Post-Irradiation Behavior of Colored PVA-Based Films Containing Ag Nanoparticles as Radiation Detectors/Exposure Indicators
Abstract
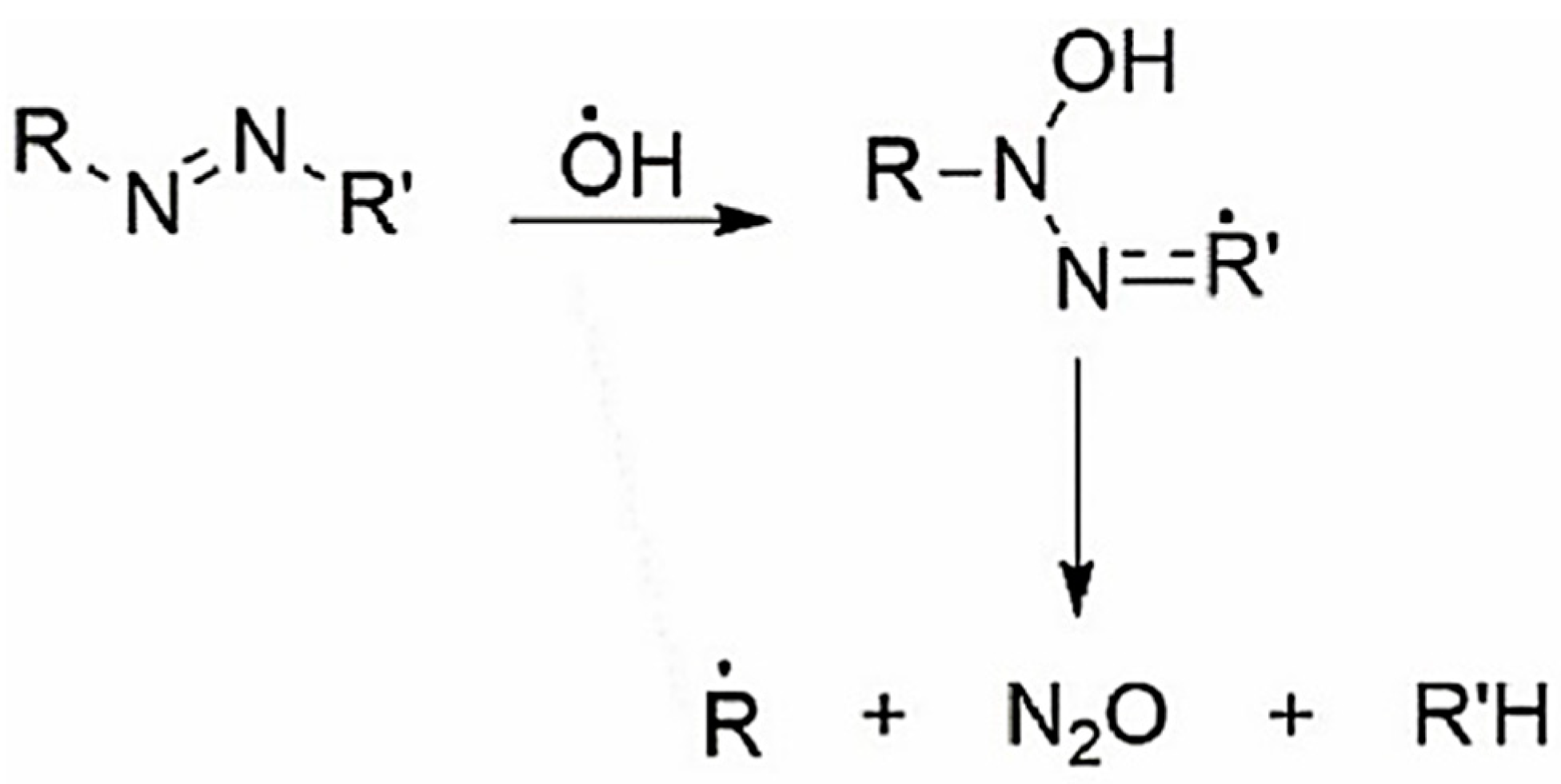
1. Introduction
2. Results and Discussion
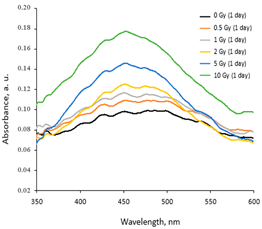
2.1. AgPVAGly Films
2.2. Silver-Enriched PVA Films with Dye Additives
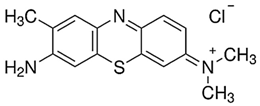 | 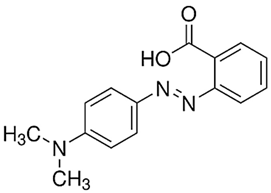 |
| Toluidine Blue O | Methyl red |
2.3. AgPVAGlyTBO Films
2.4. AgPVAGlyMR Films
3. Conclusions
4. Materials and Methods
4.1. Film Fabrication
4.2. Irradiation of Experimental Films
4.3. Characterization of Experimental Films
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adliene, D.; Urbonavicius, B.G.; Laurikaitiene, J.; Puiso, J. New application of polymer gels in medical radiation dosimetry: Plasmonic sensors. Radiat. Phys. Chem. 2020, 168, 108609. [Google Scholar] [CrossRef]
- Lochard, J.; Bartlett, D.T.; Rühm, W.; Yasuda, H.; Bottollier-Depois, J.-F. ICRP Publication 132: Radiological Protection from Cosmic Radiation in Aviation. Ann. ICRP 2016, 45, 5–48. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.M.; Copeland, K.; Klöble, K.E.J.; Matthiä, D.; Plettenberg, M.C.; Schennetten, K.; Wirtz, M.; Hellweg, C.E. Radiation in the Atmosphere—A Hazard to Aviation Safety? Atmosphere 2020, 11, 1358. [Google Scholar] [CrossRef]
- Sihver, L.; Ploc, O.; Puchalska, M.; Ova, I.A.; Ak, J.K.; Kyselova, D.; Shurshakov, V. Radiation environment at aviation altitudes and in space. Radiat. Prot. Dosim. 2015, 164, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Matthiä, D.; Meier, M.M.; Schennetten, K. New operational dose quantity ambient dose H* in the context of galactic cosmic radiation in aviation. J. Radiol. Prot. 2022, 42, 021520. [Google Scholar] [CrossRef]
- Meier, M.M.; Copeland, K.; Matthiä, D.; Mertens, C.J.; Schennetten, K. First Steps Toward the Verification of Models for the Assessment of the Radiation Exposure at Aviation Altitudes During Quiet Space Weather Conditions. Space Weather 2018, 16, 1269–1276. [Google Scholar] [CrossRef]
- Casolaro, P. Radiochromic Films for the Two-Dimensional Dose Distribution Assessment. Appl. Sci. 2021, 11, 2132. [Google Scholar] [CrossRef]
- Spelleken, E.; Crowe, S.B.; Sutherland, B.; Challens, C.; Kairn, T. Accuracy and efficiency of published film dosimetry techniques using a flat-bed scanner and EBT3 film. Australas. Phys. Eng. Sci. Med. 2018, 41, 117–128. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, L.; Chen, H.; Hu, L. Recent Advances in Hydrogel-Based Sensors Responding to Ionizing Radiation. Gels 2022, 8, 238. [Google Scholar] [CrossRef]
- Macchione, M.A.; Páez, S.L.; Strumia, M.C.; Valente, M.; Mattea, F. Chemical Overview of Gel Dosimetry Systems: A Comprehensive Review. Gels 2022, 8, 663. [Google Scholar] [CrossRef]
- Farhood, B.; Geraily, G.; Abtahi, S.M.M. A systematic review of clinical applications of polymer gel dosimeters in radiotherapy. Appl. Radiat. Isot. 2019, 143, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Akimitsu, T.; Hirokawa, Y.; Mooij, R.B.; Perera, G.M. Evaluation of dose delivery accuracy of Gamma Knife by polymer gel dosimetry. J. Appl. Clin. Med. Phys. 2005, 6, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Ibbott, G.S. Clinical Applications of Gel Dosimeters. J. Phys. Conf. Ser. 2006, 56, 108–131. [Google Scholar] [CrossRef]
- Khan, M.; Heilemann, G.; Lechner, W.; Georg, D.; Berg, A.G. Basic Properties of a New Polymer Gel for 3D-Dosimetry at High Dose-Rates Typical for FFF Irradiation Based on Dithiothreitol and Methacrylic Acid (MAGADIT): Sensitivity, Range, Reproducibility, Accuracy, Dose Rate Effect and Impact of Oxygen Scavenger. Polymers 2019, 11, 1717. [Google Scholar] [CrossRef]
- Jirasek, A.; Hilts, M.; McAuley, K.B. Polymer gel dosimeters with enhanced sensitivity for use in x-ray CT polymer gel dosimetry. Phys. Med. Biol. 2010, 55, 5269–5281. [Google Scholar] [CrossRef] [PubMed]
- De Deene, Y. Radiation Dosimetry by Use of Radiosensitive Hydrogels and Polymers: Mechanisms, State-of-the-Art and Perspective from 3D to 4D. Gels 2022, 8, 599. [Google Scholar] [CrossRef] [PubMed]
- Rabaeh, K.A.; Al-Zawaydaih, H.H.; Eyadeh, M.M.; Shatnawi, M.T. High optical stability of reusable radiochromic polyvinyl alcohol-iodine gel dosimeter for radiotherapy. Radiat. Phys. Chem. 2022, 199, 110338. [Google Scholar] [CrossRef]
- Penev, K.I.; Mulligan, M.; Mequanint, K. Optimization of the Dose Rate Effect in Tetrazolium Gellan Gel Dosimeters. Gels 2023, 9, 334. [Google Scholar] [CrossRef]
- Jaszczak, M.; Sąsiadek-Andrzejczak, E.; Kozicki, M. Discolouring 3D Gel Dosimeter for UV Dose Distribution Measurements. Materials 2022, 15, 2546. [Google Scholar] [CrossRef]
- Kozicki, M.; Kwiatos, K.; Dudek, M.; Stempien’, Z. Radiochromic gels for UV radiation measurements in 3D. J. Photochem. Photobiol. A Chem. 2018, 351, 197–207. [Google Scholar] [CrossRef]
- Jaszczak, M.; Sasiadek, E.; Kadlubowski, S.; Dudek, M.; Kozicki, M. Preliminary study on a new 3D radiochromic KI-Pluronic F-127 gel dosimeter for radiotherapy. Radiat. Phys. Chem. 2021, 185, 109507. [Google Scholar] [CrossRef]
- Raouafi, A.; Jbahi, S.; Bessalah, S.; Daoudi, M.; Dridi, W.; Hamzaoui, A.H.; Dorohzkin, S.V.; Hosni, F.; Hidouri, M. Natural red dyes from Beta vulgaris L. extract for gamma-rays color indicator: Physico-chemical and biological characterizations. J. Indian Chem. Soc. 2022, 99, 100722. [Google Scholar] [CrossRef]
- Maity, T.; Sharma, S.; Chourasiya, G. The real-time gamma radiation dosimetry with TeO2 thin films. Radiat. Meas. 2012, 47, 145–148. [Google Scholar] [CrossRef]
- Patra, G.; Singh, S.; Singh, A.; Tyagi, M.; Desai, D.; Tiwari, B.; Sen, S.; Gadkari, S. Silver doped lithium tetraborate (Li2B4O7) single crystals as efficient dosimeter material with sub-micro-Gy sensitivity. J. Lumin. 2015, 157, 333–337. [Google Scholar] [CrossRef]
- Thabit, H.A.; Kabir, N.A.; Ismail, A.K.; Saleh, M.A. Dosimetric characteristics investigation of ZnO/Ag/ZnO multilayer film for radiation applications. J. Phys. Conf. Ser. 2022, 2411, 012008. [Google Scholar] [CrossRef]
- Thabit, H.A.; Kabir, N.A.; Ahmed, N.M. Synthesis & thermoluminescence characteristics & structural and optical studies of ZnO/Ag/ZnO system for dosimetric applications. J. Lumin. 2021, 236, 118097. [Google Scholar] [CrossRef]
- Thabit, H.A.; Kabir, N.A.; Ismail, A.K.; Alraddadi, S.; Bafaqeer, A.; Saleh, M.A. Development of Ag-Doped ZnO Thin Films and Thermoluminescence (TLD) Characteristics for Radiation Technology. Nanomaterials 2022, 12, 3068. [Google Scholar] [CrossRef] [PubMed]
- Kurobori, T.; Miyamoto, Y.; Maruyama, Y.; Yamamoto, T.; Sasaki, T. A comparative study of optical and radiative characteristics of X-ray-induced luminescent defects in Ag-doped glass and LiF thin films and their applications in 2-D imaging. Nucl. Instrum. Methods Phys. Res. B 2014, 326, 76–80. [Google Scholar] [CrossRef][Green Version]
- Shin, D.-S.; Kim, T.-H.; Rah, J.-E.; Kim, D.; Yang, H.J.; Lee, S.B.; Lim, Y.K.; Jeong, J.; Kim, H.; Shin, D.; et al. Assessment of a Therapeutic X-ray Radiation Dose Measurement System Based on a Flexible Copper Indium Gallium Selenide Solar Cell. Sensors 2022, 22, 5819. [Google Scholar] [CrossRef]
- Falsini, N.; Ubaldini, A.; Cicconi, F.; Rizzo, A.; Vinattieri, A.; Bruzzi, M. Halide Perovskites Films for Ionizing Radiation Detection: An Overview of Novel Solid-State Devices. Sensors 2023, 23, 4930. [Google Scholar] [CrossRef]
- Titus, D.; Samuel, E.J.J.; Srinivasan, K.; Roopan, S.M.; Madhu, C.S. Silver nitrate based gel dosimeter. J. Phys. Conf. Ser. 2017, 847, 012066. [Google Scholar] [CrossRef]
- Sabbaghizadeh, R.; Shamsudin, R.; Deyhimihaghighi, N.; Sedghi, A. Enhancement of Dose Response and Nuclear Magnetic Resonance Image of PAGAT Polymer Gel Dosimeter by Adding Silver Nanoparticles. PLoS ONE 2017, 12, e0168737. [Google Scholar] [CrossRef] [PubMed]
- Tadros, S.M.; Soliman, Y.S.; Beshir, W.B.; Saad, G.R.; Ali, L. Dosimetric investigations on radiation-induced Ag nanoparticles in a gel dosimeter. J. Radioanal. Nucl. Chem. 2021, 329, 463–473. [Google Scholar] [CrossRef]
- Ghazy, O.; Saleh, H.; Shehata, M.; Hosni, H.; Ali, Z. Electron beam radiation induced solid-state synthesis of gold nanoparticles in polyvinyl alcohol films and their Physico-chemical properties. Radiat. Phys. Chem. 2022, 191, 109848. [Google Scholar] [CrossRef]
- Guidelli, E.J.; Ramos, A.P.; Baffa, O. Silver nanoparticle films for metal enhanced luminescence: Toward development of plasmonic radiation detectors for medical applications. Sens. Actuators B Chem. 2016, 224, 248–255. [Google Scholar] [CrossRef]
- Soliman, Y. Gamma-radiation induced synthesis of silver nanoparticles in gelatin and its application for radiotherapy dose measurements. Radiat. Phys. Chem. 2014, 102, 60–67. [Google Scholar] [CrossRef]
- Yasuda, H.; Yoshida, H. Application of a Portable Colorimeter for Reading a Radiochromic Film for On-Site Dosimetry. Appl. Sci. 2023, 13, 4761. [Google Scholar] [CrossRef]
- Kudrevicius, L.; Adliene, D.; Puiso, J.; Plaga, A. Investigation of Colored Film Indicators for the Assessment of the Occasional Radiation Exposure. Gels 2023, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Funaro, M.; Di Bartolomeo, A.; Pelosi, P.; Saponetti, M.S.; Proto, A. Dosimeter based on silver-nanoparticle precursors for medical applications with linear response over a wide dynamic range. Micro Nano Lett. 2011, 6, 759–762. [Google Scholar] [CrossRef]
- Merkis, M.; Puišo, J.; Adliene, D.; Laurikaitiene, J. Development and Characterization of Silver Containing Free Standing Polymer FILMS for Dosimetry Applications. Polymers 2021, 13, 3925. [Google Scholar] [CrossRef]
- Petisiwaveth, P.; Wanotayan, R.; Damrongkijudom, N.; Ninlaphruk, S.; Kladsomboon, S. Dosimetric Performance of Poly(vinyl alcohol)/Silver Nanoparticles Hybrid Nanomaterials for Colorimetric Sensing of Gamma Radiation. Nanomaterials 2022, 12, 1088. [Google Scholar] [CrossRef] [PubMed]
- Soliman, Y.S.; Tadros, S.M.; Beshir, W.B.; Saad, G.R.; Gallo, S.; Ali, L.I.; Naoum, M.M. Study of Ag Nanoparticles in a Polyacrylamide Hydrogel Dosimeters by Optical Technique. Gels 2022, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Kaiyum, R.; Schruder, C.W.; Mermut, O.; Rink, A. Investigation of cyanine-based infrared dyes as calibrants in radiochromic films. Med. Phys. 2023, 50, 8034–8043. [Google Scholar] [CrossRef] [PubMed]
- Dhayagude, A.C.; Das, A.; Joshi, S.S.; Kapoor, S. γ-Radiation induced synthesis of silver nanoparticles in aqueous poly (N-vinylpyrrolidone) solution. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 556, 148–156. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Motellier, S.; Clavaguera, S.; Nowack, B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ. Int. 2015, 77, 132–147. [Google Scholar] [CrossRef]
- Nunes, L.H.S.; Guidelli, E.J. Hydrodynamic radius dictates sensitivity of x-ray detectors based on the radiomicrofluidic synthesis of colloidal silver. Nanotechnology 2023, 34, 335601. [Google Scholar] [CrossRef]
- Hayat, N.A.M.K.; Ramiza; Hussain, T.; Khaliq, R.; Farooq, H.; ul Haq, I. A Study of Percentage Decoloration of Aqueous Solutions of a Synthetic Dye for Gamma Dosimetry. Int. J. Sci. Eng. Res. 2017, 8, 1437–1439. [Google Scholar]
- Barakat, M.; El-Salamawy, K.; El-Banna, M.; Abdel-Hamid, M.; Taha, A.A.-R. Radiation effects on some dyes in non-aqueous solvents and in some polymeric films. Radiat. Phys. Chem. 2001, 61, 129–136. [Google Scholar] [CrossRef]
- Rauf, M.; Ashraf, S.S. Radiation induced degradation of dyes—An overview. J. Hazard. Mater. 2009, 166, 6–16. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Al-Shehri, A.S.; Zaheer, Z.; Alsudairi, A.M.; Kosa, S.A. Photo-oxidative Decolorization of Brilliant Blue with AgNPs as an Activator in the Presence of K2S2O8 and NaBH4. ACS Omega 2021, 6, 27510–27526. [Google Scholar] [CrossRef] [PubMed]

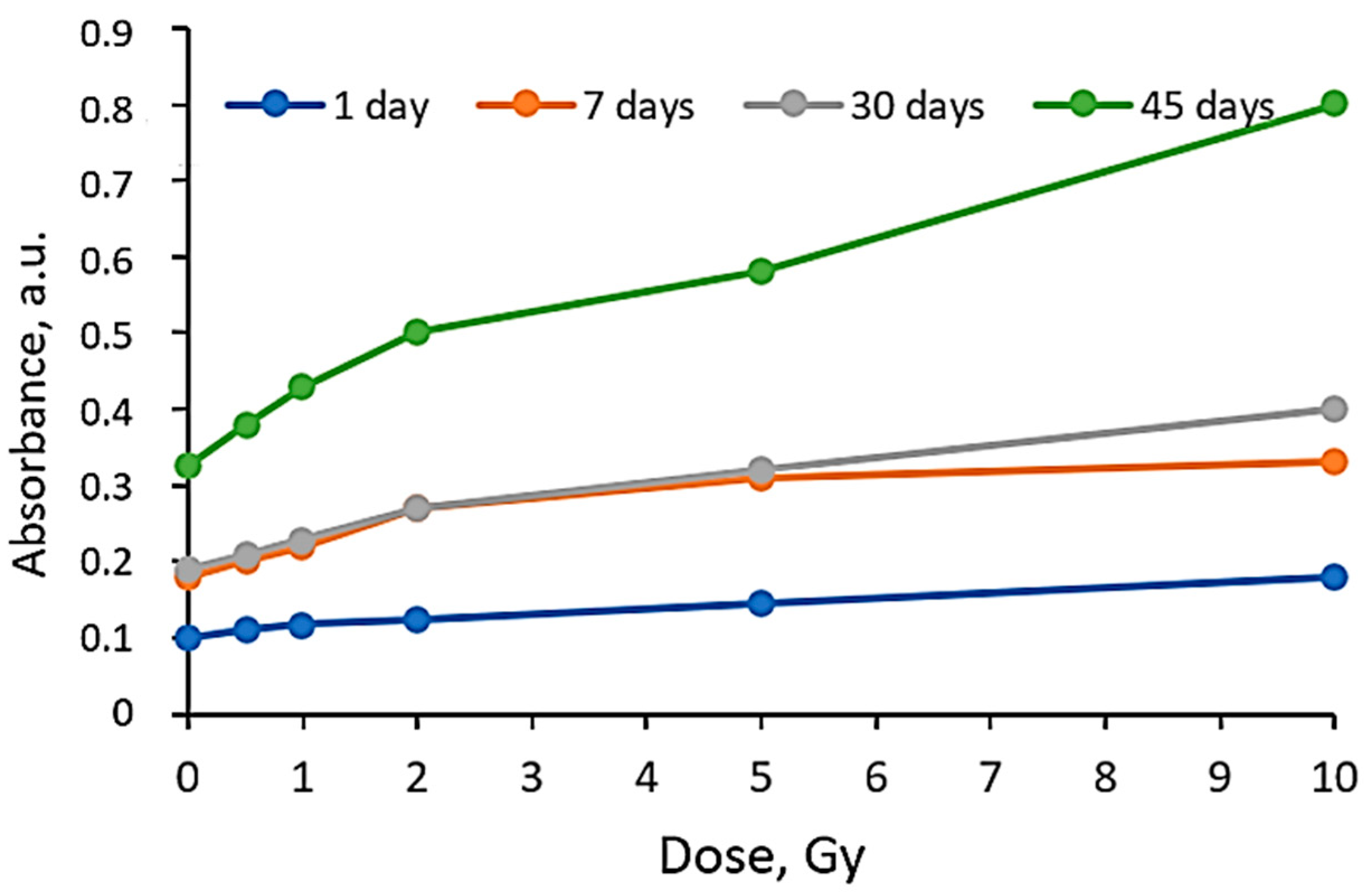
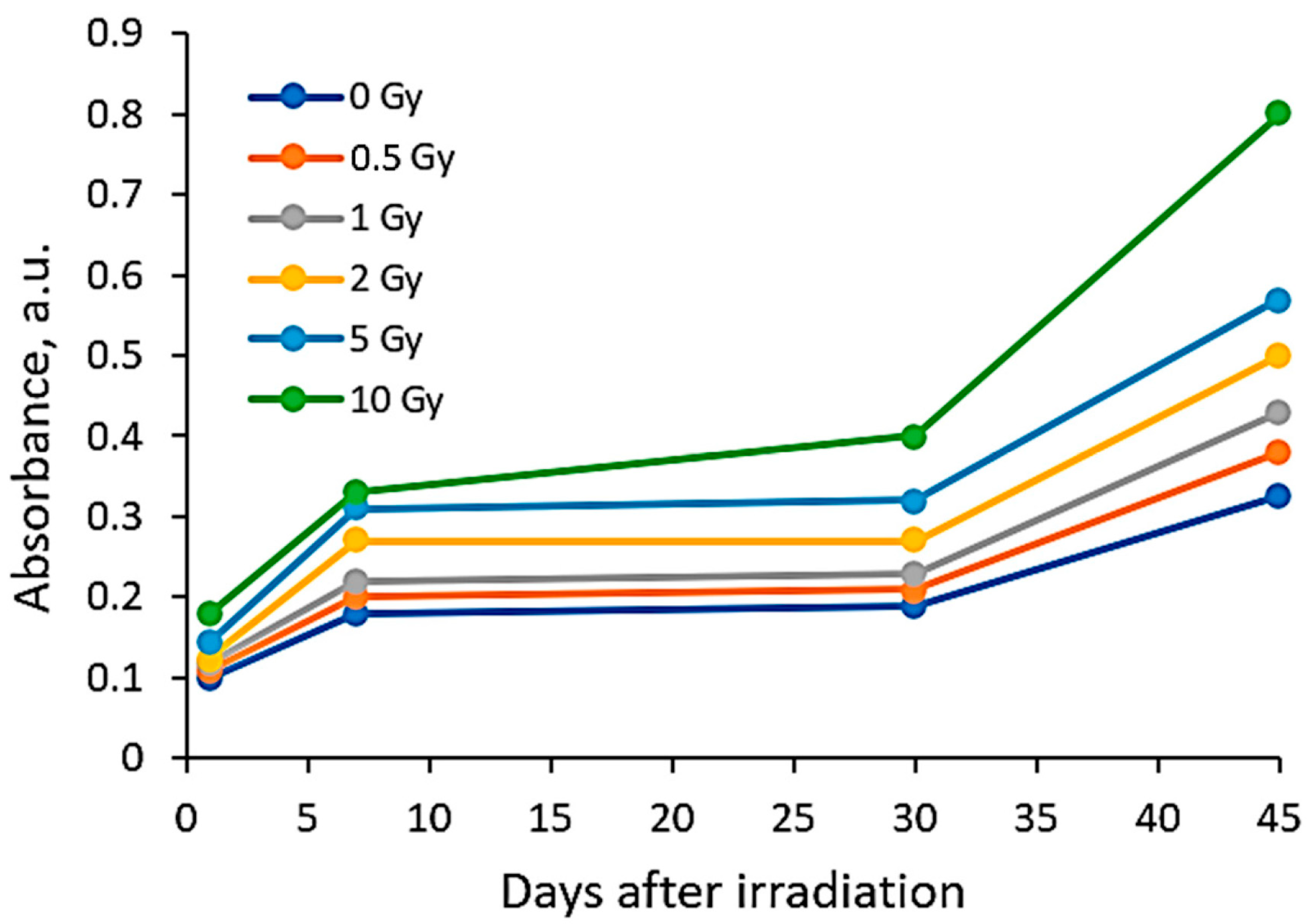

| Dose, Gy | 0 | 0.5 | 1 | 2 | 5 | 10 |
|---|---|---|---|---|---|---|
| On the same day of irradiation |  |  |  |  |  |  |
| 7 days after irradiation |  |  |  |  |  |  |
| 30 days after irradiation |  |  |  |  |  |  |
| 45 days after irradiation |  |  |  |  |  |  |
| 90 days after irradiation |  |  |  |  |  |  |
| 180 days after irradiation |  |  |  |  |  |  |
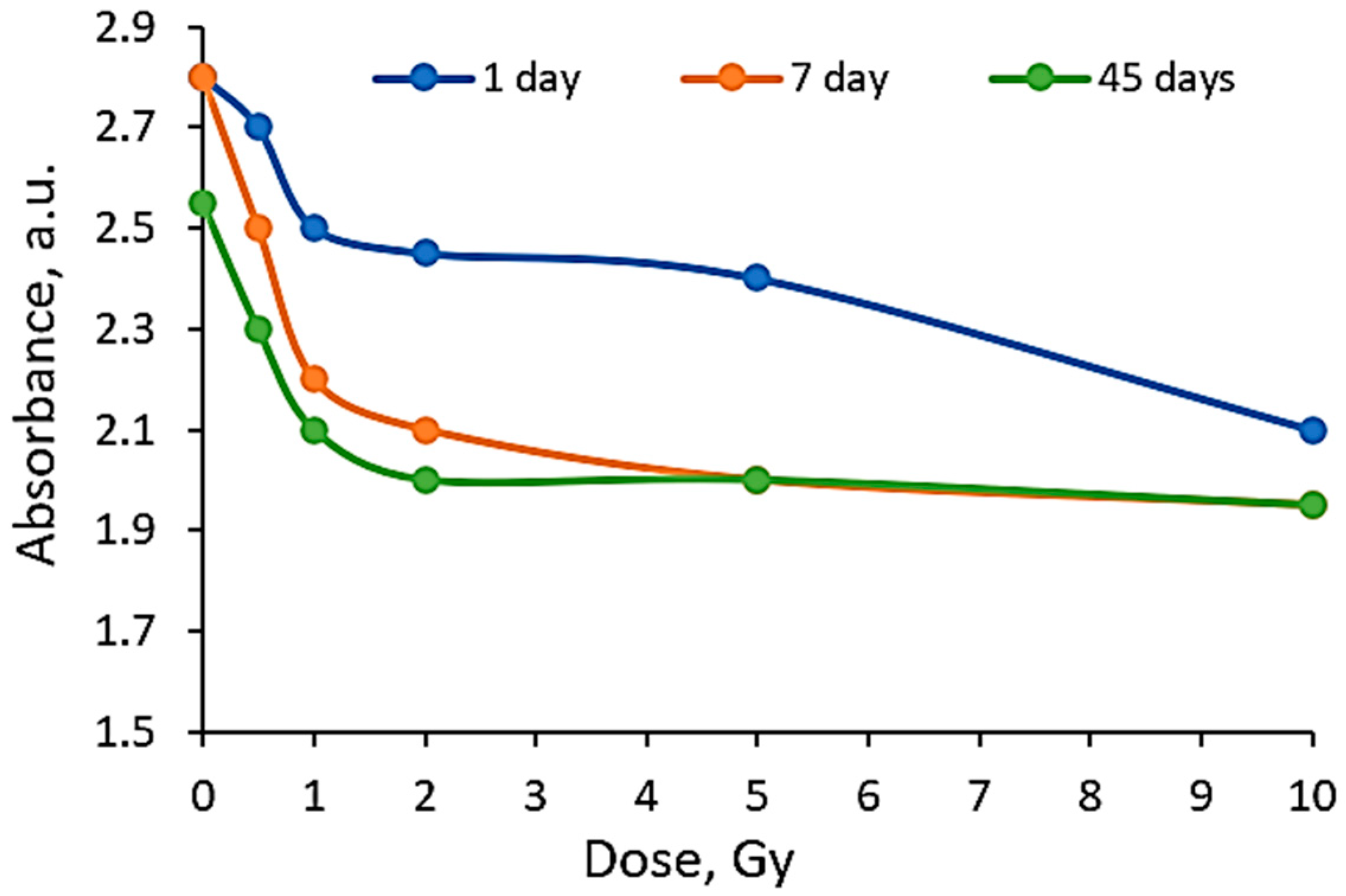
 | 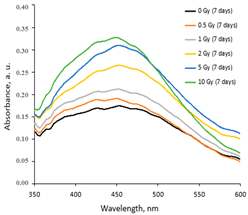 |  |
| On the same day of irradiation | After 7 days post irradiation | After 45 days post irradiation |
| Different scales on Absorbance axis for AgPVAGly films | ||
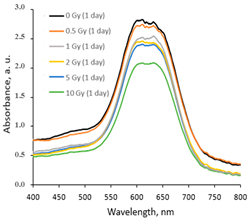 | 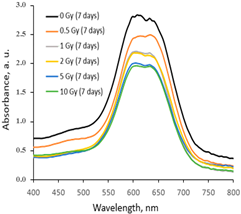 |  |
| Within the same day of irradiation | After 7 days post irradiation | After 45 days post irradiation |
| Dose, Gy | 0 | 0.5 | 1 | 2 | 5 | 10 |
|---|---|---|---|---|---|---|
| On the same day of irradiation |  |  |  |  |  |  |
| 7 days after irradiation |  |  |  |  |  |  |
| 30 days after irradiation |  |  |  |  |  |  |
| 180 days after irradiation |  |  |  |  |  |  |
| Dose, Gy | 0 | 0.5 | 1 | 2 | 5 | 10 |
|---|---|---|---|---|---|---|
| On the same day of irradiation |  |  |  |  |  |  |
| 7 days after irradiation |  |  |  |  |  |  |
| 30 days after irradiation |  |  |  |  |  |  |
| 180 days after irradiation |  |  |  |  |  |  |
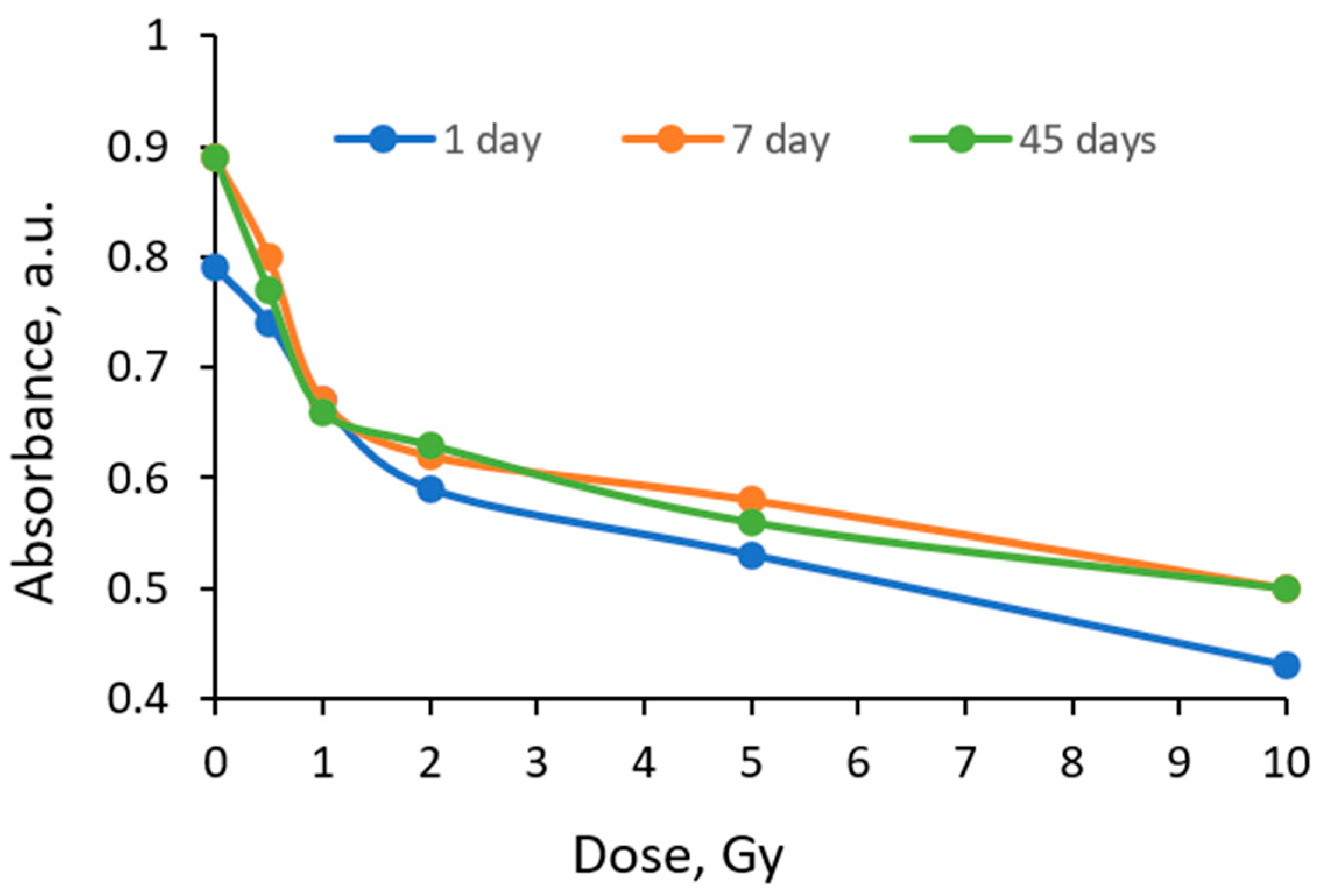
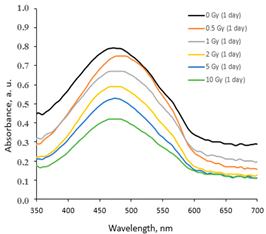 | 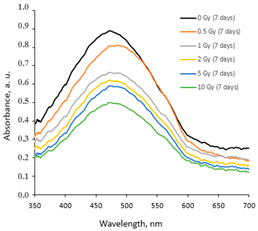 | 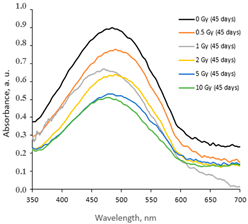 |
| On the same day of irradiation | After 7 days post irradiation | After 45 days post irradiation |
| Material | Chemical Formula | PVA-Ag-Gly, % | PVA-Ag-Gly-TBO, % | PVA-Ag-Gly-MR, % |
|---|---|---|---|---|
| Polivynyl alcohol, (PVA) | C2H8O3 | 8.62 | 8.58 | 8.58 |
| Silver nitrate | AgNO3 | 0.86 | 0.86 | 0.86 |
| Glycerol (Gly) | C3H8O3 | 4.31 | 4.28 | 4.28 |
| Ethanol (Etha) | C2H5OH | 0.5 | 0.5 | |
| Toluidine blue O (TBO) | C15H16ClN3S | 0.01 | ||
| Methyl red (MR) | C15H15N3O2 | 0.01 | ||
| Distilled water | H2O | 86.21 | 85.77 | 85.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudrevicius, L.; Jaselskė, E.; Stankus, G.; Arslonova, S.; Adliene, D. Post-Irradiation Behavior of Colored PVA-Based Films Containing Ag Nanoparticles as Radiation Detectors/Exposure Indicators. Gels 2024, 10, 290. https://doi.org/10.3390/gels10050290
Kudrevicius L, Jaselskė E, Stankus G, Arslonova S, Adliene D. Post-Irradiation Behavior of Colored PVA-Based Films Containing Ag Nanoparticles as Radiation Detectors/Exposure Indicators. Gels. 2024; 10(5):290. https://doi.org/10.3390/gels10050290
Chicago/Turabian StyleKudrevicius, Linas, Evelina Jaselskė, Gabrielius Stankus, Shirin Arslonova, and Diana Adliene. 2024. "Post-Irradiation Behavior of Colored PVA-Based Films Containing Ag Nanoparticles as Radiation Detectors/Exposure Indicators" Gels 10, no. 5: 290. https://doi.org/10.3390/gels10050290
APA StyleKudrevicius, L., Jaselskė, E., Stankus, G., Arslonova, S., & Adliene, D. (2024). Post-Irradiation Behavior of Colored PVA-Based Films Containing Ag Nanoparticles as Radiation Detectors/Exposure Indicators. Gels, 10(5), 290. https://doi.org/10.3390/gels10050290






