The Potential for Reusing Superabsorbent Polymer from Baby Diapers for Water Retention in Agriculture
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Gel Samples
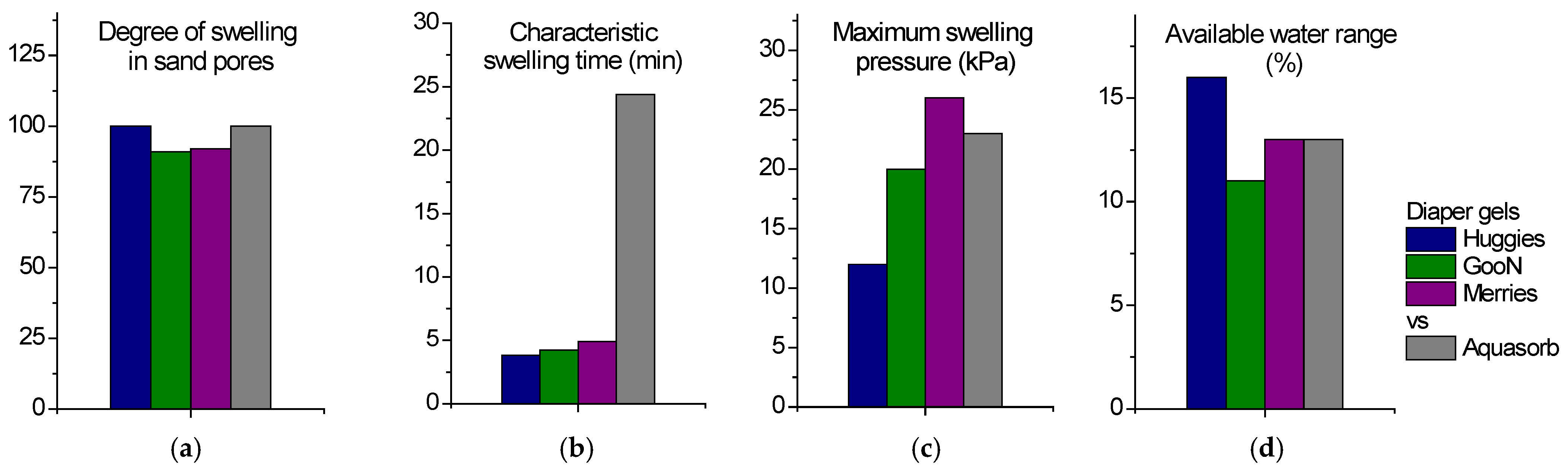
2.2. Water Retention Capacity
2.2.1. Swelling
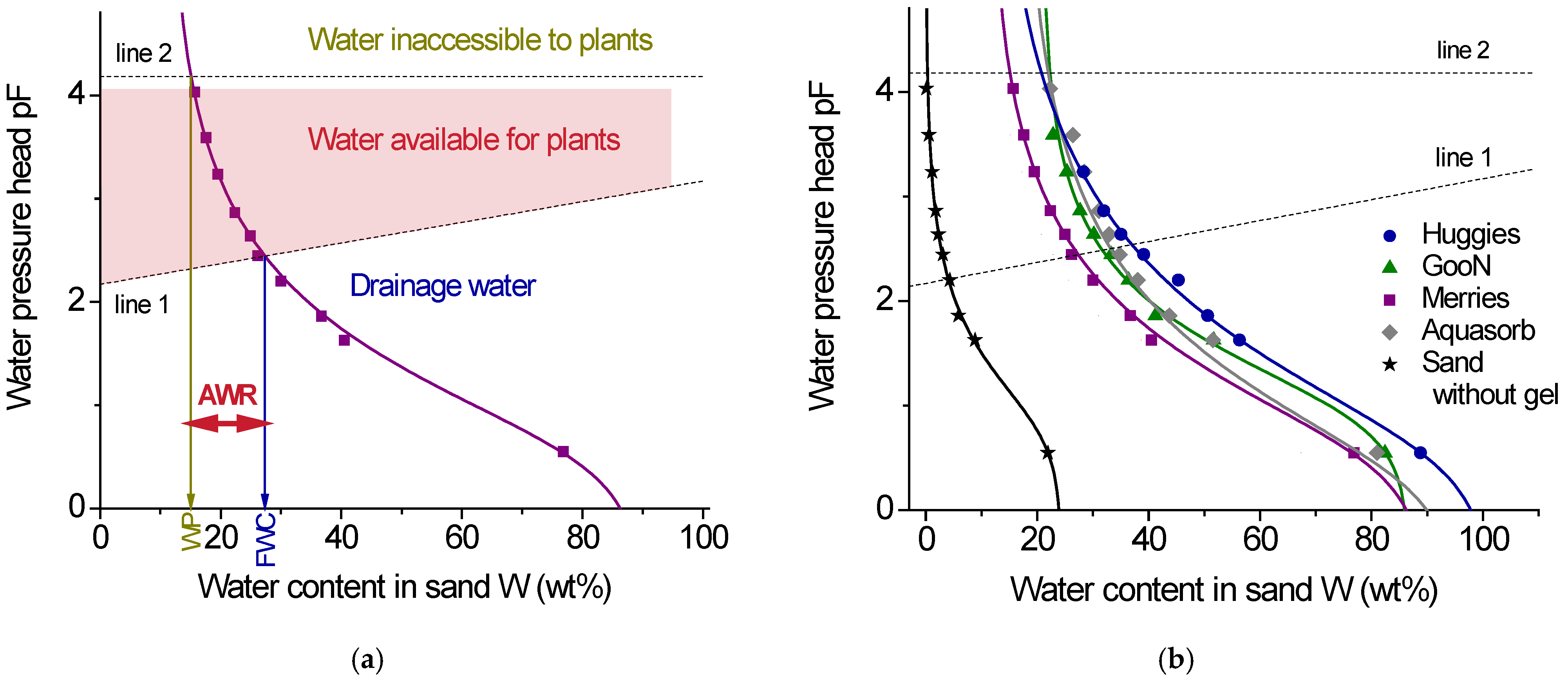
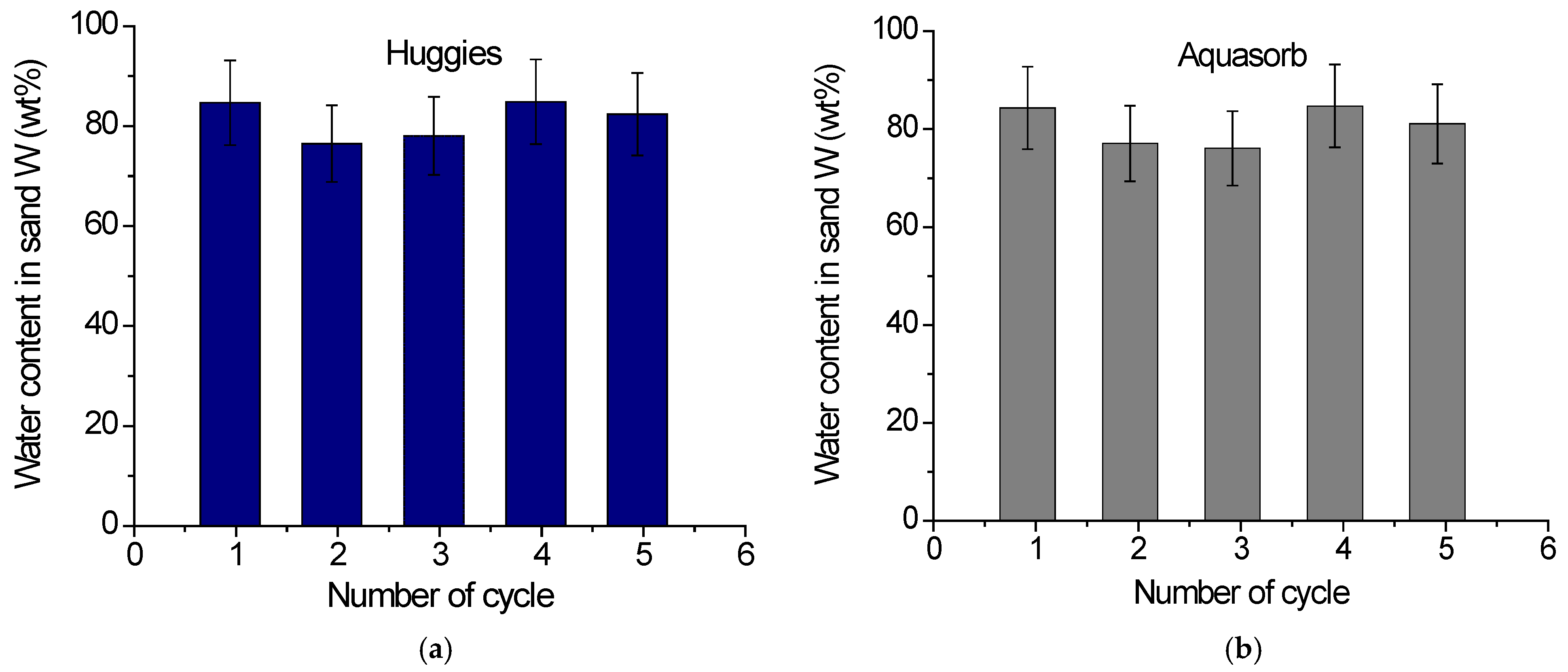
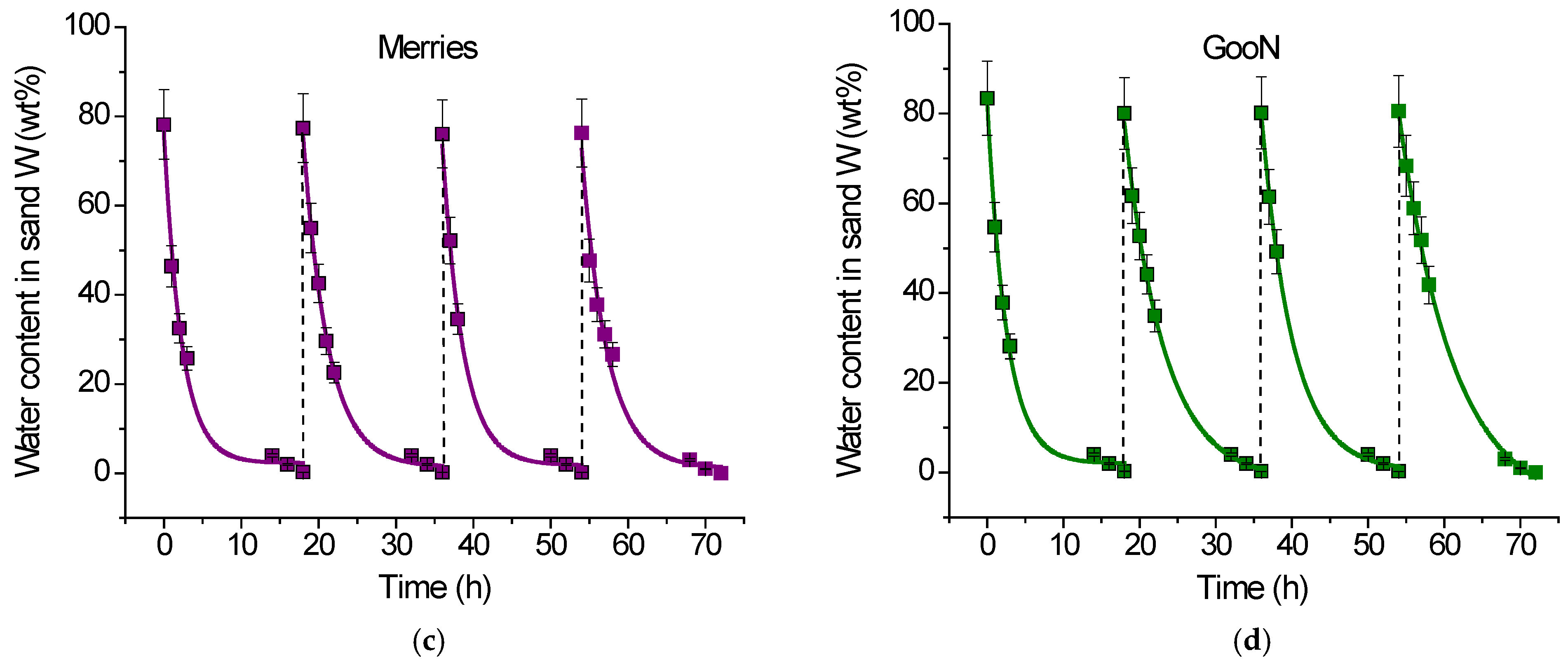
2.2.2. Water Retention
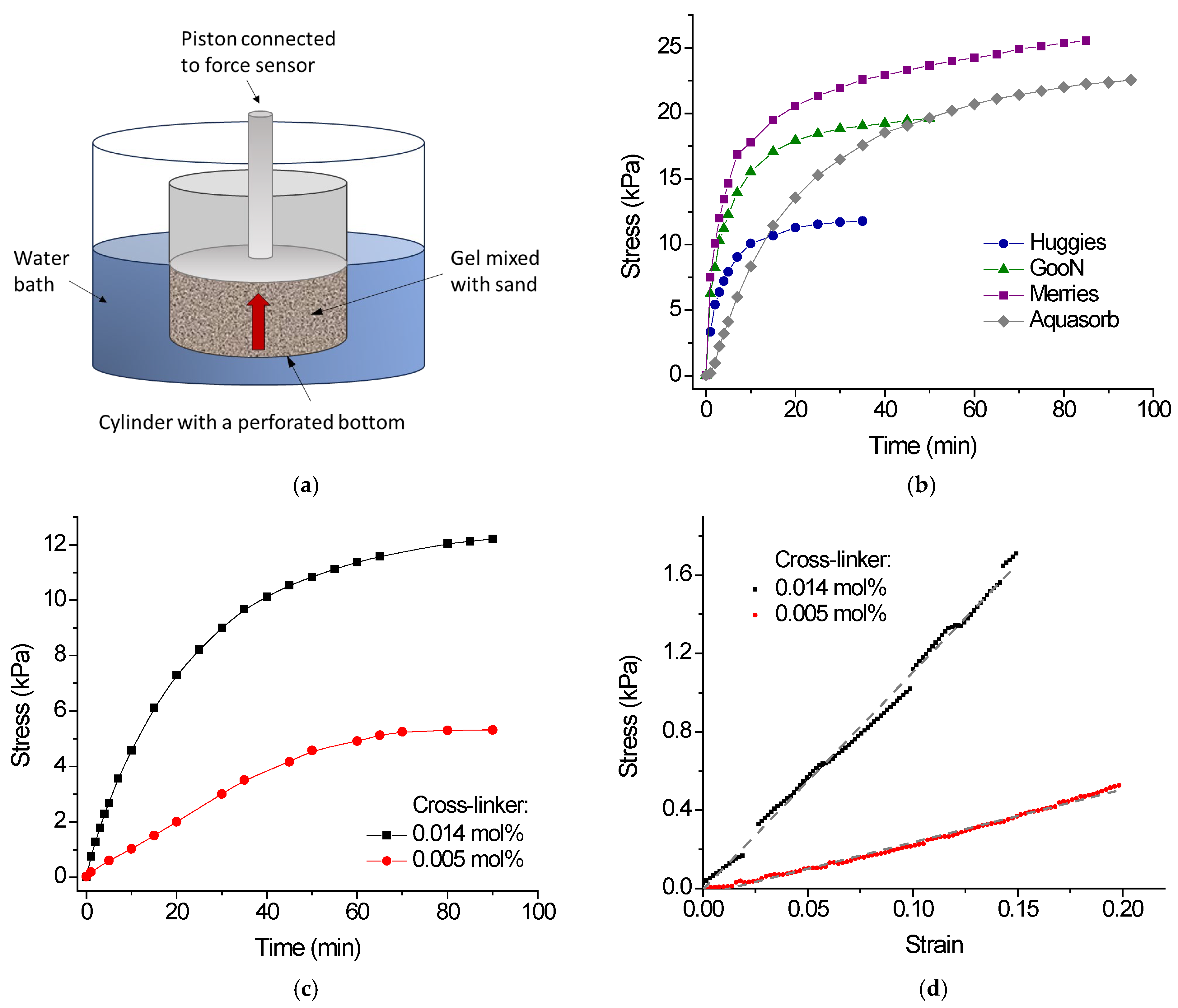
2.3. Swelling Pressure
2.4. Germination and Seedling Growth
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Gel Synthesis
4.3. Potentiometric Titration
4.4. Free Swelling
4.5. Water Retention
4.6. Swelling Pressure and Swelling in Sand Pores
4.7. Mechanical Characterization
4.8. Optical Microscopy
4.9. Germination and Seedling Growth
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, F.S.-F.; Muthu, S.S.; Li, Y.; Hui, P.C.-L. A critical review on life cycle assessment studies of diapers. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1795–1822. [Google Scholar] [CrossRef]
- Somers, M.J.; Alfaro, J.F.; Lewis, G.M. Feasibility of superabsorbent polymer recycling and reuse in disposable absorbent hygiene products. J. Clean. Prod. 2021, 313, 127686. [Google Scholar] [CrossRef]
- Superabsorbent Polymer Market Analysis. Available online: https://www.chemanalyst.com/industry-report/superabsorbent-polymer-market-727 (accessed on 7 August 2025).
- Superabsorbent polymer market revenue share by application. Available online: https://www.gminsights.com/industry-analysis/superabsorbent-polymers-sap-market (accessed on 7 August 2025).
- Takaya, C.A.; Cooper, I.; Berg, M.; Carpenter, J.; Muir, R.; Brittle, S.; Sarker, D.K. Offensive waste valorisation in the UK: Assessment of the potentials for absorbent hygiene product (AHP) recycling. Waste Manag. 2019, 88, 56–70. [Google Scholar] [CrossRef]
- Chazovachii, P.T.; Somers, M.J.; Robo, M.T.; Collias, D.I.; James, M.I.; Marsh, E.N.G.; Zimmerman, P.M.; Alfaro, J.F.; McNeil, A.J. Giving superabsorbent polymers a second life as pressure-sensitive adhesives. Nat. Commun. 2021, 12, 4524. [Google Scholar] [CrossRef]
- Misiewicz, J.; Lejcuś, K.; Dąbrowska, J.; Marczak, D. The characteristics of absorbency under load (AUL) for superabsorbent and soil mixtures. Sci. Rep. 2019, 9, 18098. [Google Scholar] [CrossRef]
- Lejcuś, K.; Śpitalniak, M.; Dąbrowska, I. Swelling behaviour of superabsorbent polymers for soil amendment under different loads. Polymers 2018, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Thombare, N.; Mishra, S.; Siddiqui, M.Z.; Jha, U.; Singh, D.; Mahajan, G.R. Design and development of guar gum based novel, superabsorbent and moisture retaining hydrogels for agricultural applications. Carbohydr. Polym. 2018, 185, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Philippova, O.E. Responsive polymer gels. Polym. Sci. 2000, 42, 208–228. [Google Scholar]
- Zohuriaan-Mehr, M.J.; Omidian, H.; Doroudiani, S.; Kabiri, K. Advances in non-hygienic applications of superabsorbent hydrogel materials. J. Mater. Sci. 2010, 45, 5711–5735. [Google Scholar] [CrossRef]
- Wei, Y.; Durian, D.J. Rain water transport and storage in a model sandy soil with hydrogel particle additives. Eur. Phys. J. E 2014, 37, 97. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Arolu, F.; Chukwu, S.C.; Salisu, M.A.; Fagbohun, I.K.; Muftaudeen, T.K.; Swaray, S.; Haliru, B.S. Superabsorbent polymer hydrogels for sustainable agriculture: A review. Horticulturae 2022, 8, 605. [Google Scholar] [CrossRef]
- Zhu, J.; Suhaimi, F.; Lim, J.Y.; Gao, Z.; Swarup, S.; Loh, C.S.; Li, J.; Ong, C.N.; Tan, W.K. A field study on using soybean waste-derived superabsorbent hydrogel to enhance growth of vegetables. Sci. Total Environ. 2022, 851, 158141. [Google Scholar] [CrossRef] [PubMed]
- Iliasov, L.; Shibaev, A.; Panova, I.; Kushchev, P.; Philippova, O.; Yaroslavov, A. Weakly cross-linked anionic copolymers: Kinetics of swelling and water-retaining properties of hydrogels. Polymers 2023, 15, 3244. [Google Scholar] [CrossRef]
- Abdelghafar, R.; Abdelfattah, A.; Mostafa, H. Effect of super absorbent hydrogel on hydro-physical properties of soil under deficit irrigation. Sci. Rep. 2024, 14, 7655. [Google Scholar] [CrossRef]
- Agbna, G.H.D.; Zaidi, S.J. Hydrogel performance in boosting plant resilience to water stress—A review. Gels 2025, 11, 276. [Google Scholar] [CrossRef]
- Niu, Q.; Xie, J.; Li, J.; An, Z.; Xiao, H.; Zhang, X.; Su, Z.; Wang, Z. Superabsorbent polymers: Innovations in ecology, environmental, and diverse applications. Materials 2025, 18, 823. [Google Scholar] [CrossRef]
- Louf, J.-F.; Lu, N.B.; O’Connell, M.G.; Cho, H.J.; Datta, S.S. Under pressure: Hydrogel swelling in a granular medium. Sci. Adv. 2021, 7, eabd2711. [Google Scholar] [CrossRef] [PubMed]
- Mikhailidi, A.; Ungureanu, E.; Tofanica, B.-M.; Ungureanu, O.C.; Fortună, M.E.; Belosinschi, D.; Volf, I. Agriculture 4.0: Polymer hydrogels as delivery agents of active ingredients. Gels 2024, 10, 368. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Z.; Zhang, R.; Zhou, S.; Yang, H.; Chen, Y.; Zhang, J.; Yin, H.; Yu, D. Research advances in superabsorbent polymers. Polymers 2024, 16, 501. [Google Scholar] [CrossRef]
- Rudzinski, W.E.; Dave, A.M.; Vaishanav, U.H.; Kumbar, S.G.; Kulkarni, A.R.; Aminabhavi, T.M. Hydrogels as controlled re-lease devices in agriculture. Des. Monomers Polym. 2002, 5, 39–65. [Google Scholar] [CrossRef]
- Campos, E.V.R.; de Oliveira, J.L.; Fraceto, L.F.; Singh, B. Polysaccharides as safer release systems for agrochemicals. Agron. Sustain. Dev. 2015, 35, 47–66. [Google Scholar] [CrossRef]
- Ramli, R.A. Slow release fertilizer hydrogels: A review. Polym. Chem. 2019, 10, 6073–6090. [Google Scholar] [CrossRef]
- Supare, K.; Mahanwar, P.A. Starch-derived superabsorbent polymers in agriculture applications: An overview. Polym. Bull. 2022, 79, 5795–5824. [Google Scholar] [CrossRef]
- Dovzhenko, A.P.; Yapryntseva, O.A.; Sinyashin, K.O.; Doolotkeldieva, T.; Zairov, R.R. Recent progress in the development of encapsulated fertilizers for time-controlled release. Heliyon 2024, 10, e34895. [Google Scholar] [CrossRef]
- Rodrigo, L.; Munaweera, I. Employing sustainable agriculture practices using eco-friendly and advanced hydrogels. RSC Adv. 2025, 15, 21212–21228. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Singh Lodhi, R.; Chourasia, S.; Das, S.; Das, P. A review on sustainable slow-release N, P, K fertilizer hydrogels for smart agriculture. Chempluschem 2025, 90, e202400643. [Google Scholar] [CrossRef]
- Chamorro, A.F.; Palencia, M.; Combatt, E.M. Starch hydrogels for slow and controlled-release fertilizers: A review. Polymers 2025, 17, 1117. [Google Scholar] [CrossRef]
- Sánchez-Orozco, R.; Timoteo-Cruz, B.; Torres-Blancas, T.; Ureña-Núñez, F. Valorization of superabsorbent polymers from used disposable diapers as soil moisture retainer. Int. J. Res. Granthaalayah 2017, 5, 105–117. [Google Scholar] [CrossRef]
- Al-Jabari, M.; Ghyadah, R.A.; Alokely, R. Recovery of hydrogel from baby diaper wastes and its application for enhancing soil irrigation management. J. Environ. Manag. 2019, 239, 255–261. [Google Scholar] [CrossRef]
- Zekry, M.; Nassar, I.; Salim, H.; Abdallah, A. The potential of super absorbent polymers from diaper wastes to enhance waterretention properties of the soil. Soil Environ. 2020, 39, 27–37. [Google Scholar] [CrossRef]
- Ilyasov, L.O.; Shibaev, A.V.; Panova, I.G.; Kushchev, P.O.; Philippova, O.E.; Yaroslavov, A.A. Relationship between swelling and mechanical properties of cross-linked polymers mixed with sand. Mendeleev Commun. 2023, 33, 80–82. [Google Scholar] [CrossRef]
- Ilyasov, L.O.; Panova, I.G.; Kushchev, P.O.; Belov, A.A.; Maksimova, I.A.; Smagin, A.V.; Yaroslavov, A.A. Sparsely cross-linked hydrogel with starch fragments as a multifunctional soil conditioner. J. Compos. Sci. 2022, 6, 347. [Google Scholar] [CrossRef]
- Philippova, O.E.; Khokhlov, A.R. Polymer gels. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 1, Ch. 1.13; pp. 339–366. [Google Scholar] [CrossRef]
- Ospennikov, A.S.; Shibaev, A.V.; Philippova, O.E. Double photocrosslinked responsive hydrogels based on hydroxypropyl guar. Int. J. Mol. Sci. 2023, 24, 17477. [Google Scholar] [CrossRef]
- Lagutina, M.A.; Dubrovskii, S.A. The swelling pressure of weakly ionic acrylamide gels. Polym. Sci. Ser. A 1996, 38, 1059–1064. [Google Scholar]
- Adjuik, T.A.; Nokes, S.E.; Montross, M.D.; Wendroth, O. The impacts of bio-based and synthetic hydrogels on soil hydraulic properties: A review. Polymers 2022, 14, 4721. [Google Scholar] [CrossRef]
- van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Voronin, A. Energy concept of the physical state of soils. Eur. Soil Sci. 1990, 23, 7–19. [Google Scholar]
- Smagin, A.; Panova, I.; Ilyasov, L.; Ogawa, K.; Adachi, Y.; Yaroslavov, A. Water retention in sandy substrates modified by cross-linked polymeric microgels and their complexes with a linear cationic polymer. J. Appl. Polym. Sci. 2021, 138, 50754. [Google Scholar] [CrossRef]
- Ehlers, W.; Goss, M. Water Dynamics in Plant Production; CABI: Wallingford, UK, 2016. [Google Scholar]
- Smagin, A.V.; Budnikov, V.I.; Sadovnikova, N.B.; Smagina, M.V.; Sidorova, M.A. Water retention in different types of protective gel compositions for plant rhizosphere. IOP Conf. Ser. Earth Environ. Sci. 2019, 368, 012049. [Google Scholar] [CrossRef]
- Kramer, P.J. Water Relations of Plants; New York Academic Press: New York, NY, USA, 1983. [Google Scholar]
- Borchard, W.; Emberger, A.; Schwarz, J. A new method to determine swelling pressure. Angew. Makromol. Chem. 1978, 66, 43–49. [Google Scholar] [CrossRef]
- Wilcox, K.G.; Kozawa, S.K.; Morozova, S. Fundamentals and mechanics of polyelectrolyte gels: Thermodynamics, swelling, scattering, and elasticity. Chem. Phys. Rev. 2021, 2, 041309. [Google Scholar] [CrossRef]
- Dubrovskii, S.A.; Lagutina, M.A.; Kazanskii, K.S. Method of measuring the swelling pressure of superabsorbent gels. Polymer Gels Netw. 1994, 2, 49–58. [Google Scholar] [CrossRef]
- Young, M.H.; Wierenga, P.J.; Warrick, A.W.; Hofmann, L.L.; Musil, S.A. Variability of wetting front velocities during a field-scale infiltration experiment. Water Resour. Res. 1999, 35, 3079–3087. [Google Scholar] [CrossRef]
- Montesano, F.F.; Parente, A.; Santamaria, P.; Sannino, A.; Serio, F. Biodegradable superabsorbent hydrogel increases water retention properties of growing media and plant growth. Agric. Agric. Sci. Procedia 2015, 4, 451–458. [Google Scholar] [CrossRef]
- Zucconi, F.; Monaco, A.; Forte, M.; de Bertoldi, M. Phytotoxins during the stabilization of organic matter. In Composting of Agricultural and Other Wastes; Gasser, J.K.R., Ed.; Elsevier Applied Science Publishers: Amsterdam, The Netherlands, 1985. [Google Scholar]
- Molchanov, V.S.; Shibaev, A.V.; Karamov, E.V.; Larichev, V.F.; Kornilaeva, G.V.; Fedyakina, I.T.; Turgiev, A.S.; Philippova, O.E.; Khokhlov, A.R. Antiseptic polymer–surfactant complexes with long-lasting activity against SARS-CoV-2. Polymers 2022, 14, 2444. [Google Scholar] [CrossRef]
- He, J.; Liu, S.; Li, L.; Piao, G. Lyotropic liquid crystal behavior of carboxylated cellulose nanocrystals. Carbohydr. Polym. 2017, 164, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Argun, A.; Can, V.; Altun, U.; Okay, O. Nonionic double and triple network hydrogels of high mechanical strength. Macromolecules 2014, 47, 6430–6440. [Google Scholar] [CrossRef]
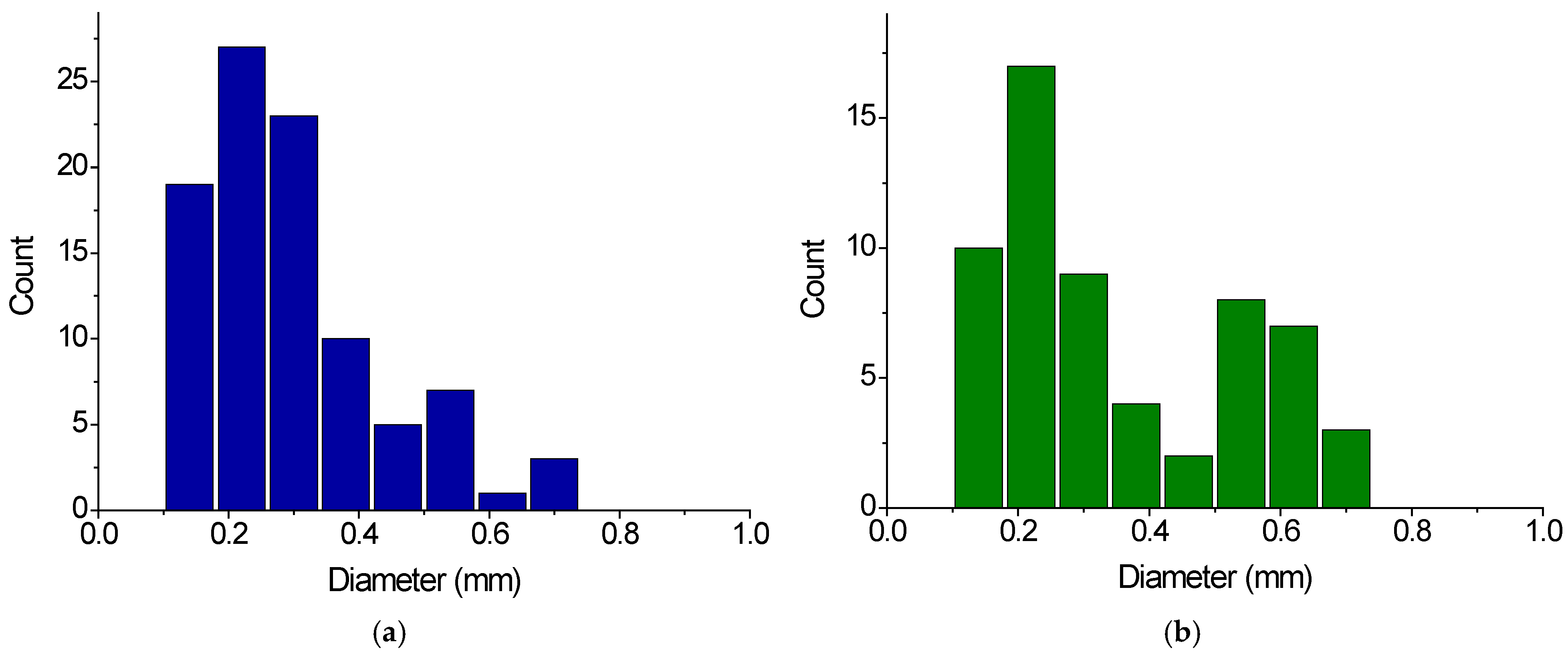
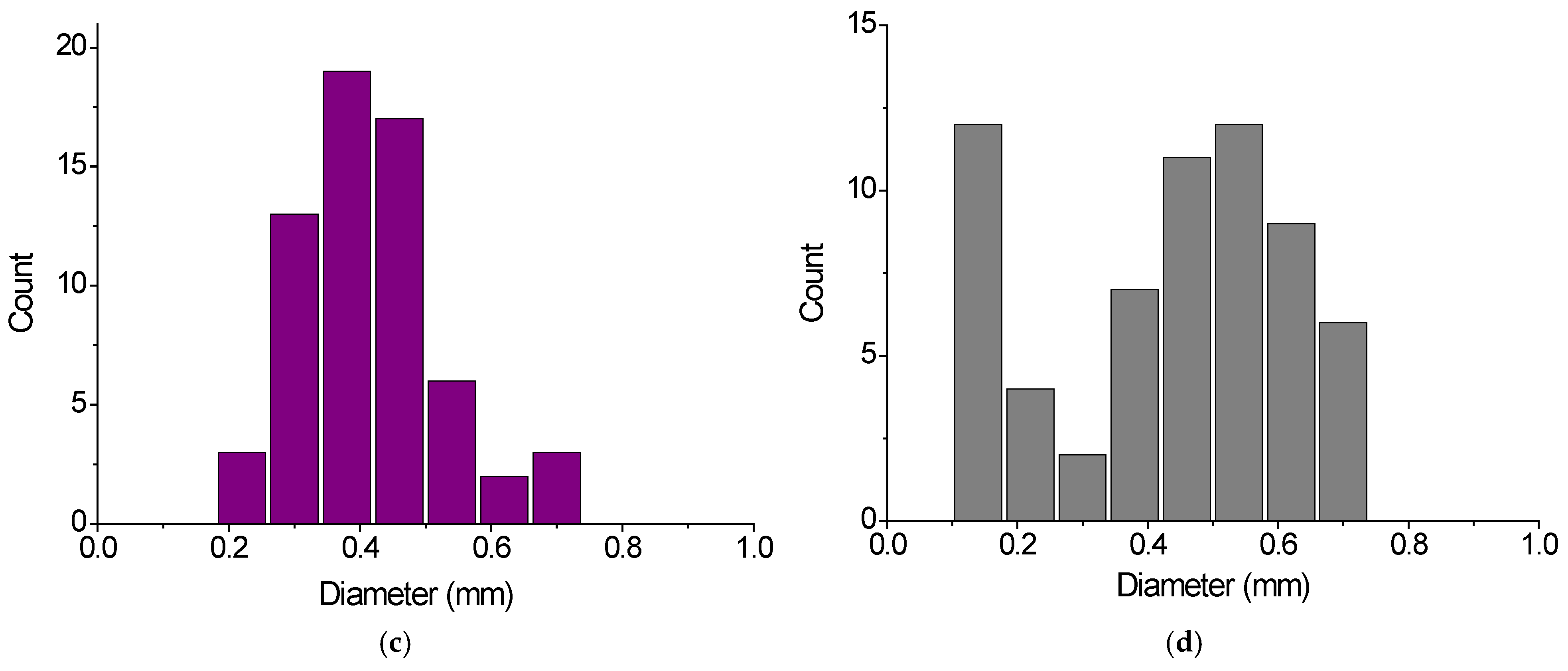
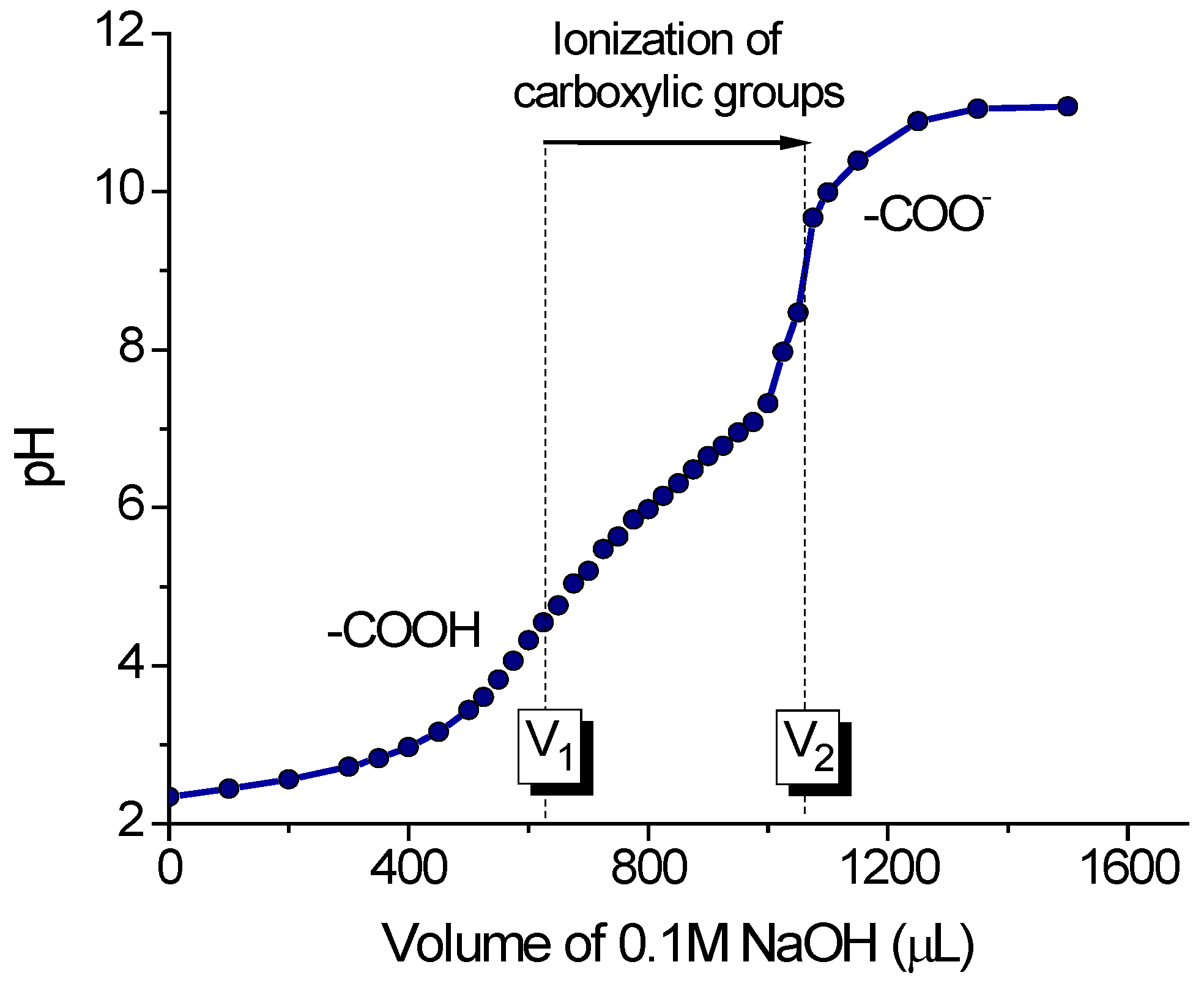
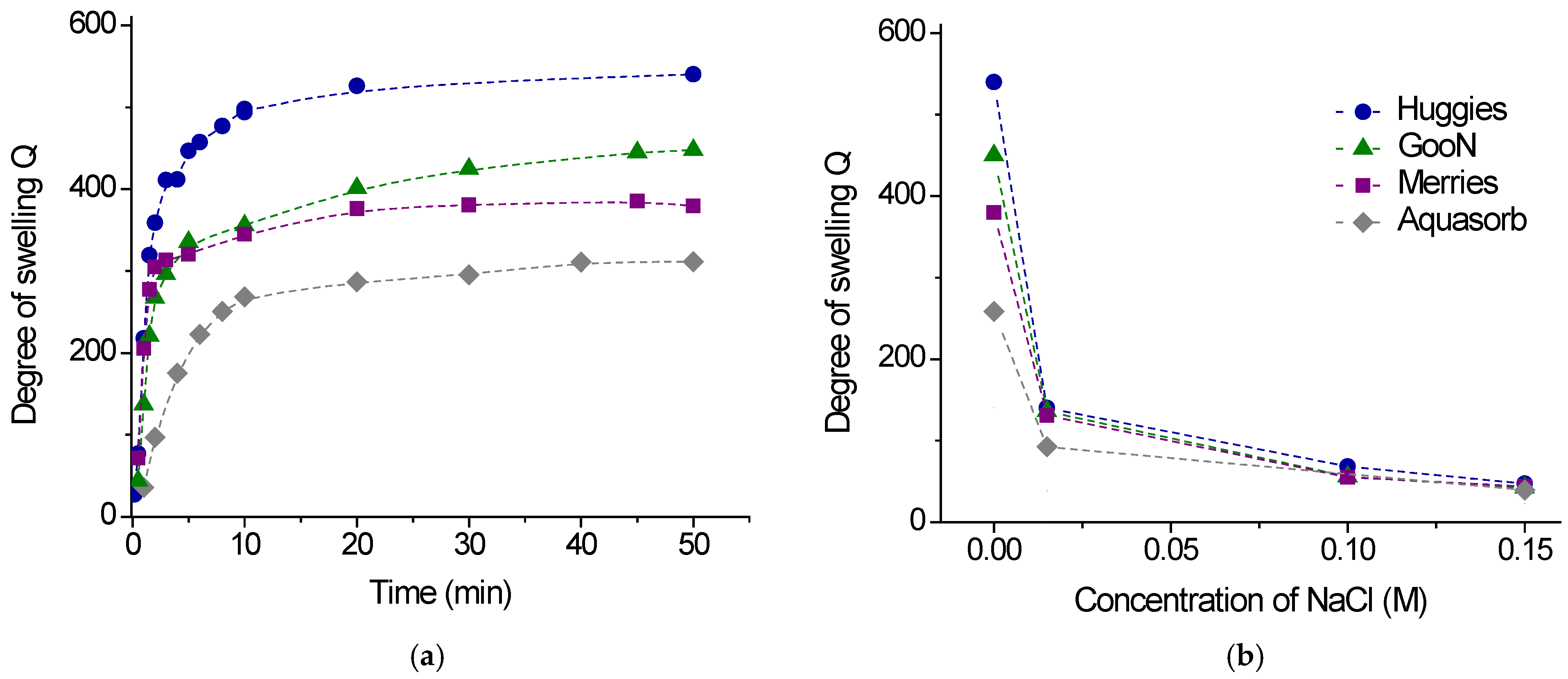
| Gel Sample | Application | Size of Dry Gel Particles, mm | Content of Charged COO- Groups, mmol/g | |
|---|---|---|---|---|
| Small Size Fraction | Large Size Fraction | |||
| Huggies | diapers | 0.2–0.25 | - | 3.6 |
| GooN | diapers | 0.2–0.25 | 0.55–0.6 | 3.7 |
| Merries | diapers | - | 0.4 | 3.5 |
| Aquasorb | agriculture | 0.15 | 0.55 | 1.8 |
| Gel Sample | Equilibrium Degree of Swelling | Maximum Swelling Pressure *, kPa | |
|---|---|---|---|
| Swelling in Sand Pores * | Free Swelling | ||
| Huggies | 100 ± 10 | 540 ± 25 | 12 ± 1 |
| GooN | 91 ± 9 | 450 ± 20 | 20 ± 2 |
| Merries | 92 ± 9 | 380 ± 20 | 26 ± 2 |
| Aquasorb | 100 ± 10 | 310 ± 15 | 23 ± 2 |
| PAAm/SA-1 | 90 ± 9 | 420 ± 20 | 5 ± 1 |
| PAAm/SA-2 | 96 ± 9 | 140 ± 10 | 12 ± 1 |
| Gel Sample | Field Water Capacity FWC, % | Wilting Point WP, % | Available Water Range AWR, % |
|---|---|---|---|
| Huggies | 37 | 21 | 16 ± 2 |
| GooN | 33 | 22 | 11 ± 2 |
| Merries | 28 | 15 | 13 ± 2 |
| Aquasorb | 35 | 22 | 13 ± 2 |
| No gel * | 4.1 | 0.4 | 3.7 ± 0.5 |
| Gel Sample | Total Fresh Weight, g | Shoot Length, mm | Germination Percentage, % | Germination Index, % |
|---|---|---|---|---|
| Huggies | 2.1 ± 0.2 | 42 ± 2 | 62 ± 2 | 137 ± 10 |
| GooN | 2.5 ± 0.2 | 52 ± 3 | 58 ± 2 | 158 ± 12 |
| Merries | 2.3 ± 0.2 | 44 ± 2 | 72 ± 3 | 163 ± 12 |
| Aquasorb | 2.0 ± 0.2 | 59 ± 3 | 54 ± 2 | 171 ± 14 |
| No gel * | 1.3 ± 0.1 | 37 ± 2 | 50 ± 2 | 100 |
| Gel Sample | C, % | H, % | N, % |
|---|---|---|---|
| Huggies | 37.67 | 4.42 | 0 |
| GooN | 38.34 | 4.55 | 0 |
| Merries | 33.67 | 5.25 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishkhanova, K.B.; Molchanov, V.S.; Prokopiv, I.V.; Khokhlov, A.R.; Philippova, O.E. The Potential for Reusing Superabsorbent Polymer from Baby Diapers for Water Retention in Agriculture. Gels 2025, 11, 795. https://doi.org/10.3390/gels11100795
Shishkhanova KB, Molchanov VS, Prokopiv IV, Khokhlov AR, Philippova OE. The Potential for Reusing Superabsorbent Polymer from Baby Diapers for Water Retention in Agriculture. Gels. 2025; 11(10):795. https://doi.org/10.3390/gels11100795
Chicago/Turabian StyleShishkhanova, Kamilla B., Vyacheslav S. Molchanov, Ilya V. Prokopiv, Alexei R. Khokhlov, and Olga E. Philippova. 2025. "The Potential for Reusing Superabsorbent Polymer from Baby Diapers for Water Retention in Agriculture" Gels 11, no. 10: 795. https://doi.org/10.3390/gels11100795
APA StyleShishkhanova, K. B., Molchanov, V. S., Prokopiv, I. V., Khokhlov, A. R., & Philippova, O. E. (2025). The Potential for Reusing Superabsorbent Polymer from Baby Diapers for Water Retention in Agriculture. Gels, 11(10), 795. https://doi.org/10.3390/gels11100795






