Thermal Insulation and Fireproof Aerogel Composites for Automotive Batteries
Abstract
1. Introduction
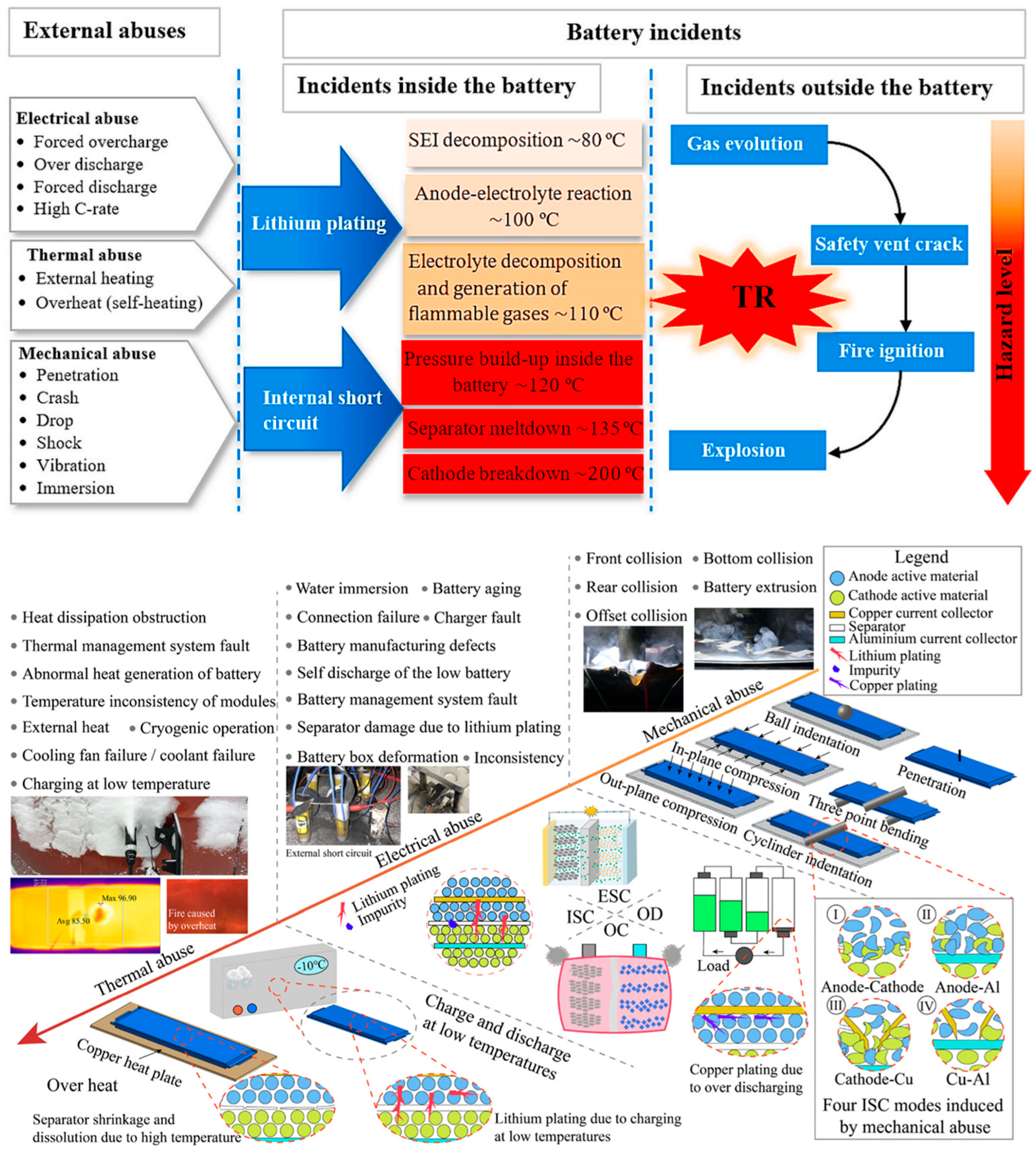
2. Thermal Runaway Phenomenon and Thermal Protection Measures of Automotive Batteries
2.1. Thermal Runaway of Automotive Batteries
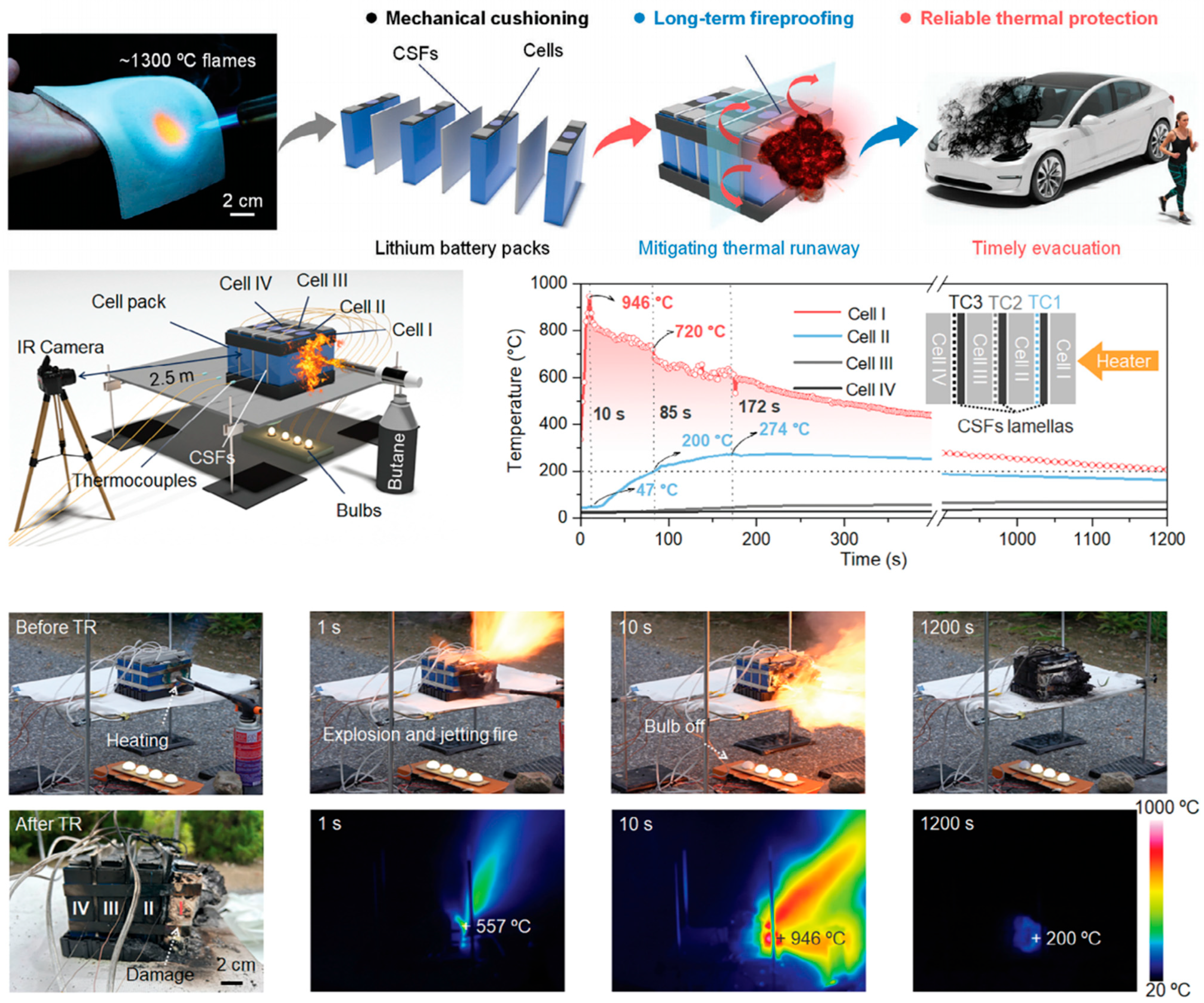
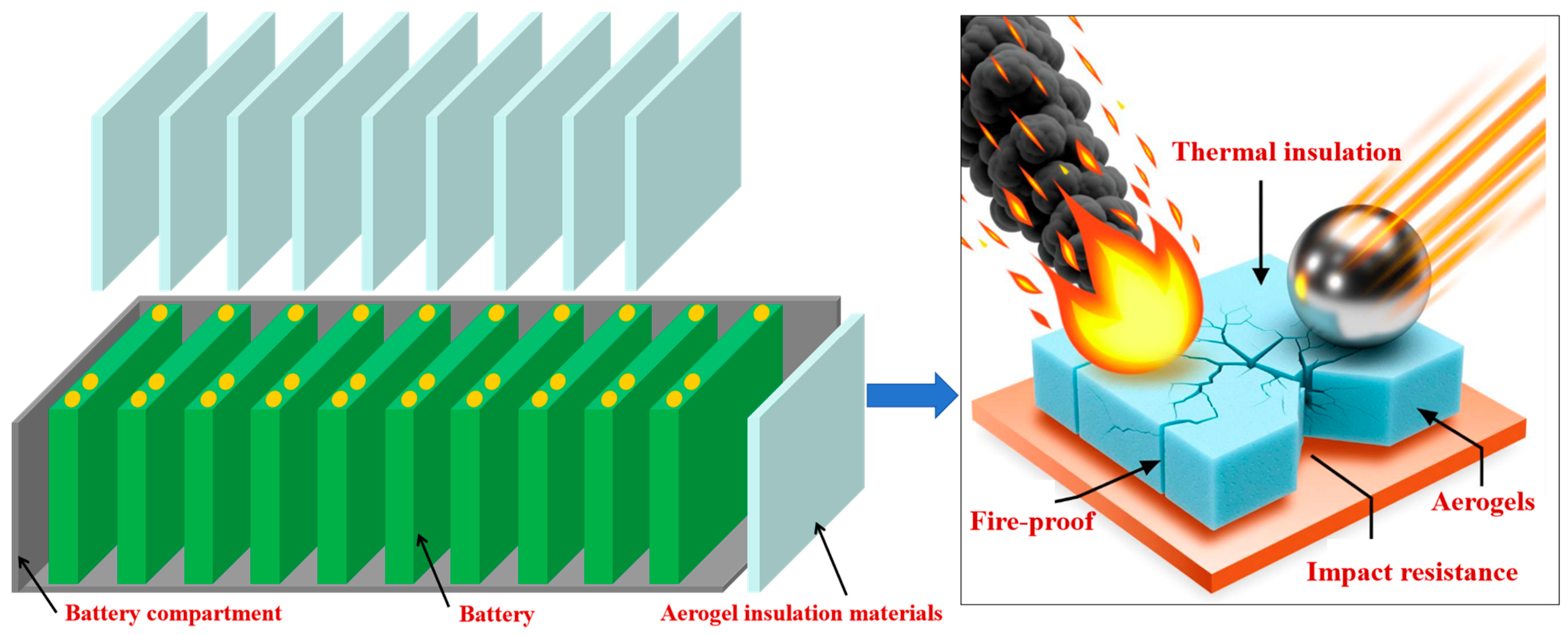
2.2. Thermal Protection of Automotive Batteries
3. Heat-Insulating and Fire-Resistant Aerogel Composite Materials
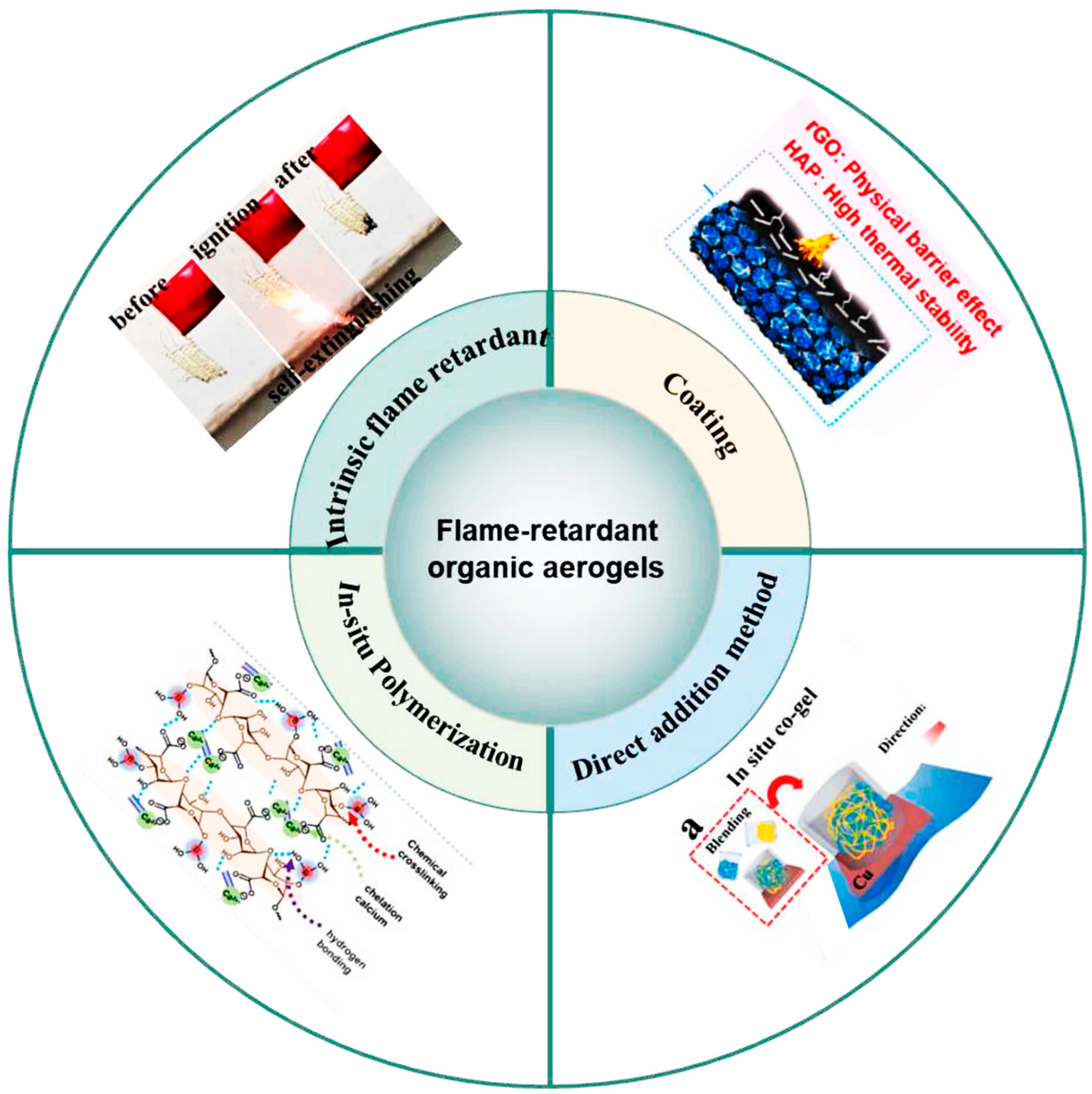
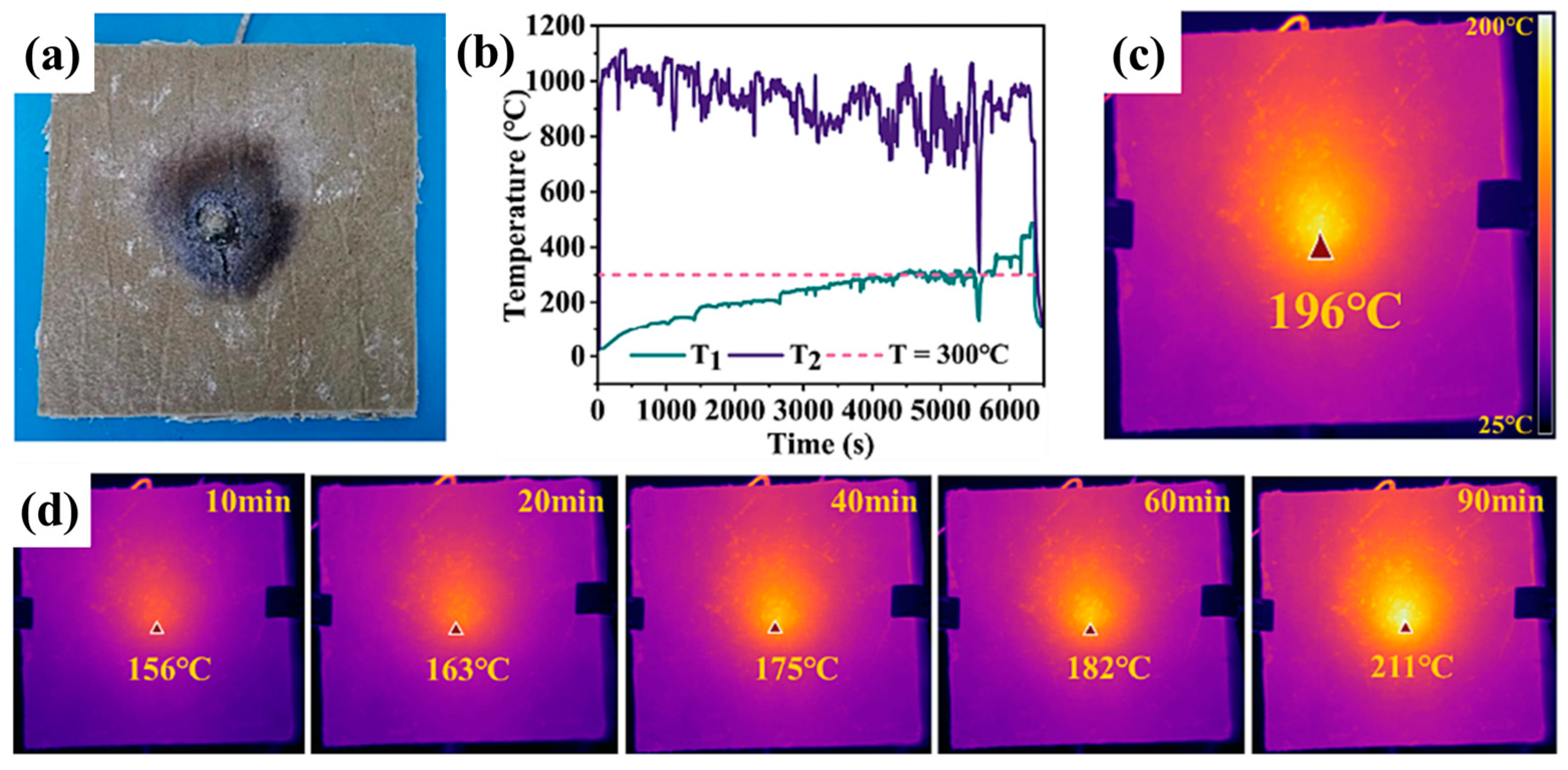
3.1. Organic Aerogels and Their Composite Materials Are Used for Protecting Against Battery Thermal Runaway
3.2. Inorganic Aerogels and Their Composite Materials for Battery Thermal Runaway Protection
4. Conclusions and Prospects
4.1. Conclusions
4.2. Prospects
- Development of low-cost and green preparation processes: Currently, the cost of aerogels, especially high-performance organic aerogels, remains relatively high. In the future, it is necessary to further develop large-scale preparation technologies with widely available raw materials, shortened process flows (such as complete atmospheric drying technology), and lower energy consumption to reduce the overall cost and meet the strict cost control requirements of the automotive industry. Comparing the current production process of aerogel, it can be seen that the production equipment and technical route of aerogel have shown a pattern of diversified development. Supercritical drying production lines can produce the most high-performance aerogel, but the high cost restricts its wide application. Atmospheric drying production lines have successfully realized low-cost, large-scale and green aerogel production through technological innovation, which has greatly promoted the industrialization process and market application of this material, making them the mainstream choice in the current civil and industrial fields. It is estimated that atmospheric drying technology is expected to reduce the production cost of aerogel by 20–40%. The production lines and surface functionalization equipment of various composite aerogel products meet the specific needs of the market for material morphology, functionality and application convenience.
- Design of organic–inorganic hybrid composite structures: Pure inorganic or organic aerogels each have their advantages and disadvantages. In the future, efforts should be made to design organic–inorganic hybrid aerogels at the microscale or multi-layer composite structures at the macroscale. For example, by combining the flexibility and hydrophobicity of organic components with the extremely high temperature resistance of inorganic components, intelligent composite materials that are “rigid yet flexible” and integrate multiple functions (such as heat insulation, flame retardancy, buffering, and sound absorption) can be developed.
- Research on full-scenario adaptability and long-term reliability: The internal environment of battery packs is complex, with conditions such as vibration, compression, and cold and hot cycles. It is necessary to deeply study the performance degradation laws and failure mechanisms of aerogel composites under long-term mechanical stress, wet heat aging, and electrolyte corrosion and establish a reliability evaluation system that matches the entire life cycle of batteries.
- Standardization and integrated design: It is vital to promote the establishment of application standards for aerogel materials in the automotive battery field and standardize their performance testing and evaluation methods. At the same time, we must strengthen the integrated design of aerogels with battery pack structural components (such as Cell to Pack/Cell to Car, etc.) to maximize their protective value and space utilization efficiency from a system perspective.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wan, H.; Xu, J.; Wang, C. Designing electrolytes and interphases for high-energy lithium batteries. Nat. Rev. Chem. 2024, 8, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Innocenti, A.; Zarrabeitia, M.; Bresser, D.; Yang, Y.; Passerini, S. Layered Oxide Cathodes for Sodium-Ion Batteries: Storage Mechanism, Electrochemistry, and Techno-economics. Acc. Chem. Res. 2023, 56, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Kumar Thakur, A.; Sathyamurthy, R.; Velraj, R.; Saidur, R.; Pandey, A.K.; Ma, Z.; Singh, P.; Hazra, S.K.; Wafa Sharshir, S.; Prabakaran, R.; et al. A state-of-the art review on advancing battery thermal management systems for fast-charging. Appl. Therm. Eng. 2023, 226, 120303. [Google Scholar] [CrossRef]
- Xu, J.; Cai, X.; Cai, S.; Shao, Y.; Hu, C.; Lu, S.; Ding, S. High-Energy Lithium-Ion Batteries: Recent Progress and a Promising Future in Applications. Energy Environ. Mater. 2023, 6, e12450. [Google Scholar] [CrossRef]
- Xin, S.; Zhang, X.; Wang, L.; Yu, H.; Chang, X.; Zhao, Y.-M.; Meng, Q.; Xu, P.; Zhao, C.-Z.; Chen, J.; et al. Roadmap for rechargeable batteries: Present and beyond. Sci. China Chem. 2024, 67, 13–42. [Google Scholar] [CrossRef]
- Yuan, S.; Lai, Q.; Duan, X.; Wang, Q. Carbon-based materials as anode materials for lithium-ion batteries and lithium-ion capacitors: A review. J. Energy Storage 2023, 61, 106716. [Google Scholar] [CrossRef]
- Rana, S.; Kumar, R.; Bharj, R.S. Current trends, challenges, and prospects in material advances for improving the overall safety of lithium-ion battery pack. Chem. Eng. J. 2023, 463, 142336. [Google Scholar] [CrossRef]
- Jaguemont, J.; Bardé, F. A critical review of lithium-ion battery safety testing and standards. Appl. Therm. Eng. 2023, 231, 121014. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, X.; Huang, W.; He, X.; Wang, L.; Ouyang, M. Challenges and Opportunities to Mitigate the Catastrophic Thermal Runaway of High-Energy Batteries. Adv. Energy Mater. 2023, 13, 2203841. [Google Scholar] [CrossRef]
- Liu, J.; Yadav, S.; Salman, M.; Chavan, S.; Kim, S.C. Review of thermal coupled battery models and parameter identification for lithium-ion battery heat generation in EV battery thermal management system. Int. J. Heat Mass Transf. 2024, 218, 124748. [Google Scholar] [CrossRef]
- Zheng, Y.; Che, Y.; Hu, X.; Sui, X.; Stroe, D.-I.; Teodorescu, R. Thermal state monitoring of lithium-ion batteries: Progress, challenges, and opportunities. Prog. Energy Combust. Sci. 2024, 100, 101120. [Google Scholar] [CrossRef]
- Cui, Z.; Manthiram, A. Thermal Stability and Outgassing Behaviors of High-nickel Cathodes in Lithium-ion Batteries. Angew. Chem. Int. Ed. 2023, 62, e202307243. [Google Scholar] [CrossRef]
- Du, H.; Wang, Y.; Kang, Y.; Zhao, Y.; Tian, Y.; Wang, X.; Tan, Y.; Liang, Z.; Wozny, J.; Li, T.; et al. Side Reactions/Changes in Lithium-Ion Batteries: Mechanisms and Strategies for Creating Safer and Better Batteries. Adv. Mater. 2024, 36, 2401482. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.; Chung, Y.-H.; Liu, J.; Bai, J.; Zhou, Y.; Chen, S.; Wang, Z.; Shu, C.-M. Characteristics and mechanisms of as well as evaluation methods and countermeasures for thermal runaway propagation in lithium-ion batteries. Prog. Energy Combust. Sci. 2025, 108, 101209. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, X.; Pang, Q.; Fowler, M.; Lian, Y.; Ouyang, M.; Burke, A.F. Battery safety: Machine learning-based prognostics. Prog. Energy Combust. Sci. 2024, 102, 101142. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, X.; Wang, X.; Chen, S.; Zhu, J.; Dai, H. Evolution mechanism and non-destructive assessment of thermal safety for lithium-ion batteries during the whole lifecycle. Nano Energy 2024, 126, 109621. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Deng, J.; Yang, W.; Zeng, Y.; Rao, Z.; Kong, F.; Li, Y. The flame retardant mechanism of composite phase change materials for battery thermal safety: A review. Energy Storage Mater. 2025, 80, 104344. [Google Scholar] [CrossRef]
- Alawi, A.; Saeed, A.; Sharqawy, M.H.; Al Janaideh, M. A Comprehensive Review of Thermal Management Challenges and Safety Considerations in Lithium-Ion Batteries for Electric Vehicles. Batteries 2025, 11, 275. [Google Scholar] [CrossRef]
- Gharehghani, A.; Rabiei, M.; Mehranfar, S.; Saeedipour, S.; Mahmoudzadeh Andwari, A.; García, A.; Reche, C.M. Progress in battery thermal management systems technologies for electric vehicles. Renew. Sustain. Energy Rev. 2024, 202, 114654. [Google Scholar] [CrossRef]
- Rojas-Alva, U.; Mancini, L.; Mauko Pranjić, A.; Marini, E.; Bozzini, B. On thermal safety characteristics of rechargeable alkaline batteries based on zinc and manganese dioxide. Process Saf. Environ. Prot. 2025, 199, 107175. [Google Scholar] [CrossRef]
- Olona, A.; Castejón, L.; Valladares, D. Influence of Traction Battery Arrangement on Risk of Thermal Runaway and Loads Suffered by Electric Vehicle Occupant during Side Collision. Energies 2023, 16, 6892. [Google Scholar] [CrossRef]
- Fu, P.; Zhao, L.; Wang, X.; Sun, J.; Xin, Z. A Review of Cooling Technologies in Lithium-Ion Power Battery Thermal Management Systems for New Energy Vehicles. Processes 2023, 11, 3450. [Google Scholar] [CrossRef]
- Bao, J.; Mao, Y.; Zhang, Y.; Xu, H.; Jiang, Y.; Yang, Y. Critical Review of Temperature Prediction for Lithium-Ion Batteries in Electric Vehicles. Batteries 2024, 10, 421. [Google Scholar] [CrossRef]
- Cui, Y.; Shen, X.; Zhang, H.; Yin, Y.; Yu, Z.; Shi, D.; Fang, Y.; Xu, R. Intrinsic Safety Risk Control and Early Warning Methods for Lithium-Ion Power Batteries. Batteries 2024, 10, 62. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, C.; Geng, M.; Meng, H. A review on models to prevent and control lithium-ion battery failures: From diagnostic and prognostic modeling to systematic risk analysis. J. Energy Storage 2023, 74, 109230. [Google Scholar] [CrossRef]
- Feng, X.; Wong, S.K.; Chen, T.; Ouyang, M. An automatic identification method of thermal physical parameter for lithium-ion batteries suffering from thermal runaway. J. Energy Storage 2024, 83, 110358. [Google Scholar] [CrossRef]
- Deng, Y.; Hao, Y.; Wang, H.; Li, W.; Qin, Q.; Pan, B.; Zhang, C. Effect of temperature and atmosphere on the fracture toughness and failure mechanisms of two-dimensional plain-woven SiCf/SiC composites: Experiments and modeling. Acta Mech. Sin. 2025, 41, 124333. [Google Scholar]
- Luo, Z.-H.; Zhang, D.; Guo, J.-X.; Jiang, F.; Shen, N.-L.; Du, Y.-F.; Jiang, Z.-J.; Wang, T.; Liu, X.; Cheng, X.-B.; et al. Recent progress on the materials design towards thermally safe sodium-ion batteries. J. Energy Chem. 2025, 102, 555–575. [Google Scholar]
- Monteiro, I.F.; Pinto, R.S.; Silva, M.M.; Fidalgo-Marijuan, A.; Costa, C.M.; Lanceros-Méndez, S.; Gonçalves, R. Lithium-ion battery high performance cathode electrode based on LiFePO4 and thermal sensitive microspheres with thermal shutdown properties. J. Power Sources 2024, 614, 234956. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, J.; Qin, J.; Zhong, Y.L.; Zhang, S.; Wang, H.; Bell, J.; Guo, Z.; Song, P. Pathways to Next-Generation Fire-Safe Alkali-Ion Batteries. Adv. Sci. 2023, 10, 2301056. [Google Scholar] [CrossRef]
- Nema Puneet, K.; Vijaya Muthukumar, P.; Thangavel, R. Recent Advancements and Future Prospects in Lithium-Ion Battery Thermal Management Techniques. Energy Storage 2024, 6, e70076. [Google Scholar] [CrossRef]
- Han, L.; Cao, Y.; Liao, C.; Kan, Y.; Hu, Y. Novel silica -coated cathode to realize internal-short-circuit proof, high-interface-stabilized, high-safety lithium batteries. J. Power Sources 2024, 602, 234273. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, F.; Gao, Z.; Liu, M.; Wang, J.; Kou, Z.; Lin, Y.; Li, Y.; Gao, L.; Chen, Y.; et al. Review of mechanical abuse related thermal runaway models of lithium-ion batteries at different scales. J. Energy Storage 2023, 64, 107145. [Google Scholar] [CrossRef]
- He, M.; Chartouni, D.; Landmann, D.; Colombi, S. Safety Aspects of Stationary Battery Energy Storage Systems. Batteries 2024, 10, 418. [Google Scholar] [CrossRef]
- Wang, C.; Bai, L.; Xu, H.; Qin, S.; Li, Y.; Zhang, G. A Review of High-Temperature Aerogels: Composition, Mechanisms, and Properties. Gels 2024, 10, 286. [Google Scholar] [CrossRef]
- Jin, R.; Zhou, Z.; Liu, J.; Shi, B.; Zhou, N.; Wang, X.; Jia, X.; Guo, D.; Xu, B. Aerogels for Thermal Protection and Their Application in Aerospace. Gels 2023, 9, 606. [Google Scholar] [CrossRef]
- Ge, L.; Shang, S.; Ma, Y.; Koudama, T.D.; Yuan, K.; Liu, W.; Cui, S. Overview of Aerogels for Thermal Insulation. ACS Appl. Mater. Interfaces 2025, 17, 26091–26116. [Google Scholar] [CrossRef]
- Parale, V.G.; Kim, T.; Choi, H.; Phadtare, V.D.; Dhavale, R.P.; Kanamori, K.; Park, H.-H. Mechanically Strengthened Aerogels through Multiscale, Multicompositional, and Multidimensional Approaches: A Review. Adv. Mater. 2024, 36, 2307772. [Google Scholar] [CrossRef]
- Oikonomou, V.K.; Dreier, T.; Sandéhn, A.; Mohammadi, M.; Christensen, J.L.; Tybrandt, K.; Dahl, A.B.; Dahl, V.A.; Bech, M.; Stavrinidou, E. Elucidating the Bulk Morphology of Cellulose-Based Conducting Aerogels with X-Ray Microtomography. Adv. Mater. Technol. 2023, 8, 2300550. [Google Scholar] [CrossRef]
- Sozcu, S.; Venkataraman, M.; Wiener, J.; Tomkova, B.; Militky, J.; Mahmood, A. Incorporation of Cellulose-Based Aerogels into Textile Structures. Materials 2024, 17, 27. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Collado, P.A.; González-Martínez, C.; Chiralt, A. Producing Aerogels from Rice Straw Cellulose Obtained by a Green Method and Its Starch Blending. Polymers 2025, 17, 1103. [Google Scholar] [CrossRef]
- Pinilla-Peñalver, E.; del Fresno, Ó.; Cantero, D.; Moreira, A.; Gomes, F.; Miranda, F.; Oliveira, M.; Ornelas, M.; Sánchez-Silva, L.; Romero, A. Enhancing Flame-Retardant Properties of Polyurethane Aerogels Doped with Silica-Based Particles. Gels 2024, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liao, R.; Jia, R.; Liu, Y.; Wu, D.; Chang, S.; Zhang, N.; Gao, G.; Wang, X.; Hu, D.; et al. A novel combustion drying synthesis route of 3D WO3–SiO2 composite aerogels for enhanced adsorption and visible light photocatalytic activity. J. Non-Cryst. Solids 2023, 609, 122259. [Google Scholar] [CrossRef]
- Hou, X.; Chen, J.; Chen, Z.; Yu, D.; Zhu, S.; Liu, T.; Chen, L. Flexible Aerogel Materials: A Review on Revolutionary Flexibility Strategies and the Multifunctional Applications. ACS Nano 2024, 18, 11525–11559. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xue, J.-Y.; Ju, Y.-Y.; Gao, Y.-J.; Cheng, X.-W.; Guan, J.-P. Multifunctional nanofiber aerogels for environmental applications: Oilwater separation, thermal insulation, fireproofing. J. Environ. Chem. Eng. 2025, 13, 115541. [Google Scholar] [CrossRef]
- Zhan, W.; Mo, J.; Shi, F.; Xu, Z.; Chen, L.; Li, L.; Zhou, R.; Chen, M.; Jiang, J. Preparation and thermo-mechanical properties of modified gelatin reinforced SiO2 aerogels. J. Non-Cryst. Solids 2024, 646, 123222. [Google Scholar] [CrossRef]
- Ding, Y.; Song, X.; Shao, F.; Li, X.; Gu, G.; Wei, Z.; Wang, J. Hierarchical Cellular Engineering toward Exceptional Mechanical and Thermal Insulating Aerogels. ACS Appl. Mater. Interfaces 2025, 17, 21875–21885. [Google Scholar] [CrossRef]
- Mandić, V.; Bafti, A.; Panžić, I.; Radovanović-Perić, F. Bio-Based Aerogels in Energy Storage Systems. Gels 2024, 10, 438. [Google Scholar] [CrossRef]
- Wang, A.; Jiang, J.; Liu, Y.; Wu, J.; Ma, Y.; Li, M.; Lu, W.; Hao, Y.; Shi, Y. Research progress of aerogel used in lithium-ion power batteries. J. Loss Prev. Process Ind. 2024, 92, 105433. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, P.; Wang, Y.; Xu, Y.; Xu, H.; Xi, K. A Versatile Method for Preparation of BrCOFs Aerogels and Efficient Functionalization via Suzuki–Miyaura Reaction. Small Methods 2025, 9, 2401373. [Google Scholar] [CrossRef]
- Zhan, W.; Tang, L.; Xu, Z.; Chen, L.; Li, L.; Zhou, R.; Chen, M.; Kong, Q.; Jiang, J. Synthesis of Lightweight and High-Strength Polyimide/Gelatin Composite Aerogels: A Thermal Insulation Material for Preventing the Thermal Runaway of Lithium Batteries. ACS Appl. Mater. Interfaces 2025, 17, 41187–41197. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Zhang, Y.; Zhang, J.; Wang, R.; Gong, Y.; He, B.; Wang, H.; Jin, J. Constructing MXene hydrogels and aerogels for rechargeable supercapacitors and batteries. J. Mater. Chem. C 2023, 11, 2414–2429. [Google Scholar] [CrossRef]
- Ma, H.; Liu, H.; Lv, T.; Xu, Y.; Zhou, X.; Zhang, L. High-Energy Laser Protection Performance of Fibrous Felt-Reinforced Aerogels with Hierarchical Porous Architectures. ACS Appl. Mater. Interfaces 2024, 16, 25568–25580. [Google Scholar] [CrossRef] [PubMed]
- Sihao, J.; Xia, C.; Hao, M.; Liu, W.; Ma, C.; Miao, Y. Preparation and Properties of Al2O3–SiO2 Aerogel Composite Mullite Fiber Felt. Glass Phys. Chem. 2024, 50, 511–520. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, J.; Ren, C.; Zhao, S.; Zhang, X.; Fan, J. Facile preparation of high-strength SiC/C aerogels from pre-reacted resorcinol–formaldehyde and siloxane. J. Ind. Eng. Chem. 2024, 134, 75–83. [Google Scholar] [CrossRef]
- Guo, J.; Chen, J.; Li, J.; Hafsa Chen, H. Structural design of the multi-layer SiC nanofiber composite aerogel felt for applications in high-temperature thermal-insulating refractories. J. Eur. Ceram. Soc. 2025, 45, 117370. [Google Scholar] [CrossRef]
- Abuhay, A.; Tadesse, M.G.; Berhanu, B.; Malengier, B.; Langenhove, L.V. Advancements in Clothing Thermal Comfort for Cold Intolerance. Fibers 2025, 13, 13. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, H.; Lei, C.; Liu, Y.; Li, W. Review of Lightweight, High-Temperature Thermal Insulation Materials for Aerospace. Materials 2025, 18, 2383. [Google Scholar] [CrossRef]
- Tian, Y.; Ding, R.; Yoon, S.S.; Zhang, S.; Yu, J.; Ding, B. Recent Advances in Next-Generation Textiles. Adv. Mater. 2025, 37, 2417022. [Google Scholar] [CrossRef]
- Sang, C.; Le, K.; Chen, K.; Luo, Q.; Li, H.; Fang, Y.; Ai, X. Temperature-switchable electrolyte with desirable phase transition behavior for thermal protection of lithium-ion batteries. Mater. Sci. Eng. R Rep. 2025, 163, 100947. [Google Scholar] [CrossRef]
- Chang, C.-H.; Gorin, C.; Zhu, B.; Beaucarne, G.; Ji, G.; Yoshida, S. Lithium-Ion Battery Thermal Event and Protection: A Review. SAE Int. J. Electrified Veh. 2023, 13, 357–397. [Google Scholar] [CrossRef]
- Shekari, M.; Naderi, G.; Khonakdar, H.A. Flexible Polymer Foams in Thermal Management Technologies: Recent Advances and Applications. In Polymer Engineering & Science; Wiley Online Library: Hoboken, NJ, USA, 2025. [Google Scholar] [CrossRef]
- Gaidhani, A.; Tribe, L.; Charpentier, P. Polystyrene carbon composite foam with enhanced insulation and fire retardancy for a sustainable future: Critical review. J. Cell. Plast. 2023, 59, 419–453. [Google Scholar] [CrossRef]
- Zhou, X.; Wei, X.; Peng, Y.; Wang, X.; Liang, Y.; Chen, S.; Niu, X. Progress on the Structure and Application of Porous Polyurethane Materials. Macromol. Rapid Commun. 2025, e00294. [Google Scholar] [CrossRef] [PubMed]
- Weldemhret, T.G.; Park, Y.T.; Song, J.I. Recent progress in surface engineering methods and advanced applications of flexible polymeric foams. Adv. Colloid Interface Sci. 2024, 326, 103132. [Google Scholar] [CrossRef]
- Liang, X.; Sun, Q.; Zhang, X.; Hu, Z.; Liu, M.; Gu, P.; Yang, X.; Zu, G. Advanced Stretchable Aerogels and Foams for Flexible Electronics and Beyond. Adv. Funct. Mater. 2024, 34, 2408707. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, Y.; Xu, X.; Dai, J.; Zhang, G.; Zhang, J.; Ma, X.; Gu, W.; Zhang, W. A novel promotion strategy for flexible and compressive performance of Al2O3 felt via aramid fiber composite. Ceram. Int. 2024, 50, 5179–5186. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Sun, C.; Zhang, P.; Xia, X. Experimental investigation on temperature-dependent effective thermal conductivity of ceramic fiber felt. Int. J. Therm. Sci. 2024, 200, 108965. [Google Scholar] [CrossRef]
- Zhang, S.-N.; Pang, H.-Q.; Fan, T.-H.; Huang, Z.; Guo, J.F.; Wu, X. Phase change extinction fiber doped aerogel vacuum insulation panels for high temperature insulation. Int. Commun. Heat Mass Transf. 2025, 162, 108650. [Google Scholar] [CrossRef]
- Yue, J.; Liu, J.; Song, X.; Yan, C. Research on the design and thermal performance of vacuum insulation panel composite insulation materials. Case Stud. Therm. Eng. 2024, 64, 105437. [Google Scholar] [CrossRef]
- Liang, W.; Di, X.; Zheng, S.; Wu, L.; Zhang, J. A study on thermal bridge effect of vacuum insulation panels (VIPs). J. Build. Eng. 2023, 71, 106492. [Google Scholar] [CrossRef]
- Chen, Z.; Miao, J.; Chen, H.; Li, H.; Wang, Q.; Zhang, H.; Yang, Y. All-in-one design and fabrication of vacuum insulation panels for ultra-efficient pipeline thermal management. Appl. Therm. Eng. 2025, 273, 126501. [Google Scholar] [CrossRef]
- Lei, B.; Shen, X.; Chen, W.; Hong, Z.; Wang, M. Orderly hybrid aerogel-based hydrate salt for wide-temperature range thermal regulation and flame retardancy in Li-ion batteries. J. Mater. Chem. A 2025, 13, 26458–26466. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, D.; Fu, H.; Wang, A.; Cao, H.; Zong, P. Conductive copper ion-reinforced sodium alginate aerogel based free-standing Si anode for Li-ion storage. Solid State Ion. 2024, 409, 116529. [Google Scholar] [CrossRef]
- Li, Z.; Hao, Y.; Cao, F.; Zhang, Y.; Wang, Y.; Zhang, S.; Tang, B. Double-Network Aerogel-Based Composite Phase Change Material Inspired by Beaver Damming for All-Weather Thermal Management of Lithium Batteries. ACS Appl. Mater. Interfaces 2025, 17, 16072–16084. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ma, H.; Chen, R.; Yan, J.; Wang, G. Experimental and Theoretical Indagation of Binder-Free N-Graphene Coupling Vanadium Tetrasulfide Aerogel Cathode for Promoting Aqueous Zn-Ion Storage. ACS Appl. Energy Mater. 2023, 6, 3808–3821. [Google Scholar] [CrossRef]
- Gültekin, S.Y.; Güler, A.; Kuruahmet, D.; Güngör, H.; Singil, M.M.; Uzun, E.; Akbulut, H.; Güler, M.O. Graphene aerogel-supported Na3V2(PO4)3/C cathodes for sodium-ion batteries. Diam. Relat. Mater. 2023, 139, 110399. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Zhao, X.; Li, N.; Guo, X.; Zhao, L.; Yin, Y. Anisotropic composite aerogel with thermal insulation and flame retardancy from cellulose nanofibers, calcium alginate and boric acid. Int. J. Biol. Macromol. 2024, 267, 131450. [Google Scholar] [CrossRef]
- Cao, M.; Feng, Y.; Wang, D.; Xie, Y.; Gu, X.; Yao, J. Construction of oxygen vacancy-rich ZnO@carbon nanofiber aerogels as a free-standing anode for superior lithium storage. J. Colloid Interface Sci. 2023, 644, 177–185. [Google Scholar] [CrossRef]
- Shaik, S.; Kumari Jha, V.; Bae, G.; Kim, D. Flow-driven directional freeze-casting of graphene aerogels on tubular components for enhanced thermal energy management. Energy Convers. Manag. 2025, 325, 119389. [Google Scholar] [CrossRef]
- Wu, S.; Cao, S.; Xie, H.; Wu, Z.; He, X. Enhanced thermal performance of 3D hybrid graphene aerogel encapsulating paraffin for battery thermal management. Int. Commun. Heat Mass Transf. 2024, 156, 107618. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Zhao, H.; Bo, X.; Zhang, Z.; Wang, L.; Daoud, W.A.; He, X. Insight Into Puncture-Induced Thermal Runaway in Lithium-Ion Batteries to Reduce Fire Risks in Electric Vehicle Collisions. In Battery Energy; Wiley Online Library: Hoboken, NJ, USA, 2005; p. e70041. [Google Scholar] [CrossRef]
- Xu, L.; Wang, S.; Li, Y.; Li, Y.; Sun, J.; Zhao, F.; Wang, H.; Wang, Y.; Xu, C.; Feng, X. Thermal runaway propagation behavior and gas production characteristics of NCM622 battery modules at different state of charge. Process Saf. Environ. Prot. 2024, 185, 267–276. [Google Scholar] [CrossRef]
- Schöberl, J.; Schumacher, J.; Urban, R.; Lienkamp, M. Impedance-based thermal runaway detection and temperature estimation for single and parallel connected large-format automotive lithium-ion batteries. eTransportation 2025, 25, 100424. [Google Scholar]
- Divakaran, A.M.; Hamilton, D.; Manjunatha, K.N.; Minakshi, M. Design, Development and Thermal Analysis of Reusable Li-Ion Battery Module for Future Mobile and Stationary Applications. Energies 2020, 13, 1477. [Google Scholar] [CrossRef]
- Zuo, K.; Li, Z.; Liang, H.; Wang, Z.; Ouyang, T. An integrated scheme to prevent the propagation of Li-ion battery thermal runaway. Int. J. Heat Mass Transf. 2025, 241, 126725. [Google Scholar] [CrossRef]
- Chombo, P.V.; Laoonual, Y. A review of safety strategies of a Li-ion battery. J. Power Sources 2020, 478, 228649. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, C.; Li, J.; Xiong, R.; Pecht, M. Challenges and outlook for lithium-ion battery fault diagnosis methods from the laboratory to real world applications. eTransportation 2023, 17, 100254. [Google Scholar] [CrossRef]
- Meng, F.; Gao, J.; Zhang, M.; Li, D.; Liu, X. Enhanced Safety Performance of Automotive Lithium-Ion Batteries with Al2O3-Coated Non-Woven Separator. Batter. Supercaps 2021, 4, 146–151. [Google Scholar] [CrossRef]
- Cianciullo, M.; Vilardi, G.; Mazzarotta, B.; Bubbico, R. Simulation of the Thermal Runaway Onset in Li-Ion Cells—Influence of Cathode Materials and Operating Conditions. Energies 2022, 15, 4169. [Google Scholar] [CrossRef]
- Li, H.; Chen, G.; Yang, Y.; Shu, B.; Liu, Z.; Peng, J. Adversarial learning for robust battery thermal runaway prognostic of electric vehicles. J. Energy Storage 2024, 82, 110381. [Google Scholar] [CrossRef]
- Kang, H.C. Experiment on Extinguishing Thermal Runaway in a Scaled-Down Model of an Electric Vehicle Battery. Int. J. Automot. Technol. 2024, 25, 989–998. [Google Scholar] [CrossRef]
- He, D.; Wang, J.; Peng, Y.; Li, B.; Feng, C.; Shen, L.; Ma, S. Research advances on thermal runaway mechanism of lithium-ion batteries and safety improvement. Sustain. Mater. Technol. 2024, 41, e01017. [Google Scholar] [CrossRef]
- Kshetrimayum, K.S.; Yoon, Y.-G.; Gye, H.-R.; Lee, C.-J. Preventing heat propagation and thermal runaway in electric vehicle battery modules using integrated PCM and micro-channel plate cooling system. Appl. Therm. Eng. 2019, 159, 113797. [Google Scholar] [CrossRef]
- Schöberl, J.; Ank, M.; Schreiber, M.; Wassiliadis, N.; Lienkamp, M. Thermal runaway propagation in automotive lithium-ion batteries with NMC-811 and LFP cathodes: Safety requirements and impact on system integration. eTransportation 2024, 19, 100305. [Google Scholar] [CrossRef]
- Held, M.; Tuchschmid, M.; Zennegg, M.; Figi, R.; Schreiner, C.; Mellert, L.D.; Welte, U.; Kompatscher, M.; Hermann, M.; Nachef, L. Thermal runaway and fire of electric vehicle lithium-ion battery and contamination of infrastructure facility. Renew. Sustain. Energy Rev. 2022, 165, 112474. [Google Scholar] [CrossRef]
- Zhu, J.; Wierzbicki, T.; Li, W. A review of safety-focused mechanical modeling of commercial lithium-ion batteries. J. Power Sources 2018, 378, 153–168. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, C.; Wang, F.; Manthiram, A. Navigating thermal stability intricacies of high-nickel cathodes for high-energy lithium batteries. Nat. Energy 2025, 10, 490–501. [Google Scholar] [CrossRef]
- Ouyang, T.; Liu, B.; Xu, P.; Wang, C.; Ye, J. Electrochemical-thermal coupled modelling and multi-measure prevention strategy for Li-ion battery thermal runaway. Int. J. Heat Mass Transf. 2022, 194, 123082. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Z.; Liu, J.; Wang, N. Thermal management with fast temperature convergence based on optimized fuzzy PID algorithm for electric vehicle battery. Appl. Energy 2023, 352, 121936. [Google Scholar] [CrossRef]
- Kim, H.; Sahebzadeh, A.; Seifert, H.J.; Ziebert, C.; Friedl, J. Needle penetration studies on automotive lithium-ion battery cells: Influence of resistance between can and positive terminal on thermal runaway. J. Power Sources 2024, 592, 233902. [Google Scholar] [CrossRef]
- Meng, X.; Liu, Y.; Guan, M.; Qiu, J.; Wang, Z. A High-Energy and Safe Lithium Battery Enabled by Solid-State Redox Chemistry in a Fireproof Gel Electrolyte. Adv. Mater. 2022, 34, 2201981. [Google Scholar] [CrossRef]
- Cui, Y.; Wan, J.; Ye, Y.; Liu, K.; Chou, L.-Y.; Cui, Y. A Fireproof, Lightweight, Polymer–Polymer Solid-State Electrolyte for Safe Lithium Batteries. Nano Lett. 2020, 20, 1686–1692. [Google Scholar] [CrossRef]
- Sundaram, M.; Appadoo, D. Traditional salt-in-water electrolyte vs. water-in-salt electrolyte with binary metal oxide for symmetric supercapacitors: Capacitive vs. faradaic. Dalton Trans. 2020, 49, 11743–11755. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Feng, X.; Zhang, Q.; Chen, L.; Li, L.; Kong, Q.; Shi, F.; Chen, M.; Du, D.; Jiang, J. Effects of silica aerogel particles on performance of the coatings for new energy vehicle battery packs. J. Dispers. Sci. Technol. 2024, 1–11. [Google Scholar] [CrossRef]
- Wu, X.; Chai, F.; Yang, J.; Li, Y.; Wu, X.; Chen, P. Artificial aluminum-doped SiO2 aerogel coating layer regulating zinc ions flow for highly reversible dendrite-free zinc anodes. Electrochim. Acta 2024, 501, 144799. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Qi, Y.; Cui, F.; Yang, X. Conformal carbon coated TiO2 aerogel as superior anode for lithium-ion batteries. Chem. Eng. J. 2018, 351, 825–831. [Google Scholar] [CrossRef]
- Pei, Z.; Zhou, J.; Chen, Q.; Wu, Z.; Xu, X.; Li, Y.; Li, Y.; Sui, Z. Rational design of three-dimensional MXene-based aerogel for high-performance lithium–sulfur batteries. J. Mater. Sci. 2022, 57, 18549–18560. [Google Scholar] [CrossRef]
- Zhu, L.; You, L.; Zhu, P.; Shen, X.; Yang, L.; Xiao, K. High Performance Lithium–Sulfur Batteries with a Sustainable and Environmentally Friendly Carbon Aerogel Modified Separator. ACS Sustain. Chem. Eng. 2018, 6, 248–257. [Google Scholar] [CrossRef]
- Yang, C.; Sunderlin, N.; Wang, W.; Churchill, C.; Keyser, M. Compressible battery foams to prevent cascading thermal runaway in Li-ion pouch batteries. J. Power Sources 2022, 541, 231666. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, B.; Hu, X.; Ren, X.; Gong, M.; Mo, R.; Yang, S. Selective recycling of spent LiFePO4 batteries Enables toward flame-retardant polyurethane foam. Sep. Purif. Technol. 2025, 361, 131491. [Google Scholar] [CrossRef]
- Alcock, K.M.; Shearer, N.; Santos, F.V.; Cai, Z.; Goh, K. An innovative approach for the passive cooling of batteries: An empirical investigation of copper deposition on polyurethane foam for the enhancement of phase change material. Appl. Mater. Today 2024, 38, 102221. [Google Scholar] [CrossRef]
- Zhao, Z.; Pan, L.; Li, Y.; Wang, J.; Luo, Z.; Chen, W.; Liu, Z.; He, H. Aluminum silicate fiber membrane: A cost-effective substitute for fiber glass separator in Li–O2 battery. Mater. Today Energy 2020, 17, 100485. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, Z.; Zhang, T.; Zhang, X.; Ma, Y.; Guo, Y.; Wang, X.; Zheng, Z.; Hou, Z.; Zi, Z. High efficient recycling of glass fiber separator for sodium-ion batteries. Ceram. Int. 2023, 49, 23598–23604. [Google Scholar] [CrossRef]
- Wei, H.; Hu, X.; Deng, Y.; Wei, X.; Yang, Z.; Han, G. Distributed activation energy treatment of polyimide aerogel and its blocking effect on thermal runaway propagation of ternary battery. J. Energy Storage 2024, 90, 111744. [Google Scholar] [CrossRef]
- Guo, D.; Mu, L.; Lin, F.; Liu, G. Mesoporous Polyimide Thin Films as Dendrite-Suppressing Separators for Lithium–Metal Batteries. ACS Nano 2024, 18, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Y.; Wu, Y.-Y.; Liu, S.-C.; Li, Y.; Guan, Z.-Q.; Cao, C.-F.; Zhang, G.-D.; Tuten, B.T.; Gao, J.-F.; Shi, Y.-Q.; et al. Pottery-Inspired Flexible Fire-Shielding Ceramifiable Silicone Foams for Exceptional Long-Term Thermal Protection. Adv. Funct. Mater. 2025, 35, 2413362. [Google Scholar] [CrossRef]
- Chen, X.; Yu, Z.; Cui, W.; Ding, Y.; Wu, J.; Hou, Y.; Yang, S.; Gong, G. Surfactant-Enabled BM particle-embedded coaxial PVDF/PEI electrospun membranes enhancing Lithium-Ion battery safety. Chem. Eng. J. 2025, 503, 158225. [Google Scholar] [CrossRef]
- Yao, X.; Hou, X.; Zhang, R. Flexible and mechanically robust polyimide foam membranes with adjustable structure for separation and recovery of oil-water emulsions and heavy oils. Polymer 2022, 250, 124889. [Google Scholar] [CrossRef]
- Ma, B.; Hou, X.; Li, Y. Mechanically strong and thermal insulating polyimide/SiO2 aerogel prepared by ambient drying. Mater. Lett. 2022, 327, 133039. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Hou, X.; Chen, L. Mechanically robust and thermal-insulated polyimide aerogel films by polymerization-regulated strategy for flexible thermal protection. Chem. Eng. J. 2024, 496, 154251. [Google Scholar] [CrossRef]
- Chen, J.; Hou, X.; Chen, Z.; Chen, L.; Nie, S.; Zhu, S.; Liu, T.; Chen, L.; Zhang, R. Double-Crosslinked Organic–Inorganic Hybrid Polyimide Aerogel Composites with Ultra-Robust Toughness for Foldable Mechanical-Thermal-Coupled Protection. Adv. Funct. Mater. 2025, 35, 2420717. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, Q.; Yang, D.; Wang, X.; Zheng, M.; Liu, Z.; Zhu, Z.; Li, Z.; Wei, H.; Cao, X.; et al. Flame Retardant Organic Aerogels: Strategies, Mechanism, and Applications. Chem. Eng. J. 2024, 500, 157355. [Google Scholar] [CrossRef]
- Wan, X.; Liu, C.; Yang, Y.; Mu, X.; Xue, T.; Fan, W.; Liu, T. Lightweight mullite fiber/polyimide aerogel composites with superior ablation resistance via energy-efficient ambient processing. Compos. Part B Eng. 2025, 307, 112884. [Google Scholar]
- Cao, J.; Wang, P.; Cai, H.; Niu, B.; Zhang, Y.; Long, D. Lightweight Nitrogen-Doped Phenolic Aerogels with Flame-Retardant and Thermal-Insulation Properties. ACS Appl. Polym. Mater. 2023, 5, 10276–10288. [Google Scholar]
- Wang, Y.-T.; Zhao, H.-B.; Guo, M.-L.; Degracia, K.; Sun, H.; Sun, M.-Z.; Zhao, Z.-Y.; Schiraldi, D.A.; Wang, Y.-Z. Rigid and Fire-Resistant All-Biomass Aerogels. ACS Sustain. Chem. Eng. 2022, 10, 12117–12126. [Google Scholar] [CrossRef]
- Yu, X.; Li, H.; Kang, N.; Lu, S.; Xu, M.; Wan, M.P. Superelastic Fire-Safe Aerogel with Hierarchical Structures via Dual Templates Aided by Microbubble Engineering. Adv. Sci. 2025, 12, e06808. [Google Scholar]
- Qian, Z.; Yang, M.; Li, R.; Li, D.; Zhang, J.; Xiao, Y.; Li, C.; Yang, R.; Zhao, N.; Xu, J. Fire-resistant, ultralight, superelastic and thermally insulated polybenzazole aerogels. J. Mater. Chem. A 2018, 6, 20769–20777. [Google Scholar] [CrossRef]
- Liu, C.; Wang, M.; Zhao, X.; Yang, C.; Wei, R.; Zeng, W.; Ding, F.; Zhang, S.; Lei, Y. High-Temperature Resistant Polyimide Aerogels With Extreme Condition Tolerance Constructed by In Situ Skeleton Encapsulation Growth. Adv. Funct. Mater. 2025, 35, 2500881. [Google Scholar] [CrossRef]
- Du, F.; Jin, Z.; Yang, R.; Hao, M.; Wang, J.; Xu, G.; Zuo, W.; Geng, Z.; Pan, H.; Li, T.; et al. Thermally insulating and fire-retardant bio-mimic structural composites with a negative Poisson's ratio for battery protection. Carbon Energy 2023, 5, e353. [Google Scholar] [CrossRef]
- Peng, Z.-C.; Zeng, F.-R.; Li, W.-X.; Chen, S.-Q.; Zeng, Z.-W.; Liu, B.-W.; Wu, B.; Wang, Y.-Z.; Zhao, H.-B. Lightweight Ambient-Dried Biobased Aerogels with Superior Fire Safety and Mechanical Durability for Thermal Insulation. ACS Appl. Mater. Interfaces 2025, 17, 34558–34568. [Google Scholar] [CrossRef]
- Zhang, R.; Gu, H.; Hou, X.; Zhou, P. High-temperature resistant Y2SiO5–TiO2 aerogel composite for efficient thermal insulation. J. Porous Mater. 2021, 28, 57–64. [Google Scholar]
- Zhou, X.; Jin, H.; Shen, Q.; Huang, W.; Li, J.; Liang, Y.; Mao, P.; Yang, Y.; Yun, S.; Chen, J. Rapid preparation of Palygorskite/Al2O3 composite aerogels with superhydrophobicity, high-temperature thermal insulation and improved mechanical properties. Ceram. Int. 2022, 48, 32994–33002. [Google Scholar] [CrossRef]
- Sun, T.; Yan, Y.; Sui, J.; Wang, X.; Rasool, G.; Zhang, K. A novel thermal-cloak-based thermal design for preventing thermal runaway propagation in lithium-ion battery packs. J. Energy Storage 2025, 123, 116828. [Google Scholar]
- Li, X.-L.; Chen, M.-J.; Chen, H.-B. Facile fabrication of mechanically-strong and flame retardant alginate/clay aerogels. Compos. Part B Eng. 2019, 164, 18–25. [Google Scholar]
- Cai, W.; Li, Z.; Xie, H.; Wang, W.; Cui, T.; Lin, B.; Qi, L.; Hu, X.; Du, Y.; Ming, Y.; et al. Thermally managed and fireproof composite aerogels for safer and year-round energy saving. Chem. Eng. J. 2024, 483, 149006. [Google Scholar] [CrossRef]
- Xiao, Y.; Yan, M.; Shi, L.; Gong, L.; Cheng, X.; Zhang, H.; Pan, Y. High-temperature resistant, super elastic aerogel sheet prepared based on in-situ supercritical separation method for thermal runaway prohibition of lithium-ion batteries. Energy Storage Mater. 2023, 61, 102871. [Google Scholar]
- Ge, S.; Ni, Y.; Zhou, F.; Yang, C.; Guo, F.; Li, J.; Shi, B. A passive fire protection method for main cables and slings of suspension bridges utilizing fiber felt/aerogel composites. Constr. Build. Mater. 2023, 408, 133822. [Google Scholar] [CrossRef]

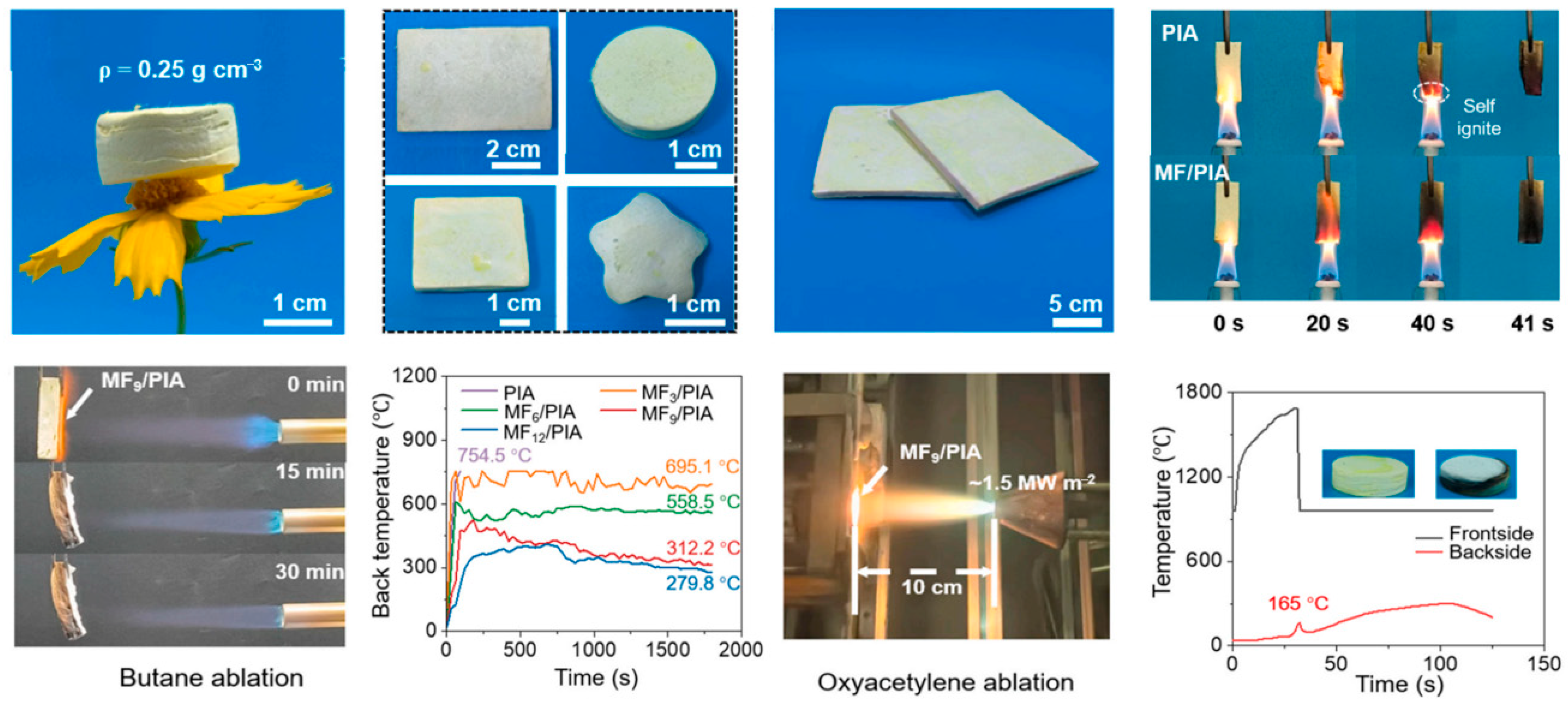
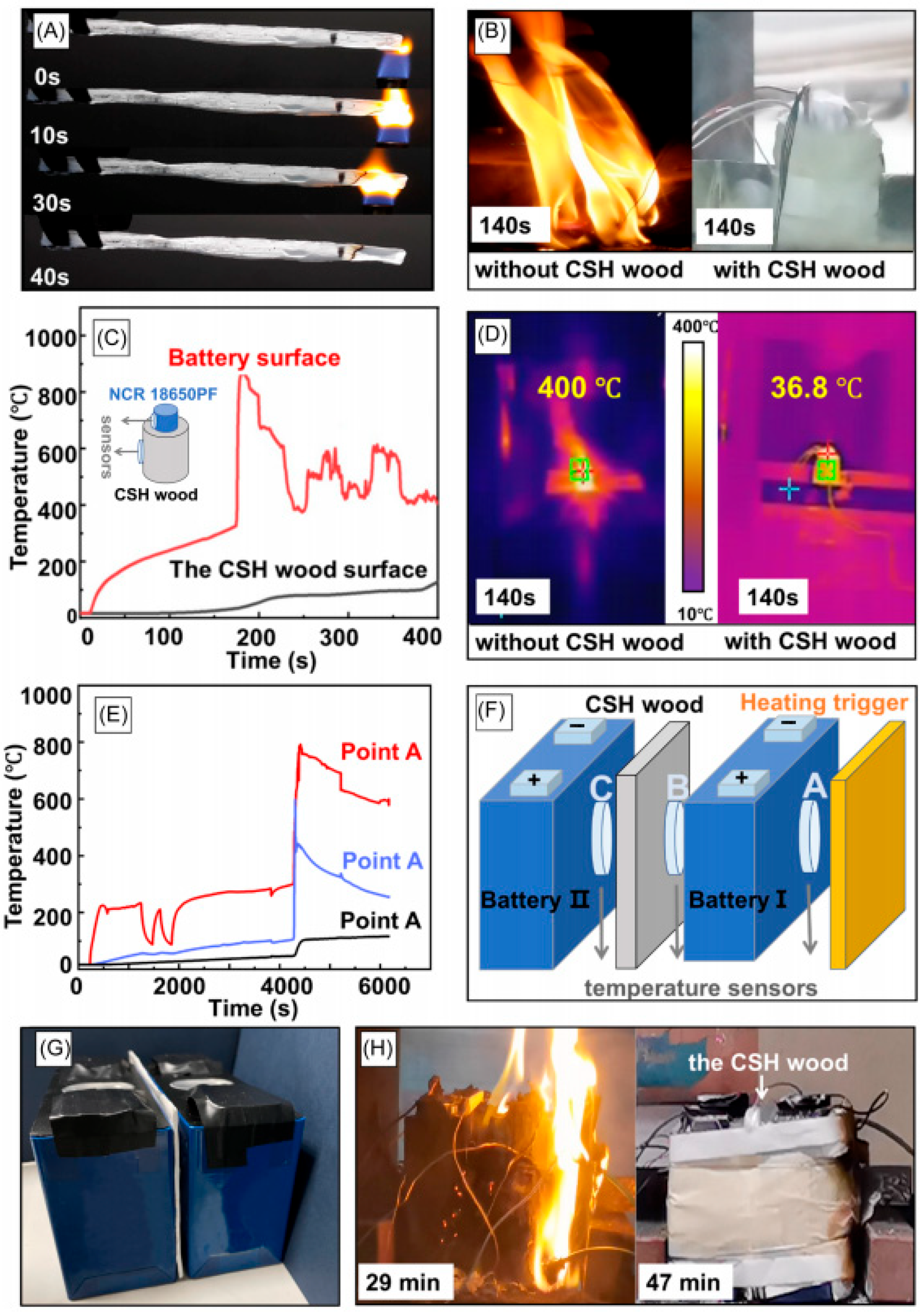

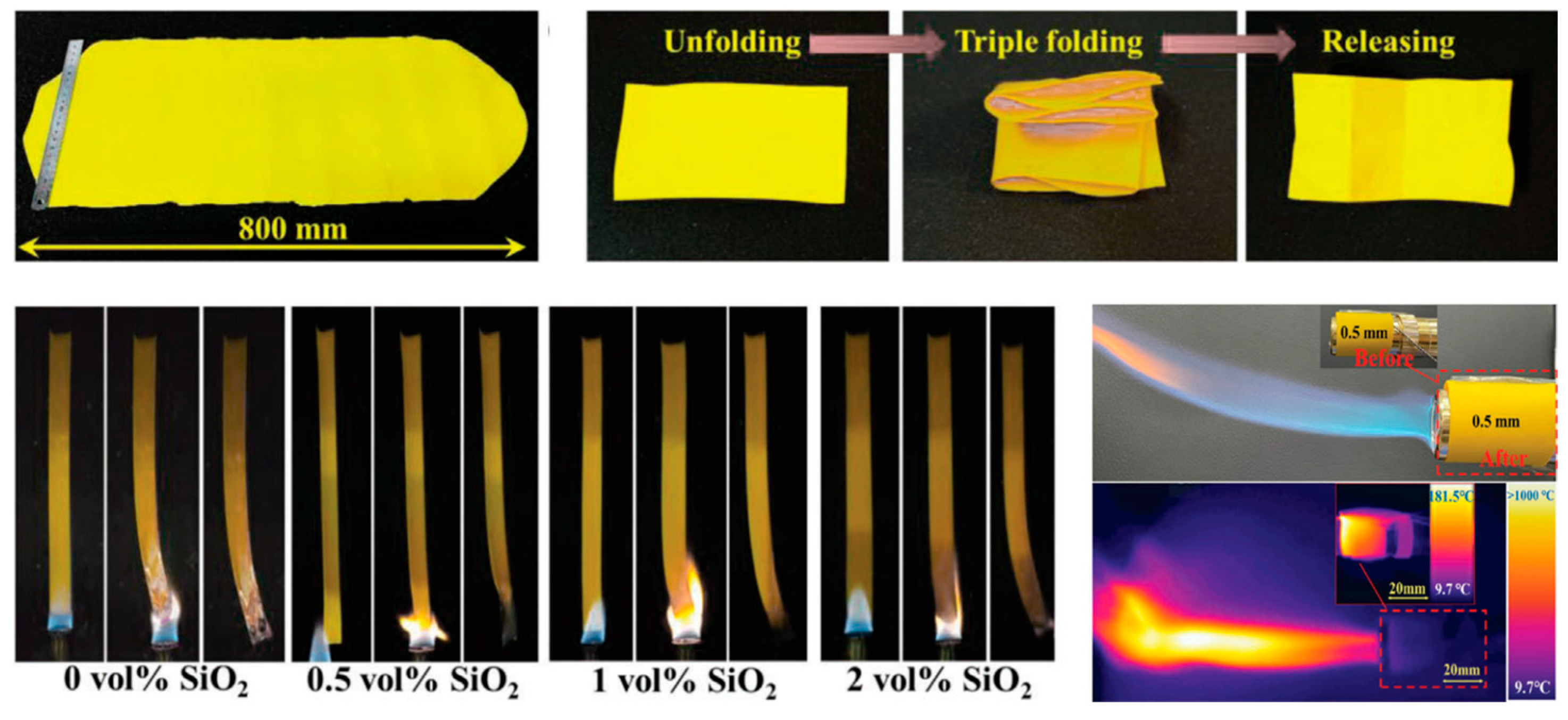
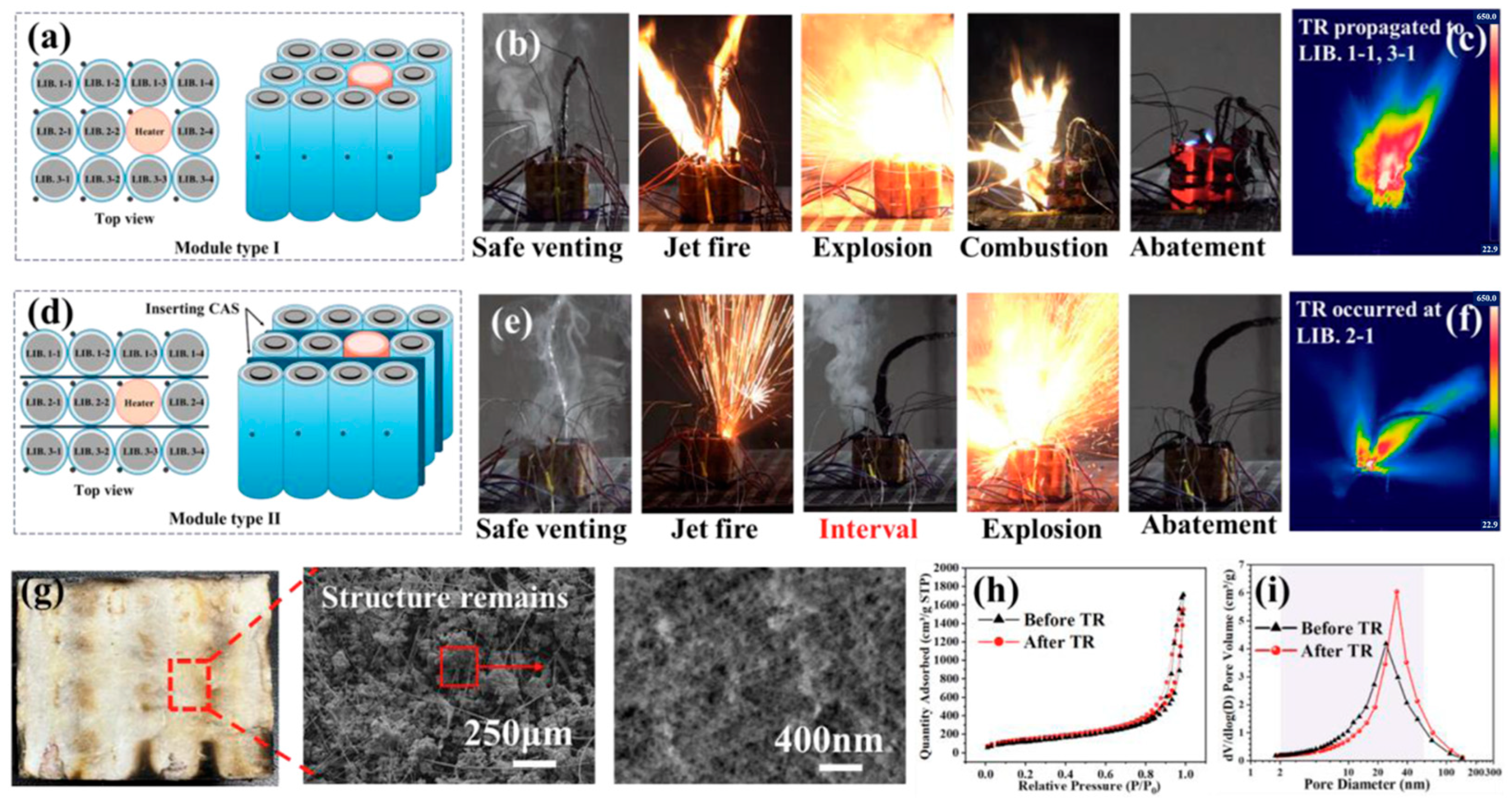

| Material Type | Thermal Conductivity (W/(m·K)) | Temperature Resistance (°C) | Density (g/cm3) | Flame- Retardant Property | Cost | Advantages | Disadvantages | Main Application |
|---|---|---|---|---|---|---|---|---|
| Traditional foam [62,63,64,65,66] | 0.02~0.04 | ≤300 | 0.03~0.05 | Non-flammable, low smoke, with a fire resistance rating up to Class B1 | Low | Low cost | Flammable, releases toxic gases, short lifespan | Early battery pack |
| Vacuum insulation panel [69,70,71,72] | <0.01 | - | ~0.2 | Class A non-combustible | Relatively high | Ultra-low thermal conductivity | The dependence on maintaining a constant vacuum level over an extended period of time | Automobile power batteries |
| Polymer aerogels [44,45] | 0.020–0.035 | −196~500 | 0.10~0.30 | Non-flammable, low smoke, with a fire resistance rating up to Class B1 | High | Flexible, foldable, and high mechanical strength | Low temperature resistance and high cost | Military industry and high-end vehicle protection |
| Inorganic Aerogels [53,54,55,56,57,58] | ~0.020 | 1200 | 0.1~0.2 | Class A non-combustible, high limit oxygen index (LOI) | Relative high | Ultra-low thermal conductivity and high-temperature resistance | Brittle, low mechanical strength | Aerospace, energy, batteries |
| Inorganic aerogel composites [54,78,79,80] | 0.015~0.025 | 650~1200 | 0.15~0.25 | Class A non-combustible, and further enhance it by adding a fireproof and flame-retardant layer | Medium | Ultra-low thermal conductivity, lightweight | High brittleness and expensive cost | High-voltage fast-charging battery system |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, X.; Chen, J.; Fang, X.; Xia, R.; Zhu, S.; Liu, T.; Zhu, K.; Chen, L. Thermal Insulation and Fireproof Aerogel Composites for Automotive Batteries. Gels 2025, 11, 791. https://doi.org/10.3390/gels11100791
Hou X, Chen J, Fang X, Xia R, Zhu S, Liu T, Zhu K, Chen L. Thermal Insulation and Fireproof Aerogel Composites for Automotive Batteries. Gels. 2025; 11(10):791. https://doi.org/10.3390/gels11100791
Chicago/Turabian StyleHou, Xianbo, Jia Chen, Xuelei Fang, Rongzhu Xia, Shaowei Zhu, Tao Liu, Keyu Zhu, and Liming Chen. 2025. "Thermal Insulation and Fireproof Aerogel Composites for Automotive Batteries" Gels 11, no. 10: 791. https://doi.org/10.3390/gels11100791
APA StyleHou, X., Chen, J., Fang, X., Xia, R., Zhu, S., Liu, T., Zhu, K., & Chen, L. (2025). Thermal Insulation and Fireproof Aerogel Composites for Automotive Batteries. Gels, 11(10), 791. https://doi.org/10.3390/gels11100791









