V.; et al. Strategies in the preparation of conductive polyvinyl alcohol hydrogels for applications in flexible strain sensors, flexible supercapacitors, and triboelectric nanogenerator sensors: An overview. Adv. Compos. Hybrid Mater. 2023, 6, 203. [Google Scholar] [CrossRef]
- Liang, X.; Zhong, H.-J.; Ding, H.; Yu, B.; Ma, X.; Liu, X.; Chong, C.-M.; He, J. Polyvinyl alcohol (PVA)-based hydrogels: Recent progress in fabrication, properties, and multifunctional applications. Polymers 2024, 16, 2755. [Google Scholar] [CrossRef]
- Yi, F.-L.; Meng, F.-C.; Li, Y.-Q.; Huang, P.; Hu, N.; Liao, K.; Fu, S.-Y. Highly stretchable CNT Fiber/PAAm hydrogel composite simultaneously serving as strain sensor and supercapacitor. Compos. Part B Eng. 2020, 198, 108246. [Google Scholar] [CrossRef]
- Hou, Y.; Jiang, N.; Sun, D.; Wang, Y.; Chen, X.; Zhu, S.; Zhang, L. A fast UV-curable PU-PAAm hydrogel with mechanical flexibility and self-adhesion for wound healing. RSC Adv. 2020, 10, 4907–4915. [Google Scholar] [CrossRef]
- Roy, A.; Zenker, S.; Jain, S.; Afshari, R.; Oz, Y.; Zheng, Y.; Annabi, N. A Highly Stretchable, Conductive, and Transparent Bioadhesive Hydrogel as a Flexible Sensor for Enhanced Real-Time Human Health Monitoring. Adv. Mater. 2024, 36, 2404225. [Google Scholar] [CrossRef]
- Li, Q.; Quan, X.; Hu, R.; Hu, Z.; Xu, S.; Liu, H.; Zhou, X.; Han, B.; Ji, X. A universal strategy for constructing hydrogel assemblies enabled by PAA hydrogel adhesive. Small 2024, 20, 2403844. [Google Scholar] [CrossRef] [PubMed]
- Pissis, P.; Kyritsis, A.; Konsta, A.; Daoukaki, D. Polymer–water interactions in PAA hydrogels. Colloids Surf. A Physicochem. Eng. Asp. 1999, 149, 253–262. [Google Scholar] [CrossRef]
- Tan, H.; Park, S.-Y. Poly (acrylic acid) hydrogel microspheres for a metal-ion sensor. ACS Sens. 2021, 6, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Mi, H.-Y.; Peng, X.-F.; Turng, L.-S. Biocompatible, self-healing, highly stretchable polyacrylic acid/reduced graphene oxide nanocomposite hydrogel sensors via mussel-inspired chemistry. Carbon 2018, 136, 63–72. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, H.; Zhao, Y.; Huyan, C.; Wang, Z.; Torun, H.; Guo, Z.; Dai, S.; Xu, B.B.; Chen, F. A strand entangled supramolecular PANI/PAA hydrogel enabled ultra-stretchable strain sensor. Small 2022, 18, 2203258. [Google Scholar] [CrossRef]
- Gao, D.; Lv, J.; Lee, P.S. Natural polymer in soft electronics: Opportunities, challenges, and future prospects. Adv. Mater. 2022, 34, 2105020. [Google Scholar] [CrossRef]
- Fan, L.H.; Pan, X.R.; Zhou, Y.; Chen, L.Y.; Xie, W.G.; Long, Z.H.; Zheng, H. Preparation and characterization of crosslinked carboxymethyl chitosan–oxidized sodium alginate hydrogels. J. Appl. Polym. Sci. 2011, 122, 2331–2337. [Google Scholar] [CrossRef]
- Zhuo, H.; Dong, X.; Liu, Q.; Hong, L.; Zhang, Z.; Long, S.; Zhai, W. Bamboo-inspired ultra-strong nanofiber-reinforced composite hydrogels. Nat. Commun. 2025, 16, 980. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, Z.; Chen, S.; Shen, Y.; Yin, J.; Wang, R. Phytic Acid-Induced Gradient Hydrogels for Highly Sensitive and Broad Range Pressure Sensing. Adv. Mater. 2025, 37, 2417978. [Google Scholar] [CrossRef]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Camci-Unal, G.; Cuttica, D.; Annabi, N.; Demarchi, D.; Khademhosseini, A. Synthesis and characterization of hybrid hyaluronic acid-gelatin hydrogels. Biomacromolecules 2013, 14, 1085–1092. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Ghilan, A.; Rusu, A.G.; Tudorachi, N.; Timpu, D. Alginate enriched with phytic acid for hydrogels preparation. Int. J. Biol. Macromol. 2021, 181, 561–571. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Zhang, J.; Duan, L.; Gao, G. A highly conductive hydrogel driven by phytic acid towards a wearable sensor with freezing and dehydration resistance. J. Mater. Chem. A 2021, 9, 22615–22625. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Bao, R.; Wang, C.; Dong, L.; Shen, C.; Zhao, K.; Pan, C. CdS nanorods/organic hybrid LED array and the piezo-phototronic effect of the device for pressure mapping. Nanoscale 2016, 8, 8078–8082. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chee, P.L.; Sugiarto, S.; Yu, Y.; Shi, C.; Yan, R.; Yao, Z.; Shi, X.; Zhi, J.; Kai, D. Hydrogel-based flexible electronics. Adv. Mater. 2023, 35, 2205326. [Google Scholar] [CrossRef]
- Peng, Q.; Chen, J.; Wang, T.; Peng, X.; Liu, J.; Wang, X.; Wang, J.; Zeng, H. Recent advances in designing conductive hydrogels for flexible electronics. InfoMat 2020, 2, 843–865. [Google Scholar] [CrossRef]
- Ding, X.; Yu, Y.; Li, W.; Bian, F.; Gu, H.; Zhao, Y. Multifunctional carbon nanotube hydrogels with on-demand removability for wearable electronics. Nano Today 2024, 54, 102124. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhang, Y.; You, L.; Song, Y.; Li, T.; Fang, Z.; Gui, A.; Li, Y.; Liao, L.; et al. Graphene-Doped Hydrogels with Enhanced Conductivity and Stretchability for All-Weather Wearable Devices. Adv. Funct. Mater. 2025, 35, 2425014. [Google Scholar] [CrossRef]
- Li, S.; Yang, H.; Zhu, N.; Chen, G.; Miao, Y.; Zheng, J.; Cong, Y.; Chen, Y.; Gao, J.; Jian, X.; et al. Biotissue-inspired anisotropic carbon fiber composite hydrogels for logic gates, integrated soft actuators, and sensors with ultra-high sensitivity. Adv. Funct. Mater. 2023, 33, 2211189. [Google Scholar] [CrossRef]
- Ma, F.; Wu, Y.; Dai, S.; Lin, P.; Sun, J.; Dong, L. A soft-contact hybrid electromagnetic–triboelectric nanogenerator for self-powered water splitting towards hydrogen production. Nano Res. 2024, 17, 6567–6574. [Google Scholar] [CrossRef]
- Wei, H.; Kong, D.; Li, T.; Xue, Q.; Wang, S.; Cui, D.; Huang, Y.; Wang, L.; Hu, S.; Wan, T.; et al. Solution-processable conductive composite hydrogels with multiple synergetic networks toward wearable pressure/strain sensors. ACS Sens. 2021, 6, 2938–2951. [Google Scholar] [CrossRef] [PubMed]
- Kougkolos, G.; Golzio, M.; Laudebat, L.; Valdez-Nava, Z.; Flahaut, E. Hydrogels with electrically conductive nanomaterials for biomedical applications. J. Mater. Chem. B 2023, 11, 2036–2062. [Google Scholar] [CrossRef]
- Lin, M.; Zheng, Z.; Yang, L.; Luo, M.; Fu, L.; Lin, B.; Xu, C. A High-Performance, Sensitive, Wearable Multifunctional Sensor Based on Rubber/CNT for Human Motion and Skin Temperature Detection. Adv. Mater. 2022, 34, 2107309. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, H.; Pan, F.; Zhang, H.; Yang, K.; Yuan, T.; Fang, Y.; Ping, H.; Wang, Q.; Fu, Z. Reusable Liquid Metal-Based Hierarchical Hydrogels with Multifunctional Sensing Capability for Electrophysiology Electrode Substitution. ACS Nano 2025, 19, 15554–15564. [Google Scholar] [CrossRef]
- Rahmani, P.; Shojaei, A.; Sakorikar, T.; Wang, M.; Mendoza-Apodaca, Y.; Dickey, M.D. Liquid metal nanoparticles physically hybridized with cellulose nanocrystals initiate and toughen hydrogels with piezoionic properties. ACS Nano 2024, 18, 8038–8050. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Xue, P.; Valenzuela, C.; Zhang, X.; Chen, Y.; Liu, Y.; Yang, L.; Xu, X.; Wang, L. Highly stretchable and conductive MXene-encapsulated liquid metal hydrogels for bioinspired self-sensing soft actuators. Adv. Funct. Mater. 2024, 34, 2309899. [Google Scholar] [CrossRef]
- Zhou, T.; Yuk, H.; Hu, F.; Wu, J.; Tian, F.; Roh, H.; Shen, Z.; Gu, G.; Xu, J.; Lu, B.; et al. 3D printable high-performance conducting polymer hydrogel for all-hydrogel bioelectronic interfaces. Nat. Mater. 2023, 22, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, H.; Wang, Y.; Fan, X.; Li, Z.; Zhang, X.; Liu, T. Highly Stretchable, Ultra-Soft, and Fast Self-Healable Conductive Hydrogels Based on Polyaniline Nanoparticles for Sensitive Flexible Sensors. Adv. Funct. Mater. 2022, 32, 2204366. [Google Scholar] [CrossRef]
- He, H.; Li, H.; Pu, A.; Li, W.; Ban, K.; Xu, L. Hybrid assembly of polymeric nanofiber network for robust and electronically conductive hydrogels. Nat. Commun. 2023, 14, 759. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced carbon for flexible and wearable electronics. Adv. Mater. 2019, 31, 1801072. [Google Scholar] [CrossRef]
- Zhang, W.; Lou, Q.; Sun, J.; Liao, J.; Zheng, G.; Jiao, F.; Chen, W.; Li, X.; Meng, J.; Shan, C.-X.; et al. Carbon nanodot-based flexible and self-powered white displays. Nano Res. 2025, 18, 94907117. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, B.; Zhang, D.; Yang, M.; Huang, X.; Han, L.; Chen, K.; Li, X.; Pang, R.; Shang, Y.; et al. Conductive hydrogels incorporating carbon nanoparticles: A review of synthesis, performance and applications. Particuology 2023, 83, 212–231. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, T.; Zhu, Z.; Yuan, H.; Tan, L.; Yao, P.; Qiao, Y.; Zhu, C.; Xu, J. Mussel-inspired adhesive and carbon fiber conductive hydrogel for flexible sensors. ACS Appl. Polym. Mater. 2023, 5, 5707–5715. [Google Scholar] [CrossRef]
- Lu, W.; Zu, M.; Byun, J.H.; Kim, B.S.; Chou, T.W. State of the art of carbon nanotube fibers: Opportunities and challenges. Adv. Mater. 2012, 24, 1805–1833. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Zhao, F.; Ping, J.; Ying, Y. Recent advances in nanomaterial-enabled wearable sensors: Material synthesis, sensor design, and personal health monitoring. Small 2020, 16, 2002681. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, X.; Lou, Q.; Zhang, Y.; Chen, Y.; Lu, Y.; Dong, L.; Shan, C.-X. Fabry-Perot interference and piezo-phototronic effect enhanced flexible MoS2 photodetector. Nano Res. 2022, 15, 4395–4402. [Google Scholar] [CrossRef]
- Liao, G.; Hu, J.; Chen, Z.; Zhang, R.; Wang, G.; Kuang, T. Preparation, properties, and applications of graphene-based hydrogels. Front. Chem. 2018, 6, 450. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, B.; Jiang, Q.; Li, Y.; Feng, Y.; Zhang, L.; Zhao, Y.; Xiong, X. Flexible and wearable sensor based on graphene nanocomposite hydrogels. Smart Mater. Struct. 2020, 29, 075027. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, M.; Huang, L.; Li, Y.; Chen, J.; Li, C.; Shi, G. High-quality graphene ribbons prepared from graphene oxide hydrogels and their application for strain sensors. ACS Nano 2015, 9, 12320–12326. [Google Scholar] [CrossRef]
- Wu, L.; Fan, M.; Qu, M.; Yang, S.; Nie, J.; Tang, P.; Pan, L.; Wang, H.; Bin, Y. Self-healing and anti-freezing graphene–hydrogel–graphene sandwich strain sensor with ultrahigh sensitivity. J. Mater. Chem. B 2021, 9, 3088–3096. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, K.; Qian, R.; Yu, Z.; Ye, C. Interfacial engineering of liquid metal nanoparticles for the fabrication of conductive hydrogels: A review. Chem. Eng. J. 2024, 486, 150197. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, H.; Yun, G.; Gong, L.; Chen, Z.; Jin, S.; Du, H.; Jiang, Z.; Li, W. A laminated gravity-driven liquid metal-doped hydrogel of unparalleled toughness and conductivity. Adv. Funct. Mater. 2024, 34, 2308113. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, S.; Xiong, J.; Liu, Z.; Li, Q.; Li, W.; Yan, F. Stretch-induced conductivity enhancement in highly conductive and tough hydrogels. Adv. Mater. 2024, 36, 2313845. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, Y.; Xiao, J.; Chen, S.-W.; Tu, Q.; Yuan, M.-S.; Wang, J. Liquid metal-doped conductive hydrogel for construction of multifunctional sensors. Anal. Chem. 2023, 95, 3811–3820. [Google Scholar] [CrossRef]
- Park, J.-E.; Kang, H.S.; Baek, J.; Park, T.H.; Oh, S.; Lee, H.; Koo, M.; Park, C. Rewritable, printable conducting liquid metal hydrogel. ACS Nano 2019, 13, 9122–9130. [Google Scholar] [CrossRef]
- Xu, Q.; Chu, N.; Wang, Y.; Wang, H.; Xu, T.; Li, X.; Huang, S.; Li, X.; Luo, Y.; Yang, H.Y. 3D Printed Low-Tortuosity and Ultra-Thick Hierarchical Porous Electrodes for High-Performance Wearable Quasi-Solid-State Zn-VOH Batteries. Adv. Sci. 2025, 12, 2401660. [Google Scholar] [CrossRef]
- Xu, H.; Lu, J.; Xi, Y.; Wang, X.; Liu, J. Liquid metal biomaterials: Translational medicines, challenges and perspectives. Natl. Sci. Rev. 2024, 11, nwad302. [Google Scholar] [CrossRef]
- Zhou, Z.; Qian, C.; Yuan, W. Self-healing, anti-freezing, adhesive and remoldable hydrogel sensor with ion-liquid metal dual conductivity for biomimetic skin. Compos. Sci. Technol. 2021, 203, 108608. [Google Scholar] [CrossRef]
- Wang, J.; Huo, X.; Huang, W.; Xu, J.; Yu, P.; Zhang, X.; Cong, Z.; Niu, J. Skin-inspired laminated liquid metal doped hydrogel with mechanical toughness and high electrical conductivity. J. Mater. Chem. C 2024, 12, 19412–19423. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Li, H.; Huo, P.; Teng, P.; Ding, H.; Shen, X. Recent progress in fabrications, properties and applications of multifunctional conductive hydrogels. Eur. Polym. J. 2024, 208, 112895. [Google Scholar] [CrossRef]
- Hou, Y.; Li, Z.; Wang, Z.; Zhang, X.; Li, Y.; Li, C.; Guo, H.; Yu, H. Programmable and Surface-Conformable Origami Design for Thermoelectric Devices. Adv. Sci. 2024, 11, 2309052. [Google Scholar] [CrossRef]
- Xu, Y.; Rothe, R.; Voigt, D.; Hauser, S.; Cui, M.; Miyagawa, T.; Patino Gaillez, M.; Kurth, T.; Bornhäuser, M.; Pietzsch, J.; et al. Convergent synthesis of diversified reversible network leads to liquid metal-containing conductive hydrogel adhesives. Nat. Commun. 2021, 12, 2407. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Juxiang, Y.; Ruifeng, S.; Zhen, L. Research Progress on Preparation and Application of Conductive Polymer Materials. Eng. Plast. Appl. 2021, 49, 167–171. [Google Scholar]
- Tingzhou, N.; Jingzhi, Z.; Ling, F. Research Progress of Conductive Polymer Materials in Electronic Devices. Eng. Plast. Appl. 2019, 47, 162–167. [Google Scholar]
- Ocheje, M.U.; Charron, B.P.; Nyayachavadi, A.; Rondeau-Gagne, S. Stretchable electronics: Recent progress in the preparation of stretchable and self-healing semiconducting conjugated polymers. Flex. Print. Electron. 2017, 2, 043002. [Google Scholar] [CrossRef]
- Smith, Z.C.; Wright, Z.M.; Arnold, A.M.; Sauve, G.; McCullough, R.D.; Sydlik, S.A. Increased Toughness and Excellent Electronic Properties in Regioregular Random Copolymers of 3-Alkylthiophenes and Thiophene. Adv. Electron. Mater. 2017, 3, 1600316. [Google Scholar] [CrossRef]
- Guo, B.; Ma, Z.; Pan, L.; Shi, Y. Properties of conductive polymer hydrogels and their application in sensors. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 1606–1621. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, M.; Yu, A.; Liu, S.; Zhao, Q. Annelid Skin Inspired Microstructure Strain Sensor Based on Conductive Polymer Hydrogel for Human Motion Monitoring and Gesture Recognition. ACS Appl. Electron. Mater. 2025, 7, 6158–6165. [Google Scholar] [CrossRef]
- Wenjing, J.; Jingwen, L.; Xuehui, Z.; Yanqin, W. Classification of conductive composite hydrogels and their application in flexible wearable devices. Acta Mater. Compos. Sin. 2023, 40, 1879–1895. [Google Scholar]
- Jiao, F.; Lin, C.; Dong, L.; Mao, X.; Wu, Y.; Dong, F.; Zhang, Z.; Sun, J.; Li, S.; Yang, X.; et al. Silicon Vacancies Diamond/Silk/PVA Hierarchical Physical Unclonable Functions for Multi-Level Encryption. Adv. Sci. 2024, 11, 2308337. [Google Scholar] [CrossRef]
- Tang, J.; Shu, H.; Lu, J.; Zhang, T.; Huang, S.; Zhang, L. Highly stretchable and conductive liquid metal-reinforced interpenetrating polymer network hydrogel for wearable devices. Polymer 2025, 336, 158243. [Google Scholar] [CrossRef]
- Zhao, B.; Bai, Z.; Lv, H.; Yan, Z.; Du, Y.; Guo, X.; Zhang, J.; Wu, L.; Deng, J.; Zhang, D.W.; et al. Self-Healing Liquid Metal Magnetic Hydrogels for Smart Feedback Sensors and High-Performance Electromagnetic Shielding. Nano-Micro Lett. 2023, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhao, Y.; Xu, J.; Qian, R.; Yu, Z.; Ye, C. Stretchable, adhesive and self-healing conductive hydrogels based on PEDOT:PSS-stabilized liquid metals for human motion detection. Chem. Eng. J. 2024, 494, 152971. [Google Scholar] [CrossRef]
- Gao, M.; Liu, W.; Chen, K.; Sun, H.; Liu, X.; Xing, H.; Wang, H.; Zhu, B.; Guo, H. Piezoresistive Effect: A New Concept for Hearing Aids. Adv. Sci. 2025, 12, 2501227. [Google Scholar] [CrossRef]
- Hua, C.; Gao, J.; Liu, J. Room temperature self-healing liquid metals: Capabilities, applications and challenges. Int. J. Smart Nano Mater. 2024, 15, 469–501. [Google Scholar] [CrossRef]
- Onoda, M.; Abe, Y.; Tada, K.; Kawakita, Y.; Fujisato, T.; Uto, S. Biocompatibility of Conductive Polymers Based on Experimental Study of Cultures for Mouse Fibroblast. Kobunshi Ronbunshu 2010, 67, 590–595. [Google Scholar] [CrossRef][Green Version]
- Heo, J.S.; Hossain, M.F.; Kim, I. Challenges in Design and Fabrication of Flexible/Stretchable Carbon- and Textile-Based Wearable Sensors for Health Monitoring: A Critical Review. Sensors 2020, 20, 3927. [Google Scholar] [CrossRef]
- Qing, Z.; Shuo, L.; Guimin, L.; Yong, Z.; Yingying, Z. Graphene-Based Flexible and Wearable Sensors: Fabrication, Application and Perspective. J. Chin. Ceram. Soc. 2022, 50, 1800–1809. [Google Scholar]
- Cheng, T.; Liu, Z.-T.; Qu, J.; Meng, C.-F.; He, L.-J.; Li, L.; Yang, X.-L.; Cao, Y.-J.; Han, K.; Zhang, Y.-Z.; et al. High-Performance Organic-Inorganic Hybrid Conductive Hydrogels for Stretchable Elastic All-Hydrogel Supercapacitors and Flexible Self-Powered Integrated Systems. Adv. Sci. 2024, 11, 2403358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-F.; Guo, M.-M.; Zhang, Y.; Tang, C.Y.; Jiang, C.; Dong, Y.; Law, W.-C.; Du, F.-P. Flexible, stretchable and conductive PVA/PEDOT:PSS composite hydrogels prepared by SIPN strategy. Polym. Test. 2020, 81, 106213. [Google Scholar] [CrossRef]
- Zheng, C.; Lu, K.; Lu, Y.; Zhu, S.; Yue, Y.; Xu, X.; Mei, C.; Xiao, H.; Wu, Q.; Han, J. A stretchable, self-healing conductive hydrogels based on nanocellulose supported graphene towards wearable monitoring of human motion. Carbohydr. Polym. 2020, 250, 116905. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, H.; Sun, C.; Jing, Y.; Li, K.; Li, Y.; Zhang, Q.; Wang, H.; Luo, Y.; Hou, C. Topological MXene Network Enabled Mixed Ion-Electron Conductive Hydrogel Bioelectronics. ACS Nano 2024, 18, 4008–4018. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Jeong, C.; Ok, J.; Kim, T.-I. Materials and Structural Designs toward Motion Artifact-Free Bioelectronics. Chem. Rev. 2024, 124, 6148–6197. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Zhou, J.; Wei, C.; Ponnuchamy, A.; Bappy, M.O.; Liao, Y.; Jiang, Q.; Du, Y.; Evans, C.J.; Wyatt, B.C.; et al. A Printed Microscopic Universal Gradient Interface for Super Stretchable Strain-Insensitive Bioelectronics. Adv. Mater. 2025, 37, 2414203. [Google Scholar] [CrossRef]
- Li, W.; Li, S.-M.; Kang, M.-C.; Xiong, X.; Wang, P.; Tao, L.-Q. Multi-characteristic tannic acid-reinforced polyacrylamide/sodium carboxymethyl cellulose ionic hydrogel strain sensor for human-machine interaction. Int. J. Biol. Macromol. 2024, 254, 127434. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, A.; Zhang, Y.; Duan, H.; Zhu, P.; Mao, Y. Recent progress of nanomaterials-based composite hydrogel sensors for human-machine interactions. Discov. Nano 2025, 20, 60. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, J.; Zhang, R.; Duan, G.; Li, Y.; Li, Z. Natural polyphenolic nanodot-knotted conductive hydrogels for flexible wearable sensors. Green Chem. 2024, 26, 3329–3337. [Google Scholar] [CrossRef]
- Jiao, X.; Song, D.; Ding, J.; Li, J.; Ding, K.; Meng, F.; Zheng, H.; Xu, W. Surfactant-Enhanced Anti-Swelling Hydrogel Flexible Sensor for Machine Learning-Assisted Underwater Gesture Recognition. Small 2025, 21, 2412346. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, D.; Tao, X.; Wu, F.; You, G.; Wang, B.; Sun, J.; Shi, S. 3D Printed Sensors for Wearable Electronics and Smart Gesture Recognition. Adv. Mater. Technol. 2024, 9, 2302048. [Google Scholar] [CrossRef]
- Fu, F.; Wang, J.; Zeng, H.; Yu, J. Functional Conductive Hydrogels for Bioelectronics. ACS Mater. Lett. 2020, 2, 1287–1301. [Google Scholar] [CrossRef]
- Liao, H.; Guo, X.; Wan, P.; Yu, G. Conductive MXene Nanocomposite Organohydrogel for Flexible, Healable, Low-Temperature Tolerant Strain Sensors. Adv. Funct. Mater. 2019, 29, 1904507. [Google Scholar] [CrossRef]
- Zhu, P.; Niu, M.; Liang, S.; Yang, W.; Zhang, Y.; Chen, K.; Pan, Z.; Mao, Y. Non-hand-worn, load-free VR hand rehabilitation system assisted by deep learning based on ionic hydrogel. Nano Res. 2025, 18, 94907301. [Google Scholar] [CrossRef]
- Lu, L.; Hu, G.; Liu, J.; Yang, B. 5G nb-IoT system integrated with high-performance fiber sensor inspired by cirrus and spider structures. Adv. Sci. 2024, 11, 2309894. [Google Scholar] [CrossRef] [PubMed]
- Das, P.S.; Kim, J.W.; Park, J.Y. Fashionable wrist band using highly conductive fabric for electrocardiogram signal monitoring. J. Ind. Text. 2019, 49, 243–261. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, Y. A silver/silver chloride woven electrode with convex based on electrical impedance tomography. J. Text. Inst. 2021, 112, 1067–1079. [Google Scholar] [CrossRef]
- Gao, X.; Lin, J.; Yu, C.; Tang, C.; Huang, Y. Constructing multifunctional polyvinyl alcohol composite hydrogels with human-like skin properties using polyaniline-coated boron nitride nanofibers. Eur. Polym. J. 2024, 215, 113238. [Google Scholar] [CrossRef]
- Li, Q.; Tian, B.; Liang, J.; Wu, W. Functional conductive hydrogels: From performance to flexible sensor applications. Mater. Chem. Front. 2023, 7, 2925–2957. [Google Scholar] [CrossRef]
- Zhao, L.; Luo, B.; Gao, S.; Liu, Y.; Lai, C.; Zhang, D.; Guan, W.; Wang, C.; Chu, F. Stretchable, self-adhesive, and conductive hemicellulose-based hydrogels as wearable strain sensors. Int. J. Biol. Macromol. 2024, 282, 137313. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Sun, C.; Chang, B.; Jing, Y.; Li, K.; Li, Y.; Zhang, Q.; Wang, H.; Hou, C. MXene-Enabled Self-Adaptive Hydrogel Interface for Active Electroencephalogram Interactions. ACS Nano 2022, 16, 19373–19384. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Lou, Q.; Shen, C.; Zheng, G.; Song, R.; Hao, J.; Liu, J.; Zhu, J.; Zang, J.; Dong, L.; et al. Sensitive humidity sensor based on moisture-driven energy generation. Nano Res. 2024, 17, 5578–5586. [Google Scholar] [CrossRef]
- Dang, X.; Fu, Y.; Wang, X. Versatile Biomass-Based Injectable Photothermal Hydrogel for Integrated Regenerative Wound Healing and Skin Bioelectronics. Adv. Funct. Mater. 2024, 34, 2405745. [Google Scholar] [CrossRef]
- Li, Y.; Tan, S.; Zhang, X.; Li, Z.; Cai, J.; Liu, Y. Design Strategies and Emerging Applications of Conductive Hydrogels in Wearable Sensing. Gels 2025, 11, 1904507. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Shi, S.; Zheng, Y.; Ye, Z.; Liao, J.; Sun, Q.; Dang, B.; Shen, X. Myelin Sheath-Inspired Hydrogel Electrode for Artificial Skin and Physiological Monitoring. ACS Nano 2024, 18, 27420–27432. [Google Scholar] [CrossRef]
- Xu, J.; Pan, J.; Cui, T.; Zhang, S.; Yang, Y.; Ren, T.-L. Recent Progress of Tactile and Force Sensors for Human-Machine Interaction. Sensors 2023, 23, 1868. [Google Scholar] [CrossRef]
- Yin, R.; Wang, D.; Zhao, S.; Lou, Z.; Shen, G. Wearable Sensors-Enabled Human-Machine Interaction Systems: From Design to Application. Adv. Funct. Mater. 2021, 31, 2008936. [Google Scholar] [CrossRef]
- Zhu, P.; Mu, S.; Huang, W.; Sun, Z.; Lin, Y.; Chen, K.; Pan, Z.; Haghighi, M.G.; Sedghi, R.; Wang, J.; et al. Soft multifunctional neurological electronic skin through intrinsically stretchable synaptic transistor. Nano Res. 2024, 17, 6550–6559. [Google Scholar] [CrossRef]
- Feng, T.; Ling, D.; Li, C.; Zheng, W.; Zhang, S.; Li, C.; Emel’yanov, A.; Pozdnyakov, A.S.; Lu, L.; Mao, Y. Stretchable on-skin touchless screen sensor enabled by ionic hydrogel. Nano Res. 2024, 17, 4462–4470. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, H.; Lyu, Q.; Dai, J.; Ji, C.; Tian, Y. Anti-freeze hydrogel-based sensors for intelligent wearable human-machine interaction. Chem. Eng. J. 2024, 481, 148526. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Z.; Zhou, Y.; Li, Z.; Zhao, D.; Guan, X.; Lan, T.; Gong, Y.; Zhou, B.; Zhong, J. Thin and Flexible Breeze-Sense Generators for Non-Contact Haptic Feedback in Virtual Reality. Nano-Micro Lett. 2025, 17, 144. [Google Scholar] [CrossRef]
- Oh, J.; Kim, S.; Lee, S.; Jeong, S.; Ko, S.H.; Bae, J. A Liquid Metal Based Multimodal Sensor and Haptic Feedback Device for Thermal and Tactile Sensation Generation in Virtual Reality. Adv. Funct. Mater. 2021, 31, 2007772. [Google Scholar] [CrossRef]
- Zhu, J.; Ji, S.; Yu, J.; Shao, H.; Wen, H.; Zhang, H.; Xia, Z.; Zhang, Z.; Lee, C. Machine learning-augmented wearable triboelectric human-machine interface in motion identification and virtual reality. Nano Energy 2022, 103, 107766. [Google Scholar] [CrossRef]
- Liu, H.; Qin, J.; Yang, X.; Lv, C.; Huang, W.; Li, F.; Zhang, C.; Wu, Y.; Dong, L.; Shan, C. Highly sensitive humidity sensors based on hexagonal boron nitride nanosheets for contactless sensing. Nano Res. 2023, 16, 10279–10286. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, B.; Wang, H.; Wu, Y.; Cao, H.; He, L.; Li, C.; Luo, X.; Li, X.; Mao, Y. 3D printed triboelectric nanogenerator as self-powered human-machine interactive sensor for breathing-based language expression. Nano Res. 2022, 15, 7460–7467. [Google Scholar] [CrossRef]
- Zhang, N.; Qin, C.; Feng, T.; Li, J.; Yang, Z.; Sun, X.; Liang, E.; Mao, Y.; Wang, X. Non-contact cylindrical rotating triboelectric nanogenerator for harvesting kinetic energy from hydraulics. Nano Res. 2020, 13, 1903–1907. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, N.; Tang, Y.; Wang, M.; Chao, M.; Liang, E. A paper triboelectric nanogenerator for self-powered electronic systems. Nanoscale 2017, 9, 14499–14505. [Google Scholar] [CrossRef]
- Li, W.; Wu, S.; Kang, M.; Zhang, X.; Zhong, X.; Qiao, H.; Chen, J.; Wang, P.; Tao, L. Wearable one-handed keyboard using hydrogel-based mechanical sensors for human-machine interaction. J. Mater. Sci. Technol. 2024, 201, 130–138. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, P.; Zhang, F.; Li, P.; Huang, W.; Li, C.; Han, N.; Mu, S.; Zhou, H.; Mao, Y. Intrinsically stretchable polymer semiconductor based electronic skin for multiple perceptions of force, temperature, and visible light. Nano Res. 2023, 16, 1196–1204. [Google Scholar] [CrossRef]
- Ullah, A.; Kim, D.Y.; Lim, S.I.; Lim, H.-R. Hydrogel-Based Biointerfaces: Recent Advances, Challenges, and Future Directions in Human-Machine Integration. Gels 2025, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wu, J.; Zhang, Y.; Liu, C.; Hu, Y.; Chen, B.; Zhu, Y.; Mao, Y. Noncontact 3D Gesture Recognition Enabled VR Human-Machine Interface via Electret-Nanofiber-Based Triboelectric Sensor. Nano Res. 2025. [Google Scholar] [CrossRef]
- Smith, J.; Parikh, D.; Tate, V.; Siddicky, S.F.; Hsiao, H.-Y. Validity of Valor Inertial Measurement Unit for Upper and Lower Extremity Joint Angles. Sensors 2024, 24, 5833. [Google Scholar] [CrossRef]
- Wu, S.; Xu, C.; Zhao, Y.; Shi, W.; Li, H.; Cai, J.; Ding, F.; Qu, P. Recent Advances in Chitosan-Based Hydrogels for Flexible Wearable Sensors. Chemosensors 2023, 11, 39. [Google Scholar] [CrossRef]
- Liu, D.; Ji, S.; Zheng, L.; Shi, L.; Chen, H.; Yan, X.; Su, L.; Zhu, Z.; Yin, X. Mussel-inspired breathable and antibacterial strain sensors based on polyurethane fibrous membrane for human motion monitoring, human-machine interaction, and acupoint photothermal therapy. Chem. Eng. J. 2025, 507, 160397. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Bo, L.; Qi, J.; Zhu, Y. Research Progress on Applying Intelligent Sensors in Sports Science. Sensors 2024, 24, 7338. [Google Scholar] [CrossRef]
- Chang, S.; Deng, Y.; Li, N.; Wang, L.; Shan, C.-X.; Dong, L. Continuous synthesis of ultra-fine fiber for wearable mechanoluminescent textile. Nano Res. 2023, 16, 9379–9386. [Google Scholar] [CrossRef]
- Huang, F.; Wei, W.; Fan, Q.; Li, L.; Zhao, M.; Zhou, Z. Super-stretchable and adhesive cellulose Nanofiber-reinforced conductive nanocomposite hydrogel for wearable Motion-monitoring sensor. J. Colloid Interface Sci. 2022, 615, 215–226. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, Y.; Wang, X.; Ran, R. Melanin-Inspired Conductive Hydrogel Sensors with Ultrahigh Stretchable, Self-Healing, and Photothermal Capacities. ACS Appl. Polym. Mater. 2021, 3, 1899–1911. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Z.; Wu, C.; Cong, Y.; Zhang, R.; Fu, J. Highly Sensitive Pressure and Strain Sensors Based on Stretchable and Recoverable Ion-Conductive Physically Cross-Linked Double-Network Hydrogels. ACS Appl. Mater. Interfaces 2020, 12, 51969–51977. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Yang, W.; Lu, B.; Xu, Y.; Chen, J.; Zheng, X.; Liu, S.; Lin, C.; Zeng, H.; Huang, B. Muscle-Inspired Robust Anisotropic Cellulose Conductive Hydrogel for Multidirectional Strain Sensors and Implantable Bioelectronics. Adv. Funct. Mater. 2024, 35, 2416419. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Lian, L.; Liu, K.; Lu, M.; Chen, Y.; Zhang, L.; Zhang, X.; Wan, P. Flexible Accelerated-Wound-Healing Antibacterial MXene-Based Epidermic Sensor for Intelligent Wearable Human-Machine Interaction. Adv. Funct. Mater. 2022, 32, 2208141. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Sufu, A.; Liu, F. Stretchable and self-healing carboxymethyl cellulose/polyacrylic acid conductive hydrogels for monitoring human motions and electrophysiological signals. Int. J. Biol. Macromol. 2025, 293, 138900. [Google Scholar] [CrossRef]
- Niu, L.; Bai, B.; Zhao, X.; Zhang, X.; Su, Z. From nature to nanotechnology: The synergistic integration of biomimetic nanomaterials and conductive hydrogels for next-generation applications. Nanoscale 2025, 17, 14558–14586. [Google Scholar] [CrossRef]
- Wang, Z.; Cong, Y.; Fu, J. Stretchable and tough conductive hydrogels for flexible pressure and strain sensors. J. Mater. Chem. B 2020, 8, 3437–3459. [Google Scholar] [CrossRef]
- Ma, J.; Han, Z.; Li, M.; Liu, Z.; He, W.; Ge, S.S. Conductive hydrogels-based self-sensing soft robot state perception and trajectory tracking. J. Field Robot. 2024, 42, 510–524. [Google Scholar] [CrossRef]

| Polymer Matrix | Conductive Fillers | Tensile Strain % | Breaking Strength | Electrical Conductivity | Young’s Modulus | Gauge Factor (GF) | Adhesion Strength | Toughness | Reference |
|---|---|---|---|---|---|---|---|---|---|
| XSBR/SS | CNTs | 217 | 12.58 MPa | 0.071 S/m | 1.19 MPa | 25.98 | / | 6.23 MJ/m3 | [78] |
| LG/TA | Graphene | 1860 | 200 kPa | 28 S/m | / | 4.61–346 | 51.3 kPa | / | [73] |
| PNIPAM | CF | 1200 | 3.0 ± 0.3 MPa | 670 S/m | 74 ± 7.0 MPa | 6–647 | / | 0.9 MJ/m3 | [74] |
| PAA/TA | EGaIn | 2000 | / | 28.3 mS/m | 6.24 kPa | 1.878 | 0.96 MPa | / | [79] |
| CNCs/PAA | EGaIn | 2000 | 70 kPa | 3.8 S/m | 49–98 kPa | / | / | 1.8 MJ/m3 | [80] |
| MXene/PNIPAM | EGaIn | 610 | 0.05 MPa | / | / | 3.25–8.92 | 1–4 kPa | / | [81] |
| P (PEG-co-AA) | PANI | 580–1030 | 25 kPa | 74.32 mS/cm | 6 kPa | 1.46–2.43 | / | 75–110 kJ/m3 | [82] |
| PU | PEDOT:PSS | 400 | / | 11 S/cm | 1 MPa | / | / | >3300 J/m2 | [83] |
| PVA/ANFs | PPy | 36 | 9.4 MPa | 80 S/cm | 25–35 MPa | 0.2–0.7 | / | 2000–2500 J/m2 | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Zhang, Y.; Duan, H.; Zhang, Y.; Lu, L.; Emel’yanov, A.; Pozdnyakov, A.S.; Zhu, P.; Mao, Y. Recent Advances in Conductive Composite Hydrogels for Electronic Skin Applications. Gels 2025, 11, 822. https://doi.org/10.3390/gels11100822
Yuan Y, Zhang Y, Duan H, Zhang Y, Lu L, Emel’yanov A, Pozdnyakov AS, Zhu P, Mao Y. Recent Advances in Conductive Composite Hydrogels for Electronic Skin Applications. Gels. 2025; 11(10):822. https://doi.org/10.3390/gels11100822
Chicago/Turabian StyleYuan, Yiqing, Yilong Zhang, Haiyang Duan, Yitao Zhang, Lijun Lu, Artem Emel’yanov, Alexander S. Pozdnyakov, Pengcheng Zhu, and Yanchao Mao. 2025. "Recent Advances in Conductive Composite Hydrogels for Electronic Skin Applications" Gels 11, no. 10: 822. https://doi.org/10.3390/gels11100822
APA StyleYuan, Y., Zhang, Y., Duan, H., Zhang, Y., Lu, L., Emel’yanov, A., Pozdnyakov, A. S., Zhu, P., & Mao, Y. (2025). Recent Advances in Conductive Composite Hydrogels for Electronic Skin Applications. Gels, 11(10), 822. https://doi.org/10.3390/gels11100822









