Gelatin-Based Scaffolds with Carrageenan and Chitosan for Soft Tissue Regeneration
Abstract
1. Introduction
2. Results and Discussion
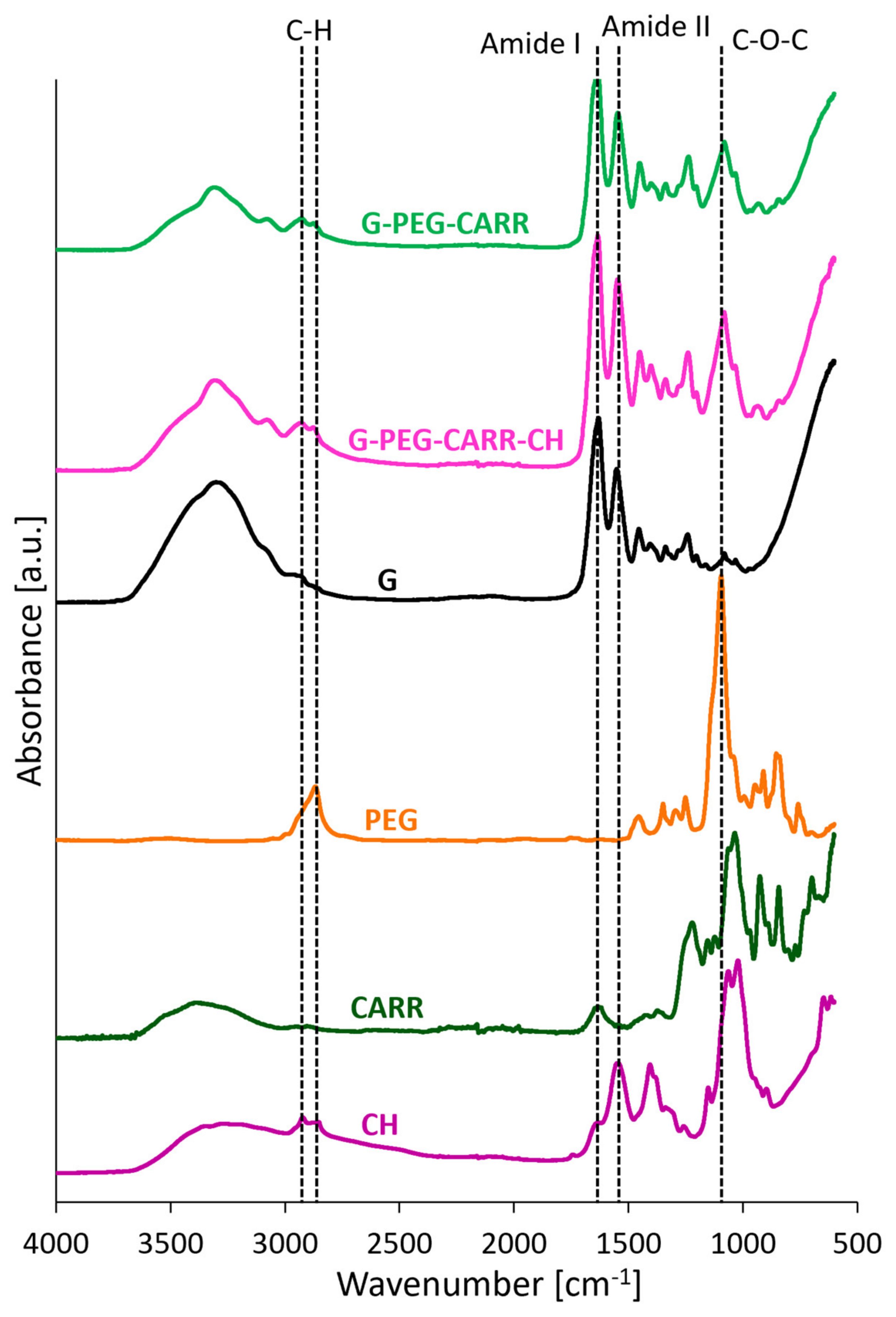
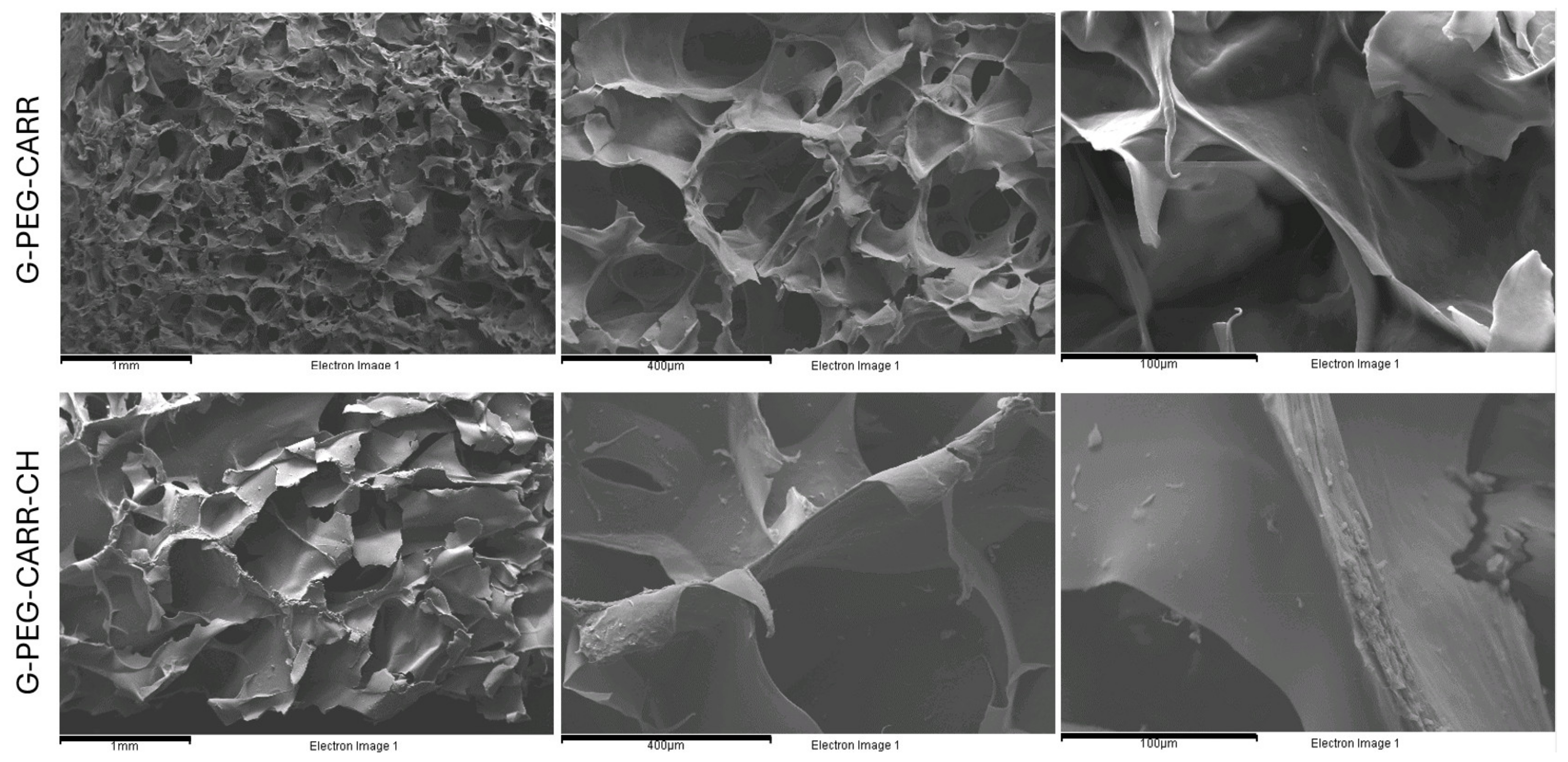
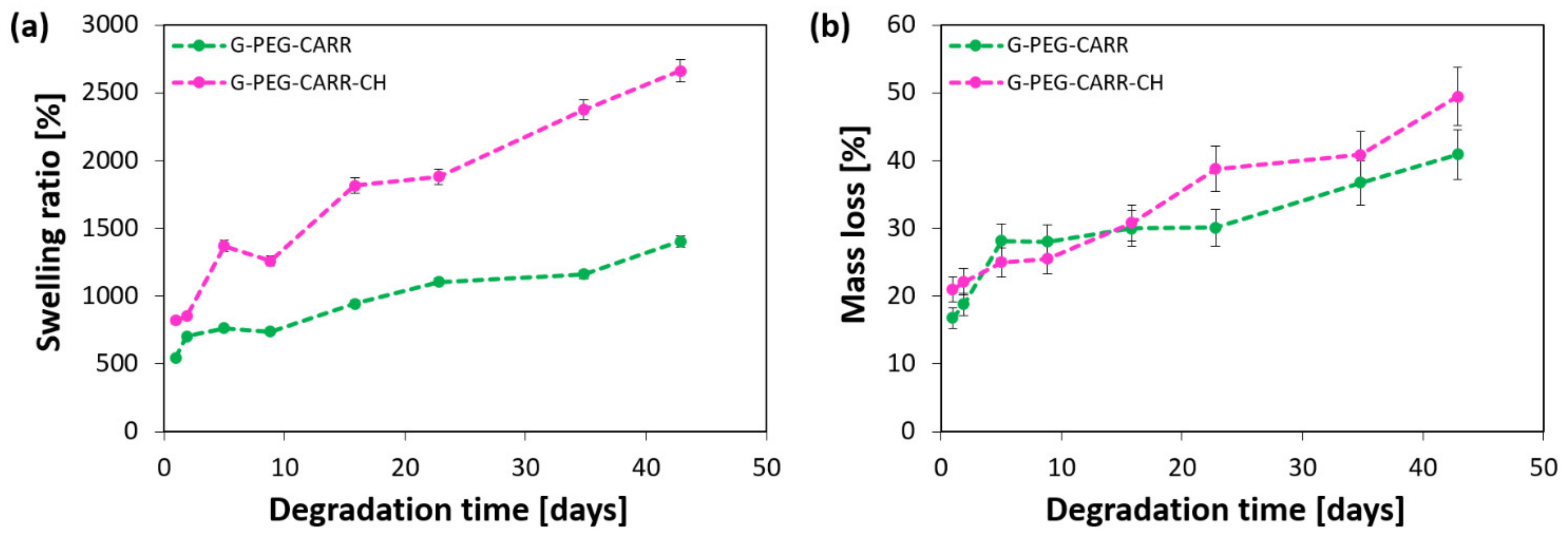
2.1. Physicochemical Properties of the Hydrogels
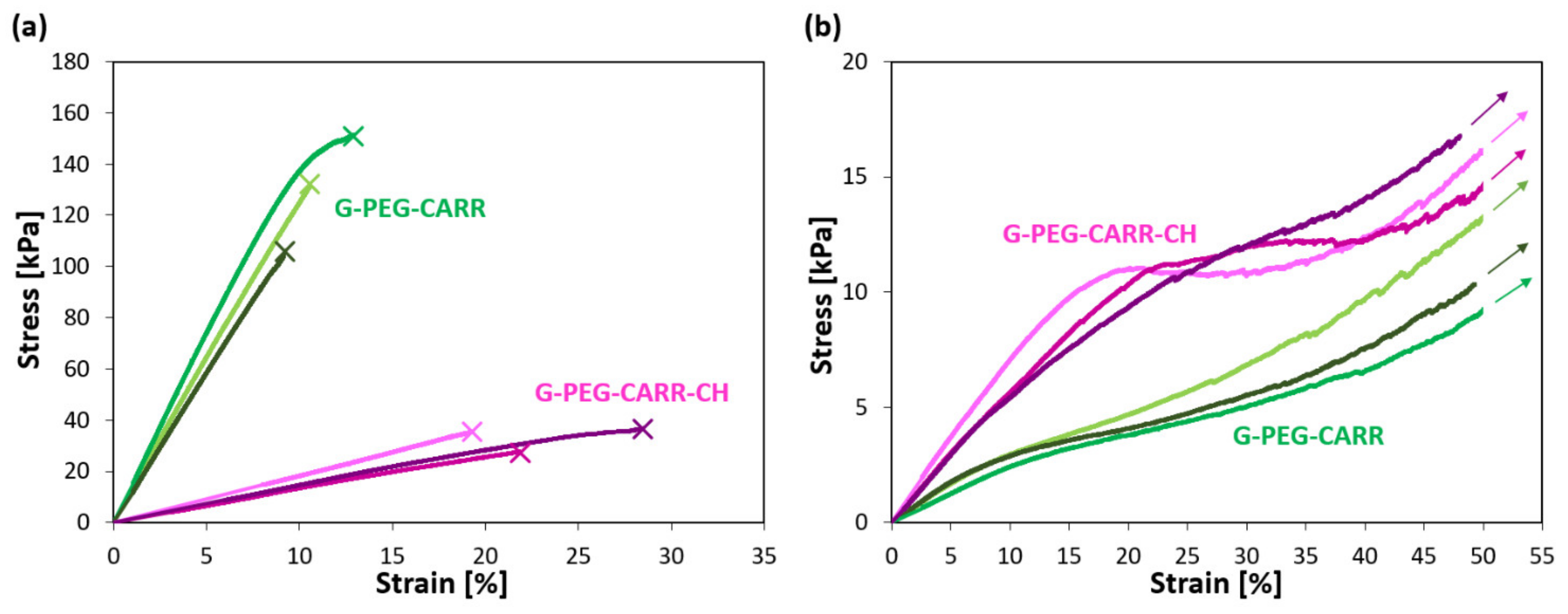
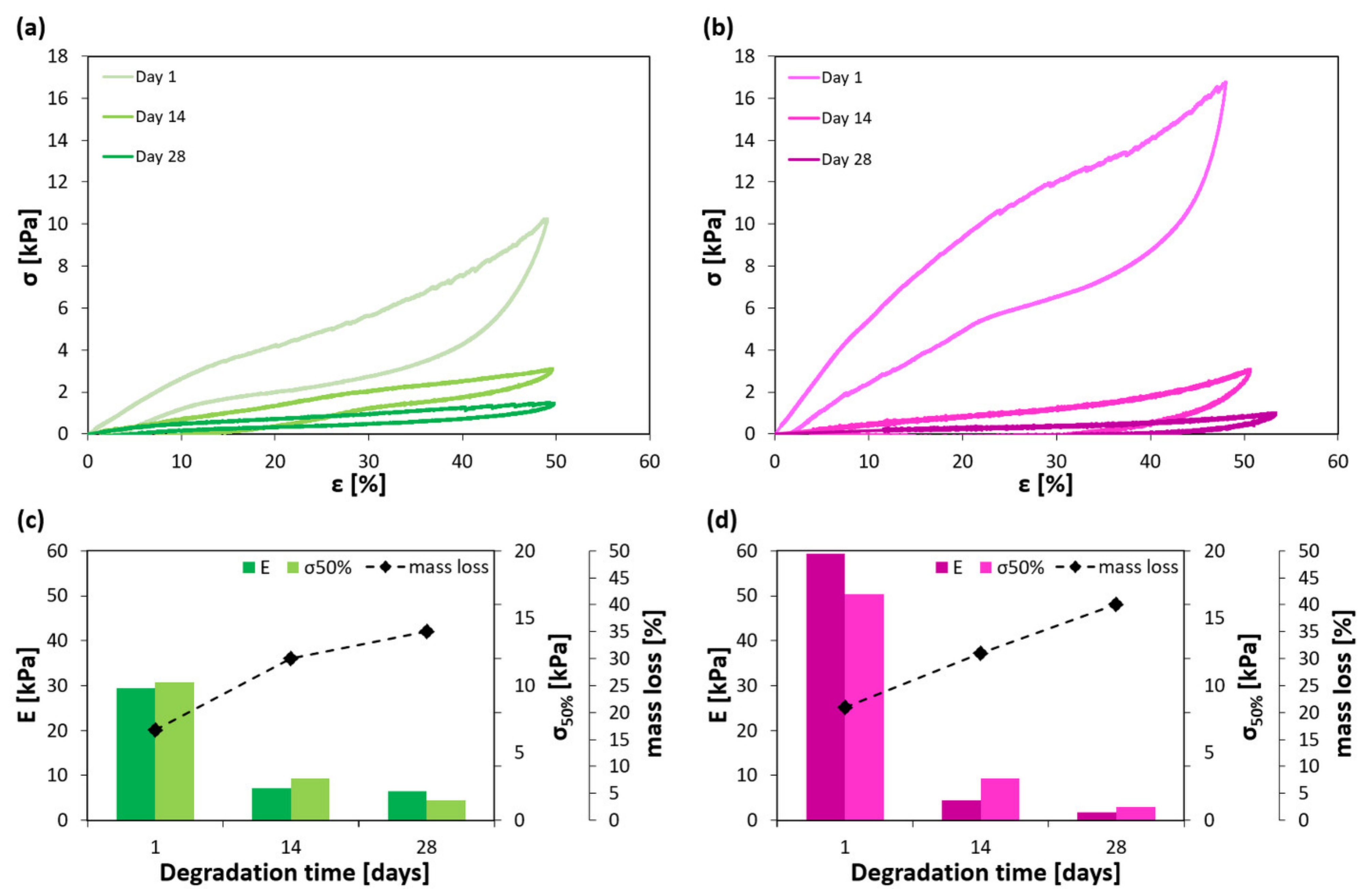
2.2. Mechanical Properties of the Hydrogels
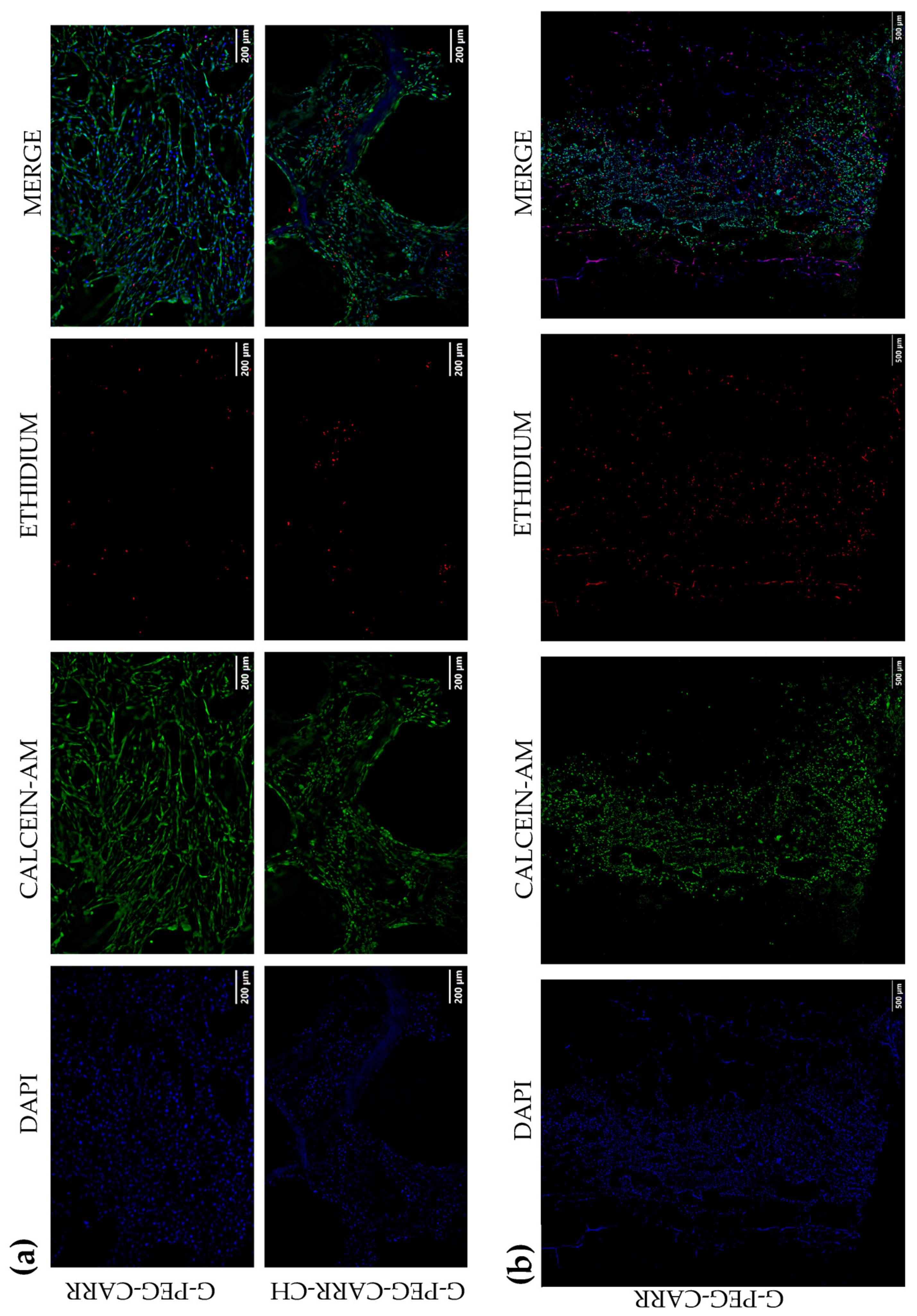
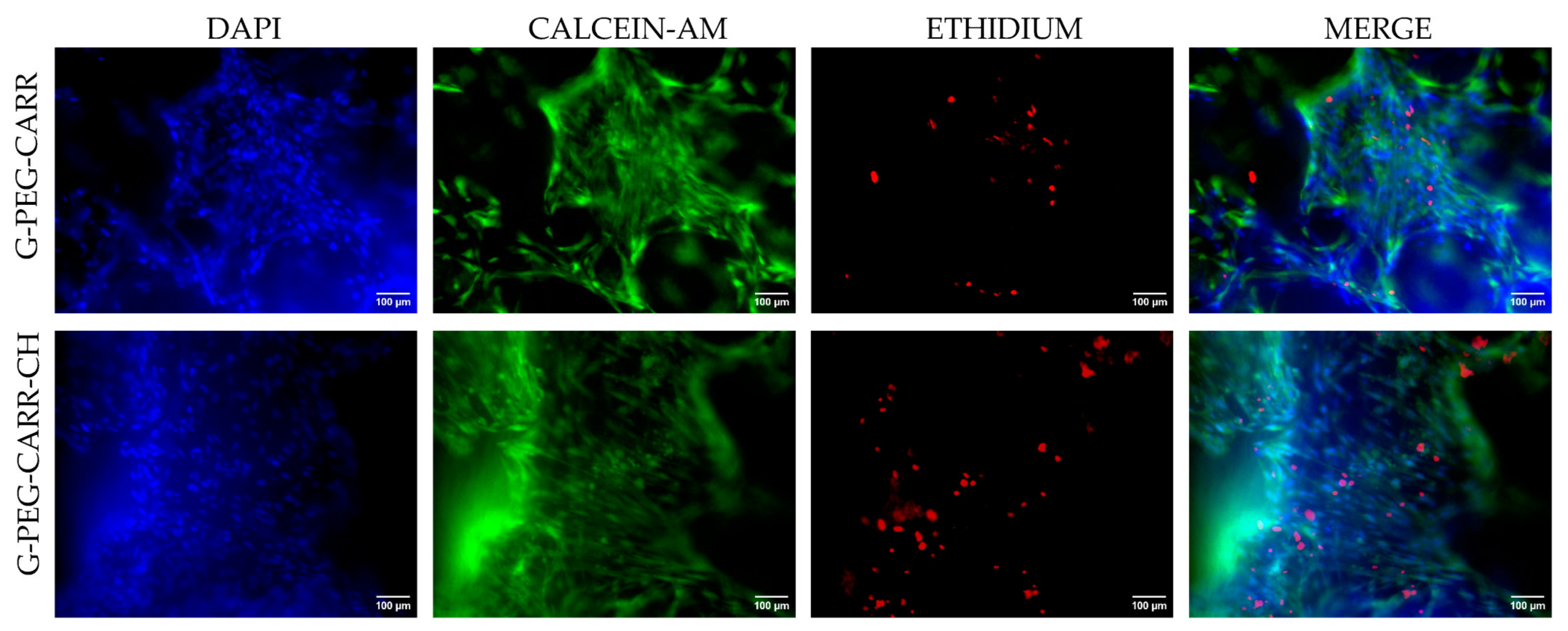
2.3. Results of In Vitro Studies on Hydrogel Scaffolds
2.3.1. hUC-MSCs Maintained Viability into the G-PEG-CARR and G-PEG-CARR-CH Scaffolds
2.3.2. hUC-MSCs Proliferate with an Increasing Trend into the Scaffolds
3. Conclusions
4. Materials and Methods
4.1. Materials
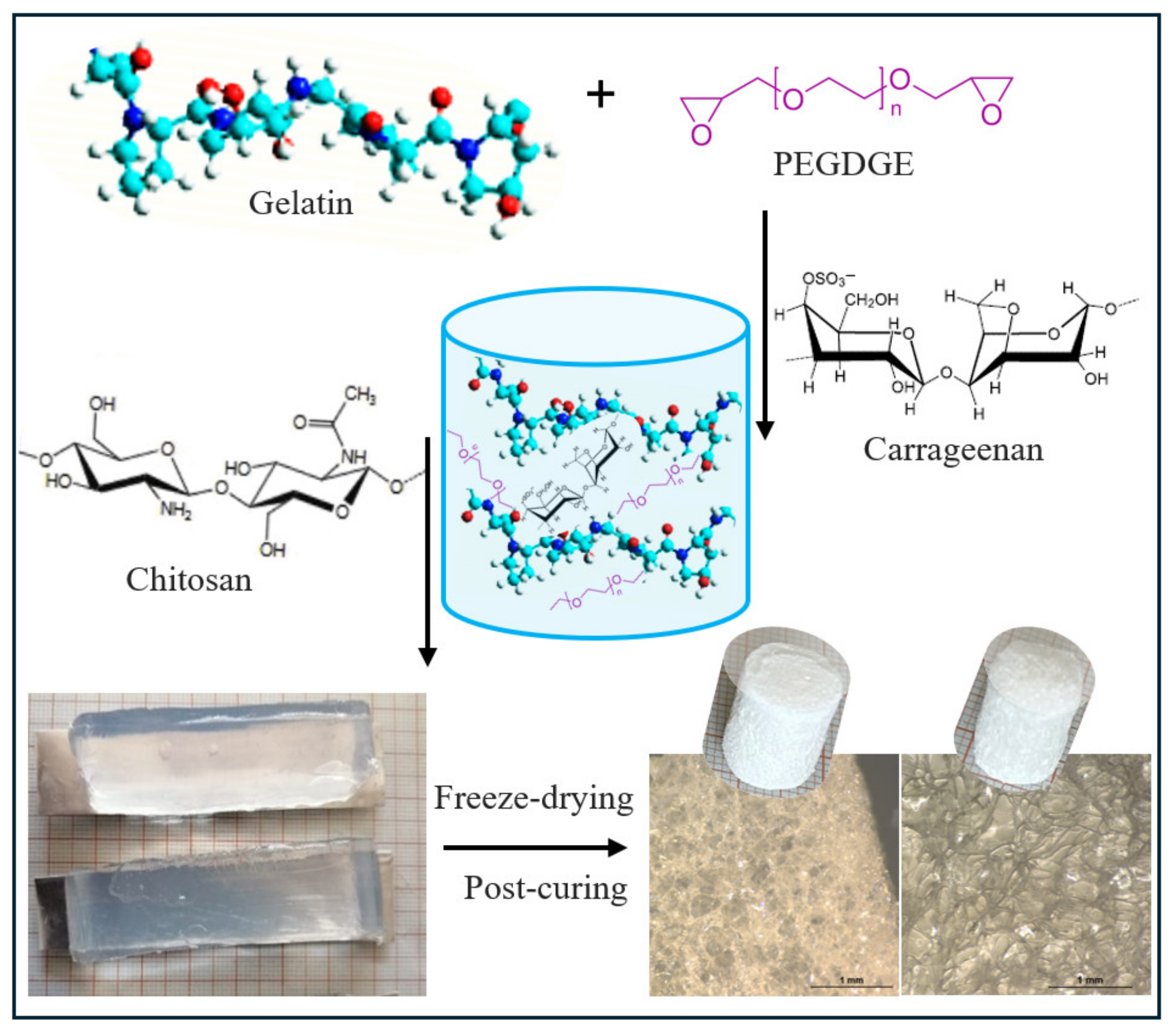
4.2. Preparation of Hydrogels
4.2.1. Preparation of the G-PEG-CARR Hydrogel
4.2.2. Preparation of the G-PEG-CARR-CH Hydrogel
4.3. Chemical Structure and Morphology Analysis
4.4. Evaluation of Apparent Density, Porosity, Swelling and Mass Loss
4.5. Mechanical Characterization
4.6. In Vitro Biological Characterization
4.6.1. Human Umbilical Cord-Derived Mesenchymal Stem Cells Culture
4.6.2. Human Platelet Lysate Production
4.6.3. hUC-MSCs Cell Proliferation and Cell Viability Assay
4.6.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abalymov, A.; Parakhonskiy, B.; Skirtach, A.G. Polymer-and Hybrid-Based Biomaterials for Interstitial, Connective, Vascular, Nerve, Visceral and Musculoskeletal Tissue Engineering. Polymers 2020, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Edgar, L.; McNamara, K.; Wong, T.; Tamburrini, R.; Katari, R.; Orlando, G. Heterogeneity of Scaffold Biomaterials in Tissue Engineering. Materials 2016, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Neves, S.C.; Moroni, L.; Barrias, C.C.; Granja, P.L. Leveling Up Hydrogels: Hybrid Systems in Tissue Engineering. Trends Biotechnol. 2020, 38, 292–315. [Google Scholar] [CrossRef] [PubMed]
- González-Díaz, E.C.; Varghese, S. Hydrogels as Extracellular Matrix Analogs. Gels 2016, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Smagina, V.; Yudaev, P.; Kuskov, A.; Chistyakov, E. Polymeric Gel Systems Cytotoxicity and Drug Release as Key Features for their Effective Application in Various Fields of Addressed Pharmaceuticals Delivery. Pharmaceutics 2023, 15, 830. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, B.; Qian, Y.; Wang, Q.; Han, R.; Hao, T.; Shu, Y.; Zhang, Y.; Yao, F.; Wang, C. Iota-carrageenan/chitosan/gelatin scaffold for the osteogenic differentiation of adipose-derived MSCs in vitro. J. Biomed. Mater. Res. Part B 2015, 103B, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Peng, J.; Xiao, H.; Xu, X.; Qian, Z. Polysaccharide Hydrogels: Functionalization, Construction and Served as Scaffold for Tissue Engineering. Carbohydr. Polym. 2022, 278, 118952. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan Based Bioactive Materials in Tissue Engineering Applications—A Review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Sairaman, S.; Nivedhitha, M.S.; Shrivastava, D.; Al Onazi, M.A.; Algarni, H.A.; Mustafa, M.; Alqahtani, A.R.; AlQahtani, N.; Teja, K.V.; Janani, K.; et al. Biocompatibility and Antioxidant Activity of a Novel Carrageenan Based Injectable Hydrogel Scaffold Incorporated with Cissus Quadrangularis: An in Vitro Study. BMC Oral Health 2022, 22, 377. [Google Scholar] [CrossRef] [PubMed]
- Graceffa, V.; Zeugolis, D.I. Carrageenan Enhances Chondrogenesis and Osteogenesis in Human Bone Marrow Stem Cell Culture. Eur. Cell Mater. 2019, 37, 310–332. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Ki, J.S. Biological Activity of Algal Derived Carrageenan: A Comprehensive Review in Light of Human Health and Disease. Int. J. Biol. Macromol. 2023, 238, 124085. [Google Scholar] [CrossRef] [PubMed]
- Rode, M.P.; Batti Angulski, A.B.; Gomes, F.A.; da Silva, M.M.; Jeremias, T.d.S.; de Carvalho, R.G.; Iucif Vieira, D.G.; Oliveira, L.F.C.; Fernandes Maia, L.; Trentin, A.G.; et al. Carrageenan Hydrogel as a Scaffold for Skin-Derived Multipotent Stromal Cells Delivery. J. Biomater. Appl. 2018, 33, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, B.; Barbu, A.; Negrea, M.O.; Berghea-Neamțu, C.Ș.; Popescu, D.; Zăhan, M.; Mireșan, V. Carrageenan-Based Compounds as Wound Healing Materials. Int. J. Mol. Sci. 2022, 23, 9117. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th Anniversary Article: Rational Design and Applications of Hydrogels in Regenerative Medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The Secretion Profile of Mesenchymal Stem Cells and Potential Applications in Treating Human Diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; LeRoux, M.A.; Danilkovitch-miagkova, A.; Leroux, M.A. Concise Review: Role of Mesenchymal Stem Cells in Wound Repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.N.; Naeem, B.K.; Ali, A.; Salim, A.; Khan, I. Expansion of Human Umbilical Cord Derived Mesenchymal Stem Cells in Regenerative Medicine. World J. Stem Cells 2024, 16, 410–433. [Google Scholar] [CrossRef] [PubMed]
- Oeller, M.; Laner-plamberger, S.; Krisch, L.; Rohde, E.; Strunk, D.; Schallmoser, K. Human Platelet Lysate for Good Manufacturing Practice-Compliant Cell Production. Int. J. Mol. Sci. 2021, 22, 5178. [Google Scholar] [CrossRef] [PubMed]
- Re, F.; Sartore, L.; Moulisova, V.; Cantini, M.; Almici, C.; Bianchetti, A.; Chinello, C.; Dey, K.; Agnelli, S.; Manferdini, C.; et al. 3D Gelatin-Chitosan Hybrid Hydrogels Combined with Human Platelet Lysate Highly Support Human Mesenchymal Stem Cell Proliferation and Osteogenic Differentiation. J. Tissue Eng. 2019, 10, 2041731419845852. [Google Scholar] [CrossRef] [PubMed]
- Dey, K.; Agnelli, S.; Re, F.; Russo, D.; Lisignoli, G.; Manferdini, C.; Bernardi, S.; Gabusi, E.; Sartore, L. Rational Design and Development of Anisotropic and Mechanically Strong Gelatin-Based Stress Relaxing Hydrogels for Osteogenic/Chondrogenic Differentiation. Macromol. Biosci. 2019, 19, 1900099. [Google Scholar] [CrossRef]
- Re, F.; Sartore, L.; Borsani, E.; Ferroni, M.; Baratto, C.; Mahajneh, A.; Smith, A.; Dey, K.; Almici, C.; Guizzi, P.; et al. Mineralization of 3D Osteogenic Model Based on Gelatin-Dextran Hybrid Hydrogel Scaffold Bioengineered with Mesenchymal Stromal Cells: A Multiparametric Evaluation. Materials 2021, 14, 3852. [Google Scholar] [CrossRef] [PubMed]
- Fung, Y.-C. Biomechanics: Mechanical Properties of Living Tissues; Springer: New York, NY, USA, 1981. [Google Scholar]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; ISBN 9781139878326. [Google Scholar]
- Ní Annaidh, A.; Bruyère, K.; Destrade, M.; Gilchrist, M.D.; Otténio, M. Characterization of the Anisotropic Mechanical Properties of Excised Human Skin. J. Mech. Behav. Biomed. Mater. 2012, 5, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Bader, D.L.; Bowker, P. Mechanical Characteristics of Skin and Underlying Tissues In Vivo. Biomaterials 1983, 4, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Little, C.J.; Bawolin, N.K.; Chen, X. Mechanical Properties of Natural Cartilage and Tissue-Engineered Constructs. Tissue Eng. Part B Rev. 2011, 17, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O. Viscoelastic Hydrogels for 3D Cell Culture. Biomater. Sci. 2017, 5, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Dey, K.; Agnelli, S.; Sartore, L. Dynamic Freedom: Substrate Stress Relaxation Stimulates Cell Responses. Biomater. Sci. 2019, 7, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Webber, R.E.; Creton, C.; Brown, H.R.; Gong, J.P. Large Strain Hysteresis and Mullins Effect of Tough Double-Network Hydrogels. Macromolecules 2007, 40, 2919–2927. [Google Scholar] [CrossRef]
- Zhan, L.; Qu, S.; Xiao, R. A Review on the Mullins Effect in Tough Elastomers and Gels. Acta Mech. Solida Sin. 2024, 37, 181–214. [Google Scholar] [CrossRef]
- Dey, K.; Agnelli, S.; Borsani, E.; Sartore, L. Degradation-Dependent Stress Relaxing Semi-Interpenetrating Networks of Hydroxyethyl Cellulose in Gelatin-PEG Hydrogel with Good Mechanical Stability and Reversibility. Gels 2021, 7, 277. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.P.; Lippens, E.; Duda, G.N.; et al. Hydrogels with Tunable Stress Relaxation Regulate Stem Cell Fate and Activity. Nat. Mater. 2015, 15, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Viidik, A. Functional Properties of Collagenous Tissues. Int. Rev. Connect. Tissue Res. 1973, 6, 127–215. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.G.; Fung, Y.-C. Mechanical Properties of the Heart Muscle in the Passive State. J. Biomech. 1973, 6, 597–616. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Han, T.; Yang, Q.; Wang, J.; Feng, B.; Jia, Y.; Wei, Z.; Xu, F. Viscoelastic Cell Microenvironment: Hydrogel-Based Strategy for Recapitulating Dynamic ECM Mechanics. Adv. Funct. Mater. 2021, 31, 2100848. [Google Scholar] [CrossRef]
- Li, H.; Lian, X.; Guan, D. Crossover Behavior in Stress Relaxations of Poroelastic and Viscoelastic Dominant Hydrogels. Soft Matter. 2023, 19, 5443–5451. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Munar, M.L.; Ishikawa, K. Effects of macropore size in carbonate apatite honeycomb scaffolds on bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110848. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Baroud, G.; Bernstein, A.; Döbelin, N.; Galea, L.; Hesse, B.; Heuberger, R.; Meille, S.; Michel, P.; von Rechenberg, B.; et al. Characterization and distribution of mechanically competent mineralized tissue in micropores of β tricalcium phosphate bone substitutes. Mater. Today 2017, 20, 106–115. [Google Scholar] [CrossRef]
- Jeyachandran, D.; Cerruti, M. Glass, Ceramic, Polymeric, and Composite Scaffolds with Multiscale Porosity for Bone Tissue Engineering. Adv. Eng. Mater. 2023, 25, 2201743. [Google Scholar] [CrossRef]
- Webber, V.; de Carvalho, S.M.; Ogliari, P.J.; Hayashi, L.; Barreto, P.L.M. Optimization of the Extraction of Carrageenan from Kappaphycus Alvarezii Using Response Surface Methodology. Food Sci. Technol. 2012, 32, 812–818. [Google Scholar] [CrossRef]
- ISO 11137-1:2006; Sterilization of Health Care Products—Radiation: Part 1: Requirements for Development, Validation and Routine Control of a Sterilization Process for Medical Devices. ISO: Geneva, Switzerland, 2006.
- Kothapalli, C.R.; Shaw, M.T.; Wei, M. Biodegradable HA-PLA 3-D Porous Scaffolds: Effect of Nano-Sized Filler Content on Scaffold Properties. Acta Biomater. 2005, 1, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Doucet, C.; Ernou, I.; Zhang, Y.; Llense, J.R.; Begot, L.; Holy, X.; Lataillade, J.J. Platelet Lysates Promote Mesenchymal Stem Cell Expansion: A Safety Substitute for Animal Serum in Cell-Based Therapy Applications. J. Cell. Physiol. 2005, 205, 228–236. [Google Scholar] [CrossRef] [PubMed]



| Hydrogel | Composition | Physical Properties | |||||
|---|---|---|---|---|---|---|---|
| G [wt%] | PEG [wt%] | CARR [wt%] | CH [wt%] | Apparent Density [g/cm3] | Porosity [%] | Swelling Ratio (24 h) [%] | |
| G-PEG-CARR | 75 | 17.5 | 7.5 | - | 0.074 ± 0.01 | 80 ± 5 | 550 ± 15 |
| G-PEG-CARR-CH | 69 | 17 | 6 | 8 | 0.080 ± 0.01 | 78 ± 7 | 830 ± 25 |
| Hydrogel | Tensile Properties | Compressive Properties | |||
|---|---|---|---|---|---|
| Modulus [kPa] | Stress at Break [kPa] | Strain at Break [%] | Modulus [kPa] | Stress at 50% [kPa] | |
| G-PEG-CARR | 1400 ± 200 | 130 ± 20 | 11 ± 2 | 31 ± 6 | 11 ± 2 |
| G-PEG-CARR-CH | 140 ± 20 | 36 ± 5 | 26 ± 9 | 63 ± 10 | 16 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasini, C.; Re, F.; Trenta, F.; Russo, D.; Sartore, L. Gelatin-Based Scaffolds with Carrageenan and Chitosan for Soft Tissue Regeneration. Gels 2024, 10, 426. https://doi.org/10.3390/gels10070426
Pasini C, Re F, Trenta F, Russo D, Sartore L. Gelatin-Based Scaffolds with Carrageenan and Chitosan for Soft Tissue Regeneration. Gels. 2024; 10(7):426. https://doi.org/10.3390/gels10070426
Chicago/Turabian StylePasini, Chiara, Federica Re, Federica Trenta, Domenico Russo, and Luciana Sartore. 2024. "Gelatin-Based Scaffolds with Carrageenan and Chitosan for Soft Tissue Regeneration" Gels 10, no. 7: 426. https://doi.org/10.3390/gels10070426
APA StylePasini, C., Re, F., Trenta, F., Russo, D., & Sartore, L. (2024). Gelatin-Based Scaffolds with Carrageenan and Chitosan for Soft Tissue Regeneration. Gels, 10(7), 426. https://doi.org/10.3390/gels10070426









