Formulation of Hydrogel Beads to Improve the Bioaccessibility of Bioactive Compounds from Goldenberry and Purple Passion Fruit and Evaluation of Their Antiproliferative Effects on Human Colorectal Carcinoma Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Load Choice
2.2. Hydrogel Particles Formulation
2.3. Antioxidant Activity of the Hydrogel Beads Loaded with Fruit Blend
2.4. Antiproliferative Effect on Human Colorectal Carcinoma Cells
2.4.1. Cell Death
2.4.2. Cell Distribution in the Different Phases of the Cell Cycle
2.4.3. Levels of Reactive Oxygen Species (ROS) and Intracellular Reduced Glutathione (GSH)
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Obtaining the Fruit Blend
4.3. Polyphenol Profile by HPLC-MS
4.4. Hydrogel Beads Obtention
4.5. Hydrogel Beads Formulation
4.5.1. Encapsulation Efficiency and Load
4.5.2. Morphology and Particle Size and Shape Descriptors
4.6. Bioaccessibility of BCs from Fruit Blend in Hydrogel Beads
4.7. Total Polyphenol Content
4.8. Analysis of Total Carotenoid Content by HPLC-DAD
4.9. Antioxidant Activity
4.9.1. Ferric Reducing Antioxidant Power (FRAP)
4.9.2. ABTS+ Anion Radical Scavenging Activity
4.9.3. ORAC
4.10. Antiproliferative Effect
4.10.1. Cell Lines and Culture Conditions
4.10.2. MTT Cytotoxicity Assay
4.10.3. Apoptosis: Annexin V/IP Assay
4.10.4. Cell Cycle Progression
4.10.5. Levels of Reactive Oxygen Species (ROS)
4.10.6. Intracellular Reduced Glutathione (GSH)
4.11. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef]
- WHO. Non-Communicable Diseases. Progress Monitor 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Hassan, H.A.; Serag, H.M.; Qadir, M.S.; Ramadan, M.F. Cape gooseberry (Physalis peruviana) juice as a modulator agent for hepatocellular carcinoma-linked apoptosis and cell cycle arrest. Biomed. Pharmacother. 2017, 94, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xin, M.; Li, L.; He, X.; Yi, P.; Tang, Y.; Li, J.; Zheng, F.; Liu, G.; Sheng, J.; et al. Characterization of the aromatic profile of purple passion fruit (Passiflora edulis Sims) during ripening by HS-SPME-GC/MS and RNA sequencing. Food Chem. 2021, 355, 129685. [Google Scholar] [CrossRef] [PubMed]
- Edge, R.; Truscott, T.G. Properties of Carotenoid Radicals and Excited States and Their Potential Role in Biological Systems. In Carotenoids: Physical, Chemical, and Biological Functions and Properties; CRC Press: Boca Raton, FL, USA, 2010; pp. 283–308. [Google Scholar]
- Cilla, A.; Lagarda, M.J.; Barberá, R.; Romero, F. Polyphenolic profile and antiproliferative activity of bioaccessible fractions of zinc-fortified fruit beverages in human colon cancer cell lines. Nutr. Hosp. 2010, 25, 561–571. [Google Scholar] [PubMed]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Castenmiller, J.J.M.; West, C.E. Bioavailability and bioconversion of carotenoids. Annu. Rev. Nutr. 1998, 18, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.R.; Appel, C.L. Polyphenols as dietary supplements: A double-edged sword. Nutr. Diet. Suppl. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Kondrashina, A.; Arranz, E.; Cilla, A.; Faria, M.A.; Santos-Hernández, M.; Miralles, B.; Hashemi, N.; Rasmussen, M.K.; Young, J.F.; Barberá, R.; et al. Coupling in vitro food digestion with in vitro epithelial absorption; recommendations for biocompatibility. Crit. Rev. Food Sci. Nutr. 2023, 64, 9618–9636. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Augustin, M.A.; Sanguansri, L.; Lockett, T. Nano- and micro-encapsulated systems for enhancing the delivery of resveratrol. Ann. N. Y. Acad. Sci. 2013, 1290, 107–112. [Google Scholar] [CrossRef]
- Laokuldilok, N.; Thakeow, P.; Kopermsub, P.; Utama-Ang, N. Optimisation of microencapsulation of turmeric extract for masking flavour. Food Chem. 2016, 194, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Massounga Bora, A.F.; Ma, S.; Li, X.; Liu, L. Application of microencapsulation for the safe delivery of green tea poly-phenols in food systems: Review and recent advances. Food Res. Int. 2018, 105, 241–249. [Google Scholar] [CrossRef] [PubMed]
- E González, R.; Tarón, A.; Morón, L.B. Formación de Microcápsulas de Tamaño Controlado por Gelación Iónica Utilizando Mezclas Biopoliméricas Binarias. Inf. Tecnol. 2015, 26, 31–38. [Google Scholar] [CrossRef]
- Nikoo, A.M.; Kadkhodaee, R.; Ghorani, B.; Razzaq, H.; Tucker, N. Electrospray-assisted encapsulation of caffeine in alginate microhydrogels. Int. J. Biol. Macromol. 2018, 116, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Donati, I.; Paoletti, S. Material Properties of Alginates. In Alginates: Biology and Applications; Rehm, B.H.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–55. [Google Scholar]
- Gayatri, A.; Hudiyono, S.; Setiasih, S. Optimization and evaluation of ionically cross-linked alginate-hpmc nanospheres for encapsulation of bromelain as antiplatelet. J. Phys. Conf. Ser. 2021, 1918, 032003. [Google Scholar] [CrossRef]
- Mancini, F.; McHugh, T.H. Fruit-alginate interactions in novel restructured products. Mol. Nutr. Food Res. 2000, 44, 152–157. [Google Scholar] [CrossRef]
- Cilla, A.; González-Sarrías, A.; Tomás-Barberán, F.A.; Espín, J.C.; Barberá, R. Availability of polyphenols in fruit beverages subjected to in vitro gastrointestinal digestion and their effects on proliferation, cell-cycle and apoptosis in human colon cancer Caco-2 cells. Food Chem. 2009, 114, 813–820. [Google Scholar] [CrossRef]
- Agudelo, C.D.; Luzardo-Ocampo, I.; Campos-Vega, R.; Loarca-Piña, G.; Maldonado-Celis, M.E. Bioaccessibility during In Vitro Digestion and Antiproliferative Effect of Bioactive Compounds from Andean Berry (Vaccinium meridionale Swartz) Juice. J. Agric. Food Chem. 2018, 66, 7358–7366. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Velázquez, O.A.; Mulero, M.; Cuevas-Rodríguez, E.O.; Mondor, M.; Arcand, Y.; Hernández-Álvarez, A.J. In vitro gastrointestinal digestion impact on stability, bioaccessibility and antioxidant activity of polyphenols from wild and commercial blackberries (Rubus spp.). Food Funct. 2021, 12, 7358–7378. [Google Scholar] [CrossRef]
- García-Martínez, E.; Camacho, M.d.M.; Martínez-Navarrete, N. In Vitro Bioaccessibility of Bioactive Compounds from Citrus. Molecules 2023, 28, 810. [Google Scholar] [CrossRef]
- Martinović, J.; Lukinac, J.; Jukić, M.; Ambrus, R.; Planinić, M.; Šelo, G.; Klarić, A.-M.; Perković, G.; Bucić-Kojić, A. In Vitro Bioaccessibility Assessment of Phenolic Compounds from Encapsulated Grape Pomace Extract by Ionic Gelation. Molecules 2023, 28, 5285. [Google Scholar] [CrossRef] [PubMed]
- Sabet, S.; Rashidinejad, A.; Melton, L.D.; Zujovic, Z.; Akbarinejad, A.; Nieuwoudt, M.; Seal, C.K.; McGillivray, D.J. The interactions between the two negatively charged polysaccharides: Gum Arabic and alginate. Food Hydrocoll. 2021, 112, 106343. [Google Scholar] [CrossRef]
- Ćujić-Nikolić, N.; Stanisavljević, N.; Šavikin, K.; Kalušević, A.; Nedović, V.; Bigović, D.; Janković, T. Application of gum Arabic in the production of spray-dried chokeberry polyphenols, microparticles characterisation and in vitro digestion method. Lek. Sirovine 2018, 38, 9–16. [Google Scholar] [CrossRef]
- Gomes, S.V.F.; Portugal, L.A.; dos Anjos, J.P.; de Jesus, O.N.; de Oliveira, E.J.; David, J.P. Accelerated solvent extraction of phenolic compounds exploiting a Box-Behnken design and quantification of five flavonoids by HPLC-DAD in Passi-flora species. Microchem. J. 2017, 132, 28–35. [Google Scholar] [CrossRef]
- Abou Baker, D.H.; Mohammed, D.M. Polyphenolic rich fraction of Physalis peruviana calyces and its nano emulsion induce apoptosis by caspase 3 up-regulation and G2/M arrest in hepatocellular carcinoma. Food Biosci. 2022, 50, 102007. [Google Scholar] [CrossRef]
- Chen, W.; Su, H.; Xu, Y.; Bao, T.; Zheng, X. Protective effect of wild raspberry (Rubus hirsutus Thunb.) extract against acrylamide-induced oxidative damage is potentiated after simulated gastrointestinal digestion. Food Chem. 2016, 196, 943–952. [Google Scholar] [CrossRef]
- Naranjo-Durán, A.M.; Quintero-Quiroz, J.; Ciro-Gómez, G.L.; Barona-Acevedo, M.-J.; Contreras-Calderón, J.d.C. Characterization of the antioxidant activity, carotenoid profile by HPLC-MS of exotic colombian fruits (goldenberry and purple passion fruit) and optimization of antioxidant activity of this fruit blend. Heliyon 2023, 9, e17819. [Google Scholar] [CrossRef] [PubMed]
- Biao, Y.; Yuxuan, C.; Qi, T.; Ziqi, Y.; Yourong, Z.; McClements, D.J.; Chongjiang, C. Enhanced performance and functionality of active edible films by incorporating tea polyphenols into thin calcium alginate hydrogels. Food Hydrocoll. 2019, 97, 105197. [Google Scholar] [CrossRef]
- Anbinder, P.S.; Deladino, L.; Navarro, A.S.; Amalvy, J.I.; Martino, M.N. Yerba Mate Extract Encapsulation with Alginate and Chitosan Systems: Interactions between Active Compound Encapsulation Polymers. J. Encapsulation Adsorpt. Sci. 2011, 01, 80–87. [Google Scholar] [CrossRef]
- Mirghani, M.E.; Elnour, A.A.; Kabbashi, N.; Alam, Z.; Musa, K.H.; Abdullah, A. Determination of antioxidant activity of gum arabic: An exudation from two different locations. ScienceAsia 2018, 44, 179–186. [Google Scholar] [CrossRef]
- Zayed, M.M.M.; Sahyon, H.A.; Hanafy, N.A.N.; El-Kemary, M.A. The Effect of Encapsulated Apigenin Nanoparticles on HePG-2 Cells through Regulation of P53. Pharmaceutics 2022, 14, 1160. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-C.; Park, K.-M.; Hong, C.R.; Kim, J.-C.; Yang, S.-H.; Yu, H.-S.; Paik, H.-D.; Pan, C.-H.; Chang, P.-S. Microfluidic assembly of liposomes dual-loaded with catechin and curcumin for enhancing bioavailability. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124670. [Google Scholar] [CrossRef]
- Farha, A.K.; Gan, R.-Y.; Li, H.-B.; Wu, D.-T.; Atanasov, A.G.; Gul, K.; Zhang, J.-R.; Yang, Q.-Q.; Corke, H. The anticancer potential of the dietary polyphenol rutin: Current status, challenges, and perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 832–859. [Google Scholar] [CrossRef] [PubMed]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic Acid–Human Pharmacokinetics and Health Benefits. Planta Medica 2021, 87, 273–282. [Google Scholar] [CrossRef]
- Ekbatan, S.S.; Li, X.Q.; Ghorbani, M.; Azadi, B.; Kubow, S. Chlorogenic acid and its microbial metabolites exert anti-proliferative effects, S-phase cell-cycle arrest and apoptosis in human colon cancer caco-2 cells. Int. J. Mol. Sci. 2018, 19, 723. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Yang, W.; Dong, J.; Li, L. Regulation of dietary polyphenols on cancer cell pyroptosis and the tumor immune microenvironment. Front. Nutr. 2022, 9, 974896. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Romero, A.; Del Toro-Barbosa, M.; Gradilla-Hernández, M.S.; Garcia-Amezquita, L.E.; García-Cayuela, T. Probiotic properties, prebiotic fermentability, and gaba-producing capacity of microorganisms isolated from mexican milk kefir grains: A clustering evaluation for functional dairy food applications. Foods 2021, 10, 2275. [Google Scholar] [CrossRef] [PubMed]
- Jilani, H.; Cilla, A.; Barberá, R.; Hamdi, M. Antiproliferative activity of green, black tea and olive leaves polyphenols subjected to biosorption and in vitro gastrointestinal digestion in Caco-2 cells. Food Res. Int. 2020, 136, 109317. [Google Scholar] [CrossRef]
- Tsai, Y.J.; Chen, B.-H. Preparation of catechin extracts and nanoemulsions from green tea leaf waste and their inhibition effect on prostate cancer cell PC-3. Int. J. Nanomed. 2016, 11, 1907–1926. [Google Scholar] [CrossRef]
- Ballesteros-Vivas, D.; Alvarez-Rivera, G.; Leon, C.; Morantes, S.J.; Ibánez, E.; Parada-Alfonso, F.; Cifuentes, A.; Valdés, A. Anti-proliferative bioactivity against HT-29 colon cancer cells of a withanolides-rich extract from golden berry (Physalis peruviana L.) calyx investigated by Foodomics. J. Funct. Foods 2019, 63, 103567. [Google Scholar] [CrossRef]
- Sznarkowska, A.; Kostecka, A.; Meller, K.; Bielawski, K.P. Inhibition of cancer antioxidant defense by natural compounds. Oncotarget 2017, 8, 15996–16016. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, B.; López-García, G.; Máñez, V.; Alegría, A.; Barberá, R.; Cilla, A. Antiproliferative effect of bioaccessible fractions of four brassicaceae microgreens on human colon cancer cells linked to their phytochemical composition. Antioxidants 2020, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Cilla, A.; Rodrigo, M.J.; Zacarías, L.; De Ancos, B.; Sánchez-Moreno, C.; Barberá, R.; Alegría, A. Protective effect of bioaccessible fractions of citrus fruit pulps against H2O2-induced oxidative stress in Caco-2 cells. Food Res Int. 2018, 103, 335–344. [Google Scholar] [CrossRef] [PubMed]
- NTC 4580:1999; Frutas Frescas. Uchuva. Especificaciones. ICONTEC: Bogotá, Colombia, 1999.
- NTC 6456:2020; Frutas Frescas. Gulupa. Especificaciones. ICONTEC: Bogotá, Colombia, 2020.
- Shanmugam, S.; Gomes, I.A.; Denadai, M.; Lima, B.d.S.; Araújo, A.A.d.S.; Narain, N.; Neta, M.T.S.L.; Serafini, M.R.; Quintans-Júnior, L.J.; Thangaraj, P. UHPLC-QqQ-MS/MS identification, quantification of polyphenols from Passiflora subpeltata fruit pulp and determination of nutritional, antioxidant, α-amylase and α-glucosidase key enzymes inhibition properties. Food Res. Int. 2018, 108, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Durán, A.M.; Quintero-Quiroz, J.; Rojas-Camargo, J.; Ciro-Gómez, G.L. Modified-release of encapsulated bioactive compounds from annatto seeds produced by optimized ionic gelation techniques. Sci. Rep. 2021, 11, 1317. [Google Scholar] [CrossRef] [PubMed]
- Piornos, J.A.; Burgos-Díaz, C.; Morales, E.; Rubilar, M.; Acevedo, F. Highly efficient encapsulation of linseed oil into algi-nate/lupin protein beads: Optimization of the emulsion formulation. Food Hydrocoll. 2017, 63, 139–148. [Google Scholar] [CrossRef]
- Londoño, C.; Rojas, J. Effect of different production variables on the physical properties of pellets prepared by extrusion-spheronization using a multivariate analysis. Thai J. Pharm. Sci. 2017, 41, 69–75. [Google Scholar]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Fredes, C.; Osorio, M.J.; Parada, J.; Robert, P. Stability and bioaccessibility of anthocyanins from maqui (Aristotelia chilensis [Mol.] Stuntz) juice microparticles. LWT Food Sci. Technol. 2018, 91, 549–556. [Google Scholar] [CrossRef]
- Nunes, M.A.; Costa, A.S.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P. Olive pomace as a valuable source of bioactive compounds: A study regarding its lipid- and water-soluble components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Jalali, S.; Heidari, Z.; de Courten, B.; Rashidkhani, B. Dietary Total Antioxidant Capacity and Odds of Breast Cancer: A Case-Control Study. Nutr. Cancer 2022, 75, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Choi, A.-H.; Kwon, M.; Joung, E.-J.; Shin, T.; Lee, S.-G.; Kim, N.-G.; Kim, H.-R. Evaluation of antioxidant activities of various solvent extract from Sargassum serratifolium and its major antioxidant components. Food Chem. 2019, 278, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Cilla, A.; Perales, S.; Lagarda, M.J.; Barberá, R.; Clemente, G.; Farré, R. Influence of storage and in vitro gastrointestinal digestion on total antioxidant capacity of fruit beverages. J. Food Compos. Anal. 2011, 24, 87–94. [Google Scholar] [CrossRef]
- Miedes, D.; Cilla, A.; Alegría, A. Chemopreventive Effect of an In Vitro Digested and Fermented Plant Sterol-Enriched Wholemeal Rye Bread in Colon Cancer Cells. Foods 2023, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- López-García, G.; Cilla, A.; Barberá, R.; Alegría, A. Anti-Inflammatory and Cytoprotective Effect of Plant Sterol and Galactooligosaccharides-Enriched Beverages in Caco-2 Cells. J. Agric. Food Chem. 2020, 68, 1862–1870. [Google Scholar] [CrossRef] [PubMed]



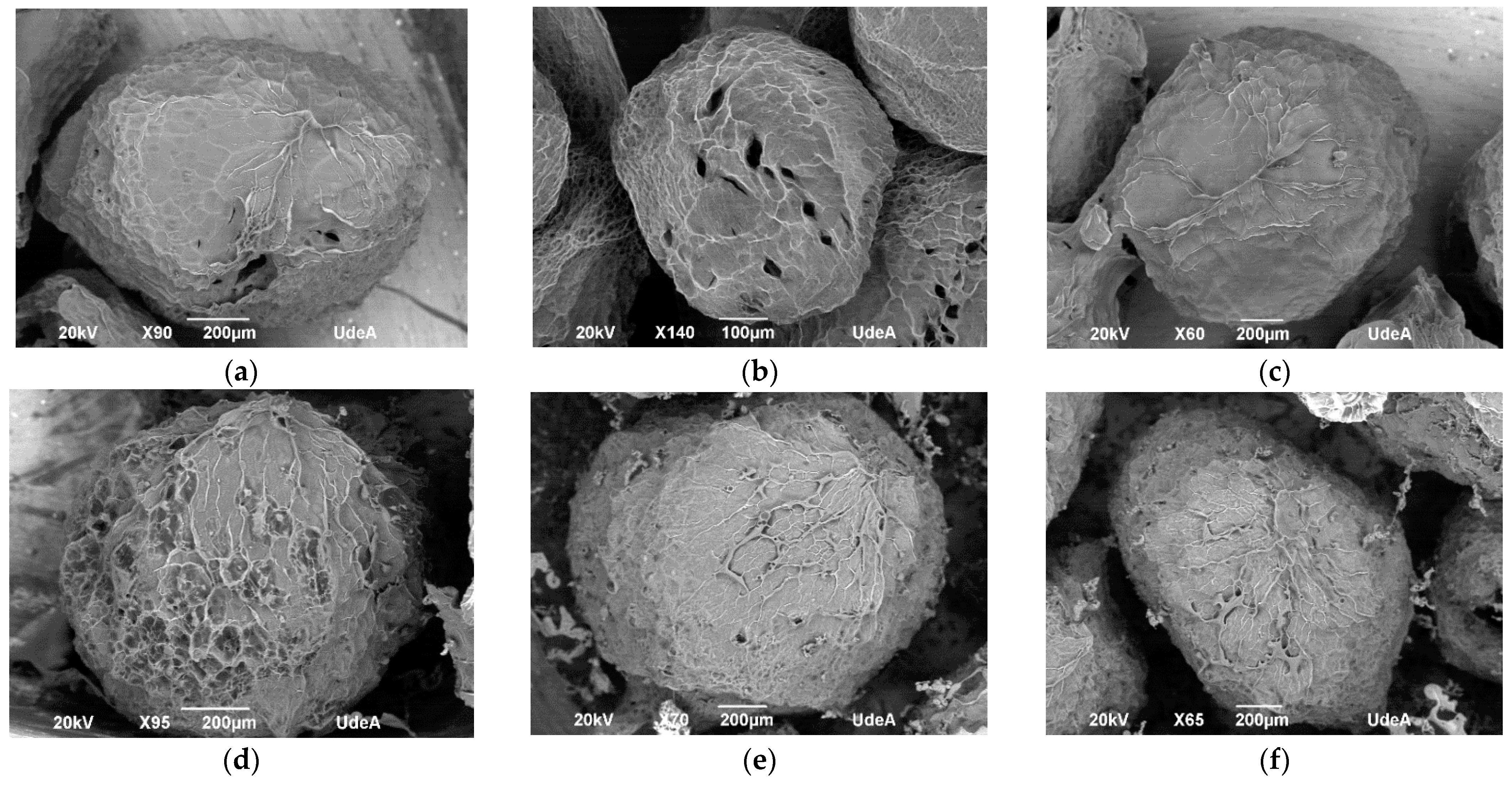


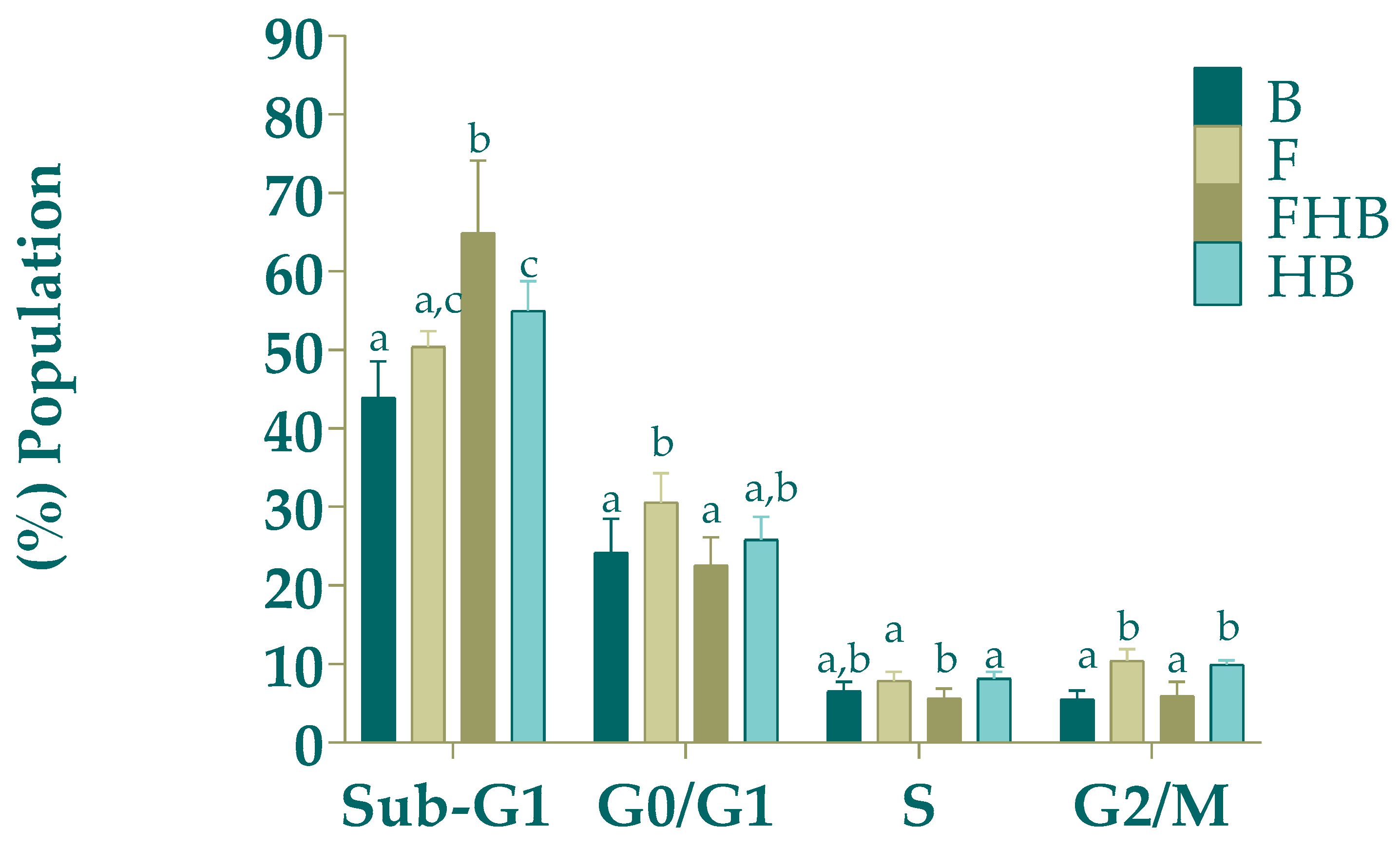
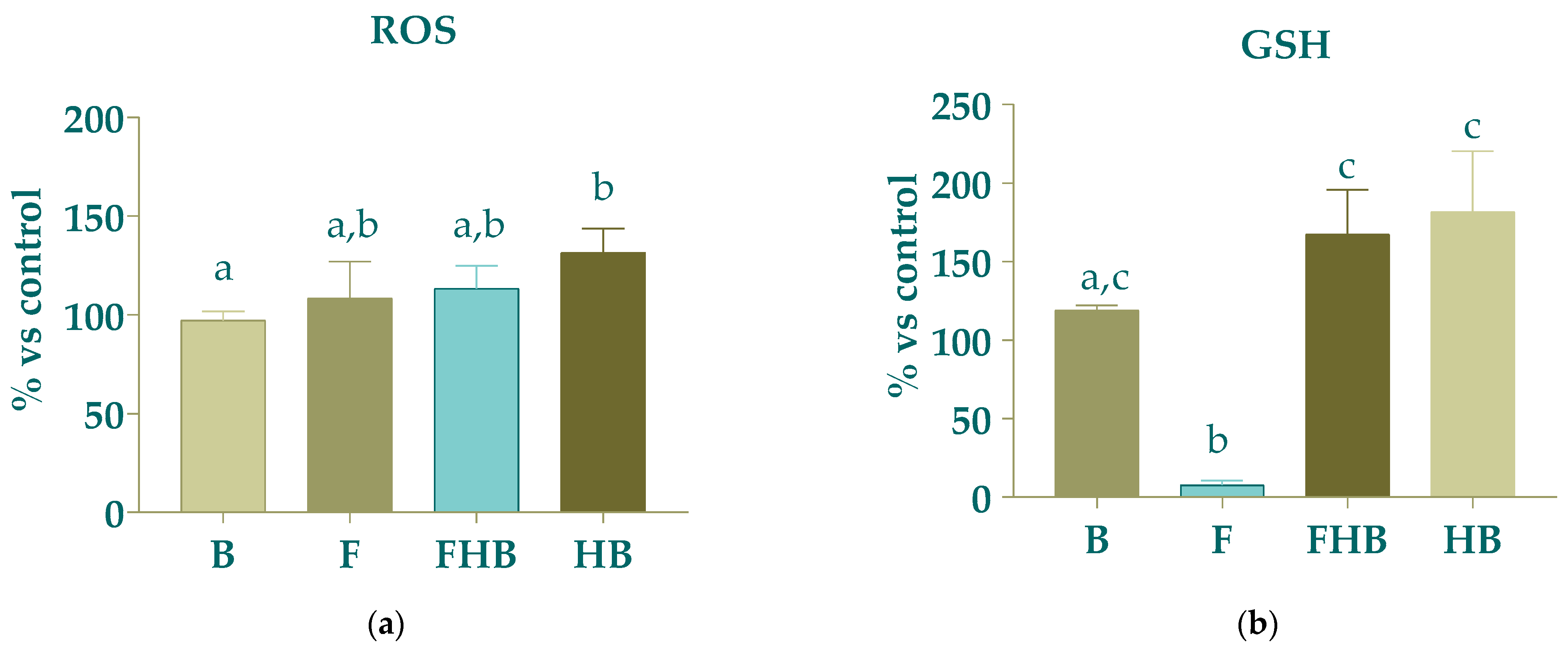
| Run | Polyphenol Bioaccessibility (%) | Carotenoid Bioaccessibility (%) | Polyphenol EE (%) | Particle Size (µm) | ABTS (%) | FRAP (%) | Sphericity |
|---|---|---|---|---|---|---|---|
| 1 | 105.16 | 75.79 | 64.7 | 1539.27 | 123.01 | 57.83 | 0.71 |
| 2 | 126.59 | 50.96 | 40.8 | 2224.47 | 44.02 | 72.7 | 0.66 |
| 3 | 110.20 | 61.34 | 49.4 | 2078.63 | 47.59 | 97.39 | 0.63 |
| 4 | 132.54 | 88.24 | 46.2 | 1625.8 | 43.78 | 51.06 | 0.66 |
| 5 | 156.93 | 105.41 | 51.4 | 1825.81 | 78.14 | 90.1 | 0.67 |
| 6 | 85.37 | 70.11 | 57.1 | 1866.22 | 129.55 | 91.24 | 0.76 |
| 7 | 73.48 | 85.42 | 58.5 | 1701.86 | 71.28 | 168.48 | 0.68 |
| 8 | 142.13 | 97.26 | 59.8 | 1592.14 | 34.41 | 106.78 | 0.66 |
| 9 | 135.70 | 55.38 | 49.6 | 1349.00 | 93.86 | 120.17 | 0.72 |
| 10 | 125.98 | 59.81 | 51.1 | 1149.88 | 95.02 | 130.33 | 0.65 |
| 11 | 77.55 | 62.50 | 55.9 | 1787.01 | 127.7 | 49.99 | 0.74 |
| 12 | 109.97 | 74.70 | 52.8 | 1720.6 | 55.79 | 170.53 | 0.69 |
| p-value | 0.0341 | 0.1823 | 0.0274 | 0.0313 | 0.0111 | 0.0374 | 0.0032 |
| r2 | 90.74 | 92.27 | 82.74 | 95.48 | 91.27 | 85.42 | 94.77 |
| r2-adjust | 79.18 | 72.93 | 68.35 | 87.95 | 82.54 | 70.84 | 89.54 |
| F | FHB | HB | |
|---|---|---|---|
| Total Polyphenols | µg GAE/100 g | ||
| Non-digested | 23,807.69 ± 783.29 a | 11,404.89 ± 335.04 c | 153.21 ± 43.33 d |
| Bioaccessible fraction | 26,946.15 ± 1201.58 b | 25,346.15 ± 1786.21 a, b | 16,330.77 ± 554.70 e |
| ABTS | µM Trolox/100 g | ||
| Non-digested | 91.50 ± 1.43 a | 69.33 ± 7.11 b | 16.81 ± 3.56 d |
| Bioaccessible fraction | 75.85 ± 16.293 b | 47.41 ± 0.00 c | 15.41 ± 2.51 d |
| FRAP | µM Trolox/100 g | ||
| Non-digested | 77.34 ± 1.31 a | 22.13 ± 1.92 c | 7.23 ± 3.18 d |
| Bioaccessible fraction | 72.85 ± 2.60 b | 20.54 ± 2.40 c | 6.70 ± 0.71 d |
| ORAC | µM Trolox/100 g | ||
| Non-digested | 47.06 ± 9.31 a | 32.10 ± 4.73 c | 7.32 ± 1.79 e |
| Bioaccessible fraction | 7865.90 ± 1238.55 b | 15,382.15 ± 2781.93 d | 7362.72 ± 2692.69 b |
| Compound | Molecular Formula | Molecular Weight (m/z) | Compound | Molecular Formula | Molecular Weight (m/z) |
|---|---|---|---|---|---|
| Apigenin | C15H10O5 | 271.06010 | kaempferol 3-glucoside | C21H20O11 | 449.10784 |
| Artepillin C | C19H24O3 | 301.17982 | Luteolin | C15H10O6 | 287.05501 |
| Caffeic acid | C9H8O4 | 181.04954 | Narigenin | C15H12O5 | 273.07575 |
| Caffein | C8H10N4O2 | 195.08765 | Orientin | C21H20O11 | 449.10784 |
| Carnosic acid | C20H28O4 | 333.20604 | p-coumaric acid | C9H8O3 | 165.05462 |
| Catechin | C15H14O6 | 291.08631 | Pelargonidin | C15H11O5+ | 271.0601 |
| Chlorogenic acid | C16H18O9 | 355.10236 | pelargonidin 3-glucoside | C21H21O10+ | 433.11292 |
| Cinnamic acid | C9H8O2 | 149.05971 | Pinocembrin | C15H12O4 | 257.08084 |
| Cyanidin | C15H11O6+ | 287.05501 | Protocatechuic acid | C7H6O4 | 155.03389 |
| Cyanidin 3-rutinoside | C27H31ClO15 | 631.14242 | Quercetin | C15H10O7 | 303.04993 |
| Epicatechin | C15H14O6 | 291.08631 | Quercetin-3-glucoside | C21H20O12 | 465.10275 |
| Epigallocatechin | C15H14O7 | 324.10778 | Rosmarinic acid | C18H16O8 | 361.09179 |
| Eriodictyol | C15H12O6 | 289.0706 | Rutin | C18H16O8 | 611.16066 |
| Ferulic acid | C10H10O4 | 195.06519 | Theobromine | C7H8N4O2 | 181.0720 |
| Gallic acid | C7H6O5 | 171.0288 | ursolic acid | C30H48O3 | 457.36762 |
| Isoorientin | C21H20O11 | 449.10784 | Vanilin | C8H8O3 | 153.05462 |
| Isovitexin | C21H20O10 | 433.11292 | Vanillic acid | C8H8O4 | 169.04954 |
| Kaempferol | C15H10O6 | 287.05501 |
| Run | SA | HPMC | AG | Run | SA | HPMC | AG |
|---|---|---|---|---|---|---|---|
| 1 | 0.50 | 0.5 | 0.00 | 7 | 0.75 | 0.25 | 0.00 |
| 2 | 0.50 | 0.00 | 0.50 | 8 | 0.75 | 0.00 | 0.25 |
| 3 | 0.50 | 0.25 | 0.25 | 9 | 0.58 | 0.33 | 0.08 |
| 4 | 0.58 | 0.08 | 0.33 | 10 | 0.67 | 0.17 | 0.17 |
| 5 | 0.83 | 0.08 | 0.08 | 11 | 1.00 | 0.00 | 0.00 |
| 6 | 1.00 | 0.00 | 0.00 | 12 | 0.75 | 0.25 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naranjo-Durán, A.M.; Miedes, D.; Patiño-Osorio, J.M.; Cilla, A.; Alegría, A.; Marín-Echeverri, C.; Quintero-Quiroz, J.; Ciro-Gómez, G.L. Formulation of Hydrogel Beads to Improve the Bioaccessibility of Bioactive Compounds from Goldenberry and Purple Passion Fruit and Evaluation of Their Antiproliferative Effects on Human Colorectal Carcinoma Cells. Gels 2025, 11, 10. https://doi.org/10.3390/gels11010010
Naranjo-Durán AM, Miedes D, Patiño-Osorio JM, Cilla A, Alegría A, Marín-Echeverri C, Quintero-Quiroz J, Ciro-Gómez GL. Formulation of Hydrogel Beads to Improve the Bioaccessibility of Bioactive Compounds from Goldenberry and Purple Passion Fruit and Evaluation of Their Antiproliferative Effects on Human Colorectal Carcinoma Cells. Gels. 2025; 11(1):10. https://doi.org/10.3390/gels11010010
Chicago/Turabian StyleNaranjo-Durán, Ana María, Diego Miedes, Juan Manuel Patiño-Osorio, Antonio Cilla, Amparo Alegría, Catalina Marín-Echeverri, Julián Quintero-Quiroz, and Gelmy Luz Ciro-Gómez. 2025. "Formulation of Hydrogel Beads to Improve the Bioaccessibility of Bioactive Compounds from Goldenberry and Purple Passion Fruit and Evaluation of Their Antiproliferative Effects on Human Colorectal Carcinoma Cells" Gels 11, no. 1: 10. https://doi.org/10.3390/gels11010010
APA StyleNaranjo-Durán, A. M., Miedes, D., Patiño-Osorio, J. M., Cilla, A., Alegría, A., Marín-Echeverri, C., Quintero-Quiroz, J., & Ciro-Gómez, G. L. (2025). Formulation of Hydrogel Beads to Improve the Bioaccessibility of Bioactive Compounds from Goldenberry and Purple Passion Fruit and Evaluation of Their Antiproliferative Effects on Human Colorectal Carcinoma Cells. Gels, 11(1), 10. https://doi.org/10.3390/gels11010010








