Detecting N-Phenyl-2-Naphthylamine, L-Arabinose, D-Mannose, L-Phenylalanine, L-Methionine, and D-Trehalose via Photocurrent Measurement
Abstract
:1. Introduction
2. Results and Discussion
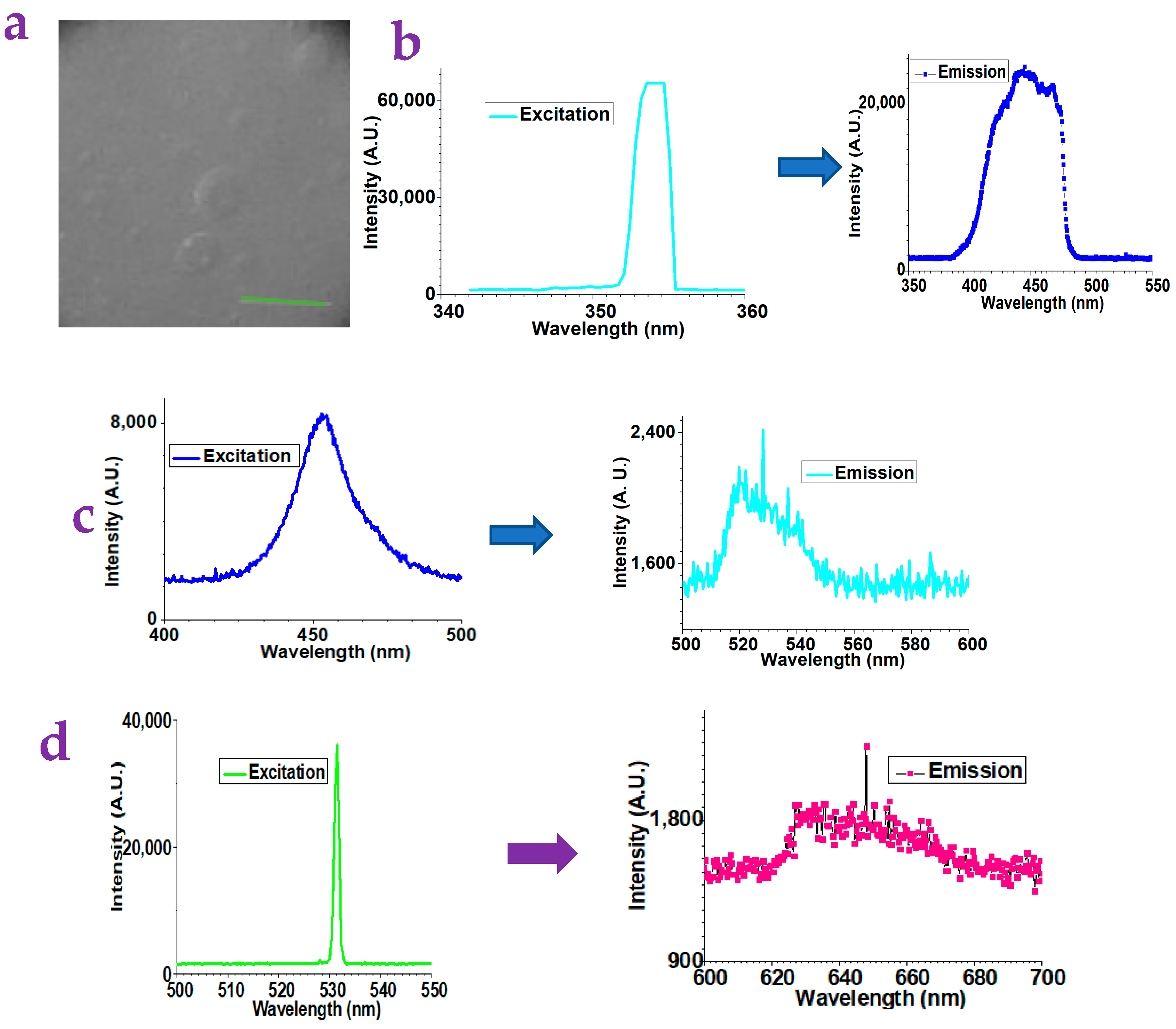
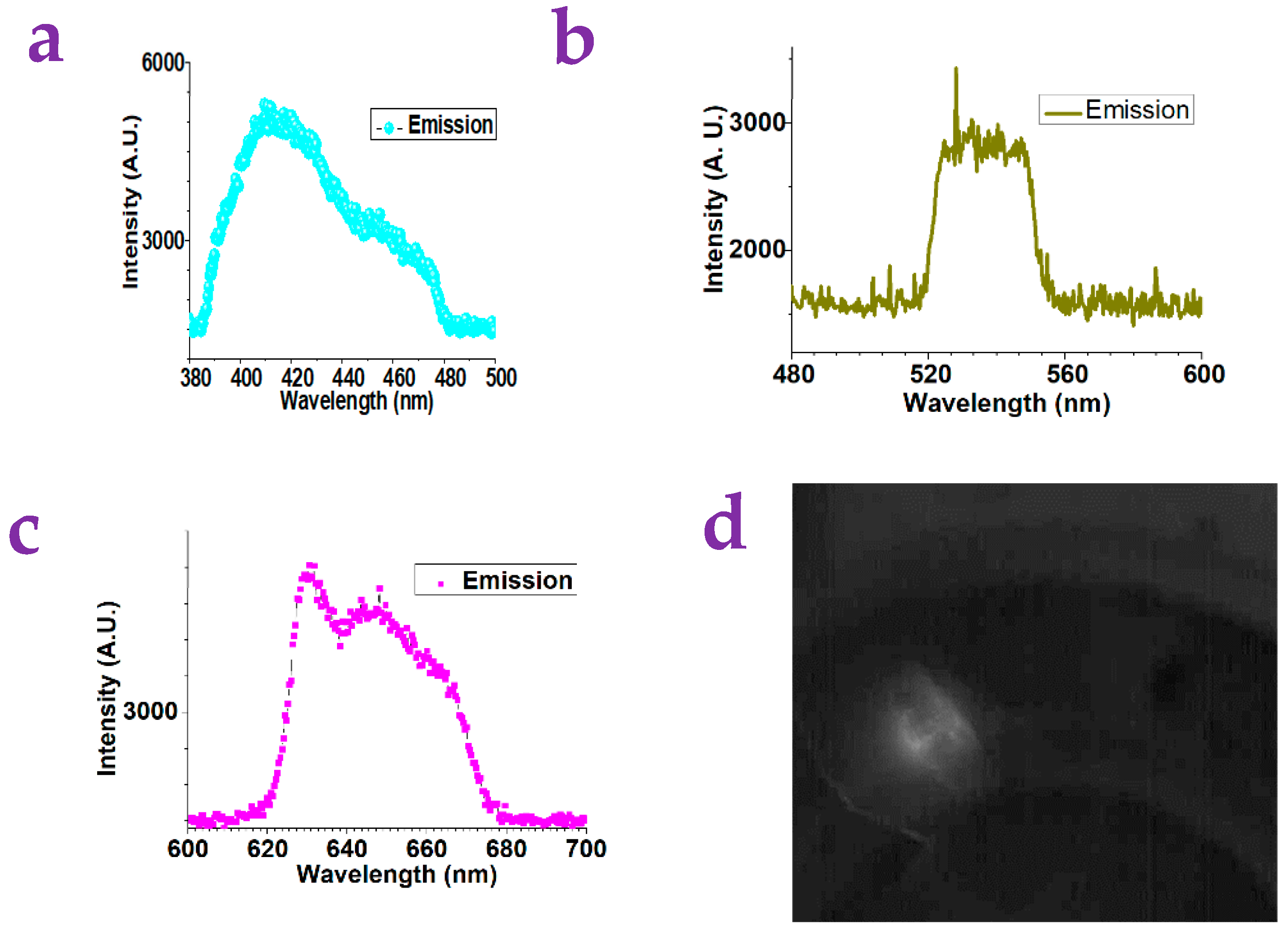
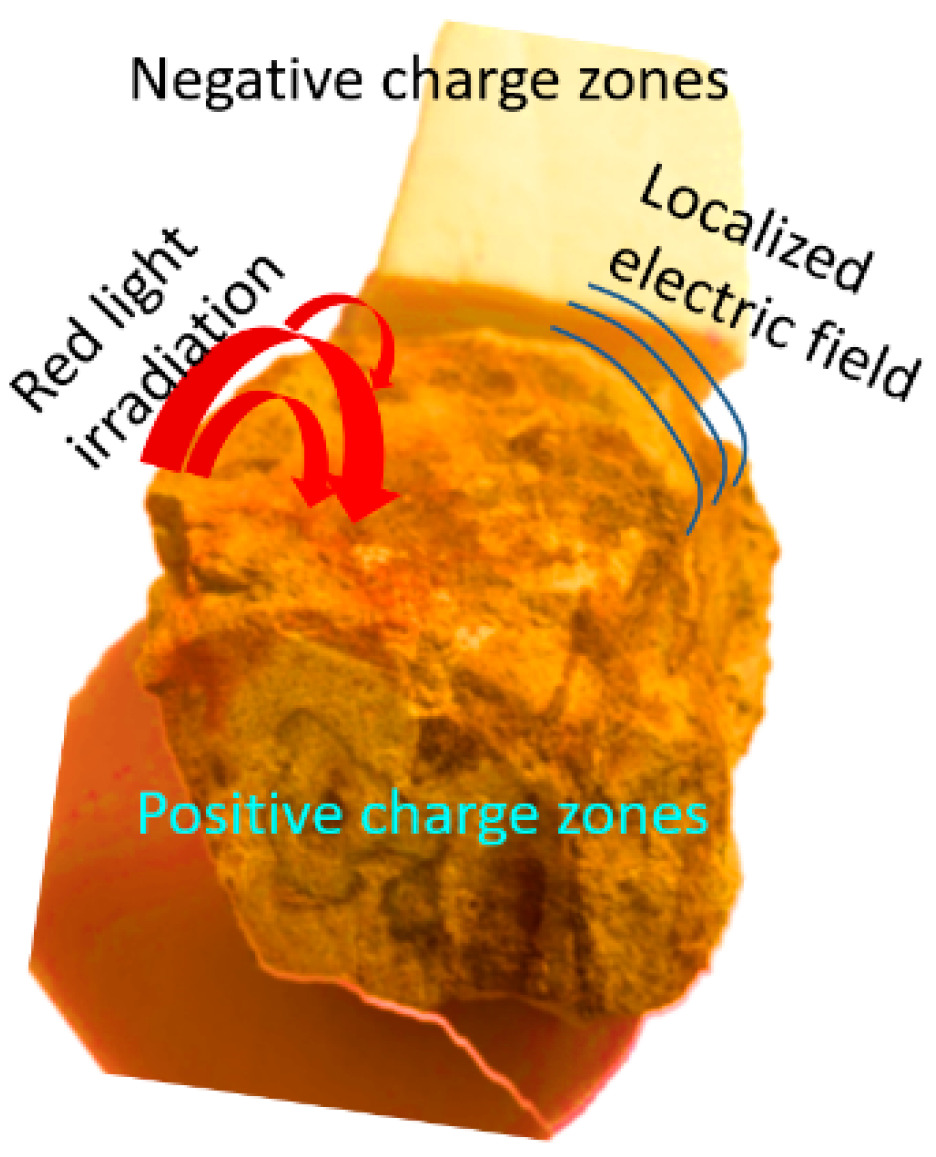
2.1. The Detection of Small Molecules via the Gel Substrates
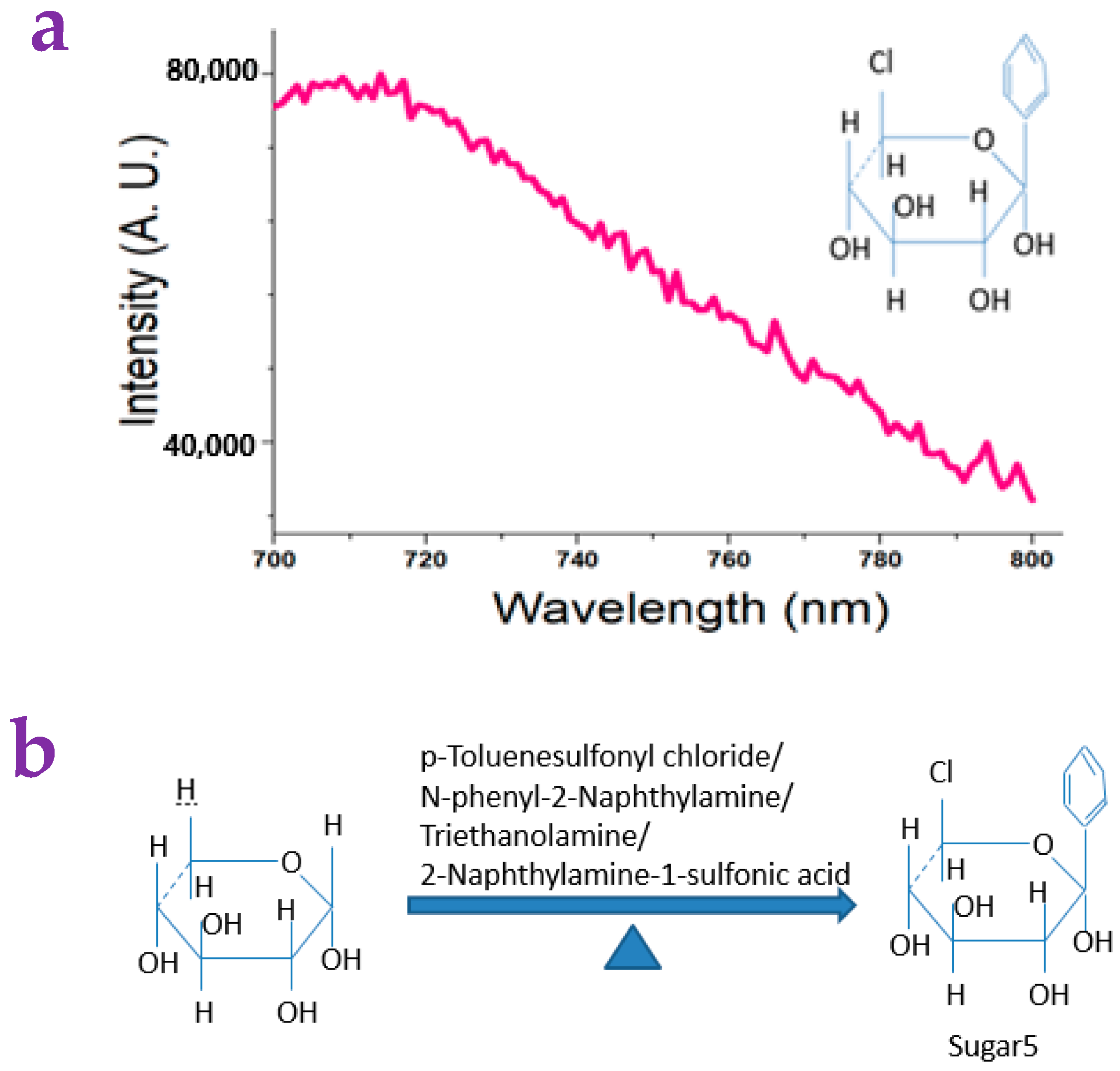
2.2. The Detection of Small Molecules via the Paper Substrates
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Gel Substrates
4.3. Preparation of the Solution of the Small Molecules for Detection
4.4. Setting up an Optical Microscope
4.5. Collection of Blue Fluorescence
4.6. Collecting Green Fluorescence
4.7. Collecting Red Fluorescence
4.8. Imaging of the Corn Bug with Red Fluorescence
4.9. Fabrication of the Device Based on the Gels for Detecting the Small Molecules
4.10. Synthesis of Sugar5
4.11. Fabrication of the Device Based on the Sugar5-Paper for Detecting the Small Molecules
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Walt, D.R. Simultaneous detection of small molecules, proteins and microRNAs using single molecule arrays. Chem. Sci. 2020, 11, 7896–7903. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Wang, L.; Duan, H. Nanomaterial based electrochemical sensors for in vitro detection of small molecule metabolites. Biotechnol. Adv. 2016, 34, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.M.; Asiri, A.M.; Rahman, M.M. Simultaneous detection of l-aspartic acid and glycine using wet-chemically prepared FeO@ZnO nanoparticles: Real sample analysis. RSC Adv. 2020, 10, 19276–19289. [Google Scholar] [CrossRef] [PubMed]
- Mohammadniaei, M.; Nguyen, H.V.; Tieu, M.V.; Lee, M.-H. 2D Materials in Development of Electrochemical Point-of-Care Cancer Screening Devices. Micromachines 2019, 10, 662. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics by Gas Chromatography-Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2016, 114, 30.4.1–30.4.32. [Google Scholar] [CrossRef]
- Brands, M.; Gutbrod, P.; Dörmann, P. Lipid Analysis by Gas Chromatography and Gas Chromatography-Mass Spectrometry. Methods Mol. Biol. 2021, 2295, 43–57. [Google Scholar]
- Swenson, T.L.; Northen, T.R. Untargeted Soil Metabolomics Using Liquid Chromatography-Mass Spectrometry and Gas Chromatography-Mass Spectrometry. Methods Mol. Biol. 2019, 1859, 97–109. [Google Scholar]
- Lo, S.F.; Pierzchalski, K.; Young, V.; Rhead, W.J. Identification of Urine Organic Acids for the Detection of Inborn Errors of Metabolism Using Urease and Gas Chromatography-Mass Spectrometry (GC/MS). Methods Mol. Biol. 2022, 2546, 335–350. [Google Scholar]
- Lin, D.Y.; Yu, C.Y.; Ku, C.A.; Chung, C.K. Design, Fabrication, and Applications of SERS Substrates for Food Safety Detection: Review. Micromachines 2023, 14, 1343. [Google Scholar] [CrossRef]
- Shard, A.G.; Wright, L.; Minelli, C. Robust and accurate measurements of gold nanoparticle concentrations using UV-visible spectrophotometry. Biointerphases 2018, 13, 061002. [Google Scholar] [CrossRef]
- Wang, M.; Gong, Q.; Liu, W.; Tan, S.; Xiao, J.; Chen, C. Applications of capillary electrophoresis in the fields of environmental, pharmaceutical, clinical, and food analysis (2019–2021). J. Sep. Sci. 2022, 45, 1918–1941. [Google Scholar] [CrossRef] [PubMed]
- Helena, H.; Ivona, V.; Roman, Ř.; František, F. Current applications of capillary electrophoresis-mass spectrometry for the analysis of biologically important analytes in urine (2017 to mid-2021): A review. J. Sep. Sci. 2022, 45, 305–324. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Cuellar, H.E.; Kuang, R.; Caurin, H.F.N.; Goswami, D.; Martinez, R.V. Self-Powered, Paper-Based Electrochemical Devices for Sensitive Point-of-Care Testing. Adv. Mater. Technol. 2017, 2, 1700130. [Google Scholar] [CrossRef]
- Lee, J.R.; Choi, J.; Shultz, T.O.; Wang, S.X. Small Molecule Detection in Saliva Facilitates Portable Tests of Marijuana Abuse. Anal. Chem. 2016, 88, 7457–7461. [Google Scholar] [CrossRef]
- Zhong, Q.; Tan, E.K.; Martin-Alonso, C.; Parisi, T.; Hao, L.; Kirkpatrick, J.D.; Fadel, T.; Fleming, H.E.; Jacks, T.; Bhatia, S.N. Inhalable point-of-care urinary diagnostic platform. Sci. Adv. 2024, 10, eadj9591. [Google Scholar] [CrossRef]
- Banbury, C.; Harris, G.; Clancy, M.; Blanch, R.J.; Rickard, J.J.; Goldberg Oppenheimer, P. Window into the mind: Advanced handheld spectroscopic eye-safe technology for point-of-care neurodiagnostic. Sci. Adv. 2023, 9, eadg5431. [Google Scholar] [CrossRef]
- Hong, J.M.; Lee, H.; Menon, N.V.; Lim, C.T.; Lee, L.P.; Ong, C.W.M. Point-of-care diagnostic tests for tuberculosis disease. Sci. Transl. Med. 2022, 14, eabj4124. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Chai, F.; Li, Q.; Wang, D.; Liu, L.; Tang, B.Z.; Jiang, X. Ultrasensitive point-of-care biochemical sensor based on metal-AIEgen frameworks. Sci. Adv. 2022, 8, eabo1874. [Google Scholar] [CrossRef]
- Ji, W.; Wu, Q.; Han, X.; Zhang, W.; Wei, W.; Chen, L.; Li, L.; Huang, W. Photosensitive hydrogels: From structure, mechanisms, design to bioapplications. Sci. China Life Sci. 2020, 63, 1813–1828. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, J.; Xing, J.; Li, C. Light-Programmable Nanocomposite Hydrogel for State-Switchable Wound Healing Promotion and Bacterial Infection Elimination. Adv. Healthc. Mater. 2023, 12, e2201565. [Google Scholar] [CrossRef]
- Lou, Z.; Tao, J.; Wei, B.; Jiang, X.; Cheng, S.; Wang, Z.; Qin, C.; Liang, R.; Guo, H.; Zhu, L.; et al. Near-Infrared Organic Photodetectors toward Skin-Integrated Photoplethysmography-Electrocardiography Multimodal Sensing System. Adv. Sci. 2023, 10, e2304174. [Google Scholar] [CrossRef] [PubMed]
- Marek, E.M.; Koslitz, S.; Weiss, T.; Fartasch, M.; Schlüter, G.; Käfferlein, H.U.; Brüning, T. Quantification of N-phenyl-2-naphthylamine by gas chromatography and isotope-dilution mass spectrometry and its percutaneous absorption ex vivo under workplace conditions. Arch. Toxicol. 2017, 91, 3587–3596. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Y.; Wang, J.; Xiong, C.; Xue, J.; Zhan, L.; Nie, Z. N-Phenyl-2-naphthylamine as a Novel MALDI Matrix for Analysis and in Situ Imaging of Small Molecules. Anal. Chem. 2018, 90, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, M.; Beton-Mysur, K.; Abramczyk, H. Biochemical changes in lipid and protein metabolism caused by mannose-Raman spectroscopy studies. Analyst 2024, 149, 2942–2955. [Google Scholar] [CrossRef]
- Hayward, G.; Mort, S.; Hay, A.D. d-Mannose for Prevention of Recurrent Urinary Tract Infection Among Women: A Randomized Clinical Trial. JAMA Intern. Med. 2024, 8, e240264. [Google Scholar] [CrossRef]
- Xiang, S.; Ge, Y.; Zhang, Y.; Bao, X.; Su, X.; Shi, L.; Xia, Y.; Han, H.; Ying, J.; Lai, S.; et al. L-arabinose exerts probiotic functions by improving gut microbiota and metabolism in vivo and in vitro. J. Funct. Foods 2024, 113, 1756–4646. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, C.; Yang, X.; Zhang, C. L-phenylalanine attenuates high salt-induced hypertension in Dahl SS rats through activation of GCH1-BH4. PLoS ONE 2021, 16, e0250126. [Google Scholar] [CrossRef]
- Cai, M.; Liu, Z.; Zhao, Z.; Wu, H.; Xu, M.; Rao, Z. Microbial production of L-methionine and its precursors using systems metabolic engineering. Biotechnol. Adv. 2023, 69, 108260. [Google Scholar] [CrossRef]
- Benavides, M.A.; Bosland, M.C.; Silva, C.P.; Gomes, S.C.T.; Oliveira, A.M.; Kemp, R.; Reis, R.B.; Martins, V.R.; Sampaio, S.V.; Bland, K.I.; et al. L-Methionine inhibits growth of human pancreatic cancer cells. Anti-Cancer Drugs 2014, 25, 200–203. [Google Scholar] [CrossRef]
- Wu, Y.; Fu, H.; Li, B.; Lin, Y. Negative-resistance and high-mobility devices based on paper. Mater. Express 2017, 7, 5–14. [Google Scholar] [CrossRef]
- Wu, Y.; Ou, P.; Fronczek, F.R.; Song, J.; Lin, Y.; Wen, H.-M.; Xu, J. Simultaneous Enhancement of Near-Infrared Emission and Dye Photodegradation in a Racemic Aspartic Acid Compound via Metal-Ion Modification. ACS Omega 2019, 4, 19136–19144. [Google Scholar] [CrossRef]
- Cai, W.; Shin, D.-W.; Chen, K.; Gheysens, O.; Cao, Q.; Wang, S.X.; Gambhir, S.S.; Chen, X. Peptide-Labeled Near-Infrared Quantum Dots for Imaging Tumor Vasculature in Living Subjects. Nano Lett. 2006, 6, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Nesvet, J.C.; Antilla, K.A.; Pancirer, D.S.; Lozano, A.X.; Preiss, J.S.; Ma, W.; Fu, A.; Park, S.-M.; Gambhir, S.S.; Fan, A.C.; et al. Giant Magnetoresistive Nanosensor Analysis of Circulating Tumor DNA Epidermal Growth Factor Receptor Mutations for Diagnosis and Therapy Response Monitoring. Clin. Chem. 2021, 67, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Shao, X.-J.; Li, Z.; Lin, C.-H.; Wang, C.-W.-Q.; Jiao, K.; Xu, J.; Pan, H.-X.; Wu, Y. Synthesis of Holmium-Oxide Nanoparticles for Near-Infrared Imaging and Dye-Photodegradation. Molecules 2022, 27, 3522. [Google Scholar] [CrossRef]
- Fu, H.; Ou, P.; Zhu, J.; Song, P.; Yang, J.; Wu, Y. Enhanced Protein Adsorption in Fibrous Substrates Treated with Zeolitic Imidazolate Framework-8 (ZIF-8) Nanoparticles. ACS Appl. Nano Mater. 2019, 2, 7626–7636. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Lin, Y.; Alam, M.Z.; Wu, Y. Synthesizing Ag+: MgS, Ag+: Nb2S5, Sm3+: Y2S3, Sm3+: Er2S3 and Sm3+:ZrS2 compound nanoparticles for multicolor fluorescence imaging of bio-tissues. ACS Omega 2020, 5, 32868–32876. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, J.; Lin, Y.; Xu, J. Synthesis of samarium-based metal organic compound nanoparticles with polychromatic-photoluminescence for bio-tissue fluorescence imaging. Molecules 2019, 24, 3657. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Y.; Xu, J. Synthesis of Ag-Ho, Ag-Sm, Ag-Zn, Ag-Cu, Ag-Cs, Ag-Zr, Ag-Er, Ag-Y and Ag-Co metal organic nanoparticles for UV-Vis-NIR wide-range bio-tissue imaging. Photochem. Photobiol. Sci. 2019, 18, 1081–1091. [Google Scholar] [CrossRef]
- Wu, Y.; Ou, P.; Song, J.; Zhang, L.; Lin, Y.; Song, P.; Xu, J. Synthesis of praseodymium-and molybdenum- sulfide nanoparticles for dye-photodegradation and near-infrared deep-tissue imaging. Mater. Res. Express 2020, 7, 036203. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Guo, R. Achieving near-infrared deep tissue imaging via metal organic complex nanoparticles. In Proceedings of the SPIE Optical Engineering + Applications 2019, Photonic Fiber and Crystal Devices: Advances in Materials and Innovations in Device Applications XIII, 111230Z, San Diego, CA, USA, 10 September 2019; SPIE: Bellingham, WA, USA, 2019. [Google Scholar]
- Li, I.L.; Wu, Y.; Zhai, J.P.; Ruan, S.C.; Tang, Z.K. Template technique-A promising approach for well-aligned quantum wires fabrication. Proc. SPIE 2007, 6724, 67241D. [Google Scholar]
- Rizzi, G.; Lee, J.-R.; Dahl, C.; Guldberg, P.; Dufva, M.; Wang, S.X.; Hansen, M.F. Simultaneous Profiling of DNA Mutation and Methylation by Melting Analysis Using Magnetoresistive Biosensor Array. ACS Nano 2017, 11, 8864–8870. [Google Scholar] [CrossRef] [PubMed]
- Cortade, D.L.; Markovits, J.; Spiegel, D.; Wang, S.X. Point-of-care testing of enzyme polymorphisms for predicting hypnotizability and postoperative pain. J. Mol. Diagn. 2023, 25, 197–210. [Google Scholar] [CrossRef] [PubMed]
- de Olazarra, A.S.; Cortade, D.L.; Wang, S.X. From saliva to SNP: Non-invasive, point-of-care genotyping for precision medicine applications using recombinase polymerase amplification and giant magnetoresistive nanosensors. Lab Chip 2022, 22, 2131–2144. [Google Scholar] [CrossRef] [PubMed]
- de Olazarra, A.S.; Chen, F.-E.; Wang, T.-H.; Wang, S.X. Rapid, point-of-care Host-Based Gene Expression Diagnostics Using Giant Magnetoresistive Biosensors. ACS Sens. 2023, 8, 2780–2790. [Google Scholar] [CrossRef]
- Ng, E.; Le, A.K.; Nguyen, M.H.; Wang, S.X. Early multiplexed detection of cirrhosis using giant magnetoresistive biosensors with protein biomarkers. ACS Sens. 2020, 5, 3049–3057. [Google Scholar] [CrossRef]
- Fu, A.; Wilson, R.J.; Smith, B.R.; Mullenix, J.; Earhart, C.; Akin, D.; Guccione, S.; Wang, S.X.; Gambhir, S.S. Fluorescent magnetic nanoparticles for magnetically enhanced cancer imaging and targeting in living subjects. ACS Nano 2012, 6, 6862–6869. [Google Scholar] [CrossRef]
- Lee, J.-R.; Bechstein, D.J.B.; Ooi, C.C.; Patel, A.; Gaster, R.S.; Ng, E.; Gonzalez, L.C.; Wang, S.X. Magneto-nanosensor platformfor probing low-affinity protein–protein interactions and identification of a low-affinity PD-L1/PD-L2 interaction. Nat. Commun. 2016, 7, 12220. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Yang, J.; Nguyen, H.V.; Poo, H.; Seo, T.S. Development of a high-throughput centrifugal microsystem for enzyme-linked immunosorbent assay to detect SARS-CoV-2. Chem. Eng. J. 2023, 472, 144808. [Google Scholar]
- Cho, C.H.; Park, C.Y.; Chun, H.S.; Park, T.J.; Park, J.P. Antibody-free and selective detection of okadaic acid using an affinity peptide-based indirect assay. Food Chem. 2023, 422, 136243. [Google Scholar] [CrossRef]
- Kadam, U.S.; Cho, Y.; Park, T.Y.; Hong, J.C. Aptamer-based CRISPR-Cas powered diagnostics of diverse biomarkers and small molecule targets. Appl. Biol. Chem. 2023, 66, 13. [Google Scholar] [CrossRef]
- Abdel-Rahman, S.A.; Zhang, L.; Gabr, M.T. Development of a high-throughput TR-FRET screening assay for LAG-3/FGL1 interaction. SLAS Discov. 2023, 28, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Ana Díaz-Fernández, A.; Ranallo, S.; Ricci, F. Enzyme-Linked DNA Displacement (ELIDIS) Assay for Ultrasensitive Electrochemical Detection of Antibodies. Angew. Chem. Int. Ed. 2024, 63, e202314818. [Google Scholar] [CrossRef]
- Ebanks, F.; Nasrallah, H.; Garant, T.M.; McConnell, E.M.; DeRosa, M.C. Colorimetric detection of aflatoxins B1 and M1 using aptamers and gold and silver nanoparticles. Adv. Agrochem 2023, 2, 221–230. [Google Scholar] [CrossRef]
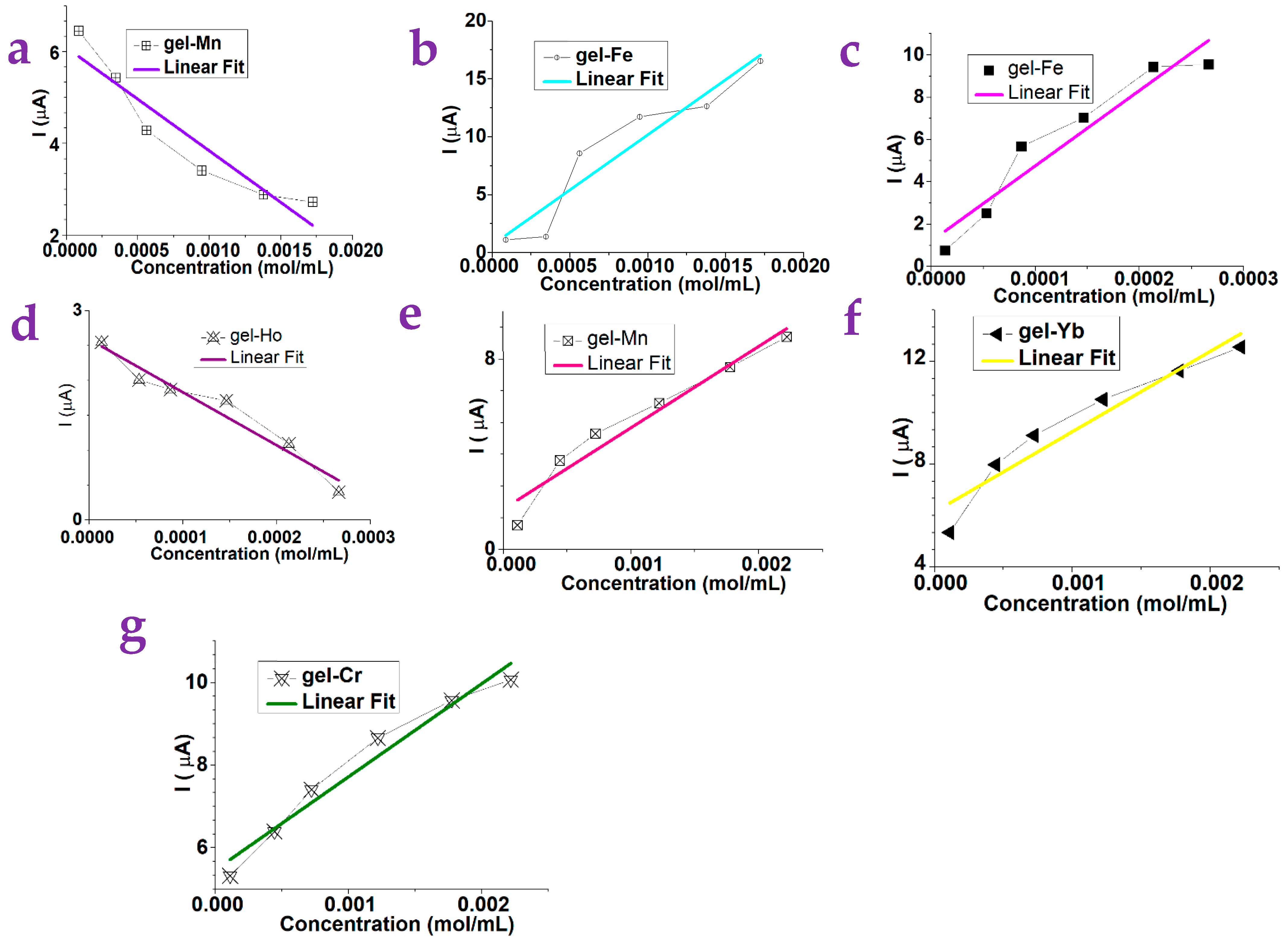


| Gels | Dependence | Gels | Dependence | Gels | Dependence |
|---|---|---|---|---|---|
| gel-Cr | Nonlinear | gel-Ce | Nonlinear | gel-Li | Nonlinear |
| gel-Na | Nonlinear | gel-Ca | Nonlinear | gel-Er | Nonlinear |
| gel-Eu | Nonlinear | gel-Zn | Nonlinear | gel-Cs | Nonlinear |
| gel-Y | Nonlinear | gel-Fe | Linear | gel-Yb | Nonlinear |
| gel-Sm | Nonlinear | gel-Ho | Nonlinear | gel-Mn | Linear |
| gel-Cu | Nonlinear | gel-Nd | Nonlinear | gel-K | Nonlinear |
| Gels | Dependence | Gels | Dependence | Gels | Dependence |
|---|---|---|---|---|---|
| gel-Cr | Nonlinear | gel-Ce | Nonlinear | gel-Li | Nonlinear |
| gel-Na | Nonlinear | gel-Ca | Nonlinear | gel-Er | Nonlinear |
| gel-Eu | Nonlinear | gel-Zn | Nonlinear | gel-Cs | Nonlinear |
| gel-Y | Nonlinear | gel-Fe | Linear | gel-Yb | Nonlinear |
| gel-Sm | Nonlinear | gel-Ho | Linear | gel-Mn | Nonlinear |
| gel-Cu | Nonlinear | gel-Nd | Nonlinear | gel-K | Nonlinear |
| Gels | Dependence | Gels | Dependence | Gels | Dependence |
|---|---|---|---|---|---|
| gel-Cr | Linear | gel-Ce | Nonlinear | gel-Li | Nonlinear |
| gel-Na | Nonlinear | gel-Ca | Nonlinear | gel-Er | Nonlinear |
| gel-Eu | Nonlinear | gel-Zn | Nonlinear | gel-Cs | Nonlinear |
| gel-Y | Nonlinear | gel-Fe | Nonlinear | gel-Yb | Linear |
| gel-Sm | Nonlinear | gel-Ho | Nonlinear | gel-Mn | Linear |
| gel-Cu | Nonlinear | gel-Nd | Nonlinear | gel-K | Nonlinear |
| Gels | Dependence | Gels | Dependence | Gels | Dependence |
|---|---|---|---|---|---|
| gel-Cr | Nonlinear | gel-Ce | Linear | gel-Li | Nonlinear |
| gel-Na | Nonlinear | gel-Ca | Nonlinear | gel-Er | Nonlinear |
| gel-Eu | Nonlinear | gel-Zn | Nonlinear | gel-Cs | Nonlinear |
| gel-Y | Nonlinear | gel-Fe | Linear | gel-Yb | Linear |
| gel-Sm | Linear | gel-Ho | Nonlinear | gel-Mn | Nonlinear |
| gel-Cu | Nonlinear | gel-Nd | Nonlinear | gel-K | Nonlinear |
| Gels | Dependence | Gels | Dependence | Gels | Dependence |
|---|---|---|---|---|---|
| gel-Cr | Linear | gel-Ce | Nonlinear | gel-Li | Nonlinear |
| gel-Na | Nonlinear | gel-Ca | Nonlinear | gel-Er | Nonlinear |
| gel-Eu | Nonlinear | gel-Zn | Nonlinear | gel-Cs | Nonlinear |
| gel-Y | Nonlinear | gel-Fe | Nonlinear | gel-Yb | Nonlinear |
| gel-Sm | Linear | gel-Ho | Nonlinear | gel-Mn | Nonlinear |
| gel-Cu | Nonlinear | gel-Nd | Nonlinear | gel-K | Nonlinear |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Yang, R.; Xu, J.; Xu, G.; Wu, Y. Detecting N-Phenyl-2-Naphthylamine, L-Arabinose, D-Mannose, L-Phenylalanine, L-Methionine, and D-Trehalose via Photocurrent Measurement. Gels 2024, 10, 808. https://doi.org/10.3390/gels10120808
Li F, Yang R, Xu J, Xu G, Wu Y. Detecting N-Phenyl-2-Naphthylamine, L-Arabinose, D-Mannose, L-Phenylalanine, L-Methionine, and D-Trehalose via Photocurrent Measurement. Gels. 2024; 10(12):808. https://doi.org/10.3390/gels10120808
Chicago/Turabian StyleLi, Feng, Ruoxi Yang, Jian Xu, Guohai Xu, and Ye Wu. 2024. "Detecting N-Phenyl-2-Naphthylamine, L-Arabinose, D-Mannose, L-Phenylalanine, L-Methionine, and D-Trehalose via Photocurrent Measurement" Gels 10, no. 12: 808. https://doi.org/10.3390/gels10120808
APA StyleLi, F., Yang, R., Xu, J., Xu, G., & Wu, Y. (2024). Detecting N-Phenyl-2-Naphthylamine, L-Arabinose, D-Mannose, L-Phenylalanine, L-Methionine, and D-Trehalose via Photocurrent Measurement. Gels, 10(12), 808. https://doi.org/10.3390/gels10120808








