Spectroscopic and Rheological Characterization of Polyvinyl Alcohol/Hyaluronic Acid-Based Systems: Effect of Polymer Ratio and Riboflavin on Hydrogel Properties
Abstract
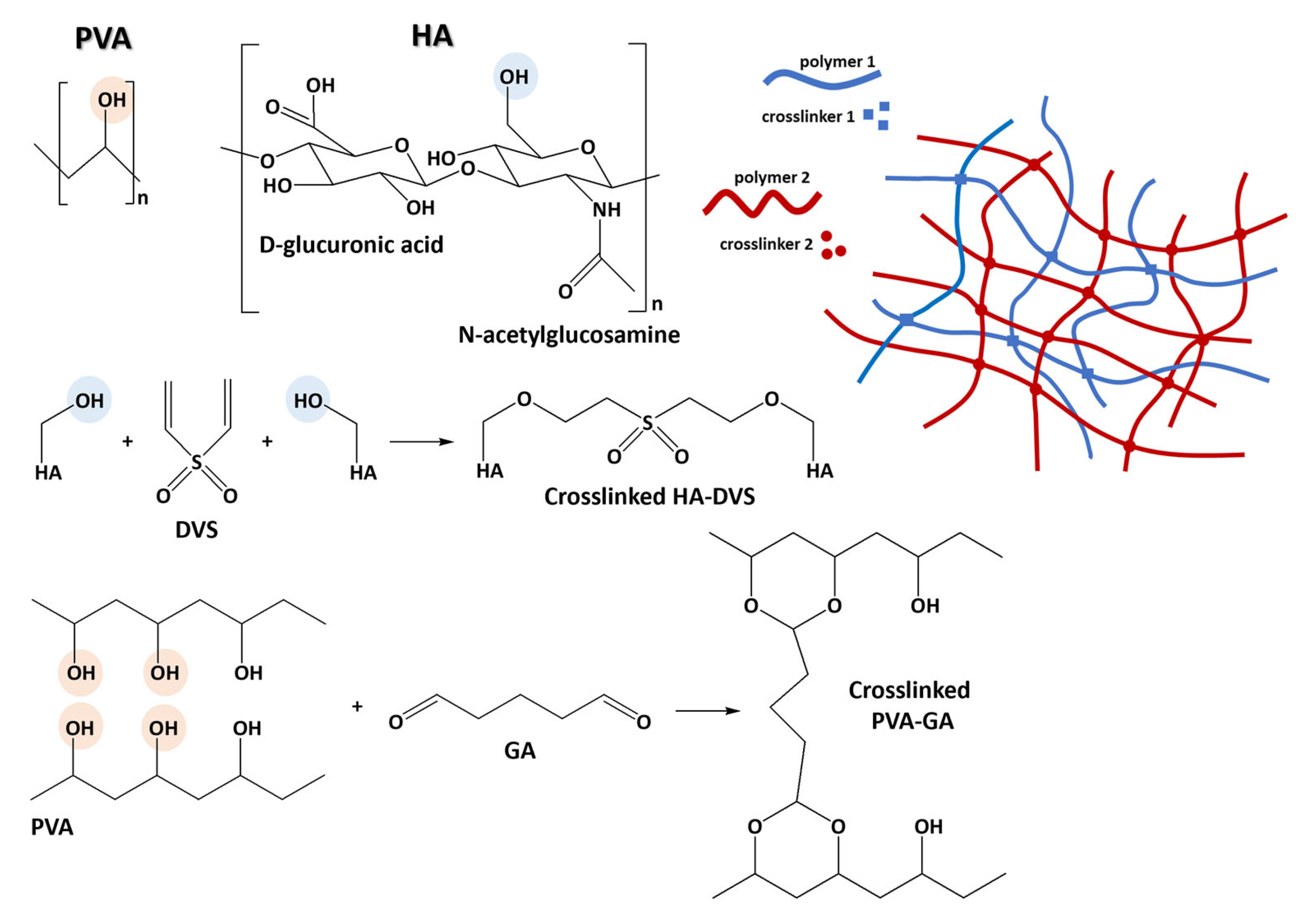
1. Introduction
2. Results and Discussion
2.1. Swelling Properties of PVA/HA Hydrogels
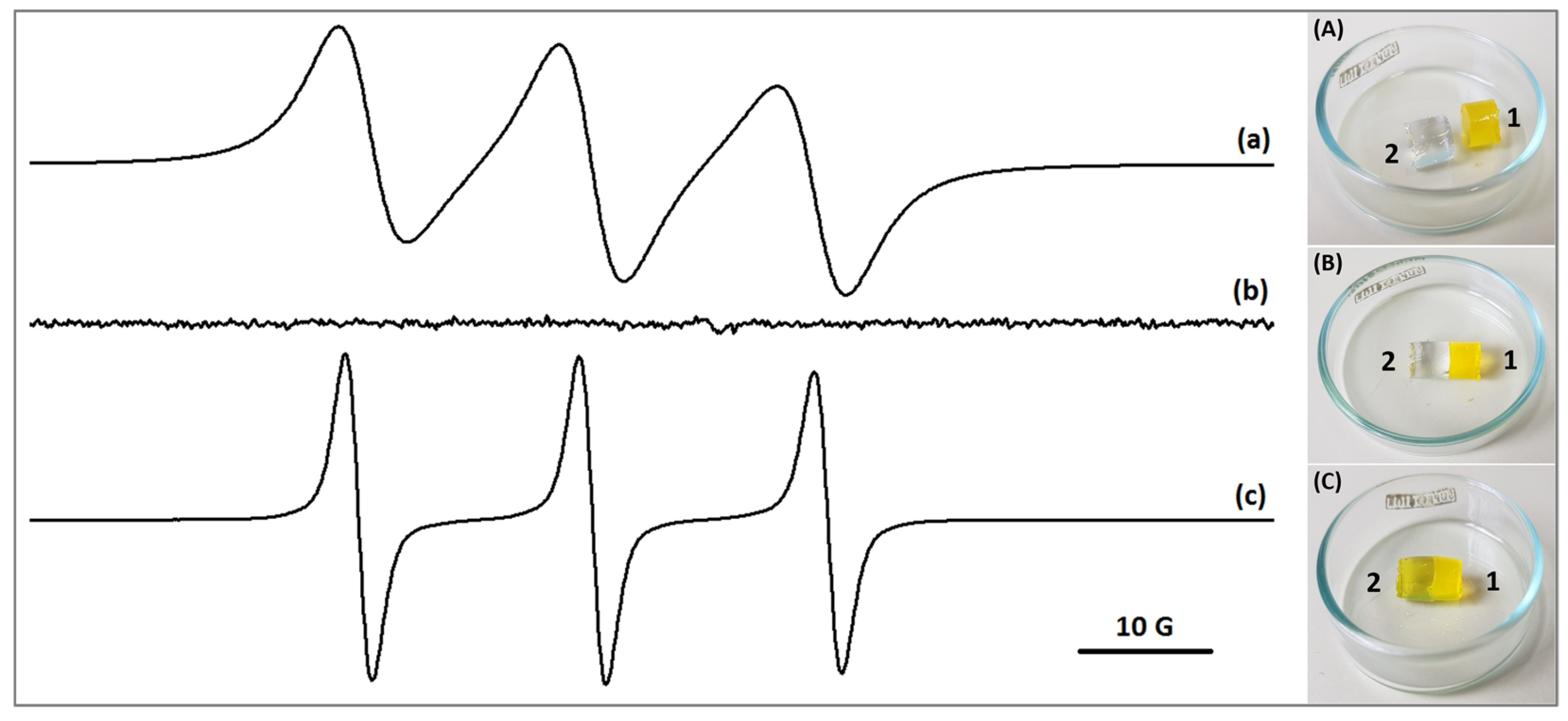
2.2. Diffusion of Low Molecular Weight Compounds in PVA/HA 4/1 Hydrogel and Gel Network Stability
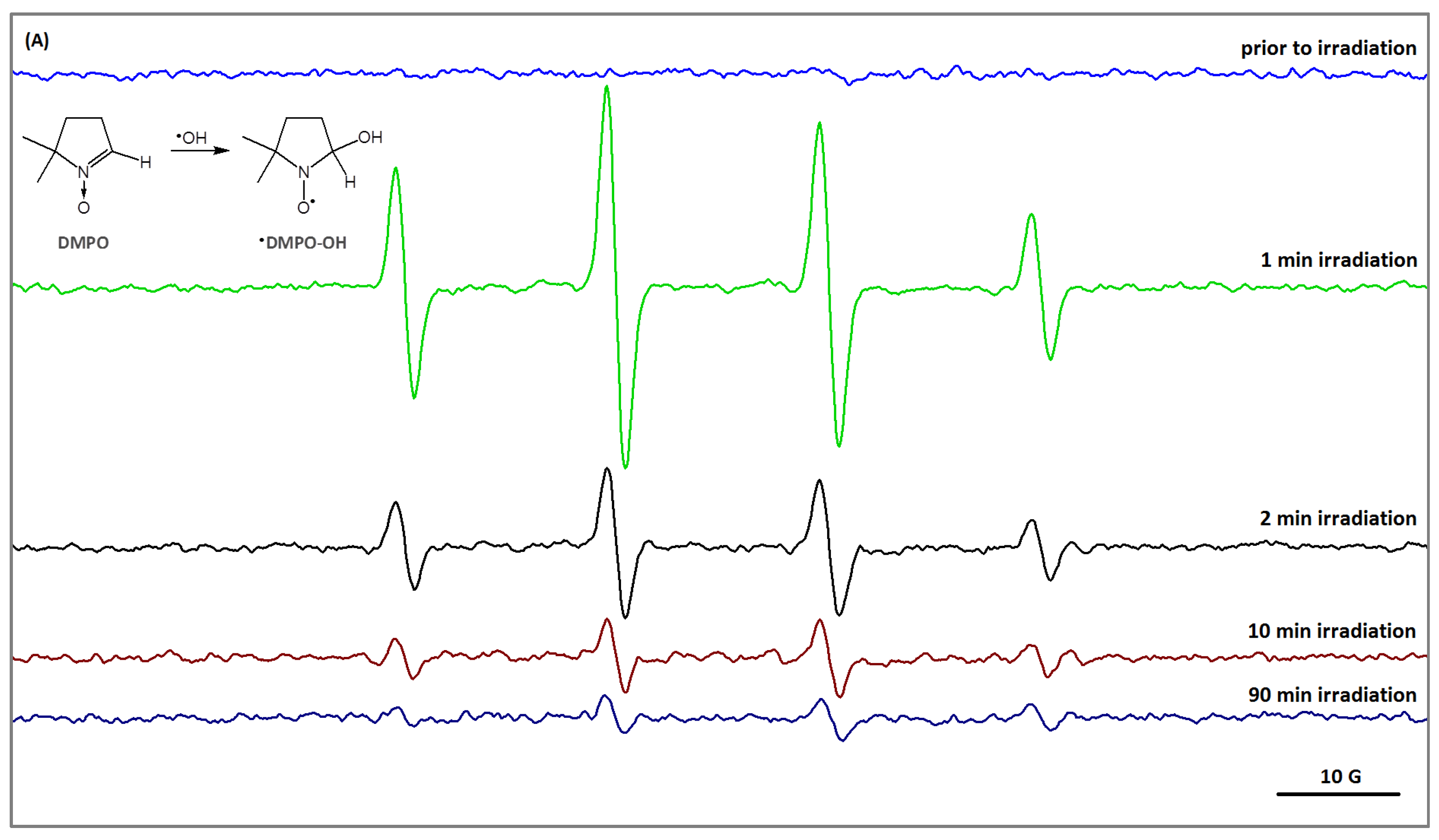
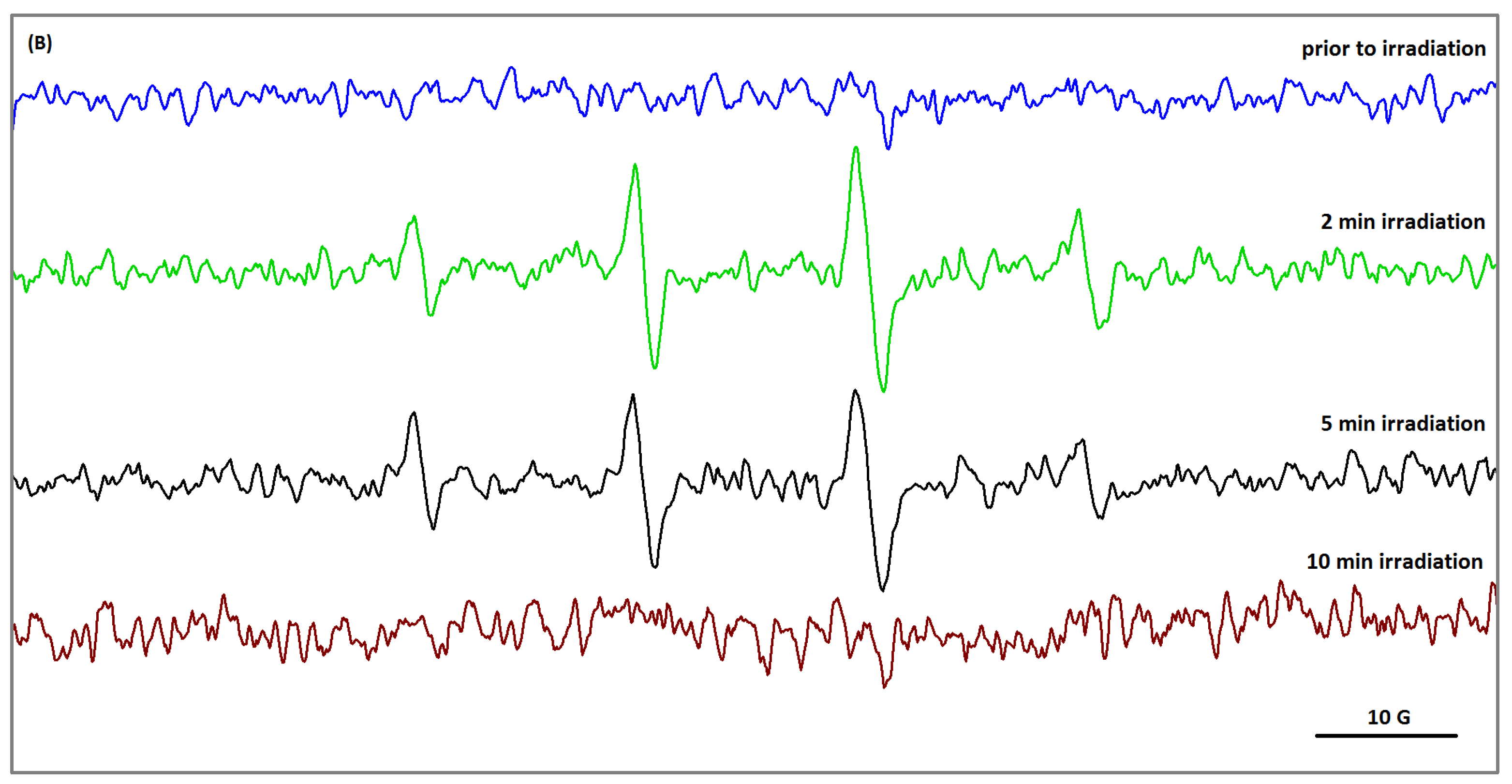
2.3. Reactive Oxygen Species Generation in PVA/HA Hydrogel
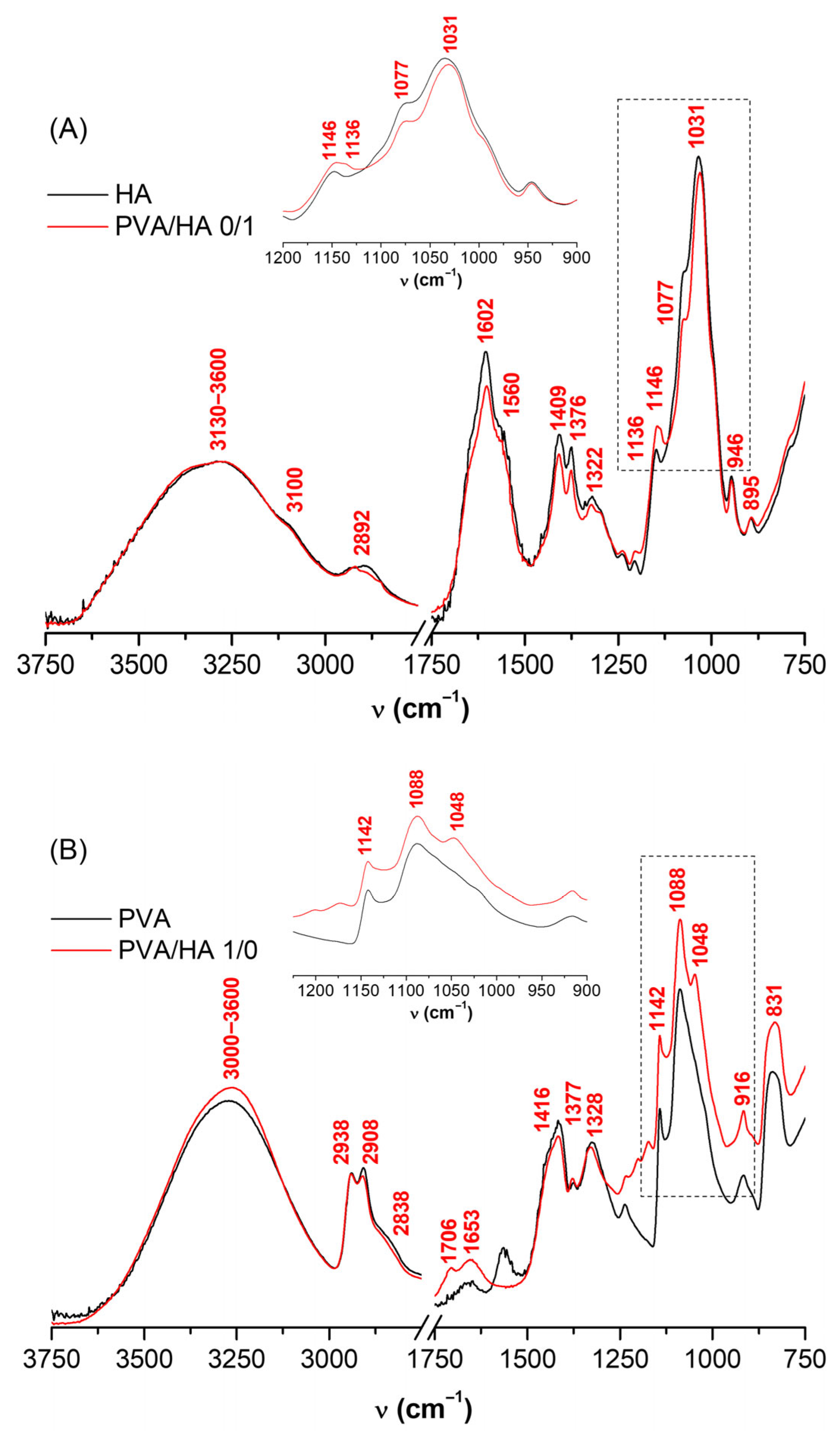
2.4. Infrared Spectroscopy of PVA/HA Hydrogels
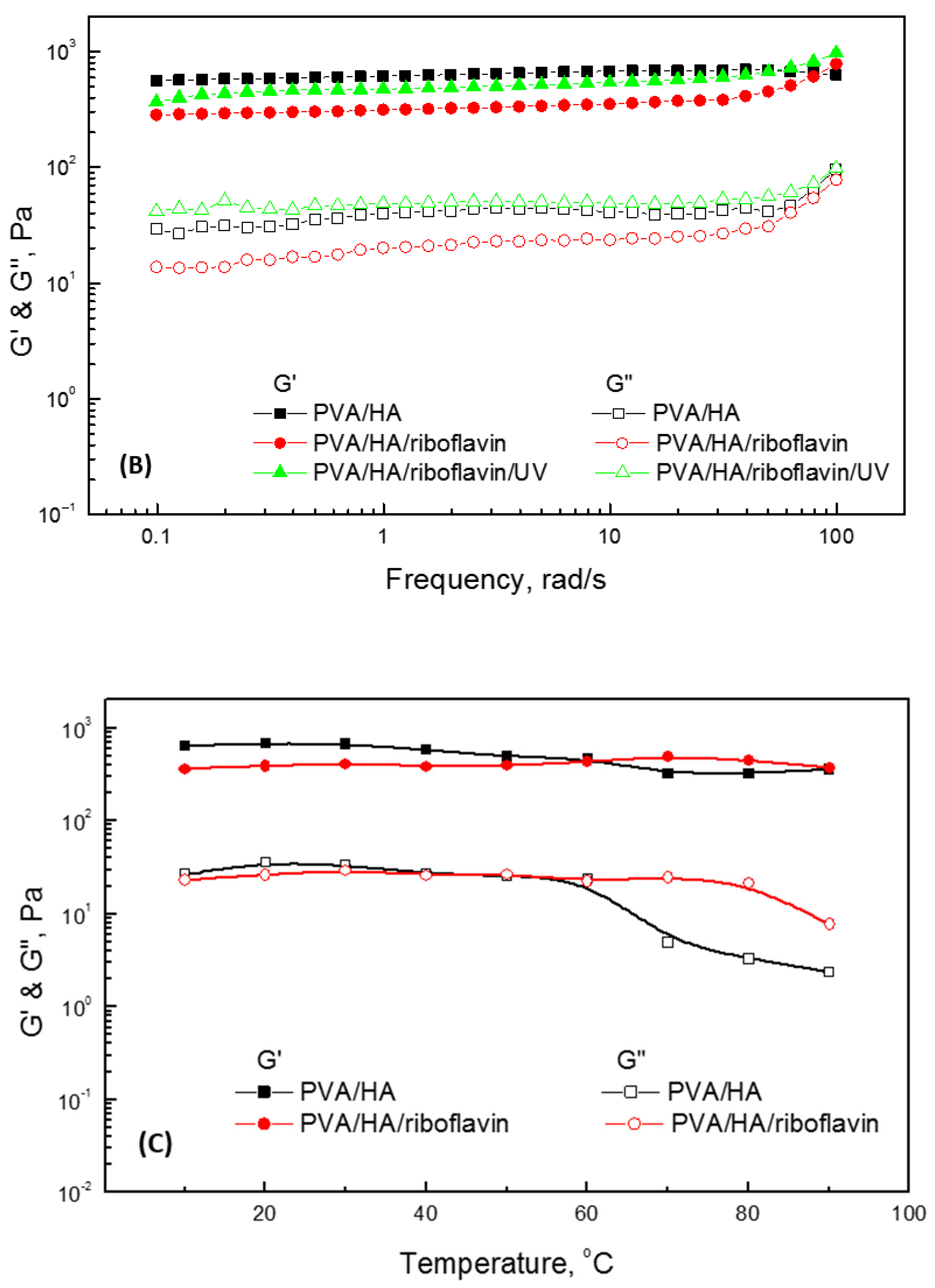
2.5. Rheological Behavior of PVA/HA Hydrogels
3. Conclusions
4. Materials and Methods
4.1. General
4.2. Preparation of PVA/HA Hydrogel Systems
4.3. Instrumentation
4.4. Swelling Capacity of PVA/HA Hydrogels
4.5. Synthesis of Spin Labeled Hyaluronic Acid
4.6. Reactive Oxygen Species Generation in PVA/HA Hydrogels
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y. Rational design of smart hydrogels for biomedical applications. Front. Chem. 2021, 8, 615665. [Google Scholar] [CrossRef]
- Frazar, E.M.; Shah, R.A.; Dziubla, T.D.; Hilt, J.Z. Multifunctional temperature-responsive polymers as advanced biomaterials and beyond. J. Appl. Polym. Sci. 2020, 137, 48770. [Google Scholar] [CrossRef]
- Carreira, A.S.; Ferreira, P.; Ribeiro, M.P.; Correia, T.R.; Coutinho, P.; Correia, I.J.; Gil, M.H. New drug-eluting lenses to be applied as bandages after keratoprosthesis implantation. Int. J. Pharmac. 2014, 477, 218–226. [Google Scholar] [CrossRef]
- Kodavaty, J.; Deshpande, A.P. Mechanical and swelling properties of poly (vinyl alcohol) and hyaluronic acid gels used in biomaterial systems—A comparative study. Def. Sci. J. 2014, 64, 222–229. [Google Scholar] [CrossRef]
- Perez, L.A.; Hernandez, R.; Alonso, J.M.; Perez-Gonzalez, R.; Saez-Martínez, V. Hyaluronic acid hydrogels crosslinked in physiological conditions: Synthesis and biomedical applications. Biomedicines 2021, 9, 1113. [Google Scholar] [CrossRef]
- Lindsey, S.; Street, G. Conductive composites from polyvinyl alcohol and polypyrrole. Synth. Met. 1984, 10, 67–69. [Google Scholar] [CrossRef]
- Mensitieri, M.; Ambrosio, L.; Nicolais, L.; Bellini, D.; O’Regan, M. Viscoelastic properties modulation of a novel autocrosslinked hyaluronic acid polymer. J. Mater. Sci. Mater. Med. 1996, 7, 695–698. [Google Scholar] [CrossRef]
- Hong, B.M.; Park, S.A.; Park, W.H. Effect of photoinitiator on chain degradation of hyaluronic acid. Biomater. Res. 2019, 23, 21. [Google Scholar] [CrossRef]
- Perez-Luna, V.H.; Gonzalez-Reynoso, O. Encapsulation of biological agents in hydrogels for therapeutic applications. Gels 2018, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- O’Brart, D.P.S. Corneal collagen cross-linking: A review. J. Optom. 2014, 7, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.M.; Corbu, C.; Mocanu, S.; Popescu, E.I.; Micutz, M.; Staicu, T.; Somoghi, R.; Trica, B.; Popa, V.T.; Precupas, A.; et al. Model systems for evidencing the mediator role of riboflavin in the UVA cross-linking treatment of keratoconus. Molecules 2022, 27, 190. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.M.; Corbu, C.G.; Tanase, C.; Codrici, E.; Mihai, S.; Popescu, I.D.; Enciu, A.M.; Mocanu, S.; Matei, I.; Ionita, G. Spin probe method of electron paramagnetic resonance spectroscopy-a qualitative test for measuring the evolution of dry eye syndrome under treatment. Anal. Methods 2019, 11, 965–972. [Google Scholar] [CrossRef]
- Joly, J.P.; Aricov, L.; Balan, G.A.; Popescu, E.I.; Mocanu, S.; Leonties, A.R.; Matei, I.; Marque, S.R.A.; Ionita, G. Formation of alginate/chitosan interpenetrated networks revealed by EPR spectroscopy. Gels 2023, 9, 231. [Google Scholar] [CrossRef]
- Matei, I.; Ariciu, A.M.; Popescu, E.I.; Mocanu, S.; Neculae, A.V.F.; Savonea, F.; Ionita, G. Evaluation of the accessibility of molecules in hydrogels using a scale of spin probes. Gels 2022, 8, 428. [Google Scholar] [CrossRef]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Fahmy, A.; Kamoun, E.A.; El-Eisawy, R.; El-Fakharany, E.M.; Taha, T.H.; El-Damhougy, B.K.; Abdelhai, F. Poly(vinyl alcohol)-hyaluronic acid membranes for wound dressing applications: Synthesis and in vitro bio-evaluations. J. Braz. Chem. Soc. 2015, 26, 1466–1474. [Google Scholar] [CrossRef]
- Buettner, G.R. Spin trapping: ESR parameters of spin adducts. Free Radic. Biol. Med. 1987, 3, 259–303. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Direct detection and identification of radicals generated during the hydroxyl radical-induced degradation of hyaluronic acid and related materials. Free Radic. Biol. Med. 1996, 21, 275–290. [Google Scholar] [CrossRef]
- Soule, B.P.; Hyodo, F.; Matsumoto, K.; Simone, N.L.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. The chemistry and biology of nitroxide compounds. Free Radic. Biol. Med. 2007, 42, 1632–1650. [Google Scholar] [CrossRef]
- Haxaire, K.; Marechal, Y.; Milas, M.; Rinaudo, M. Hydration of polysaccharide hyaluronan observed by IR spectrometry. I. Preliminary experiments and band assignments. Biopolymers 2003, 72, 10–20. [Google Scholar] [CrossRef]
- Gilli, R.; Kacurakova, M.; Mathlouthi, M.; Navarini, L.; Paoletti, S. FTIR studies of sodium hyaluronate and its oligomers in the amorphous solid phase and in aqueous solution. Carbohydr. Res. 1994, 263, 315–326. [Google Scholar] [CrossRef]
- Fonseca dos Reis, E.; Campo, F.S.; Pereira Lage, A.; Cerqueira Leite, R.; Heneine, L.G.; Vasconcelos, W.L.; Portela Lobatoa, Z.I.; Sander Mansur, H. Synthesis and characterization of poly (vinyl alcohol) hydrogels and hybrids for rMPB70 protein adsorption. Mater. Res. 2006, 9, 185–191. [Google Scholar] [CrossRef]
- Nkhwa, S.; Lauriaga, K.F.; Kemal, E.; Deb, S. Poly(vinyl alcohol): Physical approaches to designing biomaterials for biomedical applications. Confer. Paper. Sci. 2014, 2014, 403472. [Google Scholar] [CrossRef]
- Tomihata, K.; Ikada, Y. Preparation of cross-linked hyaluronic acid films of low water content. Biomaterials 1997, 18, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Tomihata, K.; Ikada, Y. Crosslinking of hyaluronic acid with water-soluble carbodiimide. J. Biomed. Mater. Res. 1997, 37, 243–251. [Google Scholar] [CrossRef]
- Tretinnikov, O.N.; Zagorskaya, S.A. Determination of the degree of crystallinity of poly(vinyl alcohol) by FTIR spectroscopy. J. Appl. Spectrosc. 2012, 79, 521–526. [Google Scholar] [CrossRef]
- Sheraz, M.A.; Kazi, S.H.; Ahmed, S.; Anwar, Z.; Ahmad, I. Photo, thermal and chemical degradation of riboflavin. Beilstein J. Org. Chem. 2014, 10, 1999–2012. [Google Scholar] [CrossRef]
- Jayasekara, R.; Harding, I.; Bowater, I.; Lonergan, G. Biodegradability of a selected range of polymers and polymer blends and standard methods for assessment of biodegradation. J. Polym. Environ. 2005, 13, 231–251. [Google Scholar] [CrossRef]
- Yui, N.; Okano, T.; Sakurai, Y. Inflammation responsive degradation of crosslinked hyaluronic acid gels. J. Control. Rel. 1992, 22, 105–116. [Google Scholar] [CrossRef]
- Ross-Murphy, S.B. Rheological characterization of polymeric gels and networks. Polym. Gel Netw. 1994, 2, 229–237. [Google Scholar] [CrossRef]
- Li, R.H.; Barbari, T.A. Protein transport through membranes based on toluene diisocyanate surface-modified poly (vinyl alcohol) gels. J. Membr. Sci. 1994, 88, 115–125. [Google Scholar] [CrossRef]
- Si, S.; Zhou, R.; Xing, Z.; Xu, H.; Cai, Y.; Zhang, Q. A study of hybrid organic/inorganic hydrogel films based on in situ-generated TiO2 nanoparticles and methacrylated gelatin. Fibers Polym. 2013, 14, 982–989. [Google Scholar] [CrossRef]
- Ionita, G.; Ariciu, A.M.; Smith, D.K.; Chechik, V. Ion exchange in alginate gels—Dynamic behaviour revealed by electron paramagnetic resonance. Soft Matter 2015, 11, 8968–8974. [Google Scholar] [CrossRef]
- Duling, D.R. PEST Winsim, version 0.96; National Institute of Environmental Health Sciences: Triangle Park, NC, USA, 1996.
- Duling, D.R. Simulation of multiple isotropic spin-trap EPR spectra. J. Magn. Reson. B 1994, 104, 105–110. [Google Scholar] [CrossRef]



| PVA/HA | 2/3 | 1/1 | 3/2 | 4/1 | 1/0 |
|---|---|---|---|---|---|
| Initial water content (%) | 96 ± 3 | 95 ± 3 | 94 ± 3 | 95 ± 2 | 94 ± 4 |
| Equilibrium swelling ratio (%) | 175 ± 5 | 165 ± 5 | 136 ± 4 | 258 ± 6 | 112 ± 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, I.; Mihai, M.A.; Leau, S.-A.; Aricov, L.; Leonties, A.R.; Alexandrescu, E.; Ionita, G. Spectroscopic and Rheological Characterization of Polyvinyl Alcohol/Hyaluronic Acid-Based Systems: Effect of Polymer Ratio and Riboflavin on Hydrogel Properties. Gels 2025, 11, 773. https://doi.org/10.3390/gels11100773
Matei I, Mihai MA, Leau S-A, Aricov L, Leonties AR, Alexandrescu E, Ionita G. Spectroscopic and Rheological Characterization of Polyvinyl Alcohol/Hyaluronic Acid-Based Systems: Effect of Polymer Ratio and Riboflavin on Hydrogel Properties. Gels. 2025; 11(10):773. https://doi.org/10.3390/gels11100773
Chicago/Turabian StyleMatei, Iulia, Marius Alexandru Mihai, Sorina-Alexandra Leau, Ludmila Aricov, Anca Ruxandra Leonties, Elvira Alexandrescu, and Gabriela Ionita. 2025. "Spectroscopic and Rheological Characterization of Polyvinyl Alcohol/Hyaluronic Acid-Based Systems: Effect of Polymer Ratio and Riboflavin on Hydrogel Properties" Gels 11, no. 10: 773. https://doi.org/10.3390/gels11100773
APA StyleMatei, I., Mihai, M. A., Leau, S.-A., Aricov, L., Leonties, A. R., Alexandrescu, E., & Ionita, G. (2025). Spectroscopic and Rheological Characterization of Polyvinyl Alcohol/Hyaluronic Acid-Based Systems: Effect of Polymer Ratio and Riboflavin on Hydrogel Properties. Gels, 11(10), 773. https://doi.org/10.3390/gels11100773







