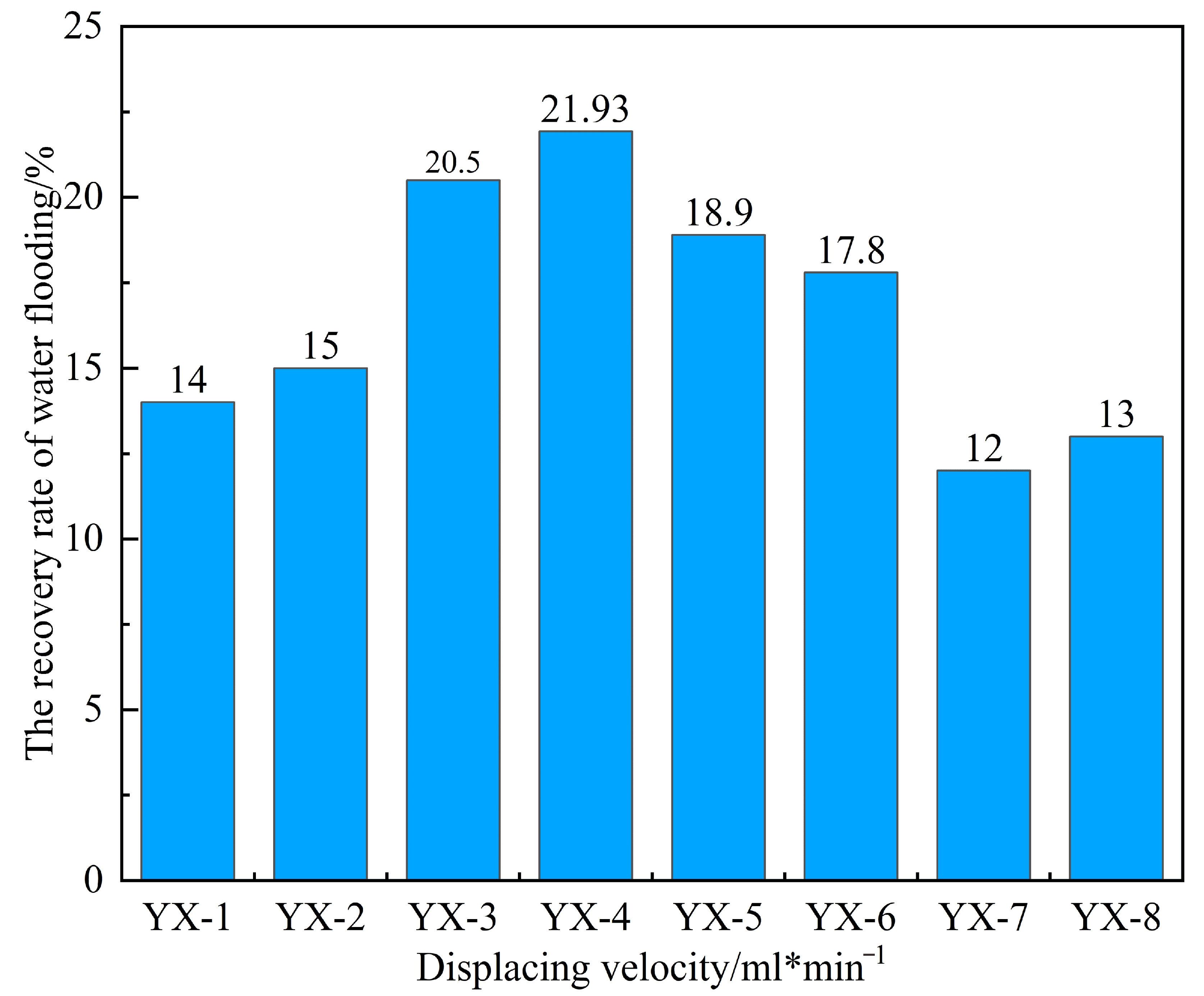
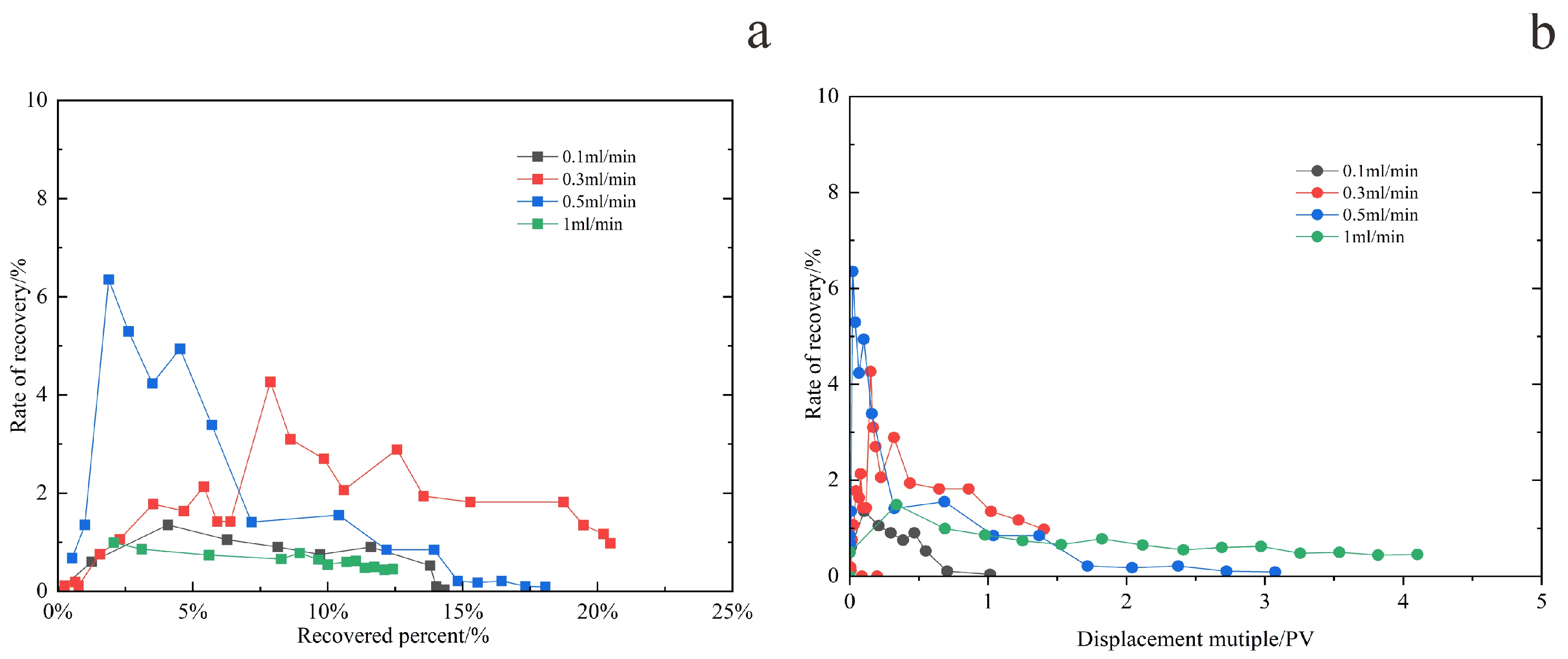
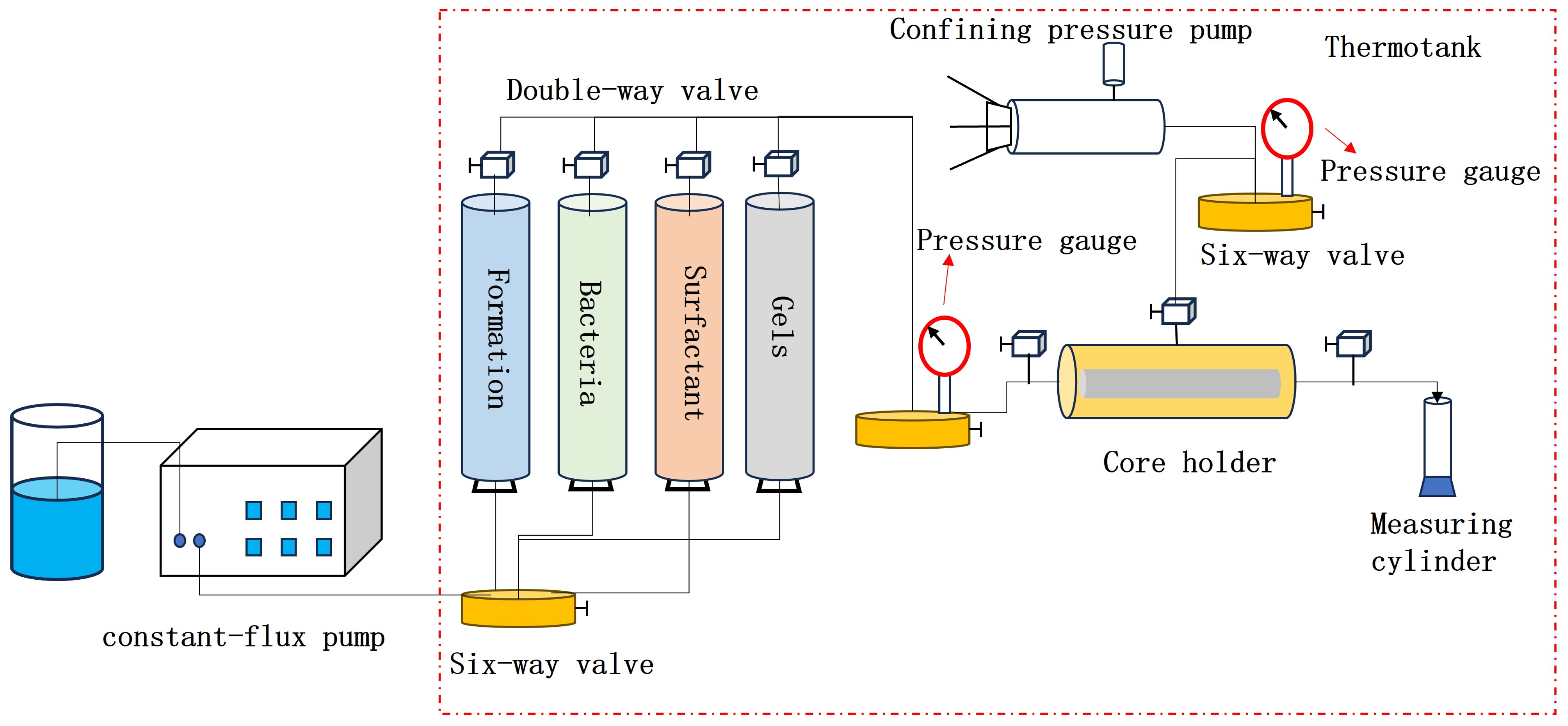
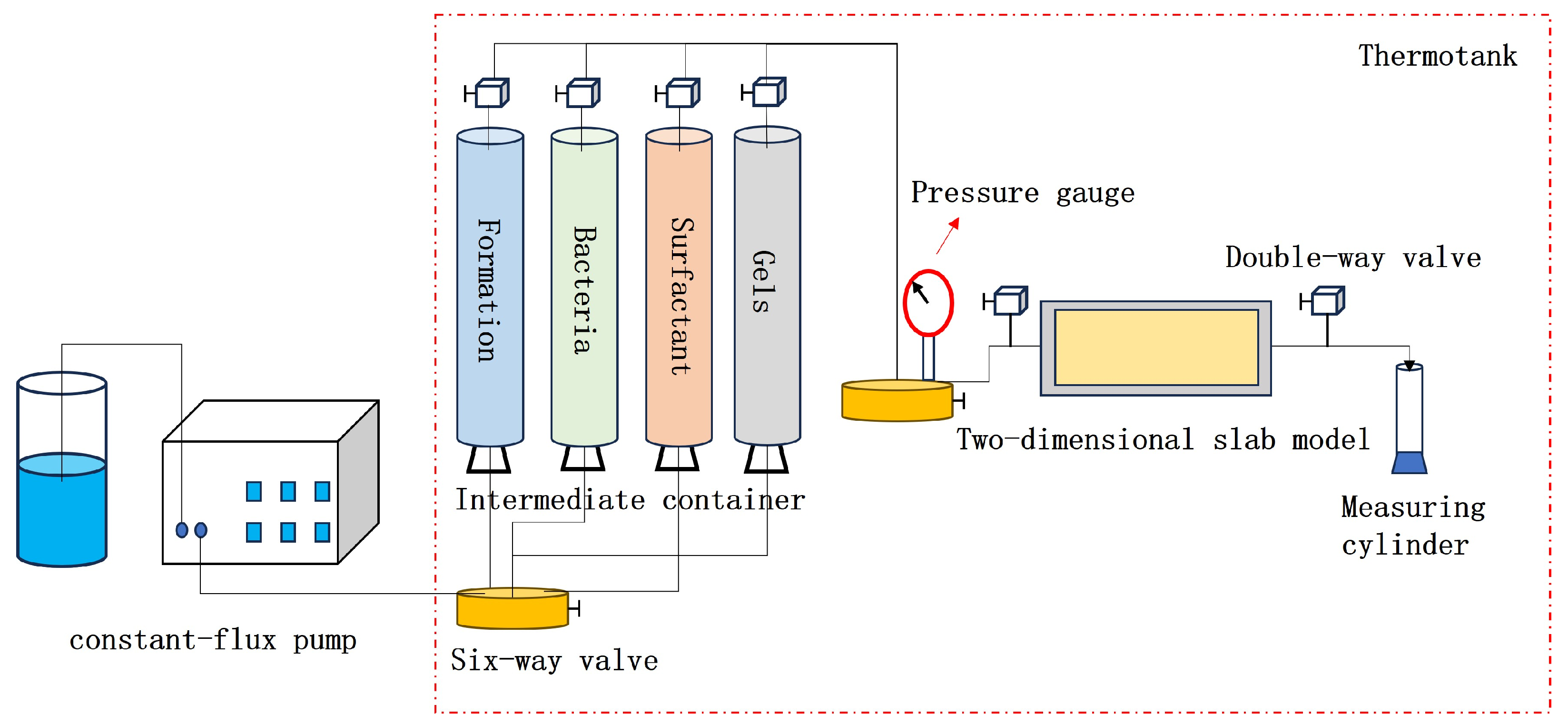
2.4.1. Determining the Optimal Oil Displacement Rate for Microorganisms
By monitoring pressure and flow rate variations during the displacement process under different injection rates, real-time data such as water cut, pressure, and recovery factor were obtained during core flooding, as shown in
Figure 9 and
Figure 10 and
Table 4.
At a low injection rate of 0.1 mL/min (
Figure 10a), the water flooding pressure increased gradually and remained at a relatively low level, not exceeding 1 MPa. The water cut rose slowly, and the recovery factor increased at a progressively slower rate with continued pore volumes injected, ultimately reaching an average value of only 14.5%. This is attributed to the high viscosity of the crude oil combined with the low displacement rate, resulting in insufficient driving force. Under these conditions, the aqueous phase fails to effectively mobilize the oil phase, leading to relatively low oil recovery efficiency.
When the displacement rate was increased to 0.3 mL/min (
Figure 10b), the injection pressure during the early stage of water flooding rose sharply. After reaching its peak, it decreased gradually while maintaining a relatively high level—neither risking formation fracture due to excessive pressure nor exhibiting inadequate drive energy. The water content initially increased in a fluctuating pattern and stabilized within a suitable range during the steady production stage. The recovery factor continued to rise throughout the experiment, reaching an average of 21.2%, which is significantly higher than that of the low-rate group.
When the displacement rate was further increased to 0.5 mL/min, the injection pressure exhibited a pattern characterized by a sharp initial rise, followed by a precipitous decline, and eventual stabilization. Throughout this process, the water cut increased rapidly, reaching the high water-cut stage within a short period. The oil production rate rose quickly during the early stage of water flooding but then dropped sharply. The resulting recovery factor was slightly lower than that achieved at 0.3 mL/min. When the displacement rate was raised to 1 mL/min, as illustrated in
Figure 10d, the excessively high flow rate caused both water cut and injection pressure to increase abruptly during the early displacement stage, indicating significant water channeling. The aqueous phase rapidly established preferential flow pathways, resulting in extensive ineffective water circulation and failure to effectively displace the remaining crude oil. Consequently, the recovery factor at this rate was the lowest among the four displacement rates tested, with an average value of only 12.5%.
As illustrated in
Figure 11, which shows the variation in oil production rate with pore volume (PV) injected and recovery factor at two displacement rates, the system with a rate of 0.3 mL/min maintained a relatively high and stable oil production rate throughout the flooding process [
15], performing more consistently than that at 0.5 mL/min.
In general, the flow rate governs the initial productivity. However, due to the high viscosity of heavy oil, an excessively high displacement rate can lead to a sharp viscosity increase, promoting “water channeling” through high-permeability zones. Conversely, an overly low rate fails to provide sufficient energy to the reservoir, resulting in poor crude oil utilization. Both scenarios ultimately impair production performance. At the displacement rate of 0.3 mL/min, parameters including pressure, flow rate, water cut, oil production rate, and ultimate recovery collectively reached their optimal balance. Therefore, this rate is identified as the optimum water flooding rate under the experimental conditions of this study.
2.4.2. Experimental Results of Reagent Dosage Optimization
This section systematically evaluates the injection performance of gel, surfactant, and composite systems through core flooding experiments, aiming to provide key insights for the design of subsequent microbial enhanced oil recovery strategies. The experimental results are analyzed as follows.
(1) Selection of gel injection volume
As shown in
Figure 12, the effect of gel injection volume (0.05–0.4 PV) on enhanced oil recovery exhibits a trend of initial increase followed by gradual saturation. When the injected volume was increased from 0.05 PV to 0.1 PV, the incremental oil recovery during subsequent water flooding improved markedly from 0.76% to 4.06% accompanied by an average increase of 5.5 MPa in water flooding pressure. This indicates that a 0.1 PV gel volume is sufficient to establish an effective deep-domain blocking system within the core, which successfully diverts subsequently injected water and expands sweep efficiency.
When the injection volume was further increased from 0.15 PV to 0.25 PV, the incremental recovery showed only a slight rise from 4.06% to 4.36%, with a markedly diminished growth rate. Concurrently, the injection pressure increased sharply, approaching the safe pressure limit of the core and raising the risk of damage formation. Therefore, 0.1 PV was identified as the optimal gel injection volume, offering an effective balance between enhanced oil recovery, operational efficiency, and injecting safety.
(2) Surfactant selection
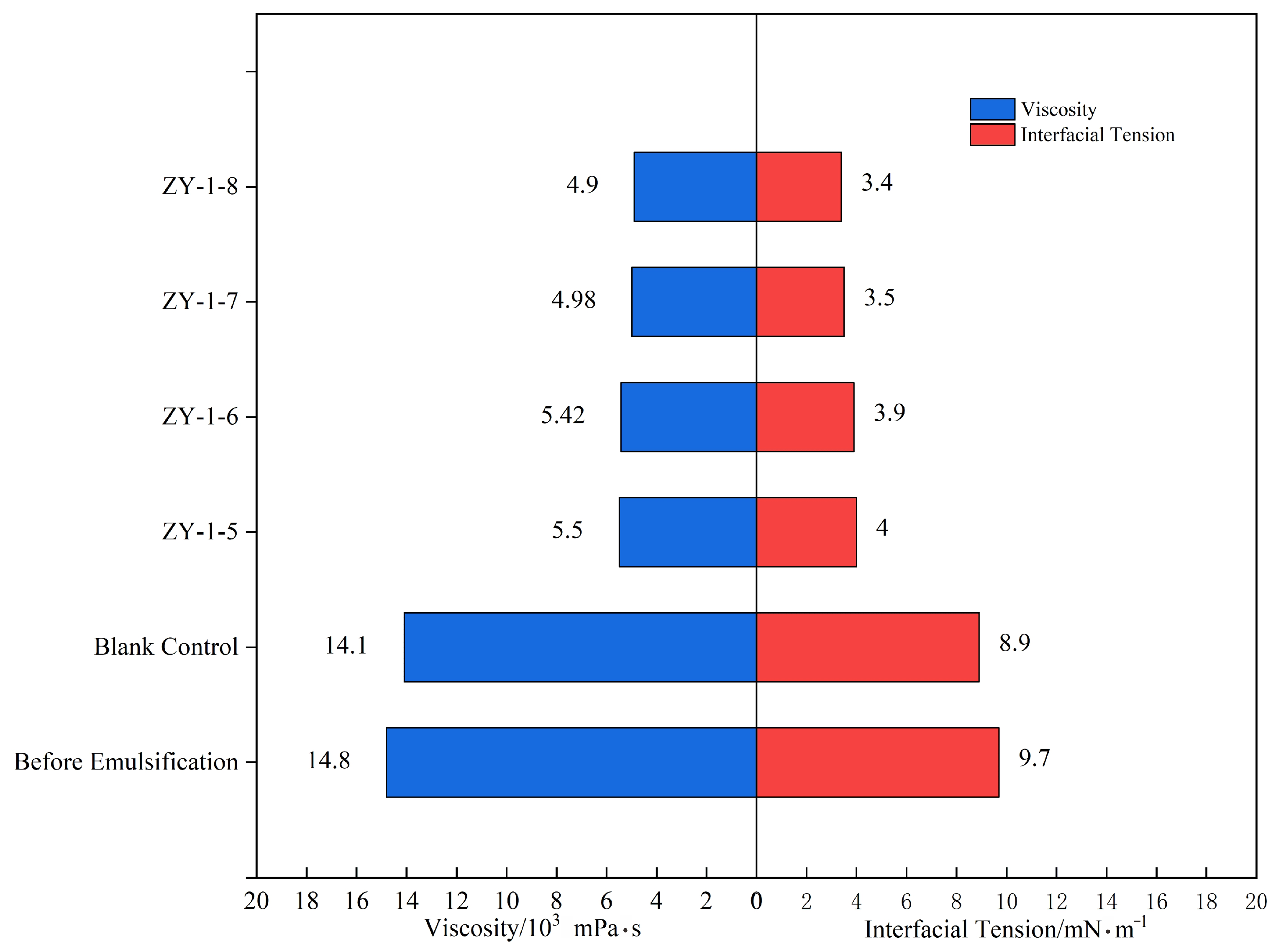
Interfacial tension measurements after 24 h of stabilization (
Figure 13) demonstrated that both surfactants significantly reduced oil–water interfacial tension, with CA601S exhibiting comprehensively superior performance. The interfacial tension of the CA601S-treated system decreased from an initial 10.5 mN/m to 6.8 mN/m, a reduction of approximately 40%, whereas the CA301 system decreased from 9.9 mN/m to 7.2 mN/m, a reduction of about 30%. The enhanced performance of CA601S is attributed to its anionic–nonionic molecular structure, which provides improved salt tolerance and interfacial activity. Based on these results, CA601S was selected as the surfactant for subsequent oil displacement experiments in this study.
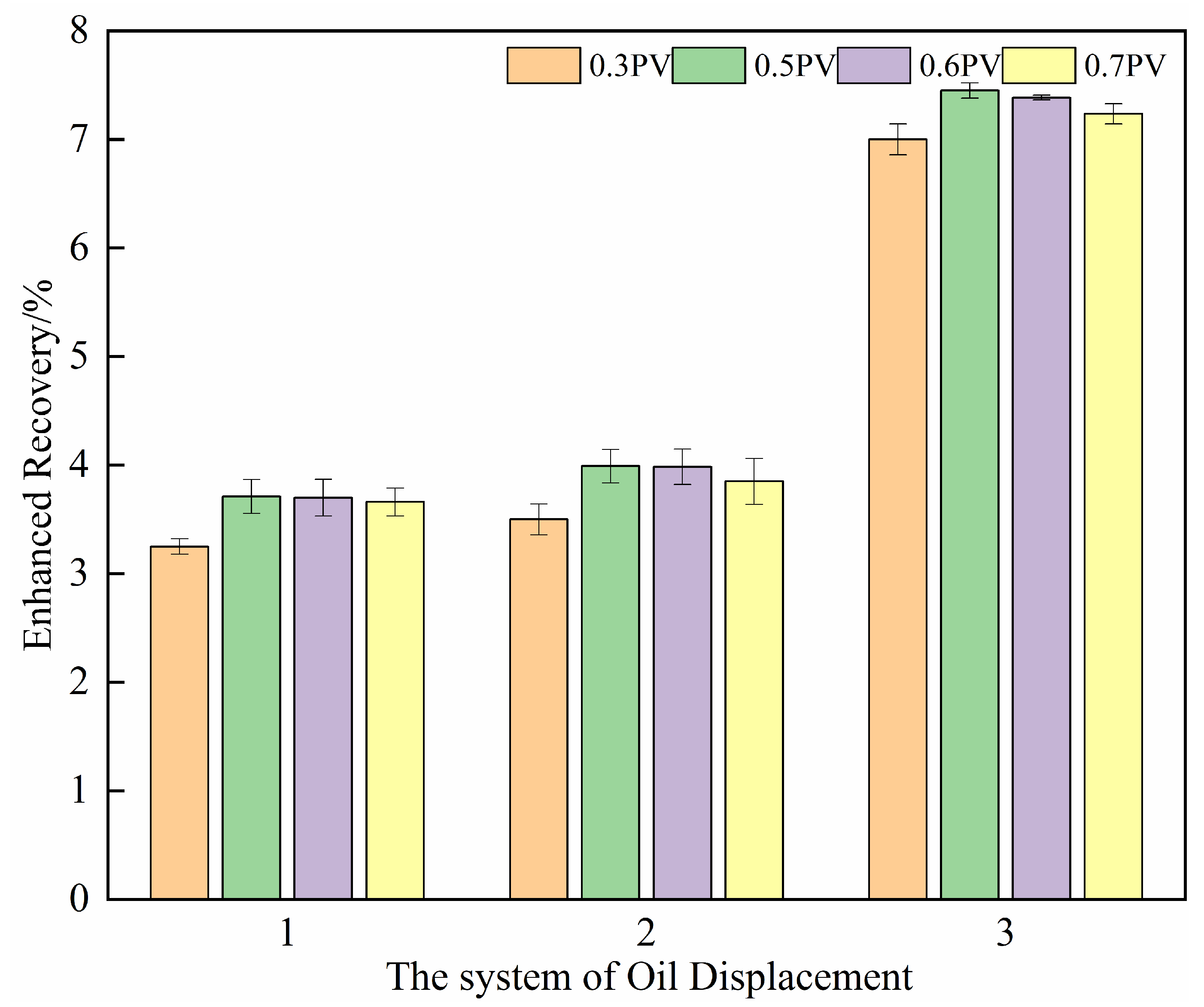
(3) Optimal injection volume for the oil displacement system
The optimization results for the injection volume of the oil displacement system are presented in
Figure 14. The incremental recovery initially rises with increasing injection volume but exhibits diminishing returns. As the injection volume increases from 0.3 PV to 0.5 PV, the recovery shows a marked improvement, with an average enhancement of 10%. This is attributed to the sufficient volume ensuring full contact and emulsification action of the composite system in the unswept oil zones.
However, when the injection volume is further increased to 0.6 PV and 0.7 PV, the incremental recovery stabilizes and even declines in Schemes 2 and 3 (as shown in
Table 5 and
Figure 14). This reduction may result from an “over-injection” effect. In addition, both Schemes 2 and 3 involve microbial growth and metabolism. At higher injection volumes, a portion of the displacing agent may be prematurely produced before microbial degradation and metabolic activity are fully expressed, leading to a lower recovery increase compared to the 0.5 PV case.
In summary, an injection volume of 0.5 PV is sufficient to achieve effective emulsification and significant recovery enhancement. Further increasing the injection volume contributes minimally to ultimate recovery and may even lead to operational inefficiencies. Therefore, 0.5 PV is recommended as the optimal injection volume for the oil displacement system in subsequent field applications.
2.4.3. Experimental Results of the One-Dimensional Model of Microbial Oil Displacement
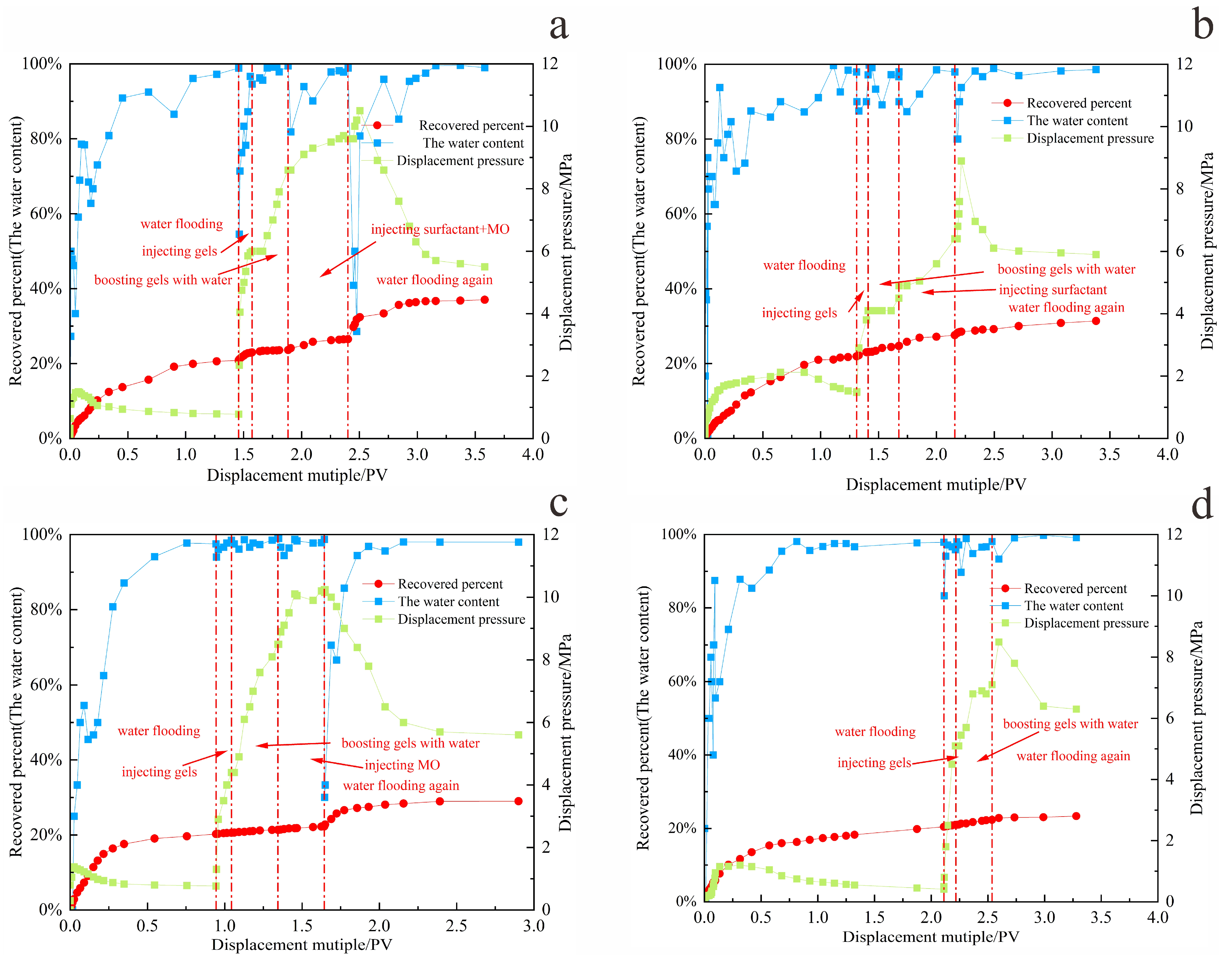
Based on the displacement rate determined in the previous section, the gel–microbial composite oil displacement experiment was carried out. By comparing the oil displacement effects of four different oil displacement systems, the gel–microbial composite oil displacement mechanism was clarified from the perspective of seepage. The experimental results of different oil displacement systems are shown in
Table 6 and
Figure 15,
Figure 16 and
Figure 17.
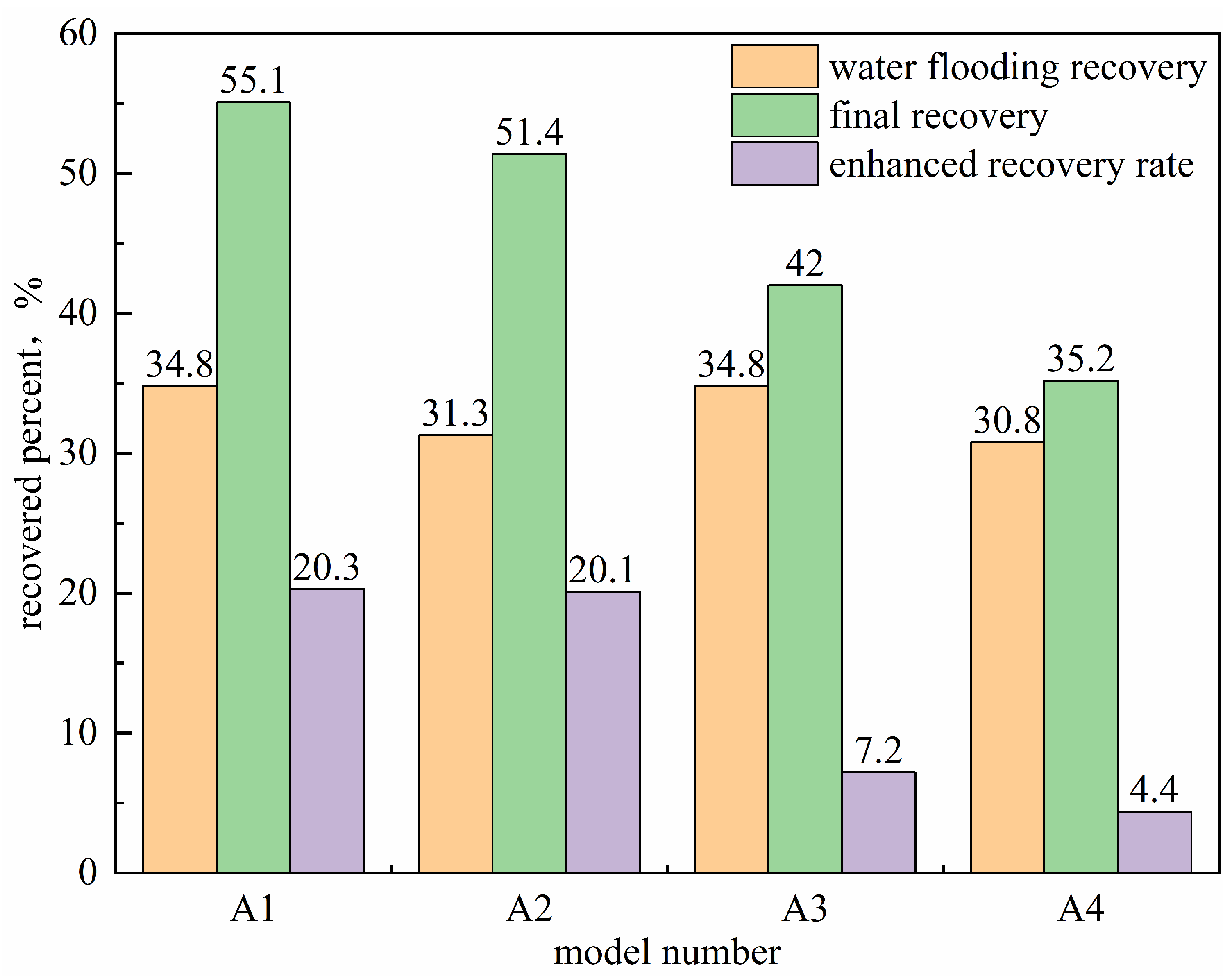
Figure 15 evaluates the enhancement in oil recovery achieved by different systems. Among all configurations, the co-injection of surfactant and microbial fermentation broth demonstrated the best performance. As shown in
Figure 17a, after a 5-day shut-in period, the water cut during subsequent secondary water flooding decreased significantly compared to the initial water flooding stage. Meanwhile, the gel plugging effect maintained sufficient reservoir pressure and improved the sweep efficiency of the displacing fluid.
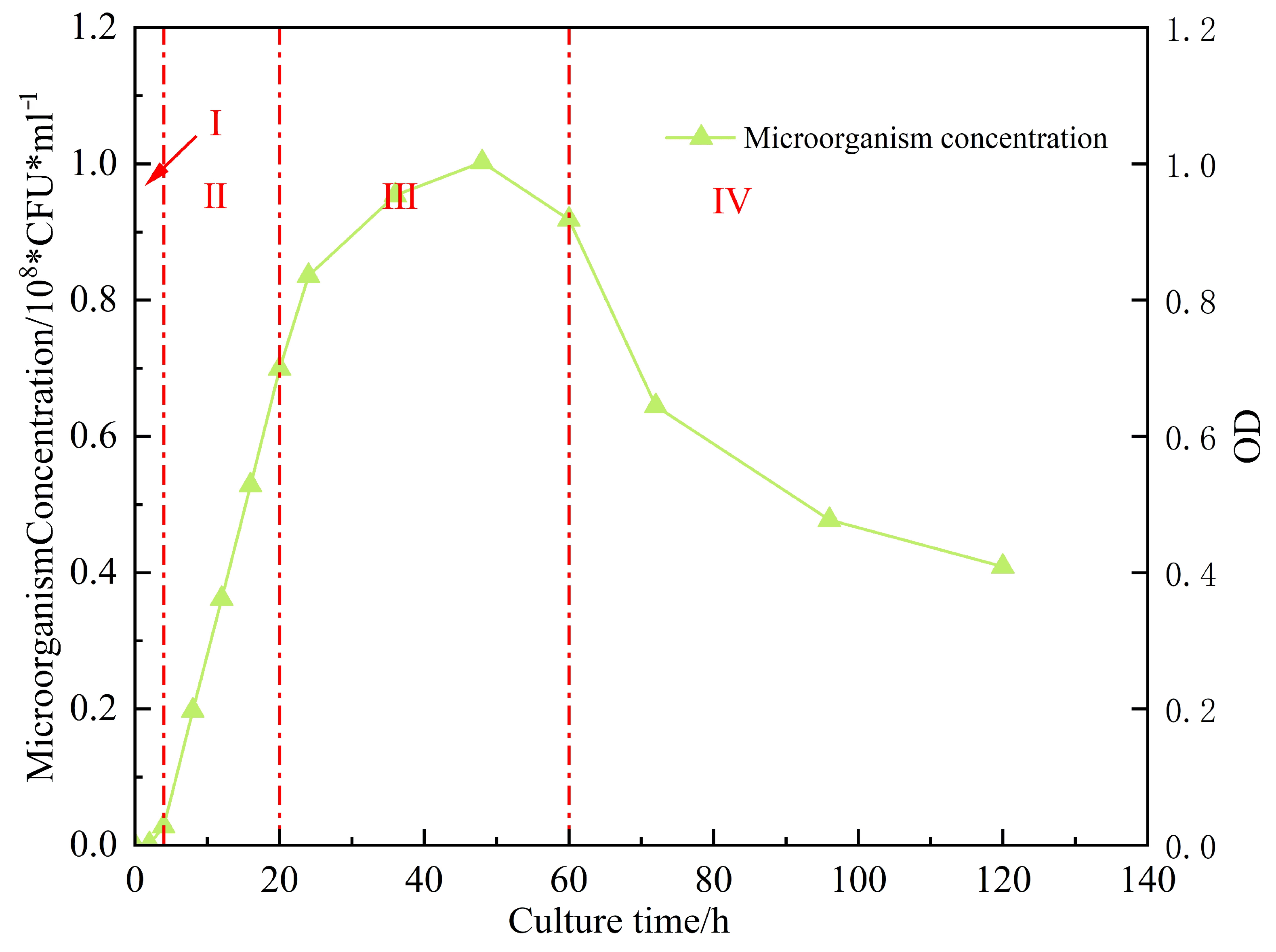
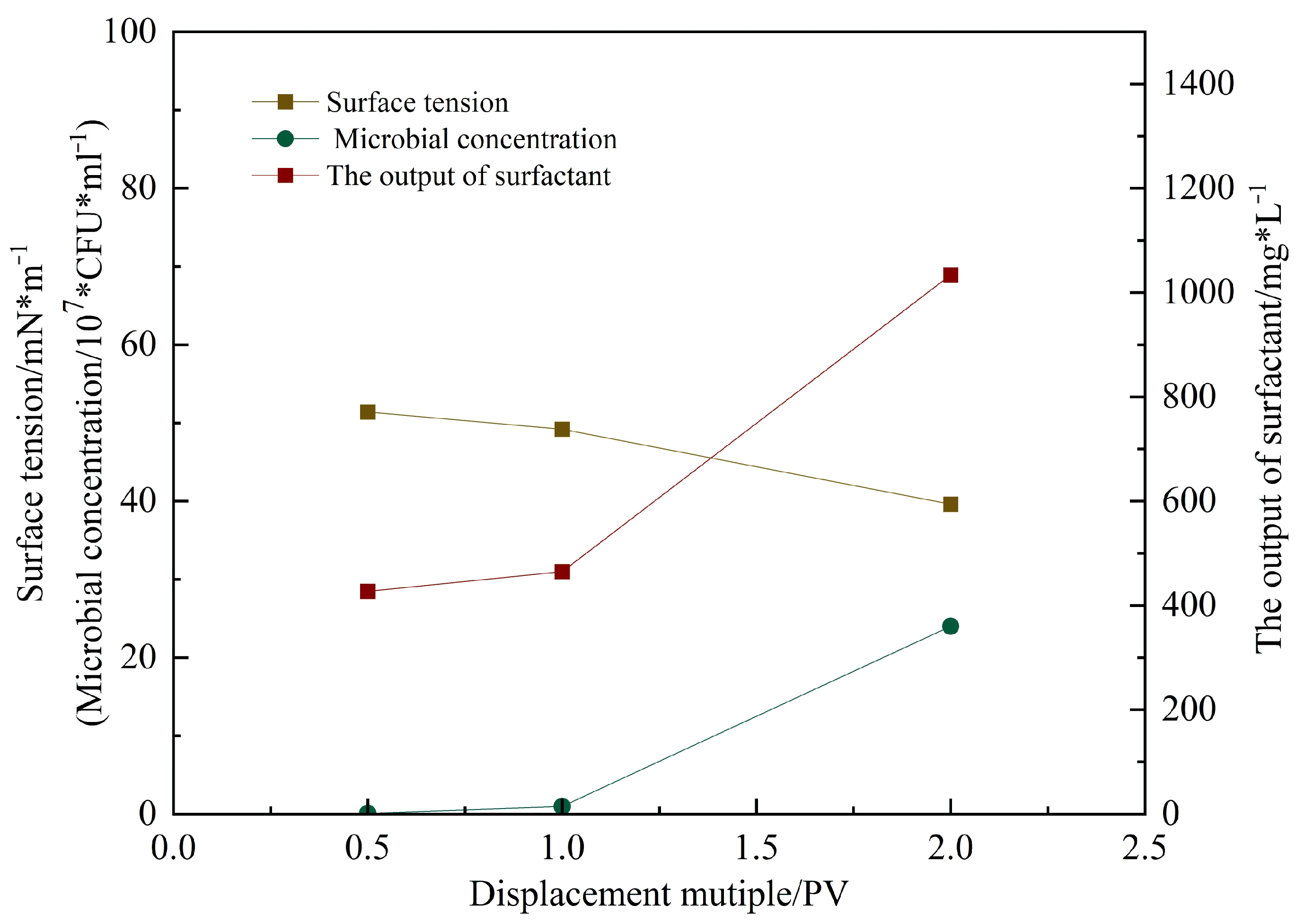
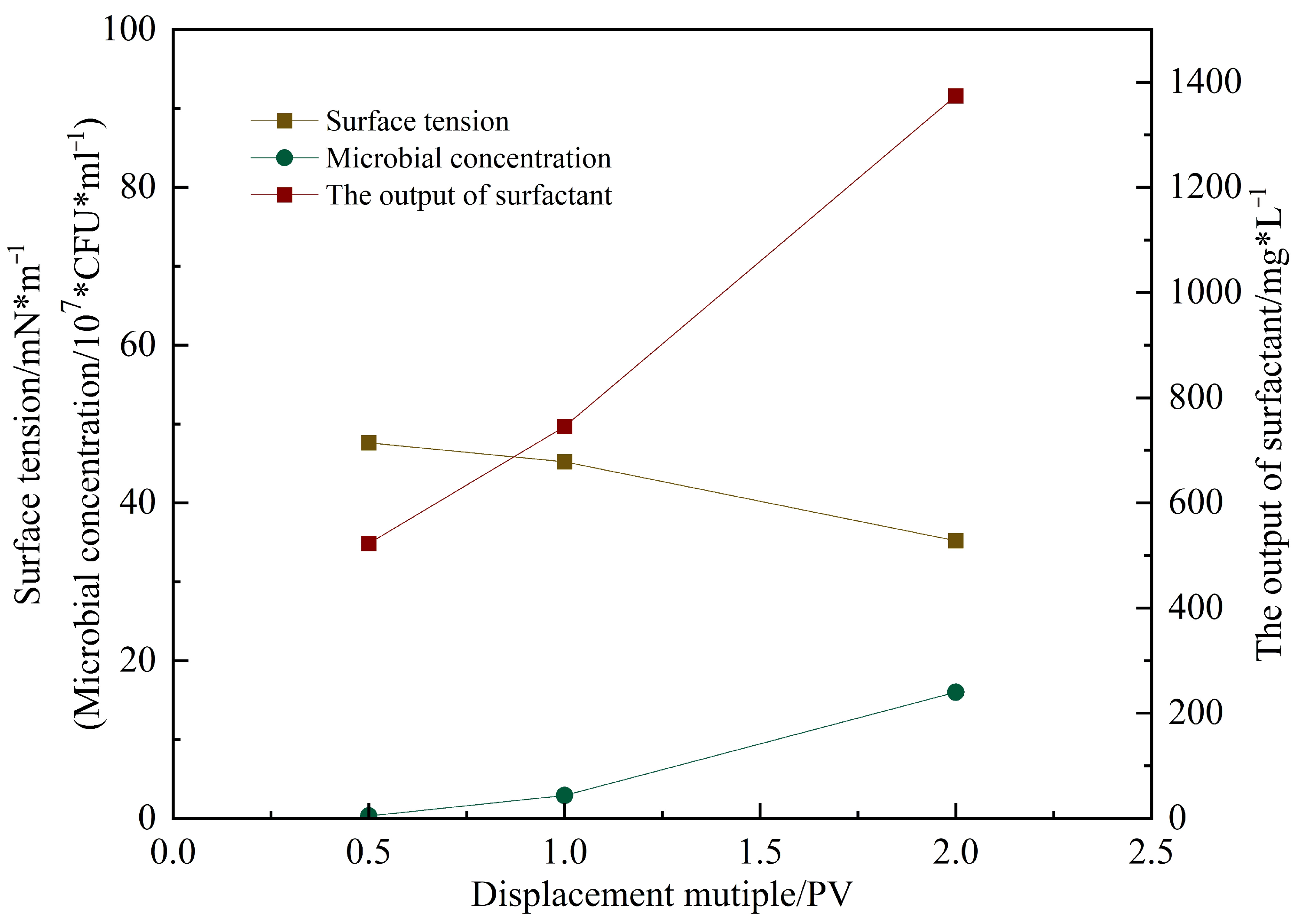
Figure 16 shows the microbial concentration, surfactant content, and surface tension measured in the produced fluid during flooding at different pore volumes injected [
16]. As the injection progressed, the surface tension decreased from 56 mN/m (initial untreated state) to 35.9 mN/m after 2 PV. The surfactant concentration reached 1100 mg/L, and the microbial concentration attained 3.6 × 10
8 CFU/mL. These results [
17] indicate that microbial metabolites rapidly reduce interfacial tension, decreasing capillary and viscous forces, thereby improving crude oil mobility.
Simultaneously, the polymer injected during the conformance control stage plugged the dominant flow channels formed during the initial water flooding, thereby expanding the sweep volume of subsequent flooding agents. This approach not only mobilizes oil in high-permeability zones—otherwise trapped by high viscosity or boundary layer effects—but also enables the displacement of fluid to access previously unswept regions. As a result, microorganisms and surfactants distribute more uniformly within pore spaces, enhancing both the operating efficiency and coverage of the flooding system.
The composite system ultimately achieved a recovery factor significantly higher than other methods, with stable displacement efficiency, delayed water breakthrough, prolonged energy retention, and more complete crude oil extraction. Compared to primary water flooding, the recovery factor was increased by 15%.
The oil displacement performance of the system injected with microorganisms alone is comparable to that of the surfactant-only system. While microbial flooding performs slightly better than surfactant flooding, both are inferior to the three composite systems that utilize synergistic mechanisms. Although microorganisms can alter crude oil properties and partially block high-permeability channels through metabolic activity, the reduction in oil–water interfacial tension by microbes alone remains limited, and their ability to mobilize higher-viscosity crude oil is insufficient, resulting in a relatively narrow swept volume.
Surfactants [
17], though effective in reducing oil viscosity and interfacial tension, lack stability and do not provide the pore-scale structural modification and blocking effects offered by microorganisms. Moreover, their emulsifying performance is inferior to that of biosurfactants, and the displacing fluid tends to channel through high-permeability pathways, leading to early water breakthrough. A significant portion of the surfactant is washed out by flowing water before fully contacting the crude oil, which not only reduces chemical utilization but also limits both sweep efficiency and the duration of enhanced oil recovery.
The hybrid injection system effectively combines the advantages of both agents, leading to significantly improved oil recovery. Thus, the recovery factors of the two single-agent systems are considerably lower than those of the synergistic and segmented injection systems. As shown in
Figure 17b–d, the system with microorganisms alone maintains low water cut for a longer period compared to surfactant-only injection, extending the duration of the oil enhancement effect by about 5%.
Overall, the synergy between surfactants and microorganisms—whether co-injected or sequentially injected—is key to enhancing oil recovery. Among the tested systems, the co-injection scheme offers real-time synergy and higher efficiency, making it the optimal oil displacement strategy under the experimental conditions.
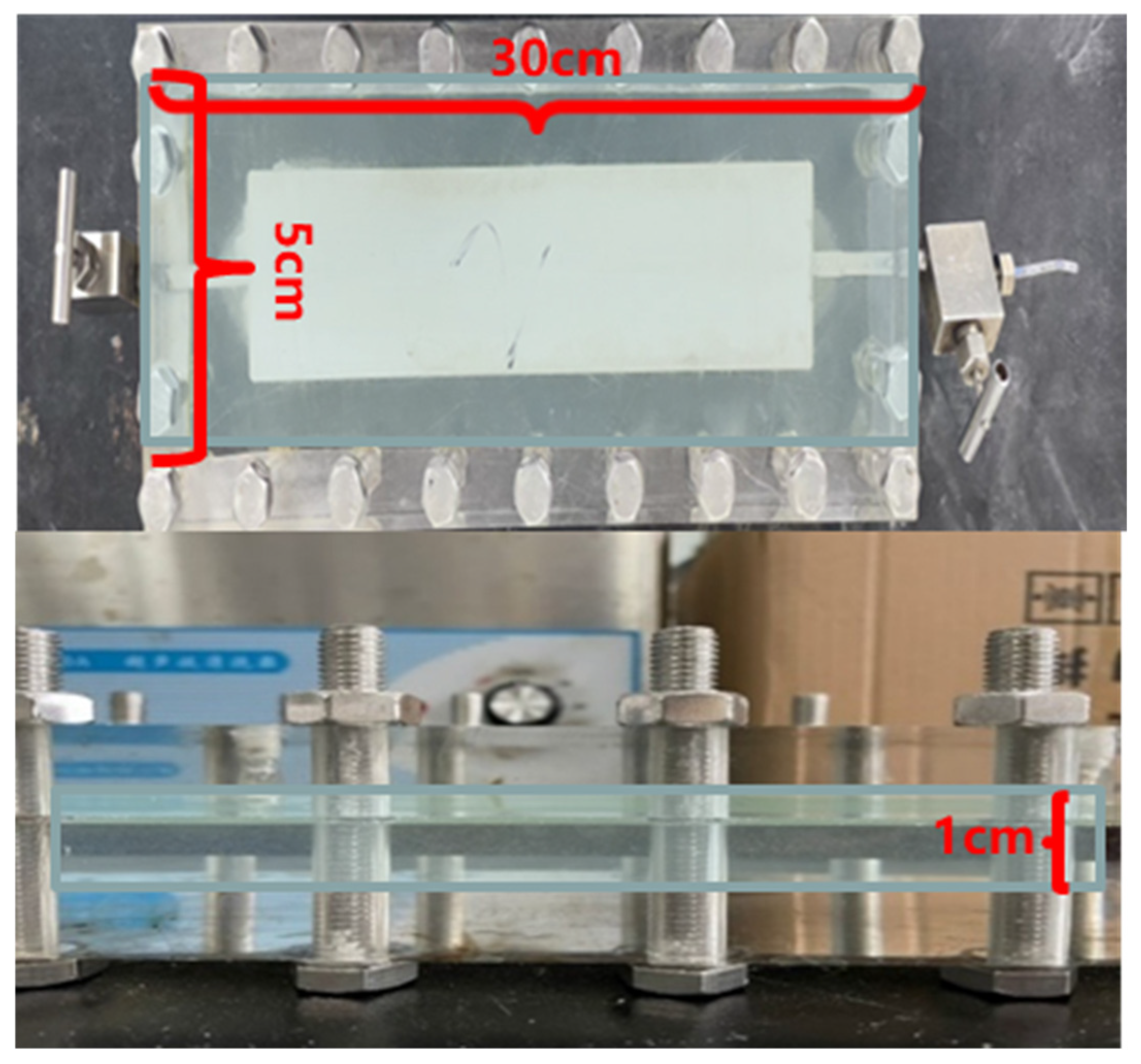
2.4.4. Research on the Compound Oil Displacement Mechanism of Two-Dimensional Model
Based on the optimal oil displacement system and injection parameters determined in
Section 2.4.1,
Section 2.4.2 and
Section 2.4.3, the mechanism of gel–microbial composite flooding was investigated using a two-dimensional slab model. As shown in
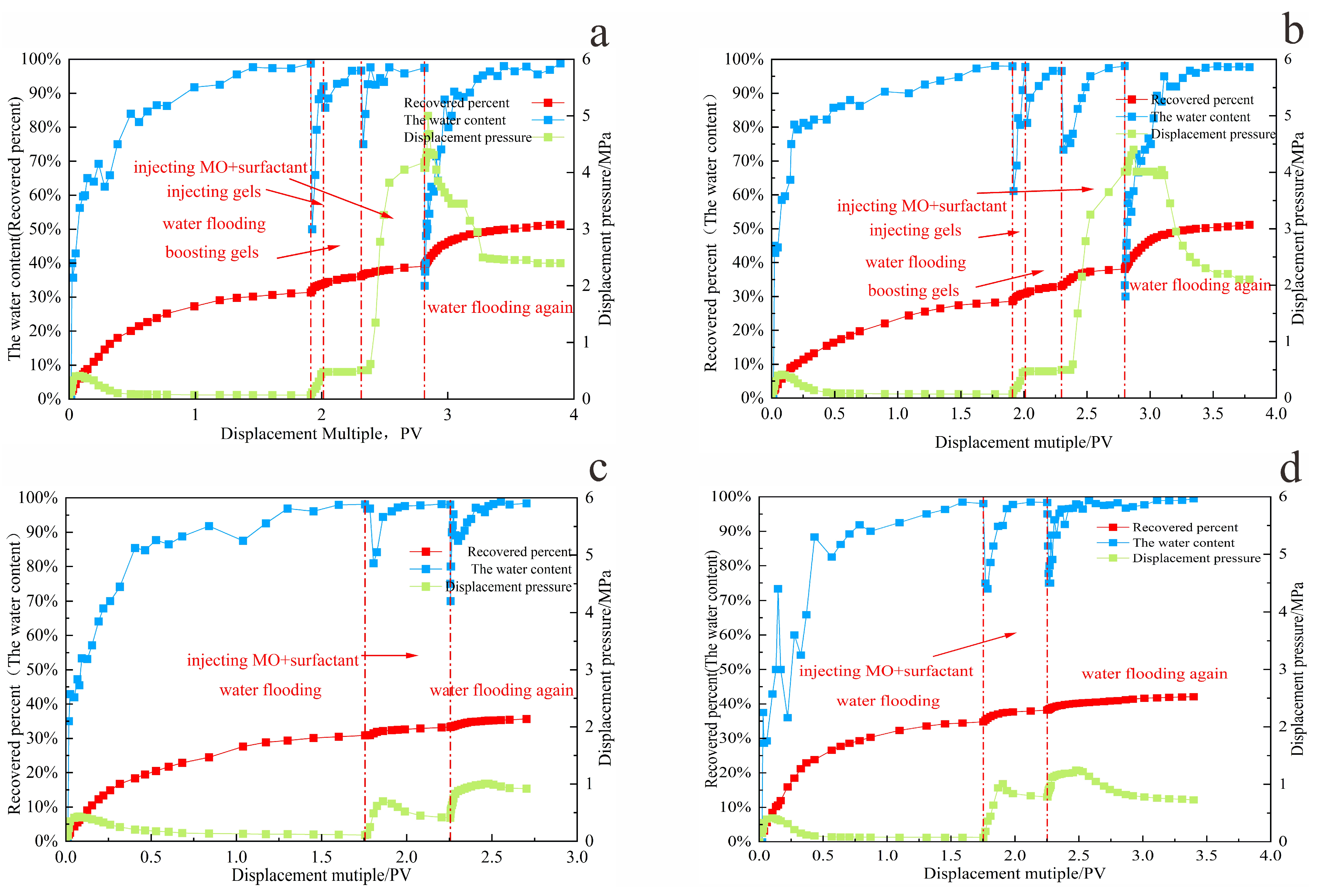
Figure 18a,b, during the gel conformance control stage, the breakthrough pressure in the model increased from 0.4 MPa during water flooding to 4 MPa—an increase of 560%—and remained stable. In contrast,
Figure 18c,d depict the process without gel treatment, where the maximum pressure during chemical injection increased by only 0.5 MPa compared to water flooding. In the subsequent water flooding stage after shut-in, the maximum pressure reached 2 MPa, whereas the gel-treated model sustained pressures up to 6 MPa and exhibited a significantly slower water cut rebound.
These results demonstrate that the gel system forms effective physical retention within high-permeability channels, reducing aqueous phase mobility in dominant flow paths. The gel accumulated at the displacement front establishes two uniformly distributed and highly stable sealing zones, diverting the subsequently injected microbe–surfactant system toward unswept crude oil in microchannels. This expands the sweep volume and enhances crude oil recovery.
Furthermore, the gel’s porous structure increases the specific surface area, providing additional attachment sites for microorganisms and prolonging the activity of metabolic products [
18]. Amino groups released during gel swelling help buffer formation pH, maintaining an optimal environment for microbial metabolism [
6,
19]. Gel conformance control also reduces ineffective surfactant loss, enabling more sustained interfacial tension reduction. In turn, organic acids generated by microbial metabolism moderately weaken gel strength, preventing long-term formation damage and establishing a dynamic “blocking–unblocking” equilibrium.
During the subsequent water flooding phase following microbial treatment, the dynamic variations in pressure and water cut reflect clear improvements in displacement efficiency. The initial injection pressure rapidly rose to 5 MPa, then gradually decreased, and stabilized around 2 MPa. This behavior indicates enhanced crude oil mobility due to the combined action of microorganisms and surfactants, while the increased mobilization of previously unswept oil raised overall flow resistance in the porous media. Water cut data show that the breakthrough water cut during the second water flooding was 32%, nearly 40% lower than that of the first water flooding after gel treatment. As shown in
Figure 19, the final average crude oil recovery increased by 20%.
These results confirm the synergistic mechanism outlined above: gel-based conformance control expands the sweep region, while microbial degradation and surfactant-mediated viscosity reduction and emulsification collectively lower crude oil flow resistance. This combination enables the effective production of residual oil from previously unswept zones during subsequent water flooding, ultimately enhancing the recovery factor.
As shown in
Figure 20, the microbial concentration during subsequent water flooding increased with injected pore volume (PV), rising from an initial 1.2 × 10
6 CFU/mL to 2.4 × 10
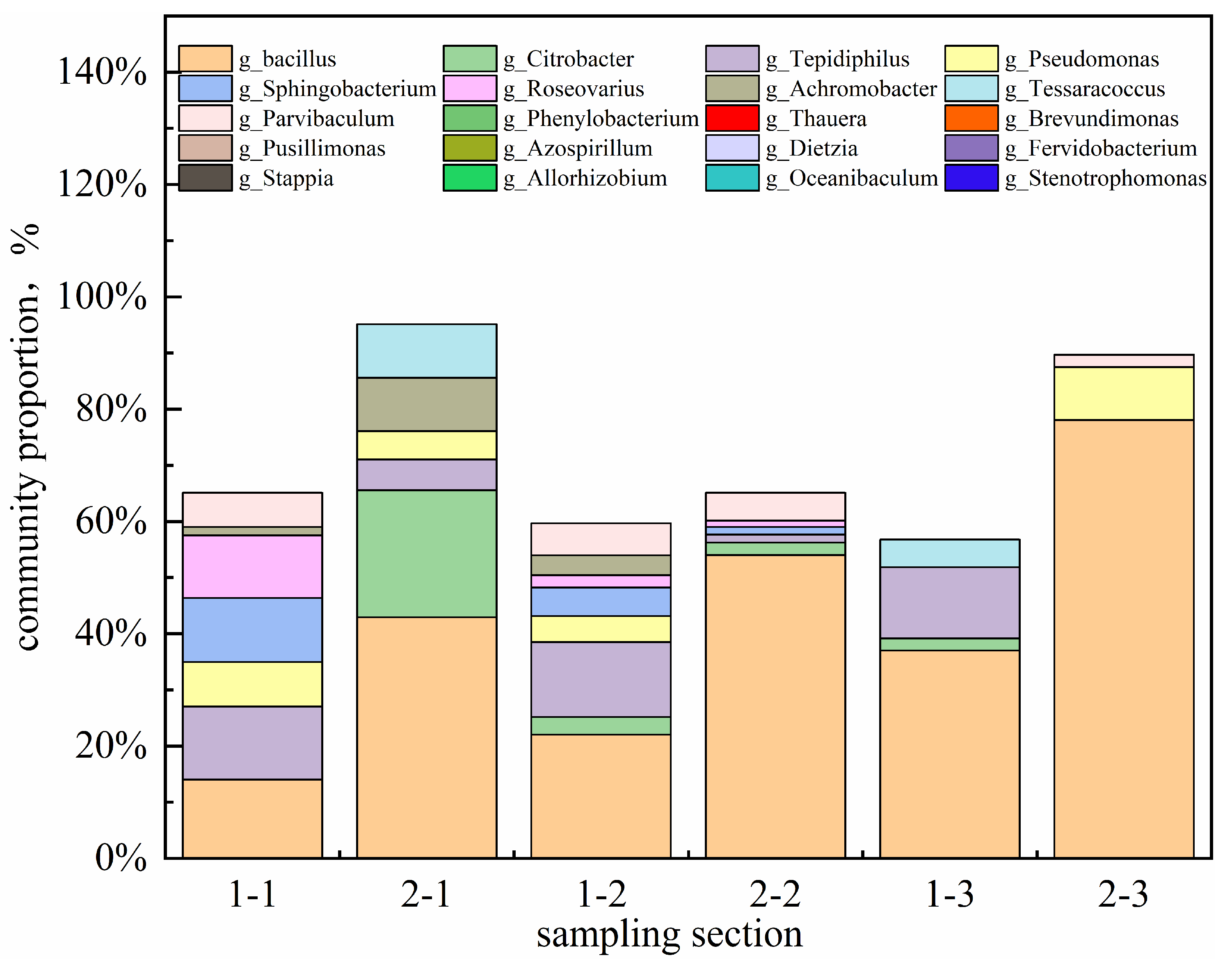
7 CFU/mL after 2 PV. Analysis of the produced fluid community structure (
Figure 21) revealed a progressive increase in the relative abundance of Bacillus, which exceeded 70% before 2 PV, establishing it as the dominant genus. This indicates successful microbial adaptation and proliferation within the reservoir model. As a hydrophobic bacterium, g_Bacillus possesses strong hydrocarbon-degrading activity [
20], providing a functional basis for oil displacement.
Surfactant concentration at the outlet was initially measured at 500 mg/L and continued to increase with further injection, demonstrating continuous in situ production of biosurfactants during microbial metabolism [
21]. These biosurfactants function synergistically with the externally injected surfactant to enhance oil mobilization. The hydrophilic carboxyl groups of the surfactants modify rock surface wettability from oil-wet to water-wet, significantly improving residual oil stripping efficiency. Concurrently, the surfactant system substantially reduces oil–water interfacial tension, weakening crude oil adhesion to rock surfaces and facilitating oil mobilization from pore spaces. This dual mechanism promotes the formation of O/W emulsions, effectively reducing crude oil flow resistance through the porous media.
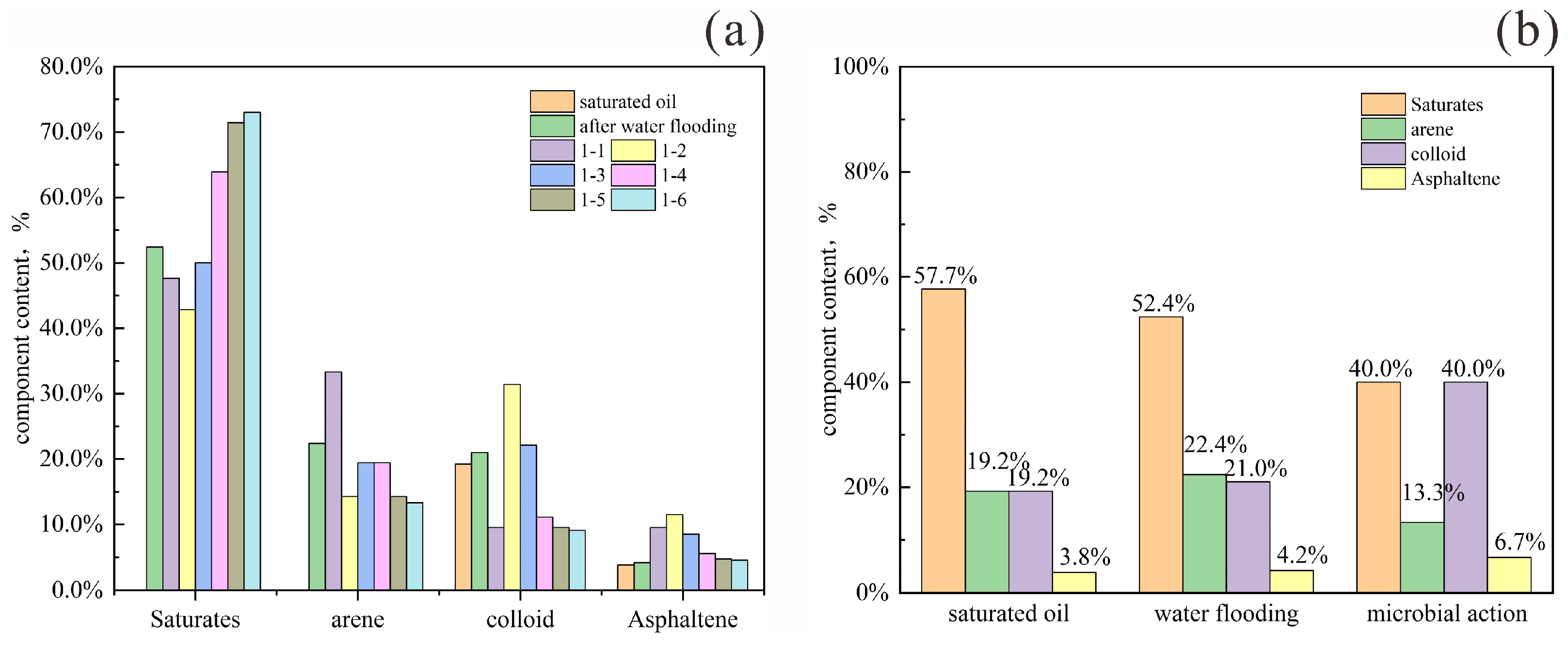
To track compositional changes during microbial oil recovery, crude oil samples were collected at three stages for component analysis: initial saturation, after water flooding, and after microbial treatment. Results in
Figure 22b show that water flooding significantly reduced saturated hydrocarbon content, while increasing the proportions of aromatics, resins, and asphaltenes. This redistribution occurs as water–oil–rock interactions preferentially mobilize less-adsorbed components, leaving polar constituents adsorbed on rock surfaces [
22]. Although core heterogeneity influences the precise composition of produced oil, it does not alter the overall four-component trend during water flooding. Pore structure directly affects the adsorption and retention of polar components, thereby altering the composition of produced crude oil.
After microbial treatment, saturated hydrocarbons and aromatics decreased by 12.4% and 9.1%, respectively, indicating preferential microbial degradation of light components (saturates and monocyclic aromatics) [
23]. Through β-oxidation, straight-chain alkanes (C
15–C
30) are broken down into short-chain fatty acids (e.g., acetic and propionic acid). Using nitrate as an electron acceptor, benzene-series compounds (toluene, xylene) are degraded into CO
2 and H
2O, and long-chain hydrocarbons are cleaved into shorter molecules. These processes collectively reduce crude oil viscosity by 65%. The relative increase in resin and asphaltene proportions reflects the consumption of saturates and aromatics. Meanwhile, microbial mild degradation or dispersion of resins and asphaltenes [
22,
24] prevents the deposition and plugging of heavy components on rock surfaces, further enhancing crude oil mobility.
Figure 22a and
Table 7 present the variations in crude oil composition at different displacement multiples during water flooding following microbial treatment. During the medium-to-low water-cut stages (samples 1-1 and 1-2), saturated hydrocarbon content continued to decline, while asphaltene content gradually increased. Aromatic hydrocarbons initially increased and then decreased, whereas resins first decreased and then increased. This pattern arises because production in these stages originates from the front and middle sections of the model, where microbial degradation is most active. Saturated hydrocarbons are preferentially consumed as microbial nutrients, while heavy components including aromatics and asphaltenes are also partially degraded and mobilized.
Beginning with sample 1-3, the system enters the high water-cut stage. Here, the influence of microorganisms and surfactants begins to diminish, and the produced oil shifts toward lighter components, showing an increase in saturates and a decrease in heavy fractions. Aromatic content begins to decline, with saturated hydrocarbons becoming the dominant component.
Upon reaching the ultra-high water-cut stage (samples 1-5 and 1-6), the injected water has largely displaced the original surfactant, and the region affected by microbial degradation has been substantially swept. The produced crude oil at this stage consists predominantly of light components, with saturated hydrocarbons accounting for nearly 70% of the total.