Functional Hydrogels Promote Vegetable Growth in Cadmium-Contaminated Soil
Abstract
1. Introduction
2. Results and Discussion
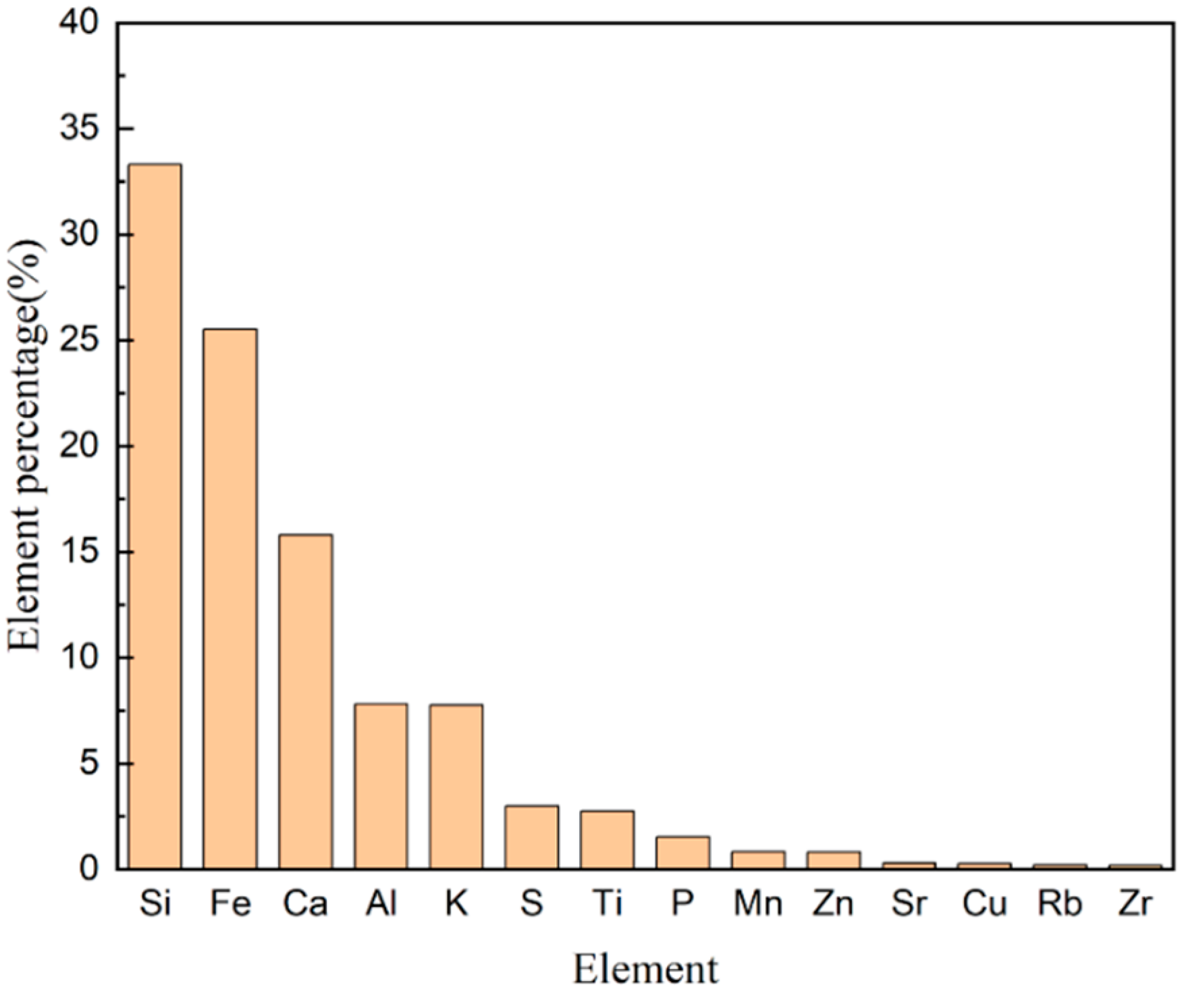
2.1. Detection of Elements in Soil
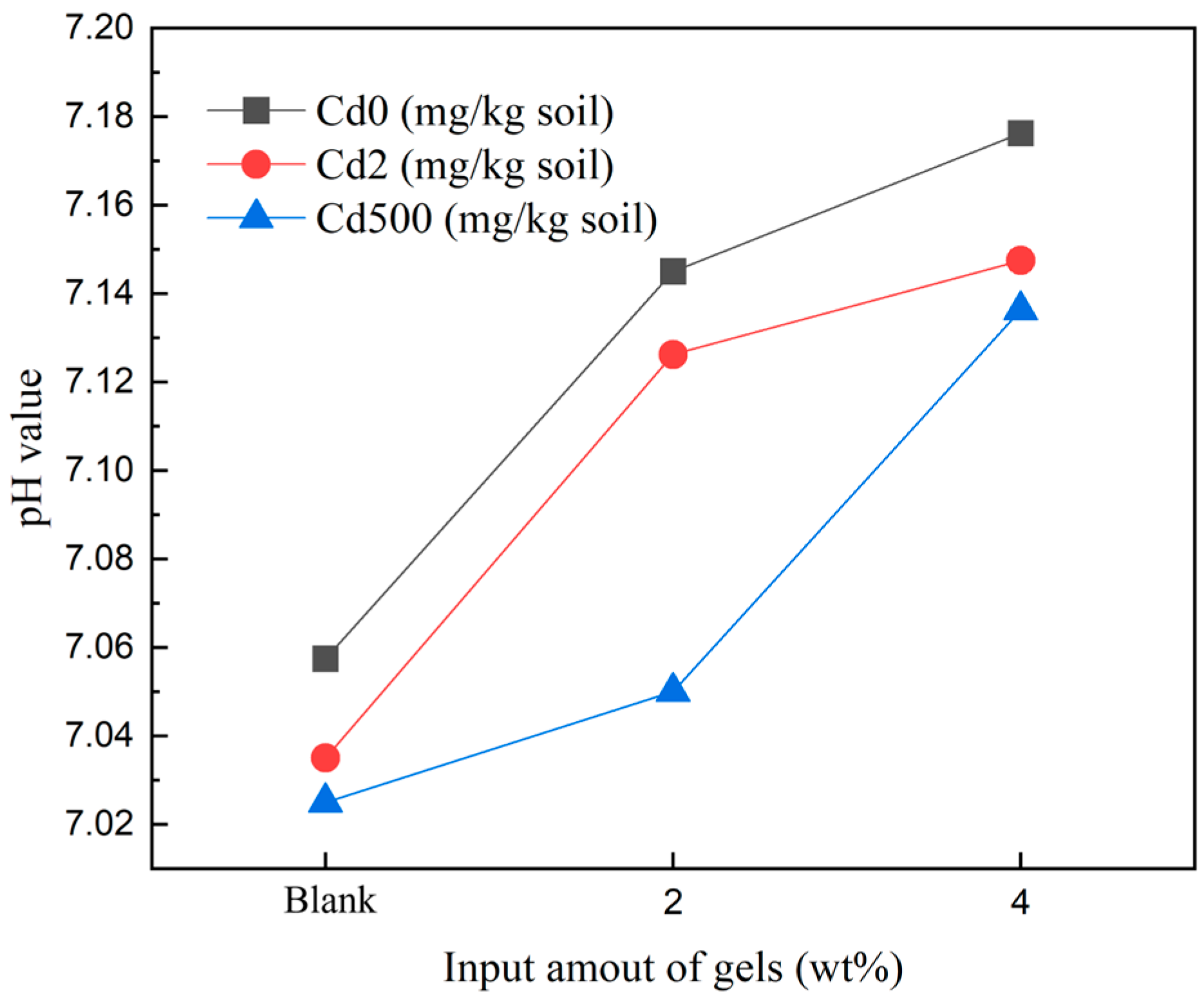
2.2. Soil pH
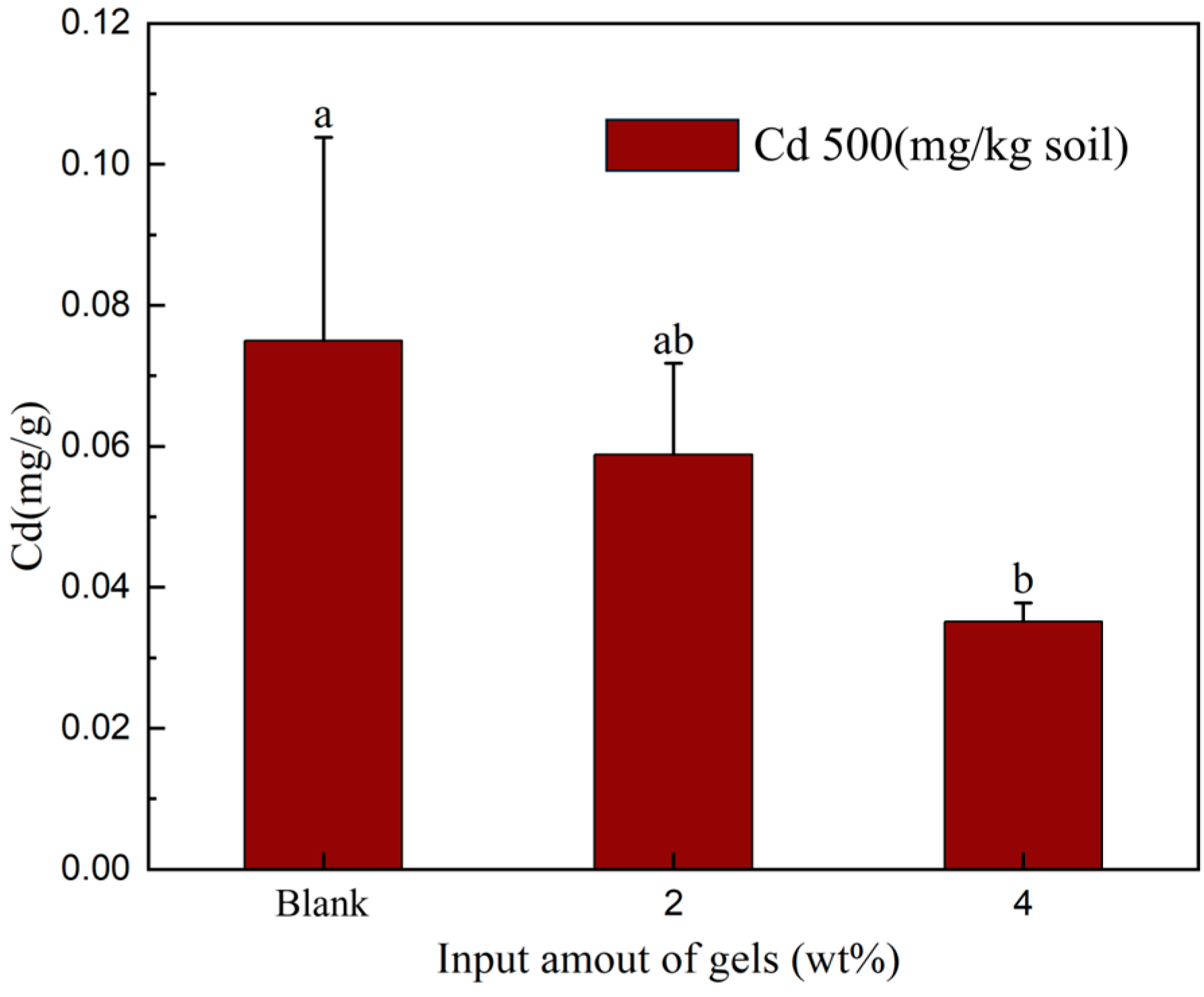
2.3. Cadmium Precipitation Experiments in Soil
2.4. Growth State of Vegetables under Cadmium Stress
2.5. Vegetable Growth Status under Two Types of Hydrogels
2.6. Cadmium Element Content in the Vegetables
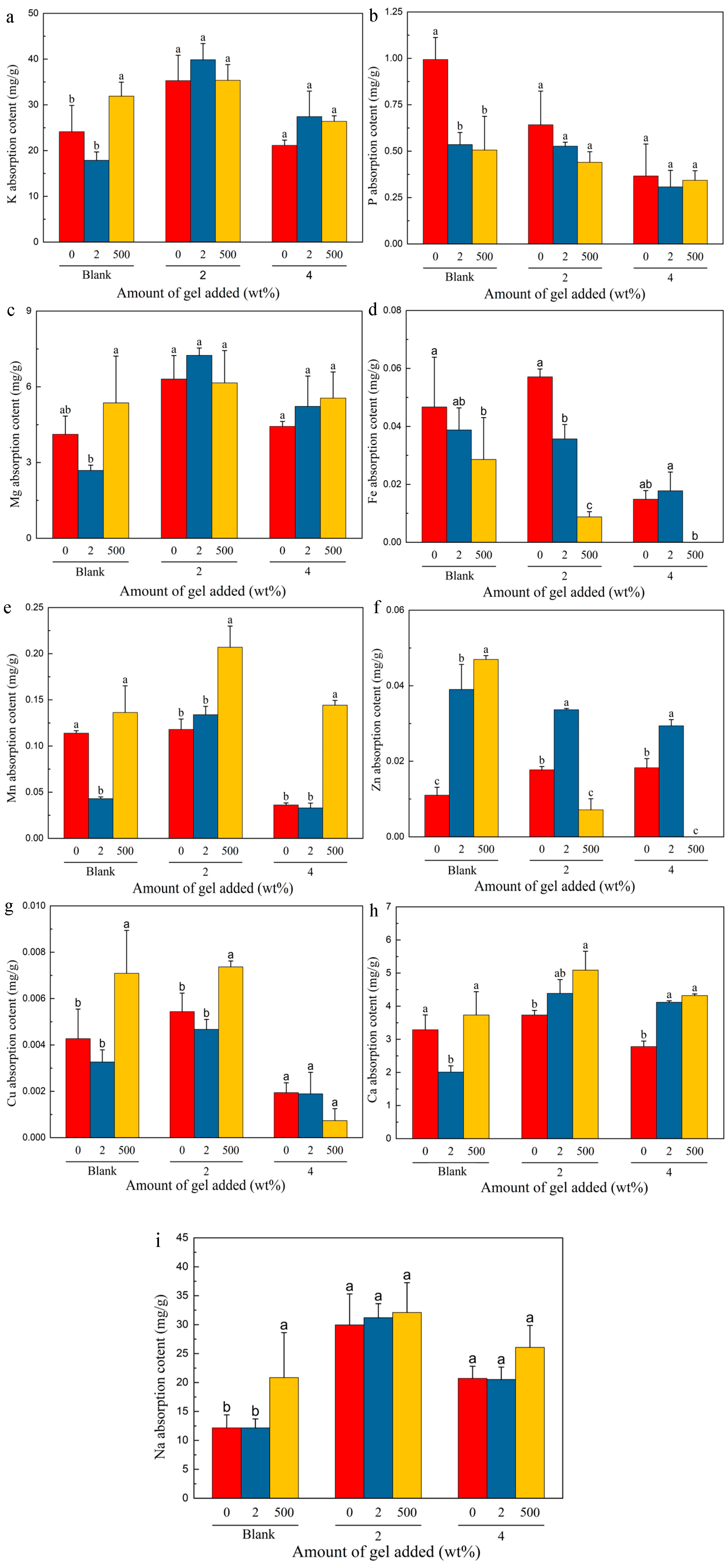
2.7. Vegetable Uptake of Different Elements
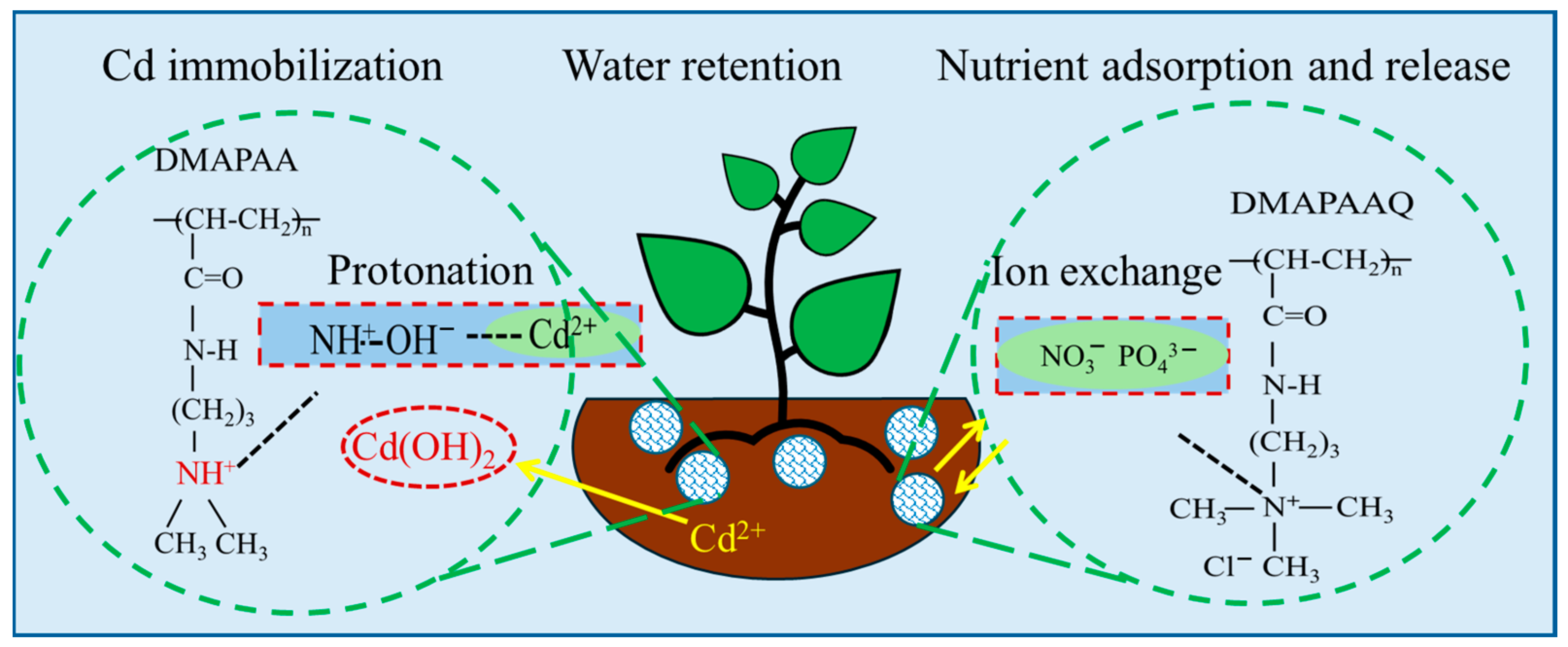
2.8. Hydrogel Repair Mechanism for Cadmium-Contaminated Soil
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of the DMAPAA/DMAPAAQ Hydrogel
4.3. Synthesis of the DMAA Hydrogel
4.4. Experimental Design
4.5. Dry Weight of the Vegetables
4.6. Plant Digestion
4.7. Experiment on Cadmium Precipitation from the Soil
4.8. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, P.; Chen, H.; Kopittke, P.M.; Zhao, F.-J. Cadmium contamination in agricultural soils of China and the impact on food safety. Environ. Pollut. 2019, 249, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Xiang, W.; Zhou, W.; Zhao, Y.; Cheng, Y.; He, M. In Situ Plant Bionic Remediation of Cadmium-Contaminated Soil Caused by a High Geological Background in Kaihua, Zhejiang Province, China. Chemosphere 2021, 269, 128693. [Google Scholar] [CrossRef]
- Che, Z.; Ahmed, W.; Weng, J.; Wenjie, L.; Mahmood, M.; Alatalo, J.M.; Wenjie, O.; Nizamani, M.M.; Lu, W.; Xian, F.X.; et al. Distribution, pollution, and human health risks of persistent and potentially toxic elements in the sediments around Hainan Island, China. Mar. Pollut. Bull. 2022, 174, 113278. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chang, Q.; Yuan, X.; Li, J.; Ayoko, G.A.; Frost, R.L.; Chen, H.; Zhang, X.; Song, Y.; Song, W. Cadmium transfer from contaminated soils to the human body through rice consumption in southern Jiangsu Province, China. Environ. Sci. Process. Impacts 2017, 19, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, G. Damage and Heavy Metal Pollution in China’s Farmland: Reality and Solutions. J. Contemp. East Asia Stud. 2017, 5, 11–25. [Google Scholar] [CrossRef]
- Li, H.; Xu, W.; Dai, M.; Wang, Z.; Dong, X.; Fang, T. Assessing Heavy Metal Pollution in Paddy Soil from Coal Mining Area, Anhui, China. Env. Monit Assess 2019, 191, 518. [Google Scholar] [CrossRef] [PubMed]
- Orooj, S.; Saleem, S.S.; Waqar, K.; Gul Kazi, A. Phytoremediation of Soils. In Soil Remediation and Plants; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–36. [Google Scholar]
- Wang, L.; Cui, X.; Cheng, H.; Chen, F.; Wang, J.; Zhao, X.; Lin, C.; Pu, X. A Review of Soil Cadmium Contamination in China Including a Health Risk Assessment. Environ. Sci. Pollut. Res. Int. 2015, 22, 16441–16452. [Google Scholar] [CrossRef] [PubMed]
- Ok, Y.S.; Lee, S.S.; Jeon, W.T.; Oh, S.E.; Usman, A.R.; Moon, D.H. Application of Eggshell Waste for the Immobilization of Cadmium and Lead in a Contaminated Soil. Environ. Geochem. Health 2011, 33 (Suppl. 1), 31–39. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.O.; Kim, S.Y.; Gutierrez, J.; Owens, V.N.; Kim, P.J. Comparison of oyster shell and calcium hydroxide as liming materials for immobilizing cadmium in upland soil. Biol. Fertil. Soils 2010, 46, 491–498. [Google Scholar] [CrossRef]
- Bi, D.; Yuan, G.; Wei, J.; Xiao, L.; Feng, L. Conversion of Oyster Shell Waste to Amendment for Immobilising Cadmium and Arsenic in Agricultural Soil. Bull. Environ. Contam. Toxicol. 2020, 105, 277–282. [Google Scholar] [CrossRef]
- Zhao, H.; Du, L.; An, T.; Wu, Y.; Zhao, Y. Evaluation of Shell Powder Remediation Effect in Cad-mium-Contaminated Soil: A Score Approach Based on Entropy Indexes. CLEAN Soil Air Water 2022, 50, 2000261. [Google Scholar] [CrossRef]
- Wu, B.; Li, J.; Sheng, M.; Peng, H.; Peng, D.; Xu, H. The application of biochar and oyster shell reduced cadmium uptake by crops and modified soil fertility and enzyme activities in contaminated soil. Soil 2022, 8, 409–419. [Google Scholar] [CrossRef]
- Noronha, F.R.; Manikandan, S.K.; Nair, V. Role of Coconut Shell Biochar and Earthworm (Eudrilus euginea) in Biore-mediation and Palak Spinach (Spinacia oleracea L.) Growth in Cadmium-Contaminated Soil. J. Environ. Manag. 2022, 302, 114057. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; He, F.; Su, B.; Sun, M.; Owens, G.; Chen, Z. The Stabilizing Mechanism of Cadmium in Contaminated Soil Using Green Synthesized Iron Oxide Nanoparticles under Long-Term Incubation. J. Hazard Mater. 2019, 379, 120832. [Google Scholar] [CrossRef] [PubMed]
- Zha, Y.; Zhao, B.; Niu, T. Bamboo Biochar and Zinc Oxide Nanoparticles Improved the Growth of Maize (Zea mays L.) and Decreased Cadmium Uptake in Cd-Contaminated Soil. Agriculture 2022, 12, 1507. [Google Scholar] [CrossRef]
- Watanabe, T.; Murata, Y.; Nakamura, T.; Sakai, Y.; Osaki, M. Effect of Zero-Valent Iron Ap-plication on Cadmium Uptake in Rice Plants Grown in Cadmium-Contaminated Soils. J. Plant Nutr. 2009, 32, 1164–1172. [Google Scholar] [CrossRef]
- Manzoor, N.; Ahmed, T.; Noman, M.; Shahid, M.; Nazir, M.M.; Ali, L.; Alnusaire, T.S.; Li, B.; Schulin, R.; Wang, G. Iron Oxide Nanoparticles Ameliorated the Cadmium and Salinity Stresses in Wheat Plants, Facilitating Photosynthetic Pigments and Restricting Cadmium Uptake. Sci. Total Environ. 2021, 769, 145221. [Google Scholar] [CrossRef]
- Fu, L.; Su, T.; Wei, D.; Wu, D.; Zhang, G.; Shen, Q. Copper Oxide Nanoparticles Al-leviate Cadmium Toxicity in Cereal Crops. Environ. Sci. Nano 2022, 9, 3502–3513. [Google Scholar] [CrossRef]
- Gray, C.W.; Wise, B.E. Can the Application of Zinc Decrease Cadmium Concentrations in Spinach in a Zinc Sufficient Soil? New Zealand J. Crop Hortic. Sci. 2020, 48, 117–129. [Google Scholar] [CrossRef]
- Sato, A.; Takeda, H.; Oyanagi, W.; Nishihara, E.; Murakami, M. Reduction of Cadmium Uptake in Spinach (Spinacia oleracea L.) by Soil Amendment with Animal Waste Compost. J. Hazard Mater. 2010, 181, 298–304. [Google Scholar] [CrossRef]
- Pinto, F.R.; Mourato, M.P.; Sales, J.R.; Fangueiro, D.; Martins, L.L. Effect of Cattle Slurry on the Growth of Spinach Plants in Cd-Contaminated Soil. Commun. Soil Sci. Plant Anal. 2020, 51, 1370–1381. [Google Scholar] [CrossRef]
- PPenido, E.S.; Martins, G.C.; Mendes, T.B.M.; Melo, L.C.A.; do Rosário Guimarães, I.; Guilherme, L.R.G. Combining biochar and sewage sludge for immobilization of heavy metals in mining soils. Ecotoxicol. Environ. Saf. 2019, 172, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.A.; Sheppard, S.C. Fertilizer Impacts on Cadmium Availability in Agricultural Soils and Crops. Hum. Ecol. Risk Assess. Int. J. 2008, 14, 210–228. [Google Scholar] [CrossRef]
- Roberts, T.L. Cadmium and Phosphorous Fertilizers: The Issues and the Science. Procedia Eng. 2014, 83, 52–59. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Abd El-Wahab, H.; Ismail, M.A.; Naser, A.M.; Abdelhai, F.; El-Damhougy, B.K.; Nady, N.; Meganid, A.S.; Alkhursani, S.A. Characterization of Starch-Based Three Components of Gamma-Ray Cross-Linked Hydrogels to Be Used as a Soil Conditioner. Mater. Sci. Eng. B 2020, 260, 114645. [Google Scholar] [CrossRef]
- Sarkar, K.; Sen, K. Polyvinyl alcohol based hydrogels for urea release and Fe(III) uptake from soil medium. J. Environ. Chem. Eng. 2018, 6, 736–744. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Zhang, C.; Li, W.; Chen, C.; Zhang, Z.; Chen, H.; Wang, J.; Li, Y.; Zhang, Y. Nano-FeS incorporated into stable lignin hydrogel: A novel strategy for cadmium removal from soil. Environ. Pollut. 2020, 264, 114739. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhao, M.; Zhao, X.; Guo, Y.; Zhao, Y. Simultaneous remediation and fertility improvement of heavy metals contaminated soil by a novel composite hydrogel synthesized from food waste. Chemosphere 2021, 275, 129984. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Seidi, F.; Wu, W.; Pan, Y.; Xiao, H. Dual-Functional Lignin-Based Hydrogels for Sustained Release of Agro-chemicals and Heavy Metal Ion Complexation. Int. J. Biol. Macromol. 2023, 235, 123701. [Google Scholar] [CrossRef]
- He, C.; Mou, H.; Hou, W.; Chen, W.; Ao, T. Drought-resistant and water-retaining tobermorite/starch composite hydrogel for the remediation of cadmium-contaminated soil. Int. J. Biol. Macromol. 2024, 255, 127534. [Google Scholar] [CrossRef]
- Du, F.; Liu, L.; Pan, Y.; Wu, C.; Wang, R.; Zhao, Z.; Fan, W.; Song, H.; Shi, Y.; Wang, J. A Novel Biochar-Based Composite Hydrogel for Removing Heavy Metals in Water and Alleviating Cadmium Stress in Tobacco Seedlings. Sci. Rep. 2023, 13, 15656. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhen, S.; Wang, J.; Ma, Y.; Wu, J.; Dai, H. FeOOH-MnO2/Sepiolite and Fe2O3-MnO2/Diatomite: Highly Efficient Adsorbents for the Removal of as(V). Appl. Clay Sci. 2022, 222, 106491. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Wen, S.-L.; Chen, P.; Zhang, L.; Cen, K.; Sun, G.-X. Mitigation of cadmium and arsenic in rice grain by applying different silicon fertilizers in contaminated fields. Environ. Sci. Pollut. Res. 2016, 23, 3781–3788. [Google Scholar] [CrossRef] [PubMed]
- Hemkemeyer, M.; Schwalb, S.A.; Heinze, S.; Joergensen, R.G.; Wichern, F. Functions of elements in soil microorganisms. Microbiol. Res. 2021, 252, 126832. [Google Scholar] [CrossRef]
- Ye, J.Y.; Tian, W.H.; Jin, C.W. Nitrogen in plants: From nutrition to the modulation of abiotic stress adaptation. Stress Biol. 2022, 2, 1–14. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, R.; Shi, X.; Lian, S.; Zhou, Q.; Chen, Y.; Liu, W.; Li, W. Synthesis of Cellulose-Based Superabsorbent Hydrogel with High Salt Tolerance for Soil Conditioning. Int. J. Biol. Macromol. 2022, 209, 1169–1178. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Ito, O.; Berry, W.L.; Wheeler, R.M. Sodium—A Functional Plant Nutrient. Crit. Rev. Plant Sci. 2010, 22, 391–416. [Google Scholar]
- Priya, A.; Gnanasekaran, L.; Dutta, K.; Rajendran, S.; Balakrishnan, D.; Soto-Moscoso, M. Biosorption of heavy metals by microorganisms: Evaluation of different underlying mechanisms. Chemosphere 2022, 307, 135957. [Google Scholar] [CrossRef] [PubMed]
- Heidari, A.; Younesi, H.; Mehraban, Z. Removal of Ni(II), Cd(II), and Pb(II) from a Ternary Aqueous Solution by Amino Functionalized Mesoporous and Nano Mesoporous Silica. Chem. Eng. J. 2009, 153, 70–79. [Google Scholar] [CrossRef]
- Dong, L.; Zhu, Z.; Ma, H.; Qiu, Y.; Zhao, J. Simultaneous adsorption of lead and cadmium on MnO2-loaded resin. J. Environ. Sci. 2010, 22, 225–229. [Google Scholar] [CrossRef]
- Zheng, L.; Peng, D.; Meng, P. Corncob-supported aluminium-manganese binary oxide composite enhanced removal of cadmium ions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561, 109–119. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Yin, D.; Peng, B.; Tan, C.; Liu, Y.; Tan, X.; Wu, S. Efficiency and Mechanisms of Cd Removal from Aqueous Solution by Biochar Derived from Water Hyacinth (Eichornia crassipes). J Environ. Manag. 2015, 153, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ayuso, E.; Garcia-Sanchez, A. Removal of Heavy Metals from Waste Waters by Natural and Na-Exchanged Bentonites. Clays Clay Miner. 2003, 51, 475–480. [Google Scholar] [CrossRef]
- Salah, T.A.; Mohammad, A.M.; Hassan, M.A.; El-Anadouli, B.E. Development of Nano-Hydroxyapatite/Chitosan Composite for Cadmium Ions Removal in Wastewater Treatment. J. Taiwan Inst. Chem. Eng. 2014, 45, 1571–1577. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Removal of cadmium(II) and zinc(II) metal ions from binary aqueous solution by rice husk ash. Colloids Surf. A Physicochem. Eng. Asp. 2008, 312, 172–184. [Google Scholar] [CrossRef]



| Adsorbent | Heavy Metal | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|
| Amino-functionalized silica | Cd | 18.25 | Heidari et al. [40] |
| MnO2-loaded resin | Cd | 21.5 | Dong et al. [41] |
| Corncob-supported Al-Mn oxides | Cd | 45.6 | Zheng et al. [42] |
| Water hyacinth BC | Cd | 70.3 | Zhang et al. [43] |
| Na-Bentonite | Cd | 30 | Ayuso et al. [44] |
| Nano-hydroxyapatite/ chitosan composite | Cd | 92 | Salah et al. [45] |
| Rice husk ash | Cd | 3.04 | Srivastava et al. [46] |
| DMAPAA/DMAPAAQ gel | Cd | 121 | This work |
| Materials | Component Type | Molar Weight (g/mol) | Concentration (mol/m3) | Mass (g) |
|---|---|---|---|---|
| DMAPAA | monomer | 156.22 | 500 | 1.953 |
| DMAPAA-Q | monomer | 206.71 | 500 | 2.584 |
| MBAA | linker | 154.17 | 50 | 0.193 |
| TEMED | accelerator | 116.21 | 20 | 0.058 |
| APS | Initiator | 228.19 | 20 | 0.114 |
| Materials | Component Type | Molar Weight (g/mol) | Concentration (mol/m3) | Mass (g) |
|---|---|---|---|---|
| DMAA | monomer | 99.13 | 1000 | 2.478 |
| MBAA | linker | 154.17 | 50 | 0.193 |
| TEMED | accelerator | 116.21 | 20 | 0.058 |
| APS | Initiator | 228.19 | 20 | 0.114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Gotoh, T.; Nakai, S.; Ueda, A. Functional Hydrogels Promote Vegetable Growth in Cadmium-Contaminated Soil. Gels 2024, 10, 348. https://doi.org/10.3390/gels10050348
Huang J, Gotoh T, Nakai S, Ueda A. Functional Hydrogels Promote Vegetable Growth in Cadmium-Contaminated Soil. Gels. 2024; 10(5):348. https://doi.org/10.3390/gels10050348
Chicago/Turabian StyleHuang, Jin, Takehiko Gotoh, Satoshi Nakai, and Akihiro Ueda. 2024. "Functional Hydrogels Promote Vegetable Growth in Cadmium-Contaminated Soil" Gels 10, no. 5: 348. https://doi.org/10.3390/gels10050348
APA StyleHuang, J., Gotoh, T., Nakai, S., & Ueda, A. (2024). Functional Hydrogels Promote Vegetable Growth in Cadmium-Contaminated Soil. Gels, 10(5), 348. https://doi.org/10.3390/gels10050348









