pH-Responsive Alginate/Chitosan Gel Films: An Alternative for Removing Cadmium and Lead from Water
Abstract
1. Introduction
2. Results and Discussion
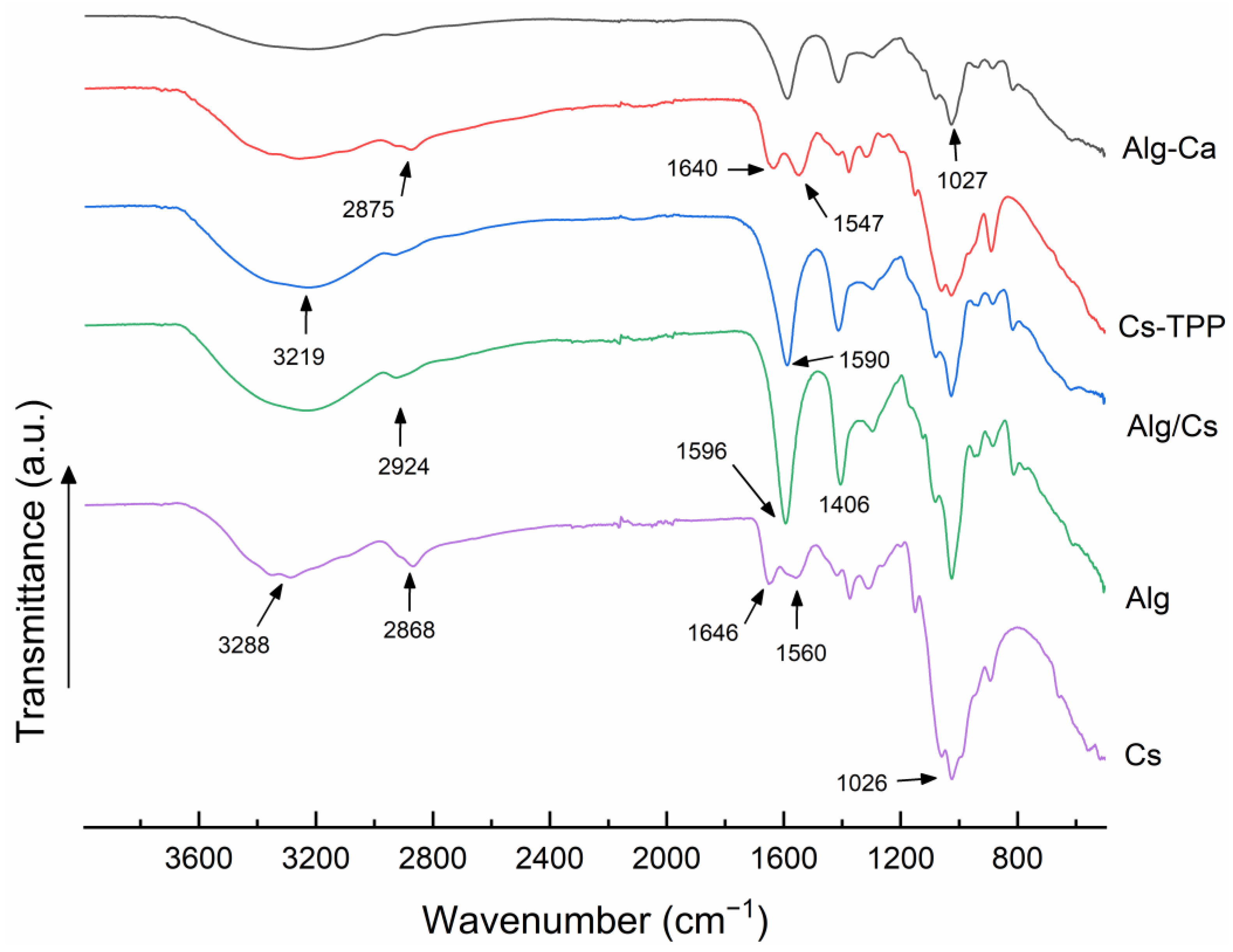
2.1. Fourier Transform Infrared Spectroscopy
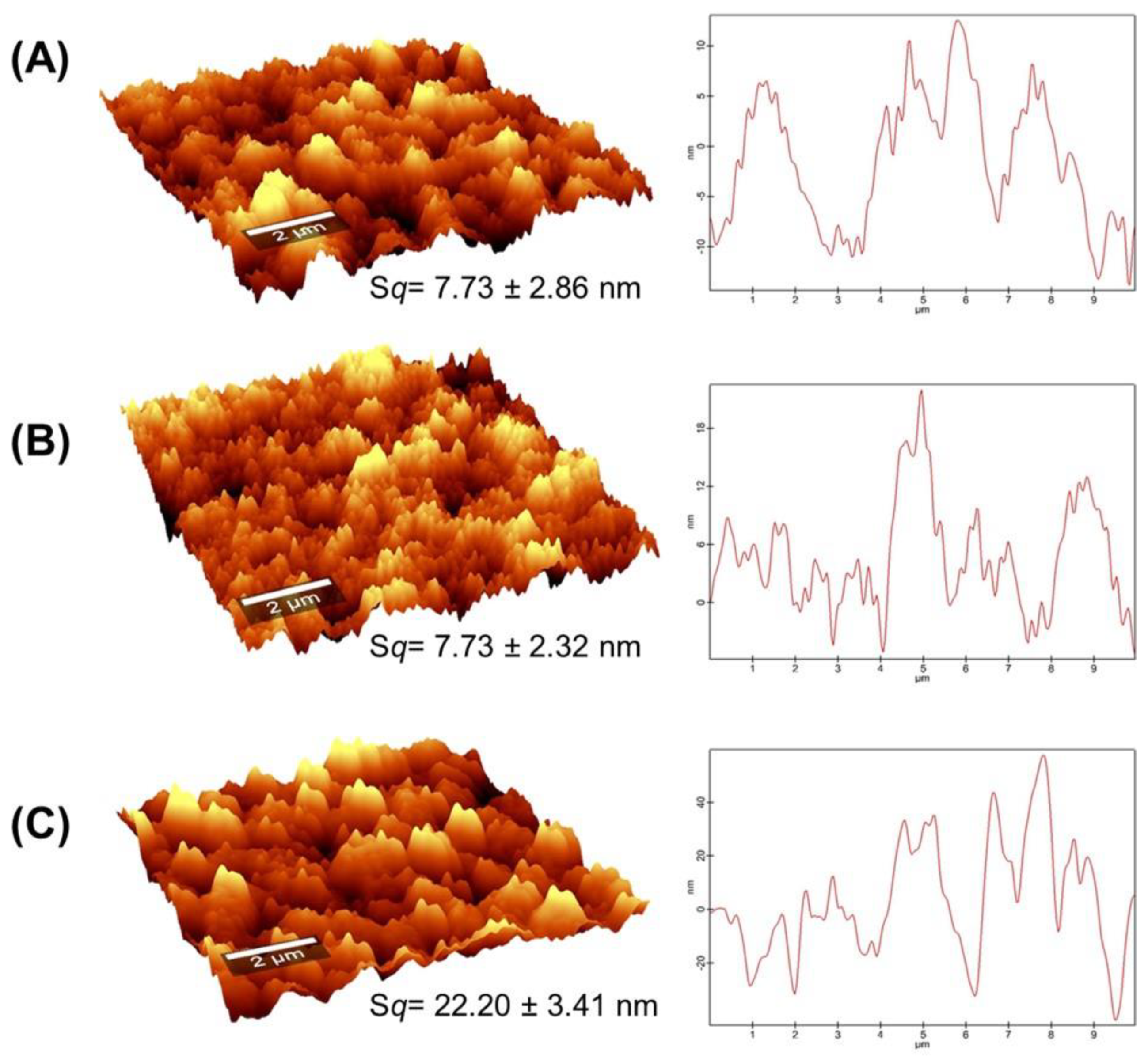
2.2. Surface Morphology of Gel Films
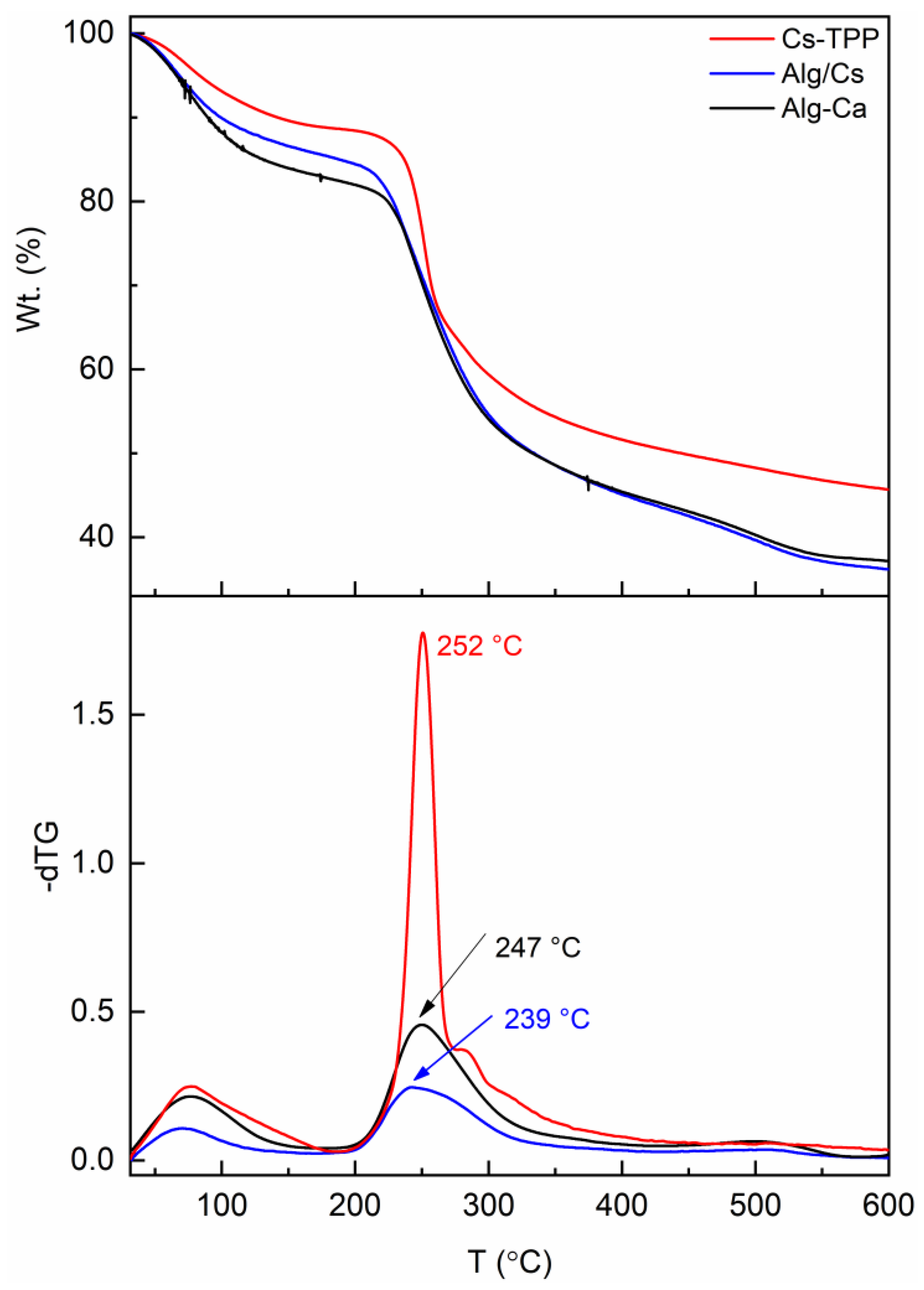
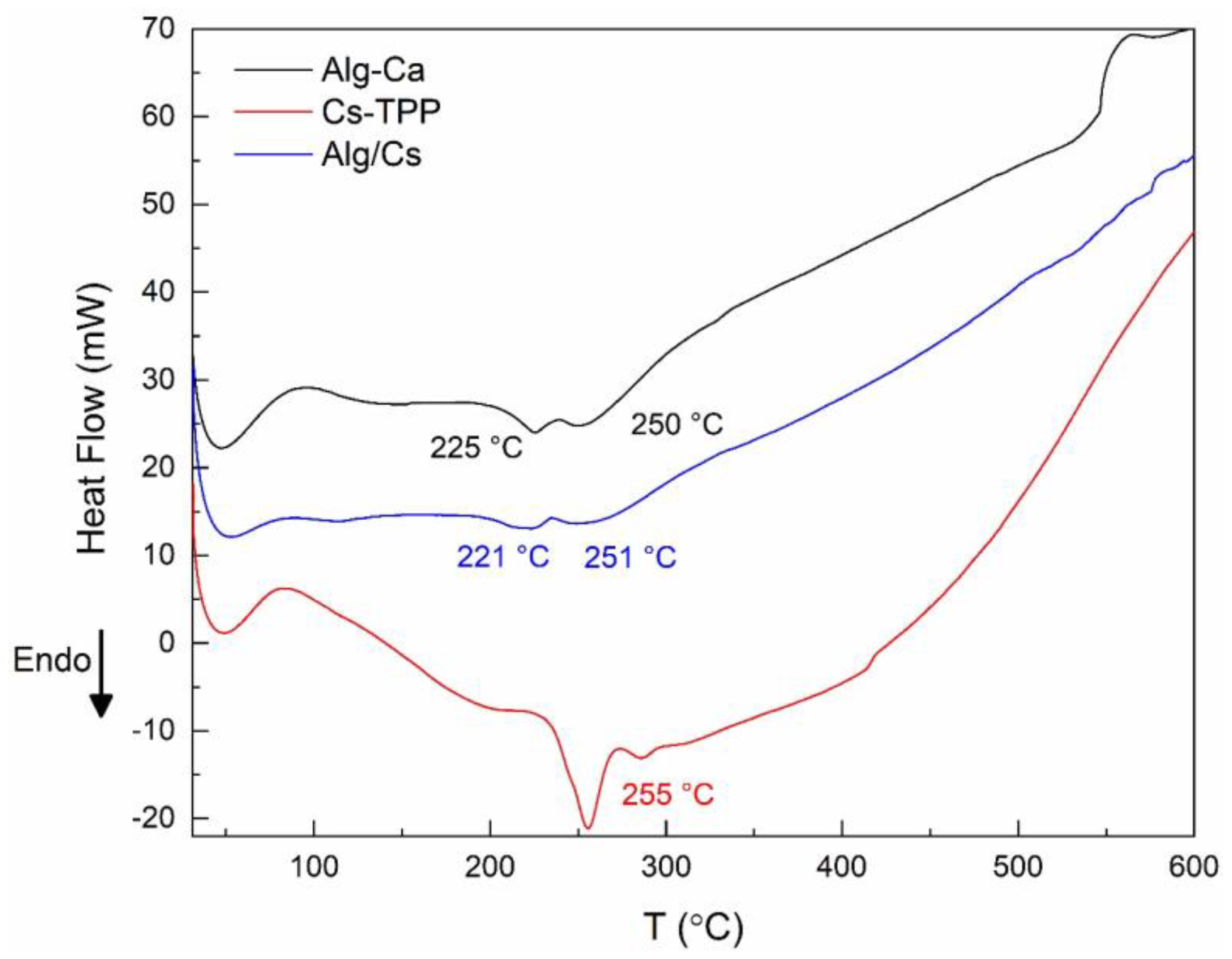
2.3. Thermal Properties of Gel Films
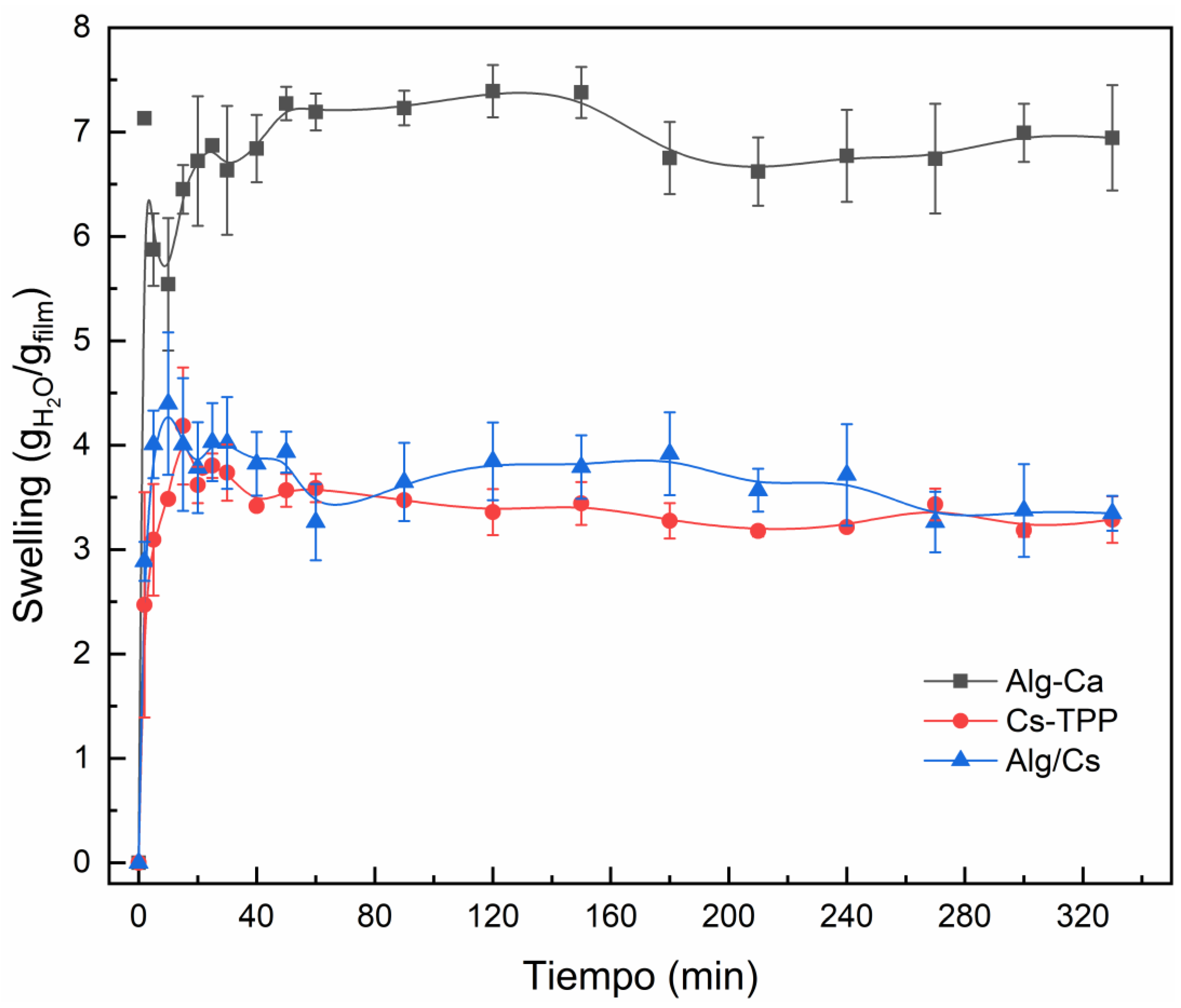
2.4. Swelling Behavior of Gel Films
2.5. Metal Adsorption Analysis
2.6. Metal Recovery and Reuse of Films
3. Conclusions
4. Materials and Methods
4.1. Film Preparation
4.2. Biopolymeric Films Characterization
4.2.1. Fourier Transform Infrared Spectroscopy (FTIR)
4.2.2. Surface Analysis
4.2.3. Thermogravimetric (TGA) and Differential Thermal Calorimetry (DSC) Analysis
4.2.4. Swelling Studies
4.3. Batch Sorption Studies
4.3.1. Cadmium and Lead Batch Removal
4.3.2. Desorption and Regeneration of Films
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Orosun, M.M.; Nwabachili, S.; Alshehri, R.F.; Omeje, M.; Alshdoukhi, I.F.; Okoro, H.K.; Ogunkunle, C.O.; Louis, H.; Abdulhamid, F.A.; Osahon, S.E.; et al. Potentially Toxic Metals in Irrigation Water, Soil, and Vegetables and Their Health Risks Using Monte Carlo Models. Sci. Rep. 2023, 13, 21220. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Shah, H.-R.; Khan, M.S.; Iqbal, A.; Potrich, E.; Amaral, L.S.; Rasheed, S.; Nawaz, H.; Ayub, A.; Naseem, K.; et al. Lead in Drinking Water: Adsorption Method and Role of Zeolitic Imidazolate Frameworks for Its Remediation: A Review. J. Clean. Prod. 2022, 368, 133010. [Google Scholar] [CrossRef]
- Brown, V.J. Baring Bone’s Secrets: Understanding How Lead Exposure Affects Skeletal Development. Environ. Health Perspect. 2007, 115, A461. [Google Scholar] [CrossRef][Green Version]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological Trends in Heavy Metals Removal from Industrial Wastewater: A Review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Wadhawan, S.; Jain, A.; Nayyar, J.; Mehta, S.K. Role of Nanomaterials as Adsorbents in Heavy Metal Ion Removal from Waste Water: A Review. J. Water Process Eng. 2020, 33, 101038. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Qin, L.; Lai, C.; Wang, Z.; Zhou, M.; Xiao, L.; Liu, S.; Zhang, M. Recent Advances in the Application of Water-Stable Metal-Organic Frameworks: Adsorption and Photocatalytic Reduction of Heavy Metal in Water. Chemosphere 2021, 285, 131432. [Google Scholar] [CrossRef]
- Benalia, M.C.; Youcef, L.; Bouaziz, M.G.; Achour, S.; Menasra, H. Removal of Heavy Metals from Industrial Wastewater by Chemical Precipitation: Mechanisms and Sludge Characterization. Arab. J. Sci. Eng. 2022, 47, 5587–5599. [Google Scholar] [CrossRef]
- Torres, E. Biosorption: A Review of the Latest Advances. Processes 2020, 8, 1584. [Google Scholar] [CrossRef]
- Ubando, A.T.; Africa, A.D.M.; Maniquiz-Redillas, M.C.; Culaba, A.B.; Chen, W.-H.; Chang, J.-S. Microalgal Biosorption of Heavy Metals: A Comprehensive Bibliometric Review. J. Hazard. Mater. 2021, 402, 123431. [Google Scholar] [CrossRef]
- Karić, N.; Maia, A.S.; Teodorović, A.; Atanasova, N.; Langergraber, G.; Crini, G.; Ribeiro, A.R.L.; Đolić, M. Bio-Waste Valorisation: Agricultural Wastes as Biosorbents for Removal of (in)Organic Pollutants in Wastewater Treatment. Chem. Eng. J. Adv. 2022, 9, 100239. [Google Scholar] [CrossRef]
- Syeda, H.I.; Sultan, I.; Razavi, K.S.; Yap, P.-S. Biosorption of Heavy Metals from Aqueous Solution by Various Chemically Modified Agricultural Wastes: A Review. J. Water Process Eng. 2022, 46, 102446. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A Critical Review of Biosorption of Dyes, Heavy Metals and Metalloids from Wastewater as an Efficient and Green Process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Ali Redha, A. Removal of Heavy Metals from Aqueous Media by Biosorption. Arab. J. Basic Appl. Sci. 2020, 27, 183–193. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Zhou, J.; Luo, X. Uranium Biosorption Mechanism Model of Protonated Saccharomyces cerevisiae. J. Hazard. Mater. 2020, 385, 121588. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Van Tran, V.; Park, D.; Lee, Y.-C. Hydrogel Applications for Adsorption of Contaminants in Water and Wastewater Treatment. Environ. Sci. Pollut. Res. 2018, 25, 24569–24599. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Russo, T.; Fucile, P.; Giacometti, R.; Sannino, F. Sustainable Removal of Contaminants by Biopolymers: A Novel Approach for Wastewater Treatment. Current State and Future Perspectives. Processes 2021, 9, 719. [Google Scholar] [CrossRef]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Simpson, N.E.; Grant, S.C.; Blackband, S.J.; Constantinidis, I. NMR Properties of Alginate Microbeads. Biomaterials 2003, 24, 4941–4948. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Nastaj, J.; Przewłocka, A.; Rajkowska-Myśliwiec, M. Biosorption of Ni(II), Pb(II) and Zn(II) on Calcium Alginate Beads: Equilibrium, Kinetic and Mechanism Studies. Pol. J. Chem. Technol. 2016, 18, 81–87. [Google Scholar] [CrossRef]
- Ngasotter, S.; Xavier, K.A.M.; Meitei, M.M.; Waikhom, D.; Madhulika; Pathak, J.; Singh, S.K. Crustacean Shell Waste Derived Chitin and Chitin Nanomaterials for Application in Agriculture, Food, and Health—A Review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100349. [Google Scholar] [CrossRef]
- Amiri, H.; Aghbashlo, M.; Sharma, M.; Gaffey, J.; Manning, L.; Moosavi Basri, S.M.; Kennedy, J.F.; Gupta, V.K.; Tabatabaei, M. Chitin and Chitosan Derived from Crustacean Waste Valorization Streams Can Support Food Systems and the UN Sustainable Development Goals. Nat. Food 2022, 3, 822–828. [Google Scholar] [CrossRef]
- Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M.; Anitha, K.L. Recent Advances in Heavy Metal Removal by Chitosan Based Adsorbents. Carbohydr. Polym. 2021, 251, 117000. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Li, T.; Wang, W.; Wang, W.; Yu, G. Novel Crosslinked Chitosan for Enhanced Adsorption of Hexavalent Chromium in Acidic Solution. Chem. Eng. J. 2018, 347, 782–790. [Google Scholar] [CrossRef]
- ALSamman, M.T.; Sánchez, J. Recent Advances on Hydrogels Based on Chitosan and Alginate for the Adsorption of Dyes and Metal Ions from Water. Arab. J. Chem. 2021, 14, 103455. [Google Scholar] [CrossRef]
- Benettayeb, A.; Ghosh, S.; Usman, M.; Seihoub, F.Z.; Sohoo, I.; Chia, C.H.; Sillanpää, M. Some Well-Known Alginate and Chitosan Modifications Used in Adsorption: A Review. Water 2022, 14, 1353. [Google Scholar] [CrossRef]
- Gkika, D.A.; Mitropoulos, A.C.; Kyzas, G.Z. Why Reuse Spent Adsorbents? The Latest Challenges and Limitations. Sci. Total Environ. 2022, 822, 153612. [Google Scholar] [CrossRef]
- de Freitas, G.R.; da Silva, M.G.C.; Vieira, M.G.A. Biosorption Technology for Removal of Toxic Metals: A Review of Commercial Biosorbents and Patents. Environ. Sci. Pollut. Res. 2019, 26, 19097–19118. [Google Scholar] [CrossRef] [PubMed]
- Goycoolea, F.M.; Lollo, G.; Remuñán-López, C.; Quaglia, F.; Alonso, M.J. Chitosan-Alginate Blended Nanoparticles as Carriers for the Transmucosal Delivery of Macromolecules. Biomacromolecules 2009, 10, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, D.; Simeonov, M.; Tzachev, C.; Apostolov, A.; Christov, L.; Vassileva, E. Polyelectrolyte Complexes of Chitosan and Sodium Alginate as a Drug Delivery System for Diclofenac Sodium. Polym. Int. 2022, 71, 668–678. [Google Scholar] [CrossRef]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on Alginate–Chitosan Complex Formed with Different Polymers Ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef]
- Lee, E.M.; Singh, D.; Singh, D.; Choi, S.M.; Zo, S.M.; Park, S.J.; Han, S.S. Novel Alginate-Gelatin Hybrid Nanoparticle for Drug Delivery and Tissue Engineering Applications. J. Nanomater. 2014, 2014, 124236. [Google Scholar] [CrossRef]
- Cho, A.R.; Chun, Y.G.; Kim, B.K.; Park, D.J. Preparation of Chitosan-TPP Microspheres as Resveratrol Carriers. J. Food Sci. 2014, 79, E568–E576. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and Characterization of Calcium Alginate Nanoparticles, Sodium Homopolymannuronate Salt and Its Calcium Nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Fernández-Quiroz, D.; Loya-Duarte, J.; Silva-Campa, E.; Argüelles-Monal, W.; Sarabia-Sainz, A.-í.; Lucero-Acuña, A.; del Castillo-Castro, T.; Román, J.S.; Lizardi-Mendoza, J.; Burgara-Estrella, A.J.; et al. Temperature Stimuli-Responsive Nanoparticles from Chitosan-Graft-Poly(N-Vinylcaprolactam) as a Drug Delivery System. J. Appl. Polym. Sci. 2019, 136, 47831. [Google Scholar] [CrossRef]
- Monteiro, R.T.; Da Silva, T.F.; de Souza Guedes, L.; Moreira Filho, R.N.F.; Soares, A.L.B.; Vasconcelos, N.F.; Andrade, F.K.; Vieira, R.S. Porous and Dense Alginate/Chitosan Composite Films Loaded with Simvastatin for Dressing Applications. Coatings 2024, 14, 278. [Google Scholar] [CrossRef]
- Ho, Y.-C.; Mi, F.-L.; Sung, H.-W.; Kuo, P.-L. Heparin-Functionalized Chitosan–Alginate Scaffolds for Controlled Release of Growth Factor. Int. J. Pharm. 2009, 376, 69–75. [Google Scholar] [CrossRef]
- Reyes-Chaparro, P.; Gutierrez-Mendez, N.; Salas-Muñoz, E.; Ayala-Soto, J.G.; Chavez-Flores, D.; Hernández-Ochoa, L. Effect of the Addition of Essential Oils and Functional Extracts of Clove on Physicochemical Properties of Chitosan-Based Films. Int. J. Polym. Sci. 2015, 2015, 714254. [Google Scholar] [CrossRef]
- Yu, J.; Xu, S.; Goksen, G.; Yi, C.; Shao, P. Chitosan Films Plasticized with Choline-Based Deep Eutectic Solvents: UV Shielding, Antioxidant, and Antibacterial Properties. Food Hydrocoll. 2023, 135, 108196. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, W.; Zhang, Z.; Zhang, D.; Guo, Z.; Ren, P.; Liu, F. Effect of Nano-Silica and Sorbitol on the Properties of Chitosan-Based Composite Films. Polymers 2023, 15, 4015. [Google Scholar] [CrossRef]
- Bierhalz, A.C.K.; Da Silva, M.A.; De Sousa, H.C.; Braga, M.E.M.; Kieckbusch, T.G. Influence of Natamycin Loading Methods on the Physical Characteristics of Alginate Active Films. J. Supercrit. Fluids 2013, 76, 74–82. [Google Scholar] [CrossRef]
- Van Rooyen, B.; De Wit, M.; Osthoff, G.; Van Niekerk, J.; Hugo, A. Microstructural and Mechanical Properties of Calcium-Treated Cactus Pear Mucilage (Opuntia Spp.), Pectin and Alginate Single-Biopolymer Films. Polymers 2023, 15, 4295. [Google Scholar] [CrossRef]
- Aljar, M.A.A.; Rashdan, S.; Almutawah, A.; El-Fattah, A.A. Synthesis and Characterization of Biodegradable Poly (Vinyl Alcohol)-Chitosan/Cellulose Hydrogel Beads for Efficient Removal of Pb(II), Cd(II), Zn(II), and Co(II) from Water. Gels 2023, 9, 328. [Google Scholar] [CrossRef]
- Yiğit, M.; Baran, E.; Kıvılcımdan Moral, C. A Polymer–Zeolite Composite for Mixed Metal Removal from Aqueous Solution. Water Sci. Technol. 2021, 83, 1152–1166. [Google Scholar] [CrossRef]
- Simpliciano, C.; Clark, L.; Asi, B.; Chu, N.; Mercado, M.; Diaz, S.; Goedert, M.; Mobed-Miremadi, M. Cross-Linked Alginate Film Pore Size Determination Using Atomic Force Microscopy and Validation Using Diffusivity Determinations. J. Surf. Eng. Mater. Adv. Technol. 2013, 3, 1–12. [Google Scholar] [CrossRef]
- Chelminiak-Dudkiewicz, D.; Smolarkiewicz-Wyczachowski, A.; Mylkie, K.; Wujak, M.; Mlynarczyk, D.T.; Nowak, P.; Bocian, S.; Goslinski, T.; Ziegler-Borowska, M. Chitosan-Based Films with Cannabis Oil as a Base Material for Wound Dressing Application. Sci. Rep. 2022, 12, 18658. [Google Scholar] [CrossRef]
- Fauzi, N.I.M.; Fen, Y.W.; Omar, N.A.S.; Saleviter, S.; Daniyal, W.M.E.M.M.; Hashim, H.S.; Nasrullah, M. Nanostructured Chitosan/Maghemite Composites Thin Film for Potential Optical Detection of Mercury Ion by Surface Plasmon Resonance Investigation. Polymers 2020, 12, 1497. [Google Scholar] [CrossRef]
- Badita, C.R.; Aranghel, D.; Burducea, C.; Mereuta, P. Characterization of Sodium Alginate Based Films. Rom. J. Phys. 2020, 65, 1–8. [Google Scholar]
- Akbarzadeh Solbu, A.; Koernig, A.; Kjesbu, J.S.; Zaytseva-Zotova, D.; Sletmoen, M.; Strand, B.L. High Resolution Imaging of Soft Alginate Hydrogels by Atomic Force Microscopy. Carbohydr. Polym. 2022, 276, 118804. [Google Scholar] [CrossRef]
- Mousa, N.E.; Simonescu, C.M.; Pătescu, R.-E.; Onose, C.; Tardei, C.; Culiţă, D.C.; Oprea, O.; Patroi, D.; Lavric, V. Pb2+ Removal from Aqueous Synthetic Solutions by Calcium Alginate and Chitosan Coated Calcium Alginate. React. Funct. Polym. 2016, 109, 137–150. [Google Scholar] [CrossRef]
- Avella, M.; Pace, E.D.; Immirzi, B.; Impallomeni, G.; Malinconico, M.; Santagata, G. Addition of Glycerol Plasticizer to Seaweeds Derived Alginates: Influence of Microstructure on Chemical–Physical Properties. Carbohydr. Polym. 2007, 69, 503–511. [Google Scholar] [CrossRef]
- Fernández-Quiroz, D.; González-Gómez, Á.; Lizardi-Mendoza, J.; Vázquez-Lasa, B.; Goycoolea, F.M.; San Román, J.; Argüelles-Monal, W.M. Effect of the Molecular Architecture on the Thermosensitive Properties of Chitosan-g-Poly(N-Vinylcaprolactam). Carbohydr. Polym. 2015, 134, 92–101. [Google Scholar] [CrossRef]
- Poon, L.; Wilson, L.D.; Headley, J.V. Chitosan-Glutaraldehyde Copolymers and Their Sorption Properties. Carbohydr. Polym. 2014, 109, 92–101. [Google Scholar] [CrossRef]
- Furuya, D.C.; da Costa, S.A.; de Oliveira, R.C.; Ferraz, H.G.; Pessoa, A.; da Costa, S.M. Fibers Obtained from Alginate, Chitosan and Hybrid Used in the Development of Scaffolds. Mat. Res. 2017, 20, 377–386. [Google Scholar] [CrossRef]
- Wang, T.; Gunasekaran, S. State of Water in Chitosan–PVA Hydrogel. J. Appl. Polym. Sci. 2006, 101, 3227–3232. [Google Scholar] [CrossRef]
- Jin, L.; Qi, H.; Gu, X.; Zhang, X.; Zhang, Y.; Zhang, X.; Mao, S. Effect of Sodium Alginate Type on Drug Release from Chitosan-Sodium Alginate–Based in Situ Film-Forming Tablets. AAPS PharmSciTech 2020, 21, 55. [Google Scholar] [CrossRef]
- Kowalski, G.; Witczak, M.; Kuterasiński, Ł. Structure Effects on Swelling Properties of Hydrogels Based on Sodium Alginate and Acrylic Polymers. Molecules 2024, 29, 1937. [Google Scholar] [CrossRef]
- Lopes, L.M.; de Moraes, M.A.; Beppu, M.M. Influence of Guluronic and Mannuronic Groups in Sodium Alginate Blends with Silk Fibroin: Phase Equilibrium and Thermodynamic Modeling. Adv. Polym. Technol. 2023, 2023, 4986453. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Bianco-Peled, H. A Quantitative Analysis of Alginate Swelling. Carbohydr. Polym. 2010, 79, 1020–1027. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Ruiz Pividal, J.; Llorens-Gámez, M. Enhancement of Water Diffusion and Compression Performance of Crosslinked Alginate Films with a Minuscule Amount of Graphene Oxide. Sci. Rep. 2017, 7, 11684. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Jalali, A.M. Chitosan-Based Hydrogels: Preparation, Properties and Applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan Based Hydrogels: Characteristics and Pharmaceutical Applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar]
- Lin, D.; Kelly, A.L.; Maidannyk, V.; Miao, S. Effect of Structuring Emulsion Gels by Whey or Soy Protein Isolate on the Structure, Mechanical Properties, and in-Vitro Digestion of Alginate-Based Emulsion Gel Beads. Food Hydrocoll. 2021, 110, 106165. [Google Scholar] [CrossRef]
- Sun, R.; Xia, Q. In Vitro Digestion Behavior of (W1/O/W2) Double Emulsions Incorporated in Alginate Hydrogel Beads: Microstructure, Lipolysis, and Release. Food Hydrocoll. 2020, 107, 105950. [Google Scholar] [CrossRef]
- Wong, S.K.; Lawrencia, D.; Supramaniam, J.; Goh, B.H.; Manickam, S.; Wong, T.W.; Pang, C.H.; Tang, S.Y. In Vitro Digestion and Swelling Kinetics of Thymoquinone-Loaded Pickering Emulsions Incorporated in Alginate-Chitosan Hydrogel Beads. Front. Nutr. 2021, 8, 752207. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, C.; Lalgee, L.; Kistow, M.; Jalsa, N.; Ward, K. On the Binding Affinity and Thermodynamics of Sodium Alginate-Heavy Metal Ion Interactions for Efficient Adsorption. Carbohydr. Polym. Technol. Appl. 2022, 3, 100203. [Google Scholar] [CrossRef]
- Martins, A.F.; de Oliveira, D.M.; Pereira, A.G.B.; Rubira, A.F.; Muniz, E.C. Chitosan/TPP Microparticles Obtained by Microemulsion Method Applied in Controlled Release of Heparin. Int. J. Biol. Macromol. 2012, 51, 1127–1133. [Google Scholar] [CrossRef]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Maghat, H. Critical of Linear and Nonlinear Equations of Pseudo-First Order and Pseudo-Second Order Kinetic Models. Karbala Int. J. Mod. Sci. 2018, 4, 244–254. [Google Scholar] [CrossRef]
- Hamza, M.F.; Hamad, N.A.; Hamad, D.M.; Khalafalla, M.S.; Abdel-Rahman, A.A.-H.; Zeid, I.F.; Wei, Y.; Hessien, M.M.; Fouda, A.; Salem, W.M. Synthesis of Eco-Friendly Biopolymer, Alginate-Chitosan Composite to Adsorb the Heavy Metals, Cd(II) and Pb(II) from Contaminated Effluents. Materials 2021, 14, 2189. [Google Scholar] [CrossRef] [PubMed]
- Mashkoor, F.; Shoeb, M.; Mashkoor, R.; Anwer, A.H.; Zhu, S.; Jeong, H.; Baek, S.-S.; Jung, J.; Jeong, C. Synergistic Effects of Tungstate Trioxide Hemihydrate Decorated Reduced Graphene Oxide for the Adsorption of Heavy Metals and Dyes and Postliminary Application in Supercapacitor Device. J. Clean. Prod. 2023, 418, 138067. [Google Scholar] [CrossRef]
- Rostami, P.; Moradi, M.R.; Ashourzadeh Pordsari, M.; Ghaemi, A. Carboxylic Acid Functionalized Para-Xylene Based Hypercrosslinked Polymer as a Novel and High Performance Adsorbent for Heavy Metal Removal. Arab. J. Chem. 2024, 17, 105634. [Google Scholar] [CrossRef]
- Alexandratos, S.D.; Zhu, X. The Effect of Hydrogen Bonding in Enhancing the Ionic Affinities of Immobilized Monoprotic Phosphate Ligands. Materials 2017, 10, 968. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Soliva-Fortuny, R.; Martín-Belloso, O. Layer-by-Layer Assembly of Food-Grade Alginate/Chitosan Nanolaminates: Formation and Physicochemical Characterization. Food Biophys. 2017, 12, 299–308. [Google Scholar] [CrossRef]
- Picart, C.; Lavalle, P.; Hubert, P.; Cuisinier, F.J.G.; Decher, G.; Schaaf, P.; Voegel, J.-C. Buildup Mechanism for Poly(l-Lysine)/Hyaluronic Acid Films onto a Solid Surface. Langmuir 2001, 17, 7414–7424. [Google Scholar] [CrossRef]
- Hassan, M.; Liu, Y.; Naidu, R.; Du, J.; Qi, F.; Donne, S.W.; Islam, M.M. Mesoporous Biopolymer Architecture Enhanced the Adsorption and Selectivity of Aqueous Heavy-Metal Ions. ACS Omega 2021, 6, 15316–15331. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Tang, J.; Yang, Z.; Zhang, L.; Huang, X. New Insights into the Interactions between Pb(II) and Fruit Waste Biosorbent. Chemosphere 2022, 303, 135048. [Google Scholar] [CrossRef]
- Guerrero, J.D.; Marchesini, F.A.; Ulla, M.A.; Gutierrez, L.B. Effect of Biocomposite Production Factors on the Development of an Eco-Friendly Chitosan/Alginate-Based Adsorbent with Enhanced Copper Removal Efficiency. Int. J. Biol. Macromol. 2023, 253, 126416. [Google Scholar] [CrossRef]
- Worthen, A.J.; Irving, K.S.; Lapitsky, Y. Supramolecular Strategy Effects on Chitosan Bead Stability in Acidic Media: A Comparative Study. Gels 2019, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, T.; Filipkowska, U.; Szymczyk, P.; Rodziewicz, J.; Mielcarek, A. Effect of Ionic and Covalent Crosslinking Agents on Properties of Chitosan Beads and Sorption Effectiveness of Reactive Black 5 Dye. React. Funct. Polym. 2017, 114, 58–74. [Google Scholar] [CrossRef]
- Carnier, R.; Coscione, A.R.; de Abreu, C.A.; Melo, L.C.A.; Silva, A.F. da Cadmium and Lead Adsorption and Desorption by Coffee Waste-Derived Biochars. Bragantia 2022, 81, e0622. [Google Scholar] [CrossRef]
- Núñez-Delgado, A.; Romar-Gasalla, A.; Santás-Miguel, V.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M. Cadmium and Lead Sorption/Desorption on Non-Amended and By-Product-Amended Soil Samples and Pyritic Material. Water 2017, 9, 886. [Google Scholar] [CrossRef]



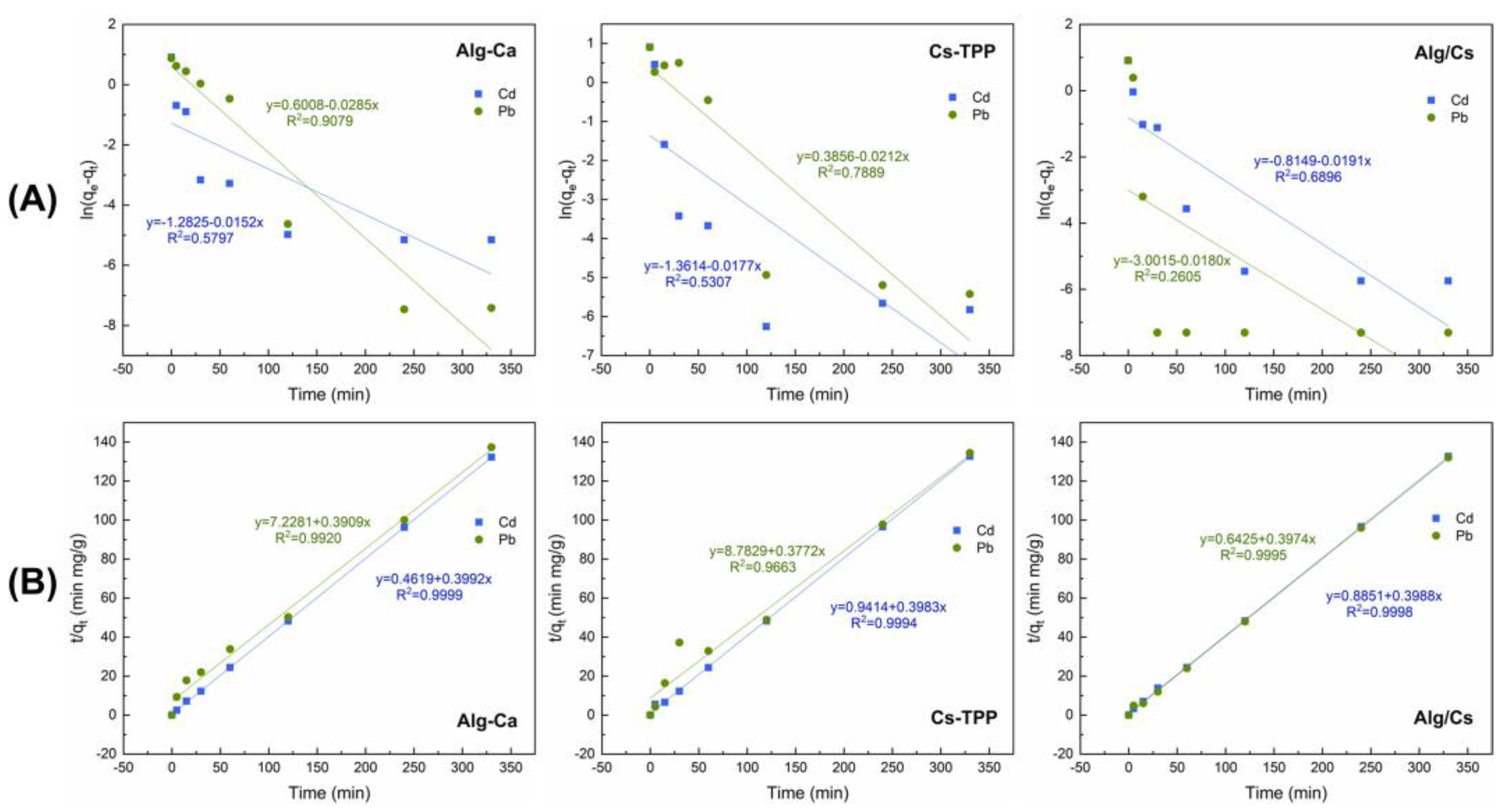
| Biosorbent Film | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| qe | k1 | R2 | qe | k2 | R2 | |
| Alg-Ca | ||||||
| Cd | 0.28 | 4.60 × 10−5 | 0.5797 | 2.51 | 0.3450 | 0.9999 |
| Pb | 1.82 | −1.0 × 10−4 | 0.9079 | 2.56 | 0.0211 | 0.9920 |
| Cs-TPP | ||||||
| Cd | 0.26 | −5.37 × 10−5 | 0.5307 | 2.51 | 0.1685 | 0.9994 |
| Pb | 1.47 | −1.0 × 10−4 | 0.7889 | 0.0162 | 0.9663 | |
| Alg/Cs | ||||||
| Cd | 0.44 | −5.78 × 10−5 | 0.6896 | 2.51 | 0.1797 | 0.9998 |
| Pb | 0.05 | −1.0 × 10−4 | 0.2605 | 2.52 | 0.2458 | 0.9995 |
| Biosorbent Film | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| R2 | qmax | KL | R2 | 1/n | KF | |
| Alg-Ca | ||||||
| Cd | 0.9875 | 198.02 | 0.0016 | 0.9702 | 0.9487 | 0.3704 |
| Pb | 0.9780 | 27.78 | 0.0080 | 0.8920 | 0.7338 | 0.4053 |
| Cs-TPP | ||||||
| Cd | 0.9665 | 12.96 | 0.0302 | 0.8795 | 0.7907 | 0.6521 |
| Pb | 0.9858 | 19.26 | 0.0075 | 0.9438 | 0.7430 | 0.2592 |
| Alg/Cs | ||||||
| Cd | 0.9944 | 60.98 | 0.0082 | 0.9515 | 0.8074 | 0.7175 |
| Pb | 0.9856 | 159.74 | 0.0029 | 0.9482 | 0.8174 | 0.6969 |
| Cycle | Desorption Efficiency (%) | |||||
|---|---|---|---|---|---|---|
| Alginate | Chitosan | Alginate–Chitosan | ||||
| Cd | Pb | Cd | Pb | Cd | Pb | |
| 1 | 97.7 | 49.0 | 94.3 | 64.6 | 98.2 | 60.0 |
| 2 | 99.2 | 56.6 | N/A * | N/A | 99.9 | 65.9 |
| 3 | 99.5 | 79.7 | N/A | N/A | 99.8 | 71.8 |
| 4 | 99.9 | 82.3 | N/A | N/A | 99.9 | 75.5 |
| 5 | 99.9 | 79.4 | N/A | N/A | 98.8 | 77.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Rivas, S.C.; Ibarra-Gutiérrez, M.J.; Fernández-Quiroz, D.; Lucero-Acuña, A.; Burgara-Estrella, A.J.; Zavala-Rivera, P. pH-Responsive Alginate/Chitosan Gel Films: An Alternative for Removing Cadmium and Lead from Water. Gels 2024, 10, 669. https://doi.org/10.3390/gels10100669
Moreno-Rivas SC, Ibarra-Gutiérrez MJ, Fernández-Quiroz D, Lucero-Acuña A, Burgara-Estrella AJ, Zavala-Rivera P. pH-Responsive Alginate/Chitosan Gel Films: An Alternative for Removing Cadmium and Lead from Water. Gels. 2024; 10(10):669. https://doi.org/10.3390/gels10100669
Chicago/Turabian StyleMoreno-Rivas, Silvia Carolina, María José Ibarra-Gutiérrez, Daniel Fernández-Quiroz, Armando Lucero-Acuña, Alexel J. Burgara-Estrella, and Paul Zavala-Rivera. 2024. "pH-Responsive Alginate/Chitosan Gel Films: An Alternative for Removing Cadmium and Lead from Water" Gels 10, no. 10: 669. https://doi.org/10.3390/gels10100669
APA StyleMoreno-Rivas, S. C., Ibarra-Gutiérrez, M. J., Fernández-Quiroz, D., Lucero-Acuña, A., Burgara-Estrella, A. J., & Zavala-Rivera, P. (2024). pH-Responsive Alginate/Chitosan Gel Films: An Alternative for Removing Cadmium and Lead from Water. Gels, 10(10), 669. https://doi.org/10.3390/gels10100669








