Microencapsulation Efficiency of Carboxymethylcellulose, Gelatin, Maltodextrin, and Acacia for Aroma Preservation in Jasmine Instant Tea
Abstract
1. Introduction
2. Results and Discussion
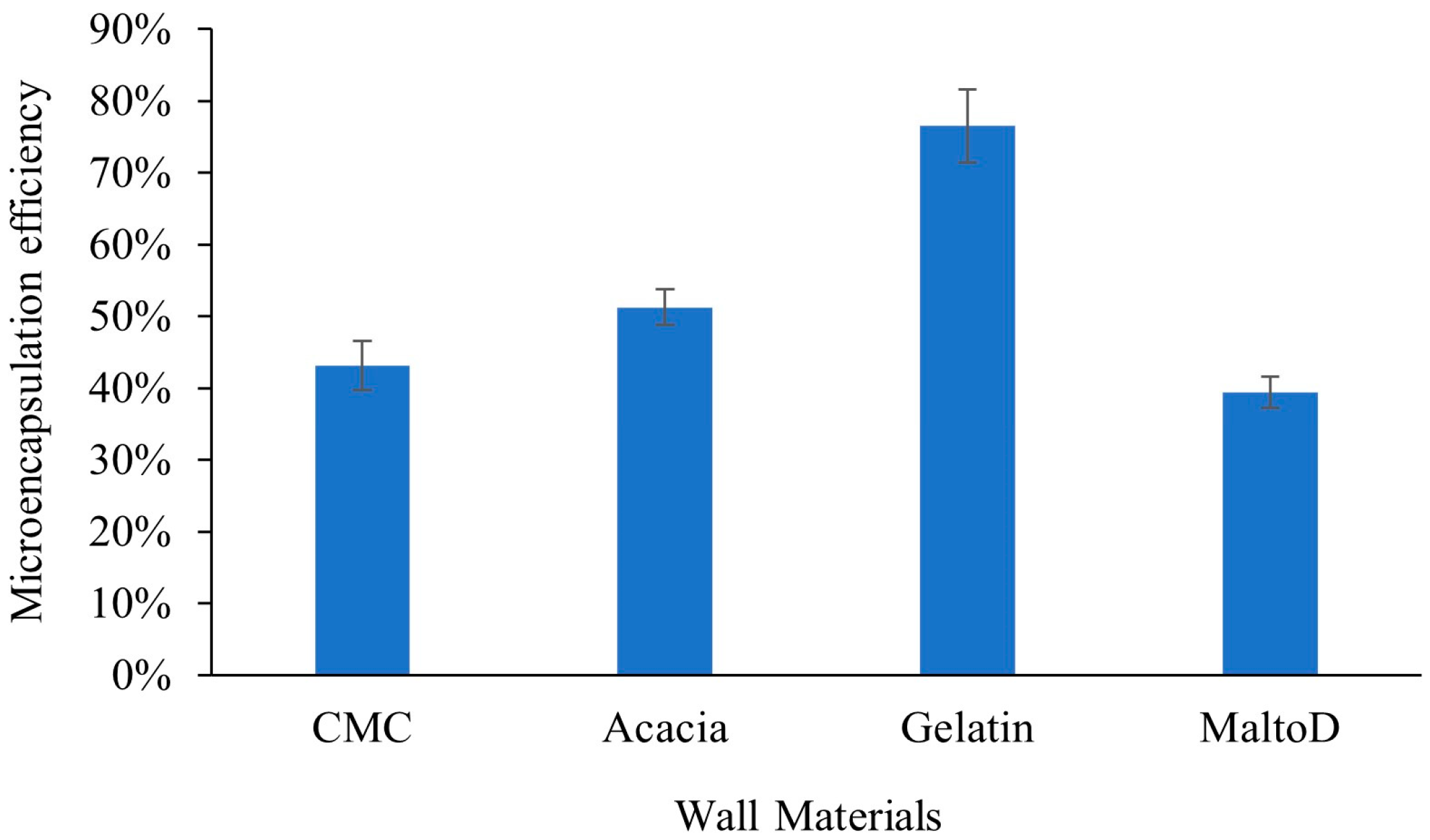
2.1. Microencapsulation Efficiency
2.2. Polyphenol Content, Flavonoid Content, and Antioxidant Capacity
2.3. Physical Characterization
2.4. Analysis of Aroma
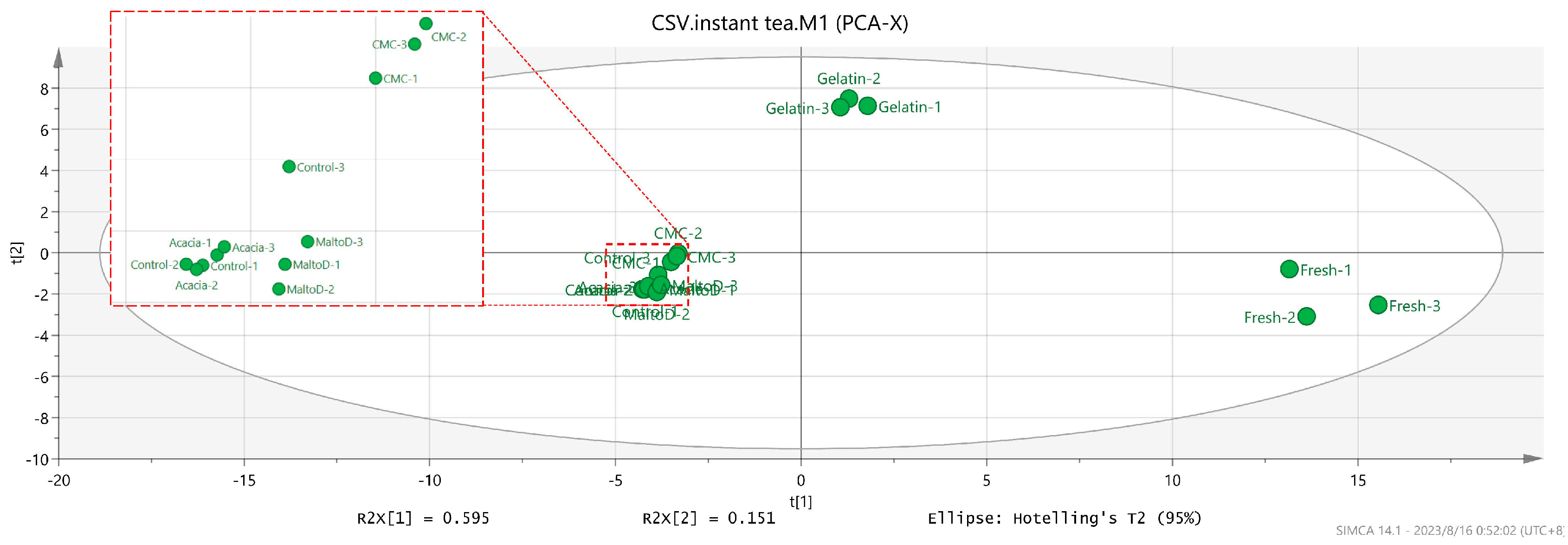
2.5. Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
2.5.1. Permuted Value
2.5.2. OPLS-DA Plots
2.5.3. Variable Importance in Projection (VIP) Analysis
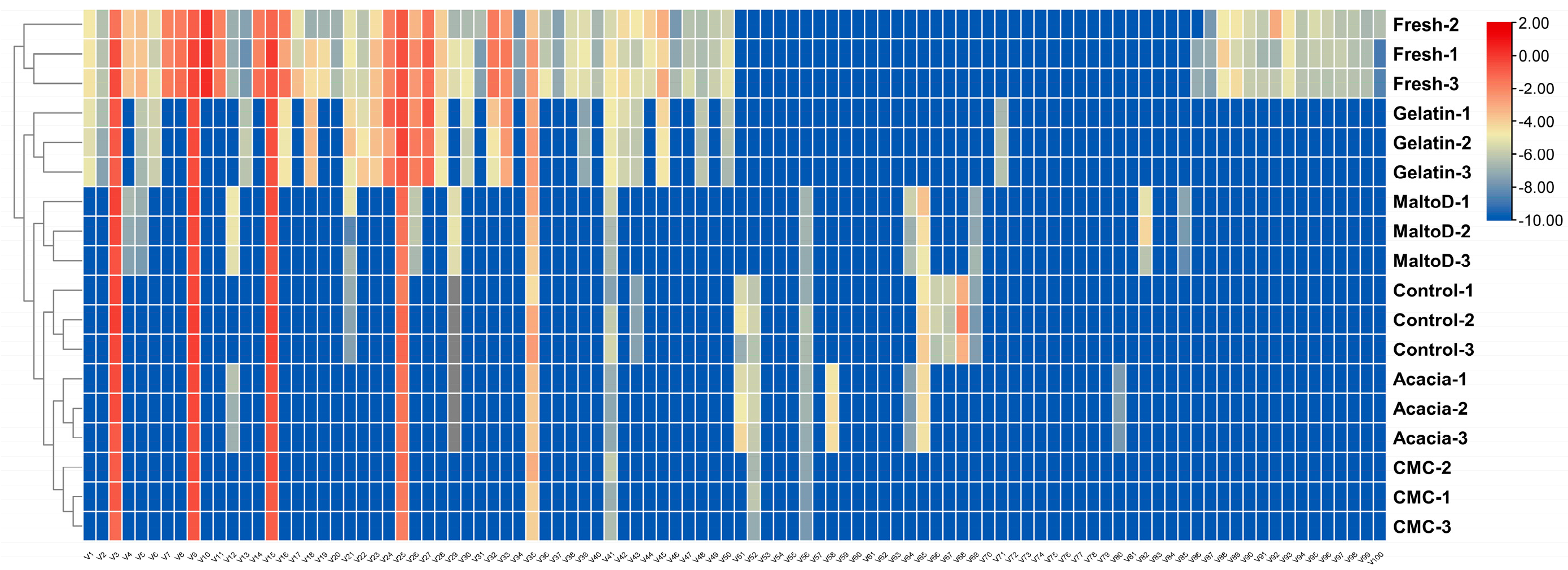
2.6. Heatmap Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Encapsulation of Jasmine Tea
4.3. Particle Size
4.4. SEM
4.5. Polyphenols and Flavonoids
4.6. Antioxidant Capacity
4.7. SPME-GC-MS Analysis of Aroma Compounds
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalpathadu, K.; Rajapakse, H.; Nissanka, S.; Jayasinghe, C. Improving the quality of instant tea with low-grade tea aroma. Arab. J. Chem. 2022, 15, 104147. [Google Scholar] [CrossRef]
- Hua, Y.; Wei, Z.; Xue, C. Chitosan and its composites-based delivery systems: Advances and applications in food science and nutrition sector. Crit. Rev. Food Sci. Nutr. 2023, 63, 4579–4598. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Zhang, L.; Granvogl, M.; Ho, C.T.; Wan, X. Flavor of tea (Camellia sinensis): A review on odorants and analytical techniques. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3867–3909. [Google Scholar] [CrossRef]
- Kumar, K.R.; Dashora, K.; Kumar, S.; Dharmaraja, S.; Sanyal, S.; Aditya, K.; Kumar, R. A review of drying technology in tea sector of industrial, non-conventional and renewable energy based drying systems. Appl. Therm. Eng. 2023, 224, 120118. [Google Scholar] [CrossRef]
- Kraujalytė, V.; Pelvan, E.; Alasalvar, C. Volatile compounds and sensory characteristics of various instant teas produced from black tea. Food Chem. 2016, 194, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, V.; Balanč, B.; Kalušević, A.; Lević, S.; Bugarski, B.; Nedović, V. Novel approaches in nanoencapsulation of aromas and flavors. Encapsulations 2016, 363–419. [Google Scholar] [CrossRef]
- Mora-Flórez, L.S.; Cabrera-Rodríguez, D.; Hernández-Carrión, M. Encapsulation of menthol and luteolin using hydrocolloids as wall material to formulate instant aromatic beverages. Foods 2023, 12, 2080. [Google Scholar] [CrossRef]
- Ma, X.; Wu, S. Oxygenated polycyclic aromatic hydrocarbons in food: Toxicity, occurrence and potential sources. Crit. Rev. Food Sci. Nutr. 2024, 64, 4882–4903. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Bora, A.F.M.; Ma, S.; Li, X.; Liu, L. Application of microencapsulation for the safe delivery of green tea polyphenols in food systems: Review and recent advances. Food Res. Int. 2018, 105, 241–249. [Google Scholar] [CrossRef]
- Gouin, S. Microencapsulation: Industrial appraisal of existing technologies and trends. Trends Food Sci. Technol. 2004, 15, 330–347. [Google Scholar] [CrossRef]
- Premjit, Y.; Pandhi, S.; Kumar, A.; Rai, D.C.; Duary, R.K.; Mahato, D.K. Current trends in flavor encapsulation: A comprehensive review of emerging encapsulation techniques, flavour release, and mathematical modelling. Food Res. Int. 2022, 151, 110879. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.M.R.; da Silva, L.M.R.; Duarte, A.S.G.; da Silva Monteiro, C.E.; Campos, A.R.; Holanda, D.K.R.; de Lima, A.C.S.; de Sousa Sabino, L.B.; de Figueiredo, R.W. Microencapsulation of green tea extract (Camellia sinensis var assamica) to increase the bioaccessibility of bioactive compounds and gastroprotective effects. Food Biosci. 2021, 42, 101190. [Google Scholar]
- Krishna, A. An integrative review of sensory marketing: Engaging the senses to affect perception, judgment and behavior. J. Consum. Psychol. 2012, 22, 332–351. [Google Scholar] [CrossRef]
- English, M.; Okagu, O.D.; Stephens, K.; Goertzen, A.; Udenigwe, C.C. Flavour encapsulation: A comparative analysis of relevant techniques, physiochemical characterisation, stability, and food applications. Front. Nutr. 2023, 10, 1019211. [Google Scholar] [CrossRef]
- Sun, B.; Wang, W.; He, Z.; Zhang, M.; Kong, F.; Sain, M.; Ni, Y. Improvement of stability of tea polyphenols: A review. Curr. Pharm. Des. 2018, 24, 3410–3423. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Ponce-Alquicira, E. The role of microencapsulation in food application. Molecules 2022, 27, 1499. [Google Scholar] [CrossRef]
- Yildirim-Yalcin, M.; Tornuk, F.; Toker, O.S. Recent advances in the improvement of carboxymethyl cellulose-based edible films. Trends Food Sci. Technol. 2022, 129, 179–193. [Google Scholar] [CrossRef]
- Luo, Q.; Hossen, M.A.; Zeng, Y.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Gelatin-based composite films and their application in food packaging: A review. J. Food Eng. 2022, 313, 110762. [Google Scholar] [CrossRef]
- Li, K.; Pan, B.; Ma, L.; Miao, S.; Ji, J. Effect of dextrose equivalent on maltodextrin/whey protein spray-dried powder microcapsules and dynamic release of loaded flavor during storage and powder rehydration. Foods 2020, 9, 1878. [Google Scholar] [CrossRef]
- Mazumder, M.A.R.; Ranganathan, T.V. Encapsulation of isoflavone with milk, maltodextrin and gum acacia improves its stability. Curr. Res. Food Sci. 2020, 2, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, R.H. The phenolic profiles and antioxidant activity in different types of tea. Int. J. Food Sci. Technol. 2013, 48, 163–171. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Zhang, J.; Kilmartin, P.A.; Quek, S.Y. Exploring the effects of microencapsulation on odour retention of fermented noni juice. J. Food Eng. 2020, 273, 109892. [Google Scholar] [CrossRef]
- Farakte, R.A.; Yadav, G.; Joshi, B.; Patwadhan, A.W.; Singh, G. Role of particle size in tea infusion process. Int. J. Food Eng. 2016, 12, 1–16. [Google Scholar] [CrossRef]
- Guo, B.; Zhu, C.; Huang, Z.; Yang, R.; Liu, C. Microcapsules with slow-release characteristics prepared by soluble small molecular starch fractions through the spray drying method. Int. J. Biol. Macromol. 2022, 200, 34–41. [Google Scholar] [CrossRef]
- Thi Anh Dao, D.; Van Thanh, H.; Viet Ha, D.; Duc Nguyen, V. Optimization of spray-drying process to manufacture green tea powder and its characters. Food Sci. Nutr. 2021, 9, 6566–6574. [Google Scholar] [CrossRef]
- Utama-Ang, N.; Kopermsub, P.; Thakeow, P.; Samakradhamrongthai, R. Encapsulation of Michelia champaca L. extract and its application in instant tea. Int. J. Food Eng. 2017, 3, 48–55. [Google Scholar] [CrossRef]
- Kang, Y.-R.; Lee, Y.-K.; Kim, Y.J.; Chang, Y.H. Characterization and storage stability of chlorophylls microencapsulated in different combination of gum Arabic and maltodextrin. Food Chem. 2019, 272, 337–346. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; Smyth, T.J.; O’Donnell, C.P. Optimization of ultrasound assisted extraction of bioactive components from brown seaweed Ascophyllum nodosum using response surface methodology. Ultrason. Sonochem. 2015, 23, 308–316. [Google Scholar] [CrossRef]
- Paini, M.; Aliakbarian, B.; Casazza, A.A.; Lagazzo, A.; Botter, R.; Perego, P. Microencapsulation of phenolic compounds from olive pomace using spray drying: A study of operative parameters. LWT-Food Sci. Technol. 2015, 62, 177–186. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of temperatures on polyphenols during extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Lund, M.N. Reactions of plant polyphenols in foods: Impact of molecular structure. Trends Food Sci. Technol. 2021, 112, 241–251. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Navarro, P.; Vallejo, A.; Olivares, M.; Etxebarria, N.; Usobiaga, A. Microencapsulation and storage stability of polyphenols from Vitis vinifera grape wastes. Food Chem. 2016, 190, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cheng, J.-R.; Liu, X.-M.; Zhu, M.-J. Effect of microencapsulated process on stability of mulberry polyphenol and oxidation property of dried minced pork slices during heat processing and storage. LWT 2019, 100, 62–68. [Google Scholar] [CrossRef]
- de Souza, V.B.; Thomazini, M.; Chaves, I.E.; Ferro-Furtado, R.; Favaro-Trindade, C.S. Microencapsulation by complex coacervation as a tool to protect bioactive compounds and to reduce astringency and strong flavor of vegetable extracts. Food Hydrocoll. 2020, 98, 105244. [Google Scholar] [CrossRef]
- Vardin, H.; Yasar, M. Optimisation of pomegranate (Punica granatum L.) juice spray-drying as affected by temperature and maltodextrin content. Int. J. Food Sci. Technol. 2012, 47, 167–176. [Google Scholar] [CrossRef]
- Jin, J.-C.; Liang, S.; Qi, S.-X.; Tang, P.; Chen, J.-X.; Chen, Q.-S.; Chen, Y.-F.; Yin, J.-F.; Xu, Y.-Q. Widely targeted metabolomics reveals the effect of different raw materials and drying methods on the quality of instant tea. Front. Nutr. 2023, 10, 1236216. [Google Scholar] [CrossRef]
- Fatima, S.; Govardhan, B.; Kalyani, S.; Sridhar, S. Extraction of volatile organic compounds from water and wastewater by vacuum-driven membrane process: A comprehensive review. Chem. Eng. J. 2022, 434, 134664. [Google Scholar] [CrossRef]
- Mamusa, M.; Resta, C.; Sofroniou, C.; Baglioni, P. Encapsulation of volatile compounds in liquid media: Fragrances, flavors, and essential oils in commercial formulations. Adv. Colloid Interface Sci. 2021, 298, 102544. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, Y.; Liu, B.; Chen, Z.; Zheng, J.; Guan, M.; Shi, H.; Wang, Y.; Yang, W. Changes in the volatiles, chemical components, and antioxidant activities of Chinese jasmine tea during the scenting processes. Int. J. Food Prop. 2017, 20, 681–693. [Google Scholar] [CrossRef]


| Var ID | Aroma Compound | VIP Value |
|---|---|---|
| V10 | Acetic acid, phenylmethyl ester | 2.93 |
| V3 | Cyclotetrasiloxane, octamethyl- | 2.37 |
| V27 | Undecanoic acid, ethyl ester | 2.18 |
| V68 | Butylated hydroxytoluene | 2.08 |
| V25 | Cycloheptasiloxane, tetradecamethyl- | 1.89 |
| V58 | Cyclotrisiloxane, hexamethyl- | 1.81 |
| V24 | 2(3H)-Furanone, dihydro-5-propyl- | 1.78 |
| V9 | Cyclopentasiloxane, decamethyl- | 1.61 |
| V15 | Cyclohexasiloxane, dodecamethyl- | 1.60 |
| V8 | Linalool | 1.58 |
| V51 | Oxime-, methoxy-phenyl-_ | 1.56 |
| V26 | 2H-Pyran, 2-[(5-cyclopropylidenepentyl)oxy]tetrahydro- | 1.56 |
| V14 | Indole | 1.49 |
| V32 | 3-Hexen-1-ol, benzoate, (Z)- | 1.47 |
| V11 | Methyl salicylate | 1.42 |
| V7 | Benzoic acid, methyl ester | 1.40 |
| V33 | Dodecanoic acid, ethyl ester | 1.38 |
| V16 | Methyl anthranilate | 1.35 |
| V82 | 2(3H)-Furanone, 5-hexyldihydro- | 1.25 |
| V65 | 2-Heptanone, 5-methyl- | 1.22 |
| V12 | Decanal | 1.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhary, M.N.; Li, X.; Yang, S.; Wang, D.; Luo, L.; Zeng, L.; Luo, W. Microencapsulation Efficiency of Carboxymethylcellulose, Gelatin, Maltodextrin, and Acacia for Aroma Preservation in Jasmine Instant Tea. Gels 2024, 10, 670. https://doi.org/10.3390/gels10100670
Chaudhary MN, Li X, Yang S, Wang D, Luo L, Zeng L, Luo W. Microencapsulation Efficiency of Carboxymethylcellulose, Gelatin, Maltodextrin, and Acacia for Aroma Preservation in Jasmine Instant Tea. Gels. 2024; 10(10):670. https://doi.org/10.3390/gels10100670
Chicago/Turabian StyleChaudhary, Muneeba Naseer, Xiaolin Li, Siyue Yang, Damao Wang, Liyong Luo, Liang Zeng, and Wei Luo. 2024. "Microencapsulation Efficiency of Carboxymethylcellulose, Gelatin, Maltodextrin, and Acacia for Aroma Preservation in Jasmine Instant Tea" Gels 10, no. 10: 670. https://doi.org/10.3390/gels10100670
APA StyleChaudhary, M. N., Li, X., Yang, S., Wang, D., Luo, L., Zeng, L., & Luo, W. (2024). Microencapsulation Efficiency of Carboxymethylcellulose, Gelatin, Maltodextrin, and Acacia for Aroma Preservation in Jasmine Instant Tea. Gels, 10(10), 670. https://doi.org/10.3390/gels10100670







