1. Introduction
High rates of oil production by waterflooding at oil fields and the geological and geophysical features of the structure of productive formations lead to the intensive and rapid watering of produced wells long before reaching a potentially possible level of oil production. The main reasons for the watering of producing wells are [
1]:
- -
A breach of tightness in the production string;
- -
Water inflow through leaky borehole space from above- or below-lying aquifers;
- -
An inflow of contour or injected water;
- -
Water inflow through fractures;
- -
Anthropogenic factors (for example, acid treatments and hydraulic fracturing).
Geologic causes of the premature watering of oil wells also include oil–water contact (OWC) movement, water cone formation, and injection water breakthrough through highly permeable interbeds. Since the inflow area (drainage zone) is large and the upward velocity of OWC is low, its rise can occur at very low vertical permeability. Cone formation occurs in vertical wells where the OWC is near lower perforations in formations with relatively high vertical permeability.
Technological methods of well water cut prevention include:
- -
The qualitative and reliable separation of productive formations during well construction;
- -
The selection of optimal underbalance on the reservoir during well development and operation;
- -
The selection of an optimal speed for oil displacement front advancement with water, corresponding to the speed of capillary water impregnation of reservoir rocks;
- -
Selection of the well operation mode (fluid withdrawal), taking into account the low mobility of the water–oil contact surface within the radius of a deep depression funnel;
- -
Equalization of the injectivity profile of injection wells by the application of fine fillers and polymer compositions;
- -
Changing the rheological properties of injected water by applying polymer flooding;
- -
Forced fluid withdrawal from the reservoir.
There are many classifications of water shut-off methods. In a general sense, these methods are divided into mechanical and physico-chemical methods. The former include running an additional production string, installing corrugated, retrievable, and polymer patches, and using a two-packer system. Various technical devices and technological methods have been proposed to prevent the formation of cone watering and extend the life of wells (e.g., joint and separate operation) [
1,
2,
3].
Physico-chemical methods of water shut-off are based on the application of special reagents and compositions that plug the pathways of water inflow to the well. Depending on the mechanism and specific features of water barrier formation, isolation methods are divided into selective and non-selective. According to the type of action, there are curing, gel-forming, and sediment-forming selective reagents, as well as water repellents and foam systems. Gel-forming and sedimentation technologies for water shut-off are of particular interest at present.
In addition, for horizontal wells, due to, as a rule, insufficient information about the sources of watering, the large length of the productive interval (which requires large volumes of injected working reagent solutions), and the likelihood of complications during the treatment process due to the formation of clogging substances already in the wellbore, the most preferable is the use of selective materials and buffer rims.
Several requirements are imposed on selective compositions for water shut-off in production wells [
1]:
- -
Selectivity of the compositions’ impact on the formation depending on fluid saturation (oil or water);
- -
Good selective filterability of compositions into zones with high water saturation, allowing the creation of water shut-off screens in the desired direction and to a sufficient depth;
- -
Adjustable timing of an isolating barrier build-up of the watered section of the formation according to the degree and duration of blockage;
- -
The ability to regulate technological properties in well conditions;
- -
Economic accessibility and non-scarcity;
- -
Environmental safety.
It is important to note that there are no completely selective materials. The selective sealant should be much more effective at blocking high-permeability, water-saturated areas with minimal impact on low-permeability, oil-saturated areas.
One of the promising reagents from the perspective of use in technologies for selective water shut-off in production wells is hydrolyzed polyacrylonitrile (HPAN) and compositions based on it.
HPAN is a water-soluble polymer containing in its macromolecule chains of acrylonitrile, acrylamide, and sodium acrylate in a ratio of 1:1:8 (
Figure 1).
In industry, HPAN can be obtained by polymerization of acrylic acid nitrile followed by hydrolysis with sodium hydroxide, as well as alkaline hydrolysis.
When a solution of HPAN interacts with ions of polyvalent metals such as Ca
2+, Mg
2+, Fe
2+, the polymer instantly coagulates to form a sediment or elastic gel of varying degrees of strength (
Figure 2).
Among the electrolytes that cause coagulation of HPAN with the formation of a sediment or elastic gel, one can mainly note calcium, magnesium, iron (II), barium chlorides, and potassium permanganate. Coagulation of HPAN with hydrochloric acid is also possible. Several works are devoted to assessing the influence of sedimentators on the coagulation of HPAN [
4,
5,
6,
7,
8,
9,
10,
11]. In [
4], the authors studied the mechanisms affecting the colmatizing properties of polymer liquids. In the laboratory, studies were carried out on a solution of HPAN with solutions of salts of polyvalent metals. The interaction of CaCl
2, widely used in the oil industry for the precipitation of gels, as well as MgCl
2 and FeCl
3 with 5 wt% HPAN solution was compared. A significant difference was found in the concentrations of these salts, causing visible gelation in the solution and its precipitation. Thus, the beginning of visible coagulation of HPAN with a concentration of 5 wt% solution occurs when more than 200 mg/L of CaCl
2 and more than 2000 mg/L of MgCl
2 are injected into the solution, and FeCl
3 causes visible coagulation when 6 mg/L is injected into the solution.
It was noted that the compositions containing partially hydrolyzed polyacrylonitrile and a crosslinking agent (aluminum chloride) under the conditions of Kogalym and Langepas oil fields are capable of forming stable gels with a high water shut-off potential. The porosity of the reservoir decreased by 50 times or more [
7].
A new composition is presented in the work in [
8] Местo для ввoда текста. based on hydrolyzed polyacrylonitrile COM-C for limiting water shut-off in wells. COM-C is a new gel-forming compound capable of forming a durable shut-off screen in highly permeable areas. Hydrogels are formed when the composition interacts with hydrochloric acid.
Polymers based on acrylic acids contain carboxyl ionogenic groups, which facilitate their dissolution in water, interaction with electrolytes, and the formation of a durable insulating screen. The chromium (III) cation is bonded to two carboxylate groups of the polymer and to two water molecules. In this case, carboxylate groups act as bidentate ligands [
12]. Thus, carboxylate groups of HPAN play a crucial role in gel formation.
When interacting with oil, coagulation of HPAN does not occur. This mechanism allows the use of HPAN in water shut-off technologies for selective isolation of watered layers [
13,
14,
15,
16,
17].
The properties of the gel or sediment formed during the interaction of HPAN with various sedimentators are influenced by many factors, from the concentration of the polymer and the nature of the sedimentator to mass transfer between the polymer and the sedimentator (
Figure 3).
Based on the available data on the use of such technologies in production wells, it becomes obvious that the efficiency of treatment is directly affected by the total mineralization of formation waters, in particular, the total content of calcium and magnesium cations. Therefore, compositions based on HPAN are, as a rule, used at sites with a total mineralization of at least 50 g/L. Technologies include mandatory pre-flushing of the well (to avoid premature gel sedimentation of HPAN) and injection of a polymer solution and a calcium chloride solution separated by a buffer liquid.
The main disadvantage of the known technologies is that the formation of an insulating screen in the form of a gel sediment when the resulting composition interacts with formation water containing polyvalent metal ions occurs almost instantly. Thus, premature sedimentation and the formation of gel sediment in the wellbore are possible, which complicates the technology of water shut-off treatment [
13,
15,
18,
19,
20].
Existing technologies for water shut-off based on HPAN do not allow for treatment at a distance from the well and require the use of technological solutions to prevent premature gel sedimentation in the well.
Therefore, the purpose of this work is to develop a gel-forming composition in the form of an invert emulsion for isolating water inflows in production wells at sites with high mineralization of formation waters, which has physical and chemical selectivity and ensures a reduction in the permeability of only water-saturated intervals.
The article presents the results of laboratory studies on the selection of the concentration of HPAN and the crosslinking agent. The influence of various factors on the coagulation of HPAN was studied. Rheological studies of the developed composition and studies of emulsions for thermal stability were carried out. Filtration tests of the developed composition were carried out on bulk reservoir models using real formation fluids.
2. Results and Discussion
The developed surfactant–polymer composition for selective water shut-off in production wells is an invert (water-in-oil) emulsion, the dispersion medium of which is represented by a hydrocarbon surfactant solution, and the dispersed phase is a solution of HPAN with a chromium crosslinker. The following sections of this work present the results of studies of the developed composition.
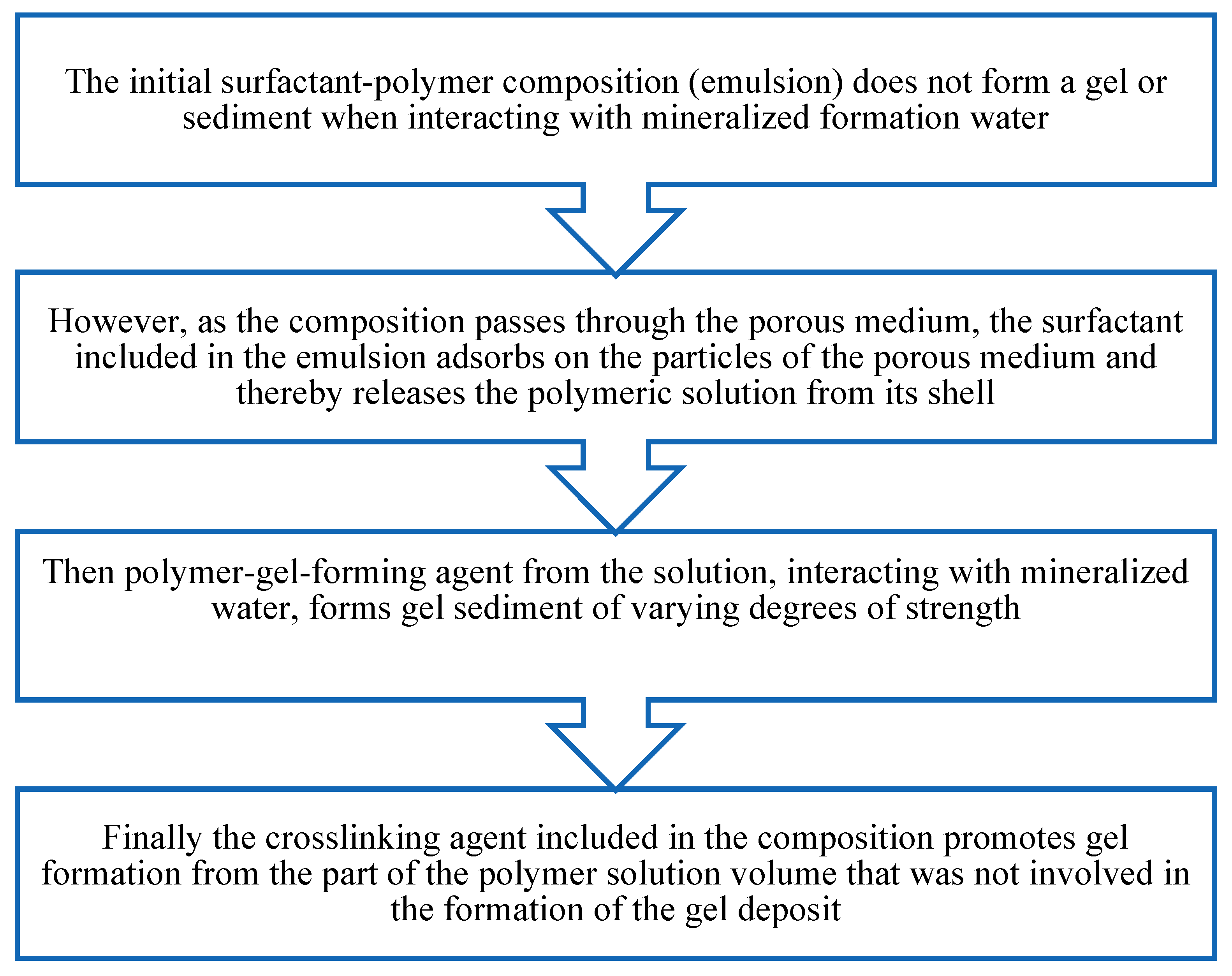
The mechanism of action of the developed surfactant–polymer composition can be represented by the diagram below (
Figure 4). Initially, the inverse emulsion is a stable system and does not form a gel or sediment when interacting with formation water. As the composition moves through the pore space of the formation, the emulsion is destroyed, and the hydrocarbon-soluble surfactant is adsorbed on the surface of the porous medium. Thus, the polymer solution with the crosslinking agent (HPAN and CA) is freed from the surfactant shell. Upon contact with formation mineralized water, HPAN coagulates to form sediment. The remaining part of the polymer, when interacting with the crosslinking agent, forms a gel.
First, it is necessary to determine the optimal concentration of HPAN and crosslinking agent.
2.1. Selection of the Conqcentration of Hydrolyzed Polyacrylonitrile
The most important property of any composition for water shut-off in production wells is its rheological characteristics. The developing composition must be sufficiently pumpable under downhole conditions. With increasing concentration of the polymer in the solution, the viscosity naturally increases. The systems are non-Newtonian fluids with pseudoplastic flow behavior, which is typical for polymer systems. Based on the Russian experience in using HPAN, it can be noted that the polymer concentration usually does not exceed 10 wt%. The use of a polymer at a concentration of 10 wt% increases the viscosity of the composition so much that it cannot be pumped into the formation using standard equipment, and also increases the load on pumping equipment when pumping such a viscous composition. Therefore, an increase in the concentration of HPAN by more than 10 wt% is impractical.
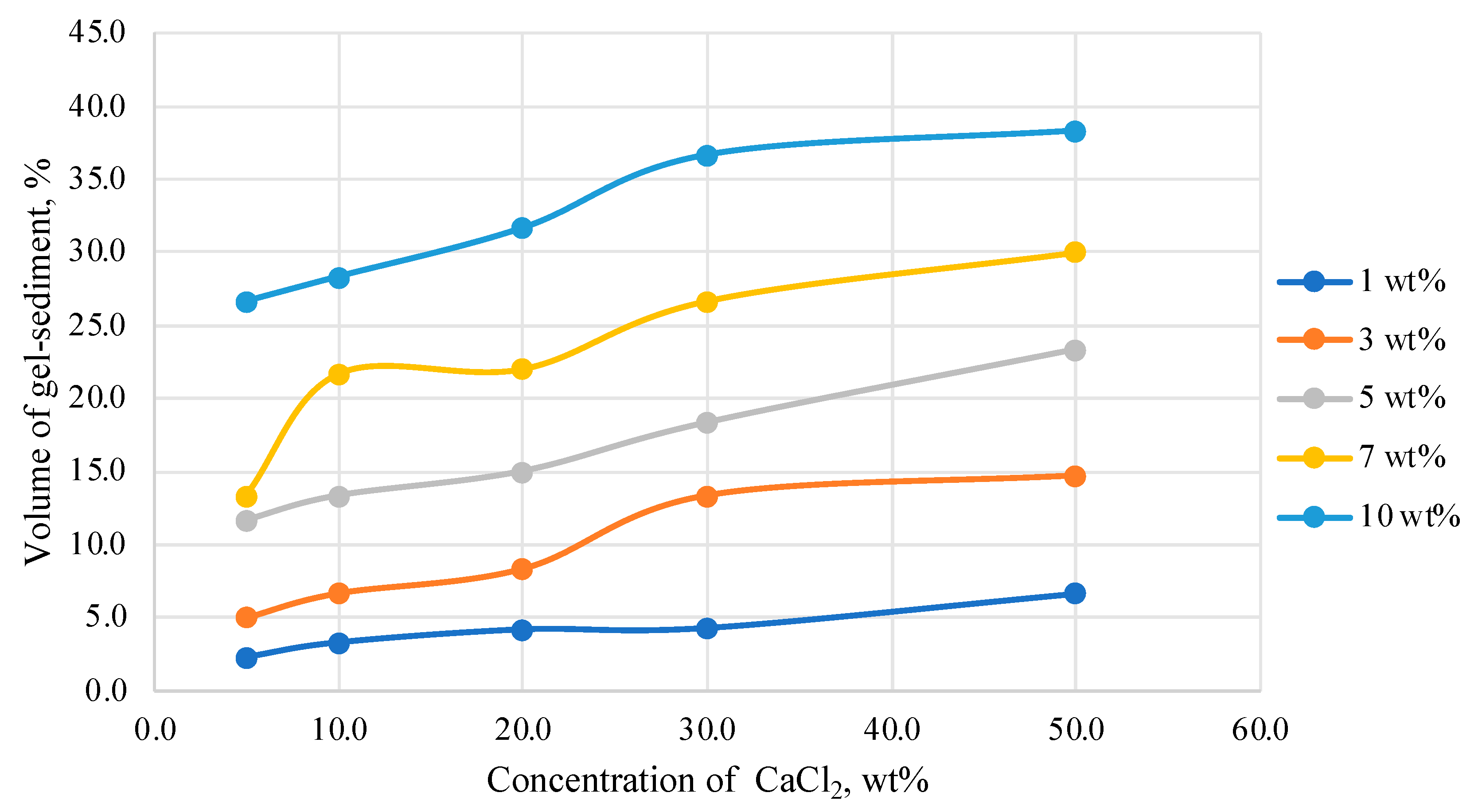
The dependence of the volume of the HPAN gel sediment on the concentration of calcium chloride in the solution is shown in
Figure 5.
From the graph data, the volume of the HPAN gel sediment directly depends on the concentration of the polymer in the solution; the higher the concentration of HPAN, the greater the volume of the resulting gel or sediment. It is very important to obtain the maximum volume of gel sediment in the system. It is assumed that the greater the volume and strength of the resulting gel sediment, the better the effect of work to limit water inflow.
And, of course, when operating wells, it is necessary to use technologically advanced and economically accessible compositions. For example, if 25–30% of the volume of gel or sediment is obtained from 100% of the composition, then this is not economically feasible.
The smallest volume of sediment is observed in the system with an HPAN concentration of 1.0 wt%. The mass formed when a polymer solution is mixed with calcium chloride is a loose sediment that cannot create a water shut-off barrier necessary when carrying out work for water shut-off (
Table 1).
However, even at relatively high polymer concentrations (10.0 wt%), the volume of the gel sediment does not exceed 40% of the total volume of the system. In technologies of water shut-off in production wells, this may not be enough for effective work. Thus, there is an interest to test the possibility of increasing the volume of the gel sediment formed by the polymer when it interacts with mineralized water.
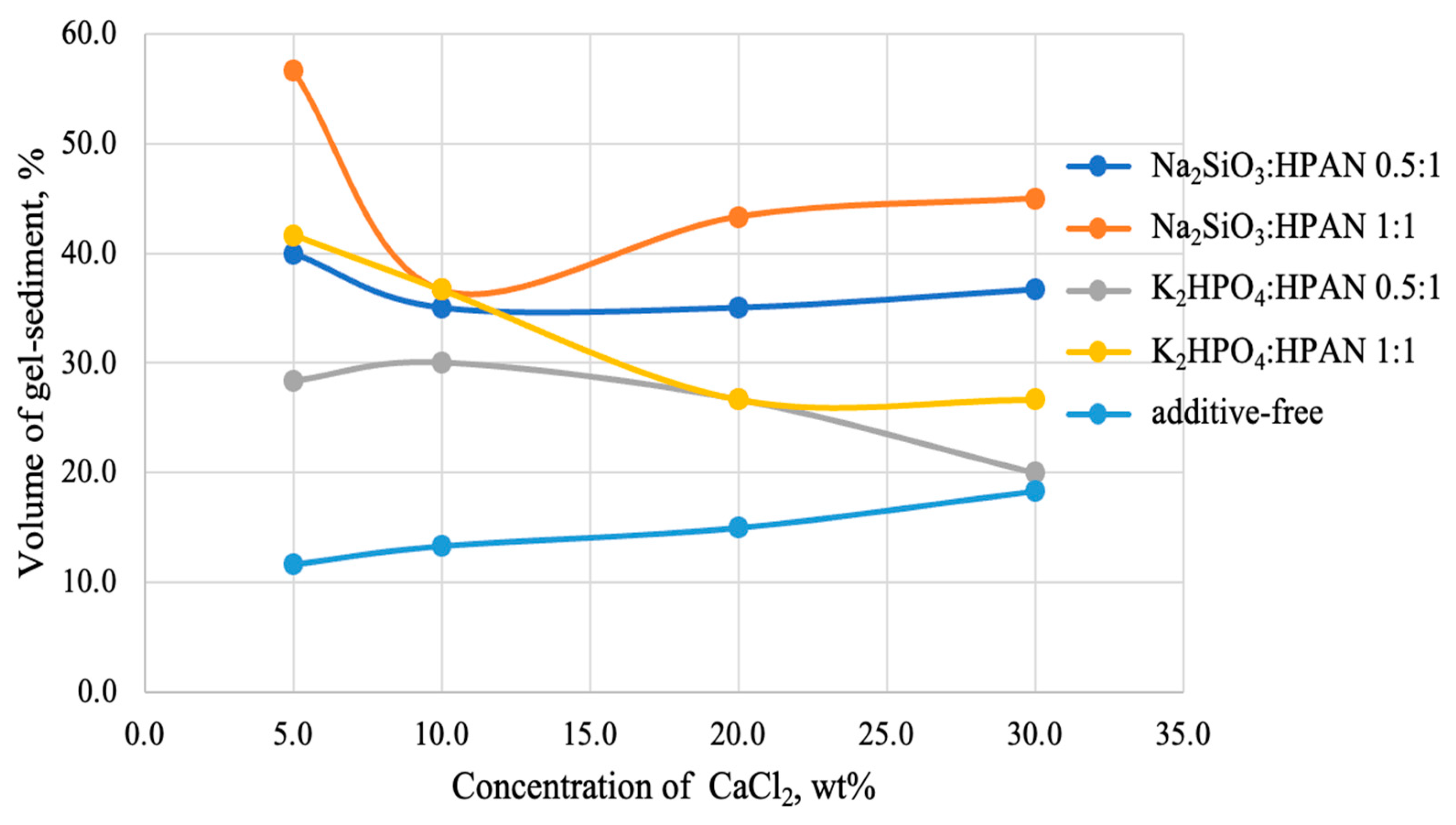
After adding salts to the polymer solution (the amount of the additive was introduced based on the dry polymer powder) and mixing the system with a solution of calcium chloride, the volume of the gel sediment actually increases (
Figure 6), but, unfortunately, to an insufficient extent (no more than 55% of total volume of the system).
The presence of carboxyl groups in the HPAN molecule makes it possible to obtain gels throughout the entire volume by reacting the polymer with chromium crosslinkers. An example of such a system is the interaction of a solution of HPAN with chromium (III) acetate. It is assumed that during the formation of a three-dimensional gel structure, the Cr
3+ cation preferentially binds with two carboxyl groups of neighboring HPAN macromolecules and two water molecules. A similar process is observed during the interaction of another polymer of the acrylic series—polyacrylamide (PA)—which also contains carboxyl groups in its macromolecule [
12,
21,
22].
By varying the ratio of polymer and crosslinking agent, it is possible to control the gelation time, as well as the structure of the resulting gels. Therefore, systems based on PA and chromium acetate have successfully found their application in technologies for water shut-off and leveling the injectivity profile [
23,
24,
25,
26,
27,
28,
29].
The ability of HPAN to form structured gels with chromium (III) acetate makes it possible to obtain an additional volume of plugging mass to form a more durable insulating screen after the initial generation of a gel sediment in contact with reservoir water.
2.2. Selection of Polymer: Crosslinking Agent Ratio
Polymer concentrations ranging from 3 wt% to 7 wt% were chosen to obtain crosslinked polymer systems throughout. The gelation process was observed at 25 °C and 70 °C to form a crosslinked three-dimensional structure. The research results are presented in
Table 2.
It is important to note that gel formation occurred in the entire volume of the system (100%) in all samples where the polymer concentration was 5.0 wt%. or more. The structure of the resulting gels depends on the temperature and content of CA in the system; the more chromium acetate in the system, the stronger the structure of the resulting gel. Based on the results of observing the samples for 30 days, the structure of the formed gels did not change, and no release of water from the gels—syneresis—was observed. During the study, all samples were stored in sealed containers with screw caps at 25 °C and atmospheric pressure for 30 days.
In samples with a polymer content of less than 5.0 wt%, the formation of a structured gel under experimental conditions did not occur within 24 h. To obtain strong gels with low concentrations of HPAN, it is necessary to increase the content of the crosslinking agent in the system (which may not be economically viable) or increase the gel holding time to more than 24 h (which is undesirable for well operations).
Thus, the optimal concentrations of the polymer and crosslinking agent were selected for preparing the dispersed phase of the emulsion that is being developed—at least 5 wt%. for HPAN and a polymer:crosslinking agent ratio = 5:1 (based on dry polymer powder) to obtain the most durable structured gel. For the HPAN concentration of 5 wt%, according to the Sydansk classifier, the gel structure had the code “H”—slightly deformable non-flowing gel. The “tongue” of the gel was not fixed; the gel was practically non-flowing. The research results provided below are given for compositions with a polymer concentration of 5 wt%.
2.3. Determination of Aggregative Stability and Thermal Stability of an Emulsion
The stability of emulsions is affected by temperature, pH, salinity, viscosity of the dispersion medium, and other factors. To increase the stability of emulsions, various methods are used (introducing stabilizing reagents, increasing the viscosity of the dispersion medium, changing the phase ratio).
Among the main factors influencing the stability of the developed system are the concentration of the surfactant stabilizer, the “dispersed phase: dispersion medium” ratio, and temperature.
Based on the results of preliminary laboratory studies, the optimal concentration of the surfactant stabilizer in the composition was determined. It amounted to 15–20% of the total volume of the hydrocarbon phase of the emulsion. The surfactant concentration was determined based on tests for the interaction of emulsion samples with mineralized water (produced water or calcium chloride solution), followed by an assessment of the system for the presence of a gel or HPAN sediment, as well as thermal stability tests of emulsion samples. An insufficient amount of surfactant stabilizer in the system also leads to low values of aggregation stability and thermal stability of the emulsion. If the emulsion is not stable enough (surfactant stabilizer content in the hydrocarbon phase of the emulsion is less than 15%), the polymer, freed from the surfactant stabilizer shell, when mixed with the formation water, instantly forms a coagulation film upon contact.
The thermal stability of the emulsion composition is the most important indicator of its applicability. This indicator characterizes the temperature limits at which the composition can be used, and shows the possible sedimentation, release of gases, and the release of phases that make up the emulsion, which indicates the unsuitability of the composition.
In this case, the composition must be sufficiently stable for successful injection into the well. Therefore, the samples were tested for thermal stability for 8 h. If the emulsion is not stable enough, it can break down in the wellbore and cause premature coagulation of HPAN upon contact with saline water. The emulsion must break down as it passes into the pore space of the formation, and HPAN must interact with the formation water after adsorption of the surfactant into the porous media.
As part of testing the compositions for thermal stability, samples of emulsions with different ratios of dispersed phase and dispersion medium were placed in graduated tubes with screw caps and thermostated in a heating cabinet at temperatures of 25 °C, 40 °C, and 70 °C for 8 h. All samples were checked every hour and preliminary results were recorded. After the exposure time had expired, samples were taken for examination under a microscope (magnification factor—40×). For each sample, the amount of hydrocarbon phase separated from above was also recorded. The results are presented in
Table 3. The rheological parameters of the emulsion samples are presented in
Figure 7. As the content of the dispersed phase in the system increases, the viscosity of the emulsion increases.
The most stable is an emulsion with a “dispersed phase:dispersion medium” ratio of 80/20.
2.4. Interaction of the Composition with Oil and Formation Mineralized Water
To assess the compatibility of the developed composition with oil, a sample of freshly prepared emulsion was mixed with oil in a ratio of 1:1 by volume and stirred using a top-drive paddle mixer at a speed of 300–350 rpm for 5 min. Next, the sample was poured into a sealed vessel with a screw cap.
When the water shut-off composition interacts with oil, the formation of flakes, sediments, emulsions, or the release of a new phase should not occur.
The selectivity of invert emulsions lies in the fact that upon contact with oil, the emulsion is diluted and its viscosity is significantly reduced compared to the original. When interacting with water, the emulsion gains viscosity, which makes it possible to create an insulating screen for use in water shut-off technologies. The proposed surfactant–polymer composition does not form any sediments with oil (
Table 4). The viscosity of the “surfactant–polymer composition:oil” system decreases by 10–15 times relative to the initial viscosity of the composition.
2.5. Compatibility of the Composition with Process Fluids
To assess the compatibility of the developed composition with displacement fluids, a sample of freshly prepared emulsion was mixed with the displacement fluid in a ratio of 1:1 by volume and stirred using an overhead stirrer at a speed of 300–350 rpm for 5 min. The samples were then placed in sealed containers with screw caps and thermostated in a drying cabinet at 25 °C for 24 h and 70 °C for 8 h. When the water shut-off composition interacts with the displacing fluids, there should be no formation of flakes, sediments, emulsions, or separation of a new phase.
The proposed surfactant–polymer composition does not form any sedimentation with the liquids under consideration. The system is completely divided into the “top layer”—the surfactant–polymer composition—and the “bottom layer”—the displacement fluid.
2.6. Laboratory Assessment of the Action Mechanism of the Composition: Filtration Studies
At the first stage of research, the action mechanism of the developed composition was assessed using a fluid loss cell for drilling fluids. A thin layer of gravel filter was poured into a clean and dry cell of the Fann LPLT model 300 fluid loss cell to prevent sand from passing through the mesh holes, then quartz sand of BC-030-B grade, fraction 0.1–0.4 mm, was poured (
Figure 8).
Freshly prepared emulsion was poured into the cell from above. The quartz sand:emulsion ratio was 2:1 (by volume). Excess pressure (about 2 atm) was supplied to the fluid loss cell. The filtrate coming out of the sand pack was a brown liquid.
After the filtering process was completed, the device cell was disassembled, visually assessing the condition of the sand pack. By the even color of the sand pack, one can indirectly judge the uniform passage of the emulsion composition.
Then, mineralized water was gradually poured into the resulting filtrate while stirring (the “filtrate:mineralized water” ratio was 1:1 by volume). At the end of mixing, the state of the system was visually assessed.
When the filtrate of the working solution of the composition is mixed with mineralized water, an elastic gel-like mass is formed, and coagulation of HPAN occurs when the polymer interacts with salts of polyvalent metals contained in the formation water (
Figure 9). There is a general increase in the viscosity of the entire system. Keeping the system for the next 24 h allows one to form an additional volume of gel due to the presence of a crosslinking agent in the composition.
Based on the results of the experiments, it can be concluded that in the process of filtering the emulsion through a dry sand pack, the process of destruction of the emulsion occurred, in addition to the adsorption of surfactants on sand particles, and as the composition passed through the filling, a polymer solution was released.
To assess the effectiveness of the technology for water shut-off using the developed surfactant–polymer composition as part of filtration studies, physical modeling of the process of injection of the composition into the bottomhole zone of the formation was carried out using bulk models of the formation with different permeability (a water-saturated model with residual oil for modeling washed intervals with high permeability, as well as an oil-saturated model with residual water for modeling unwashed areas with lower permeability).
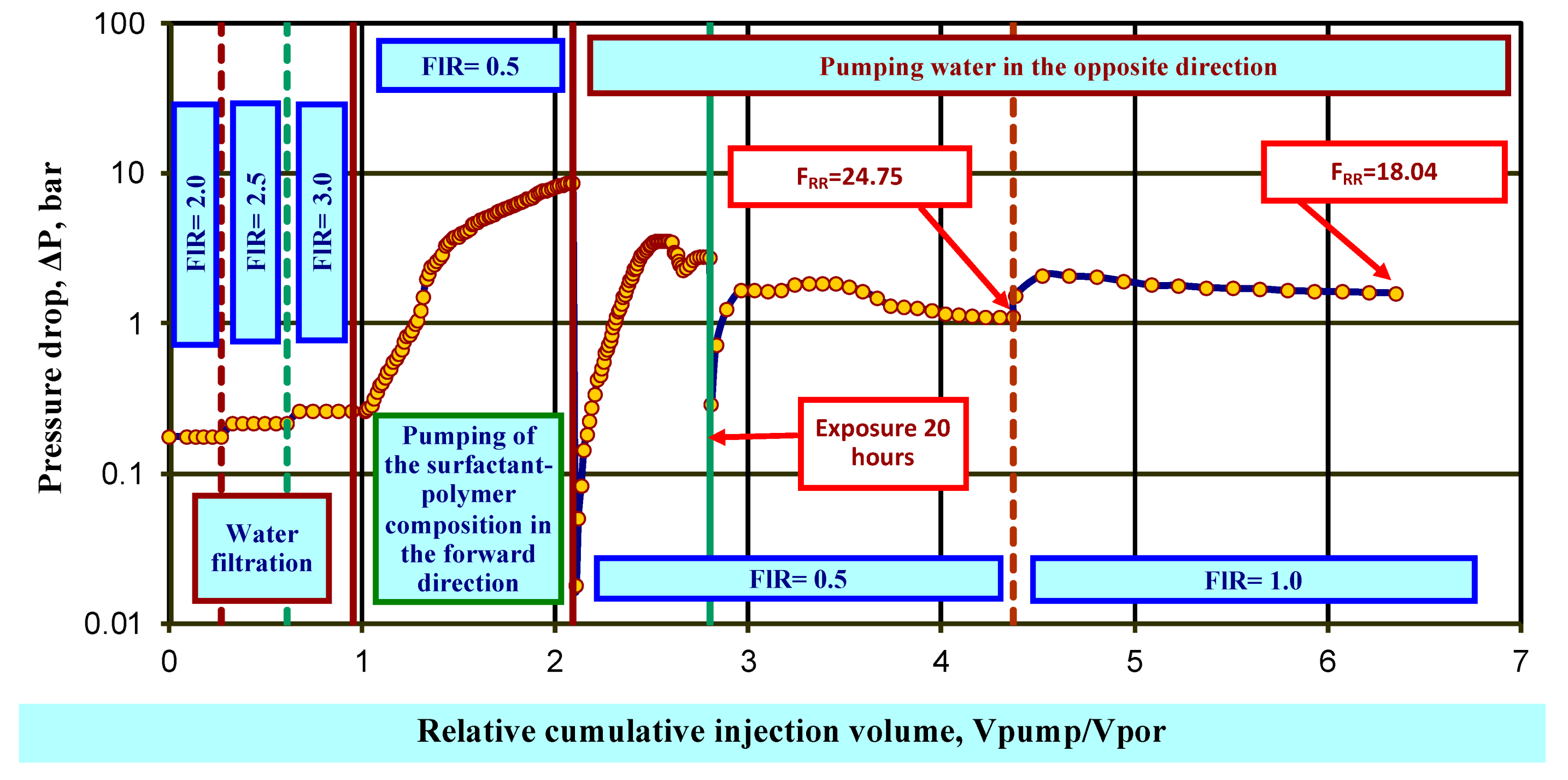
Testing of the plugging properties of the surfactant–polymer composition (emulsion with HPAN 5 wt% and HPAN:CA ratio 5:1) on a water-saturated model was carried out on a bulk reservoir model with permeability with 100% water saturation of 1.17 μm2, length 22.1 cm, and diameter 2.6 cm. Filtration was carried out at the reservoir temperature of 40 °C. Produced water from one of the fields in the Samara region with a dynamic viscosity value of 1.32 mPa·s and a density of 1156.2 kg/m3 under reservoir conditions was used as a model of the displacing agent.
The model was saturated with formation water and retained its pore volume and porosity. In the process of water filtration, the coefficient of initial water permeability is achieved at the experimental temperature .
At the next stage, 1 Vpor of a working solution of the emulsion was injected into the model without back pressure at flow rate FlR = 0.5 cm3/min in the “well-formation” direction in a maximum volume equal to the pore volume of the model, 1 Vpor, or until a specified maximum pressure drop was achieved. After holding the model for a specified time (1 h), formation water was pumped into it in the “formation-well” direction. Then, after holding for 15 h, formation water was again injected in the “formation-well” direction. The phase permeability for water kwpr1 was determined.
From the obtained values of the initial and final permeability coefficients, the residual resistance factor ((Equation (1)) was calculated:
where F
RR is residual resistance factor, units,
is initial water permeability at the experimental temperature, and
is phase permeability to water, μm
2.
After filtration of formation water in the opposite direction, the residual resistance factor F
RR was 18.04 units. (
Figure 10).
Testing of the plugging properties of the surfactant–polymer composition on an oil-saturated model with residual water saturation was carried out on a bulk reservoir model with a water permeability of 0.427 μm2, a length of 22.2 cm, and a diameter of 2.6 cm. Filtration was carried out at a reservoir temperature of 40 °C.
The model was saturated with formation water, and its pore volume and porosity were determined. In the process of water filtration, the coefficient of initial water permeability at room temperature, , was determined.
Next, residual water was created in the reservoir model by pumping oil from a field in the Samara region from a high-pressure vessel under a pressure of about 2.0 MPa into a vertically located formation model.
At the next stage, in the process of oil filtration in the “reservoir-well” direction, the phase permeability coefficient for oil was determined before the exposure of at the experimental temperature.
Then, a 1 Vpor working solution of the emulsion gel sedimentation composition was injected into the bulk model without back pressure at flow rate FlR = 0.5 cm3/min in the “well-formation” direction in a maximum volume equal to the pore volume of the model, 1 Vpor. After holding the model for a specified time (1 h), oil was injected into it in the “formation-well” direction. Then, after holding for 48 h, oil was again filtered in the “formation-well” direction. The phase permeability of oil, , was determined.
The residual resistance factor FRR (units) for an oil-saturated low-permeability model with residual water saturation is determined by the ratio of the oil permeability before injection to the oil permeability after injection of the test composition.
After oil filtration in the reverse direction, the residual resistance factor F
RR was 1.49 units (
Figure 11).
The dynamics of pressure drop during the process of sequential injection of the composition and formation oil in the opposite direction after holding into a bulk oil-saturated model with residual water are shown in
Figure 11. The obtained value of the residual resistance factor for the oil-saturated low-permeability model is much lower than the value obtained for the water-saturated high-permeability model. The ratio of the obtained values of the residual resistance factor equal to 0.08 (significantly less than 1) can characterize the surfactant–polymer composition as a selective material for water shut-off in production wells.
3. Conclusions
The work describes the stages of development and laboratory research of a surfactant–polymer composition for selective water shut-off in production wells at sites with high salinity of formation waters. The structure of the macromolecule and the chemical properties of HPAN allow the polymer to be used as a gel-forming and sediment-forming reagent. Traditional HPAN-based technologies involve alternating injection of a polymer solution and formation water (or calcium chloride solutions), separated by buffer fluids. The developed composition is an invert emulsion, which makes it possible to carry out treatment at a distance from the well and solve the problem of possible premature gel sedimentation directly in the wellbore (which is especially important in complex objects, including horizontal wells).
As part of laboratory studies, the optimal polymer concentrations (4 wt% or more) and the amount of crosslinking agent (with a polymer:crosslinking agent ratio of 5:1 (calculated on dry polymer powder)) were selected; it is possible to obtain the most durable gel structure. To ensure the stability of the developed composition, the concentration of the emulsifier and the optimal ratio of the dispersed phase and the dispersion medium were selected. To prepare a stable invert emulsion, the optimal concentration of the surfactant emulsifier was selected; this concentration was 15–20% of the entire hydrocarbon phase of the system.
Laboratory tests of the developed surfactant–polymer composition demonstrate the absence of interaction of the composition with displacement fluids, as well as with mineralized formation water (before filtration of the composition in a porous medium).
It has been experimentally proven that the composition has not only physical, but also chemical selectivity, forming a gel sediment only in water-saturated, highly permeable zones. The obtained value of the residual resistance factor for the oil-saturated low-permeability model was 1.49 units, and the value of the residual resistance factor for the water-saturated high-permeability model was 18.04 units. The ratio of the values of the residual resistance factor obtained from the results of filtration tests on a water-saturated high-permeability model and an oil-saturated low-permeability model was 0.08. When the developed composition comes into contact with oil, the process of sedimentation and gelation does not occur.
The developed composition allows us to solve the problem of premature gelation. In addition, the composition can form a blocking screen in highly permeable water-saturated zones. The development may be useful for fields with difficult conditions (high salinity of formation waters, boreholes with a horizontal end, elevated formation temperatures up to 80 °C).

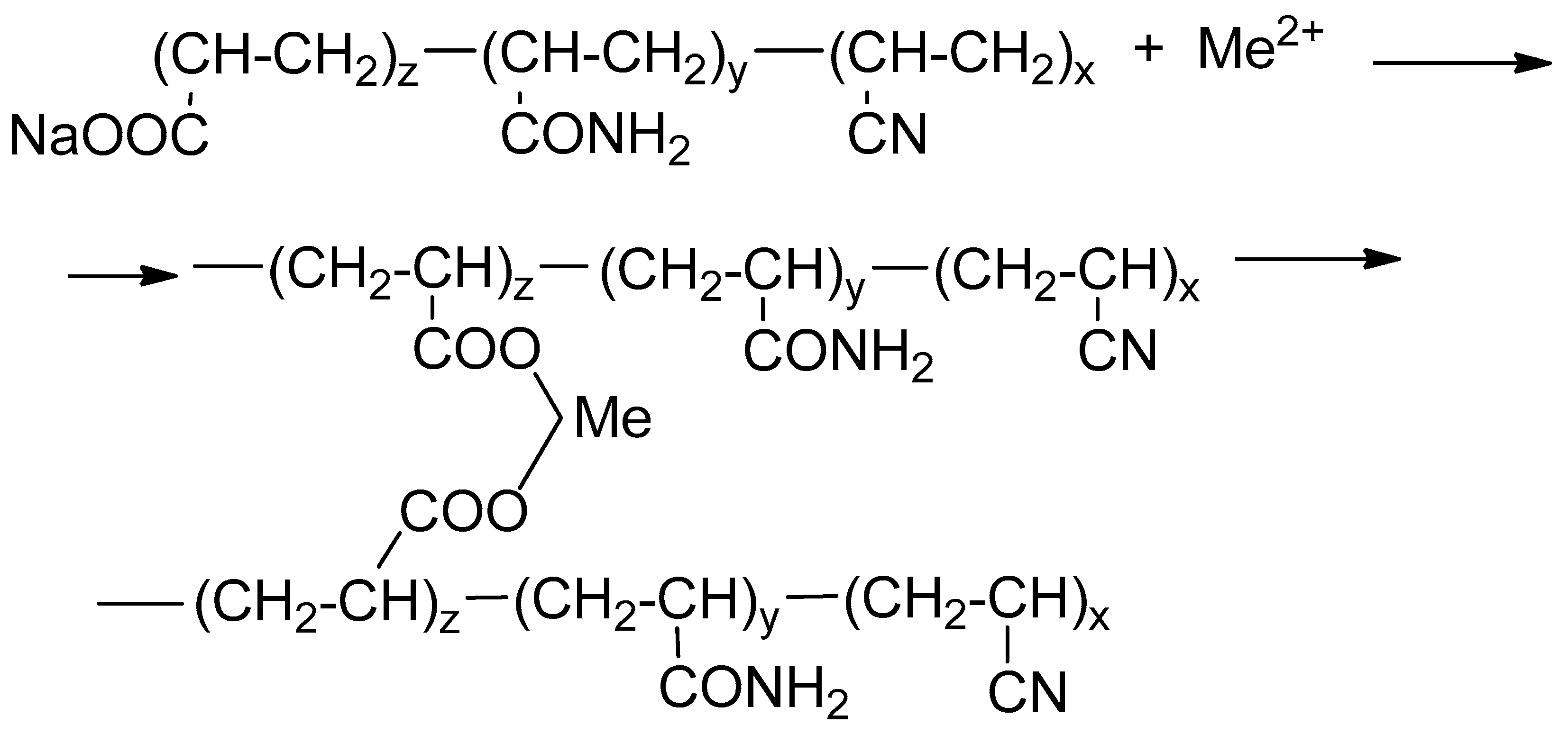





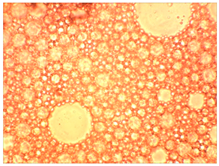 hydrocarbon phase separation < 0.5%
hydrocarbon phase separation < 0.5%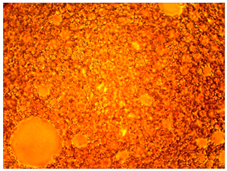 hydrocarbon phase separation < 3%
hydrocarbon phase separation < 3%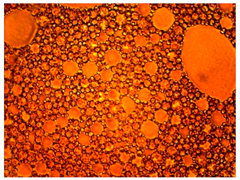 hydrocarbon phase separation < 5%
hydrocarbon phase separation < 5% hydrocarbon phase separation < 5%
hydrocarbon phase separation < 5% 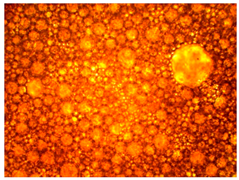 hydrocarbon phase separation < 5%
hydrocarbon phase separation < 5%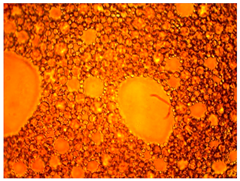 hydrocarbon phase separation > 5%
hydrocarbon phase separation > 5%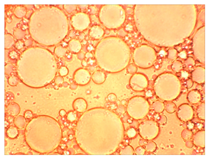 hydrocarbon phase separation > 5%The first signs of emulsion breakdown have appeared.
hydrocarbon phase separation > 5%The first signs of emulsion breakdown have appeared.











