Cross-Linked Polyimide Aerogels with Excellent Thermal and Mechanical Properties
Abstract
1. Introduction
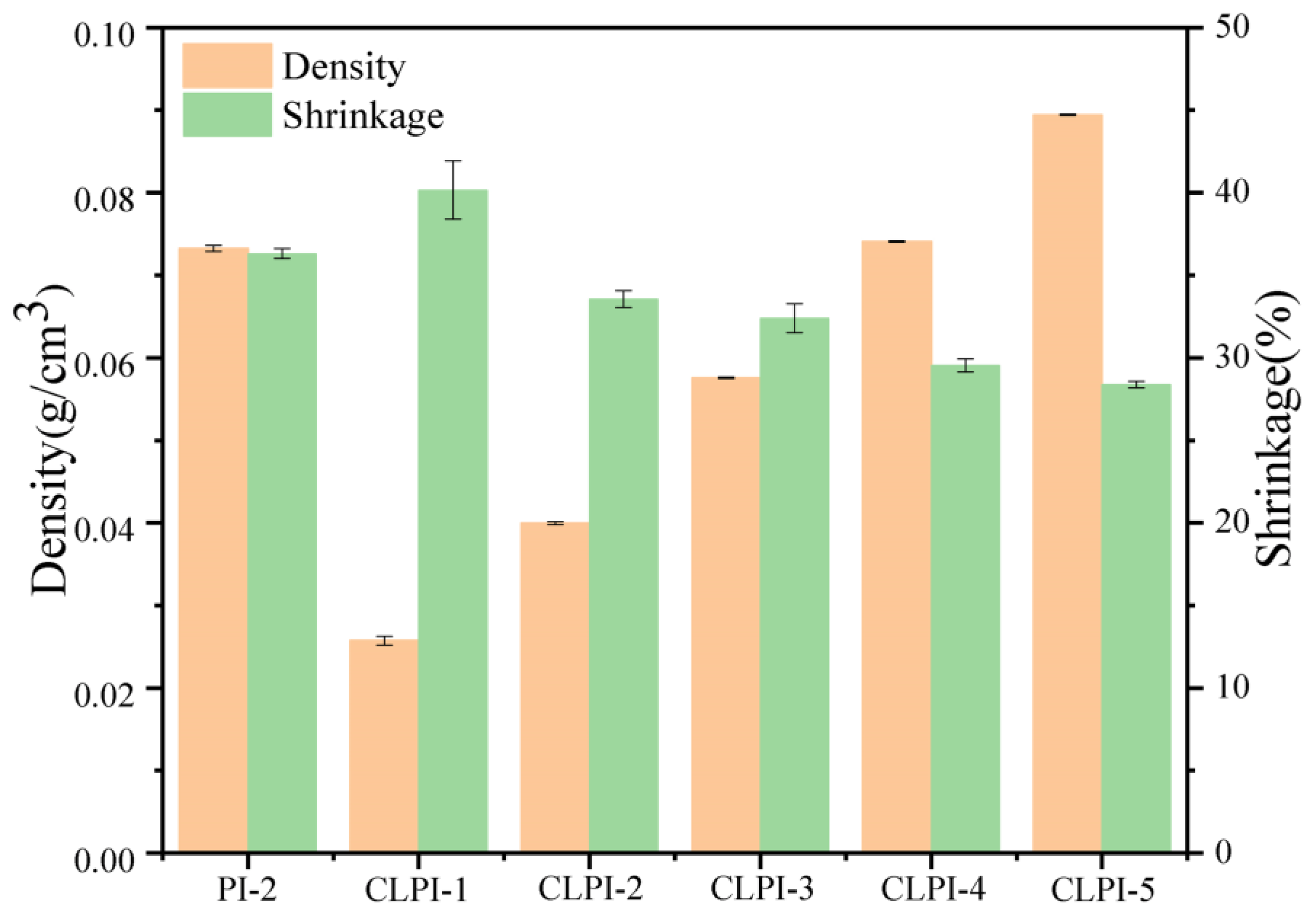
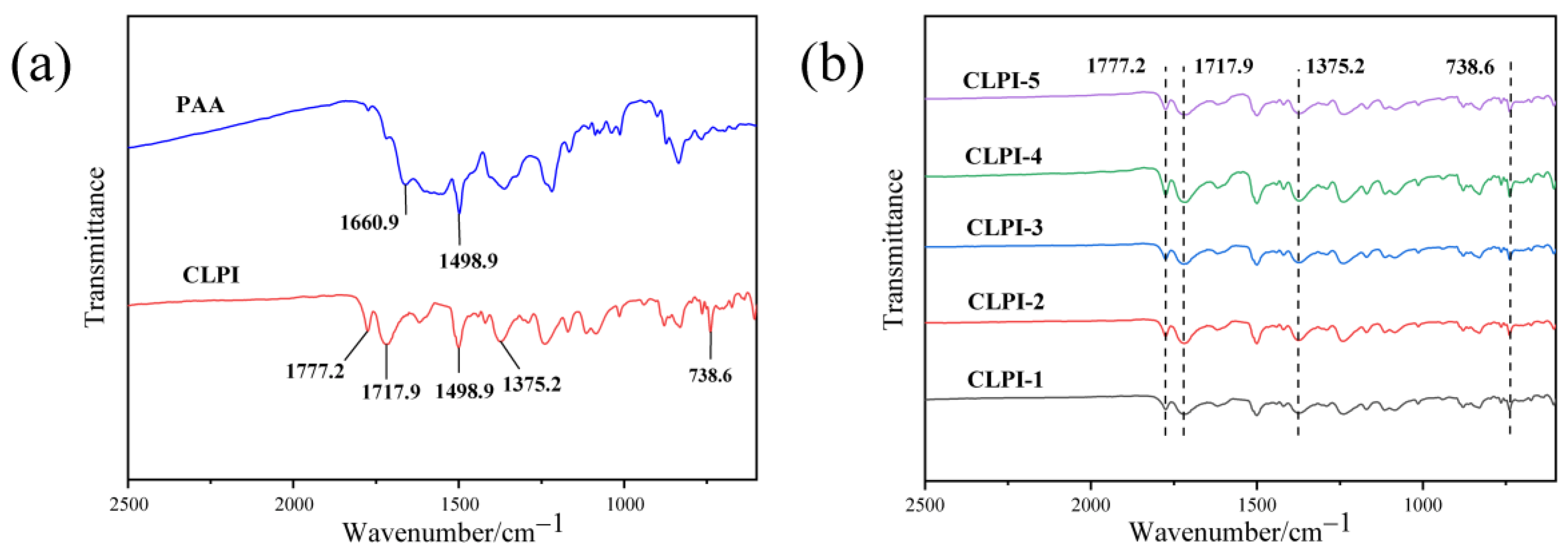
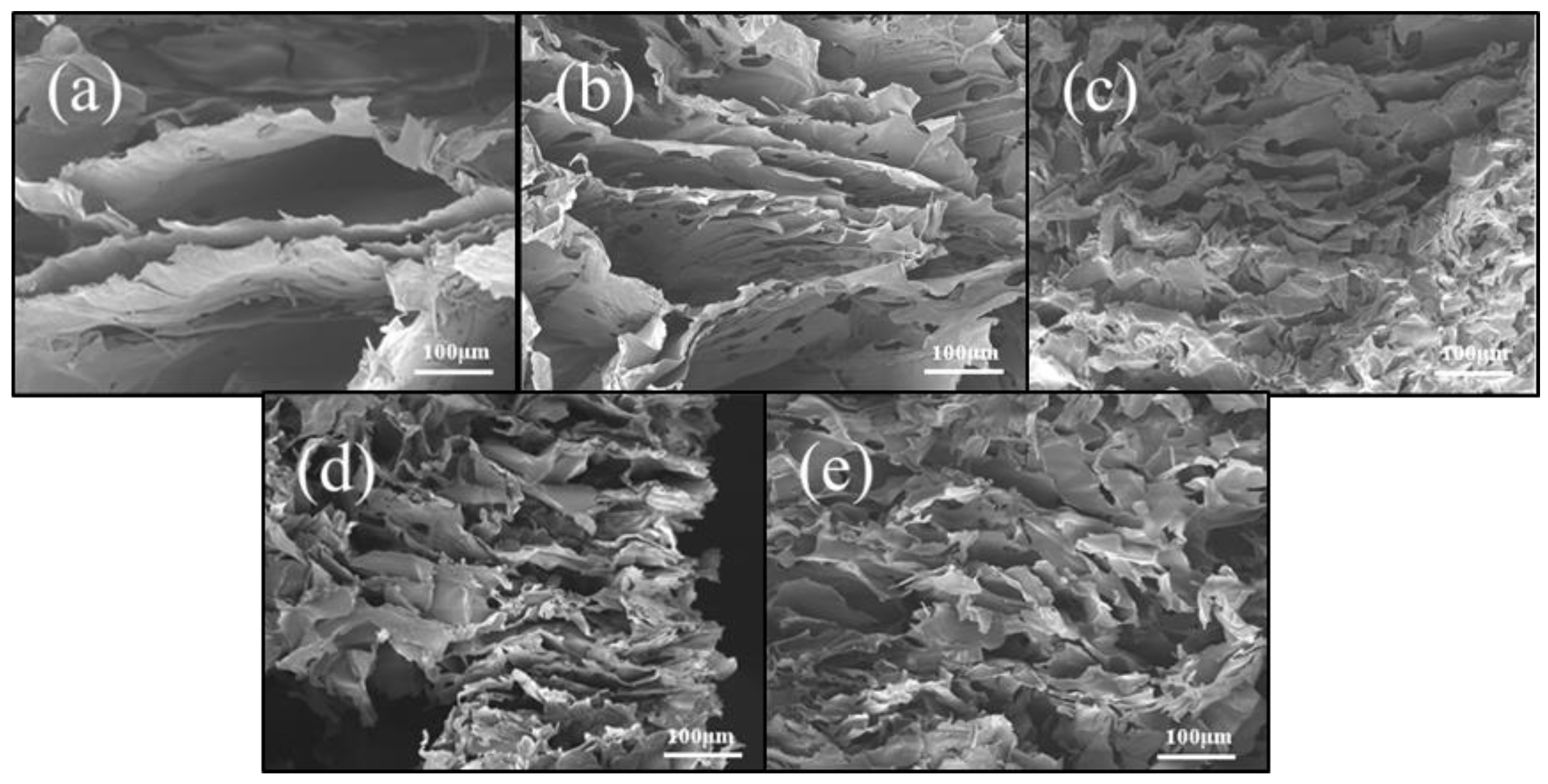
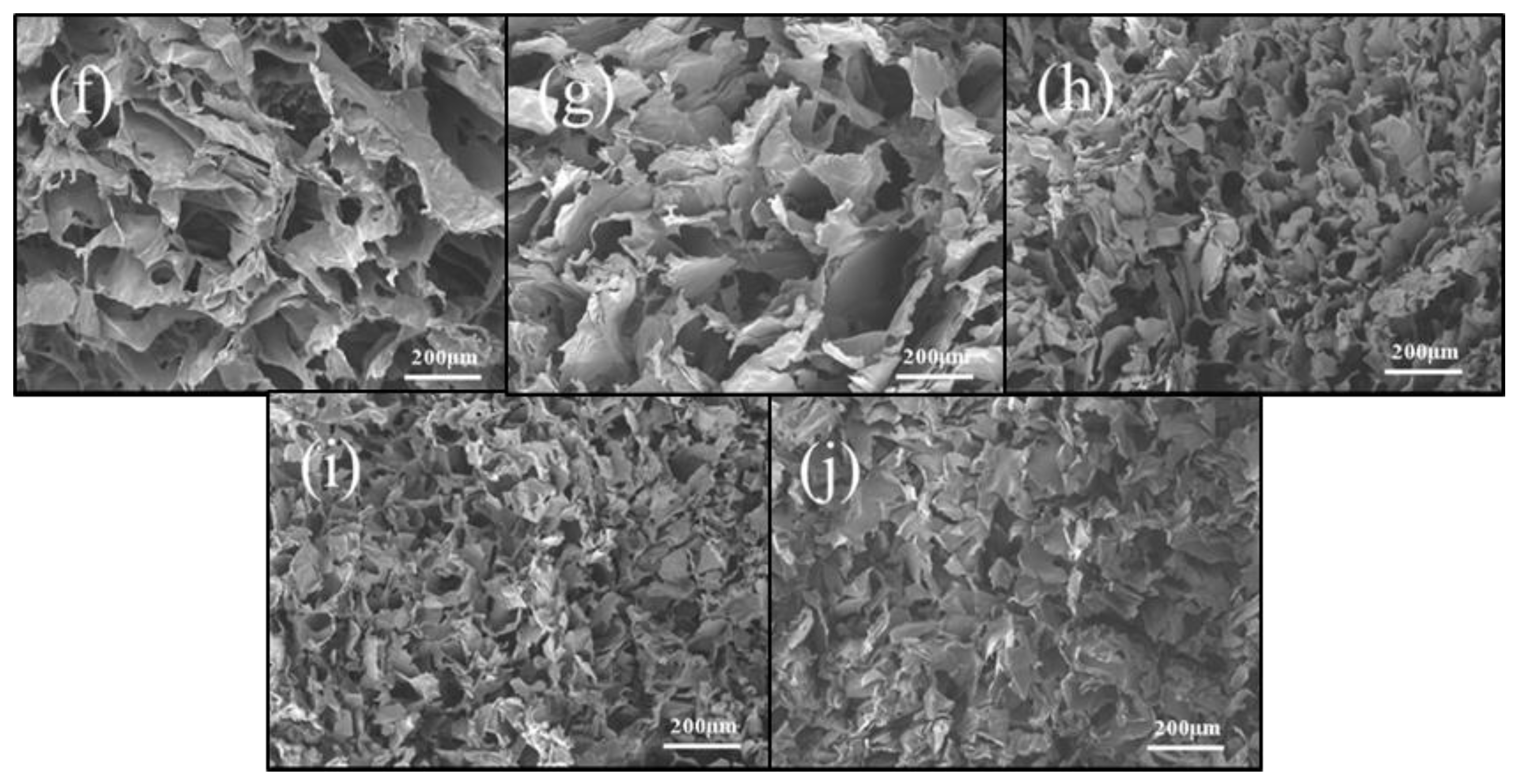
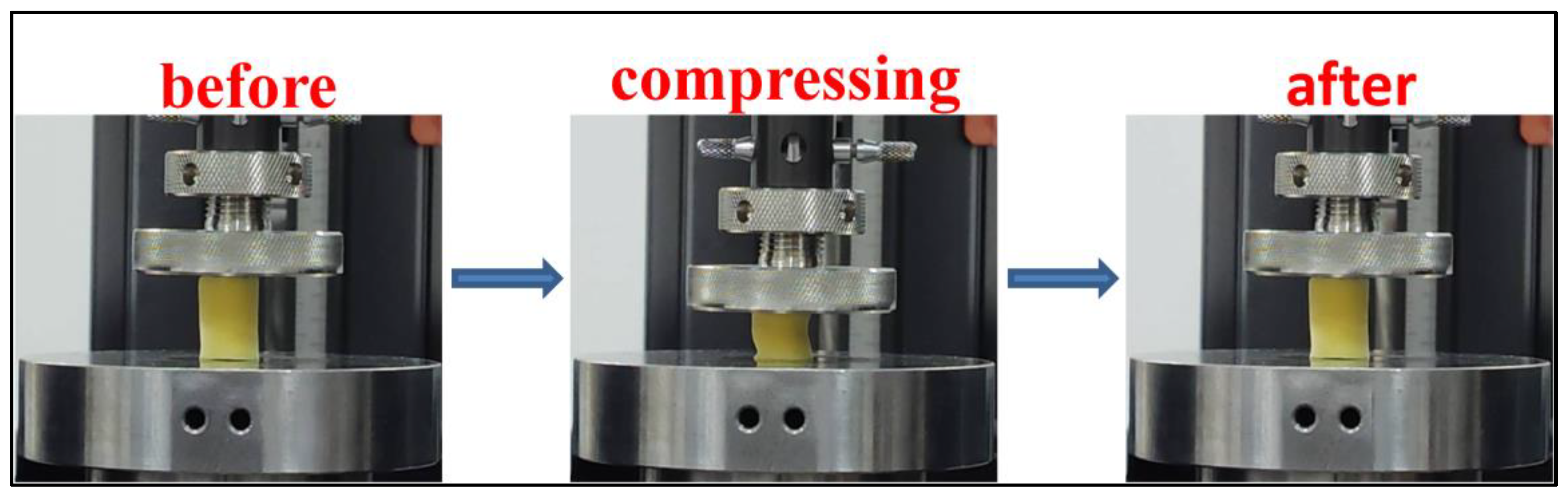
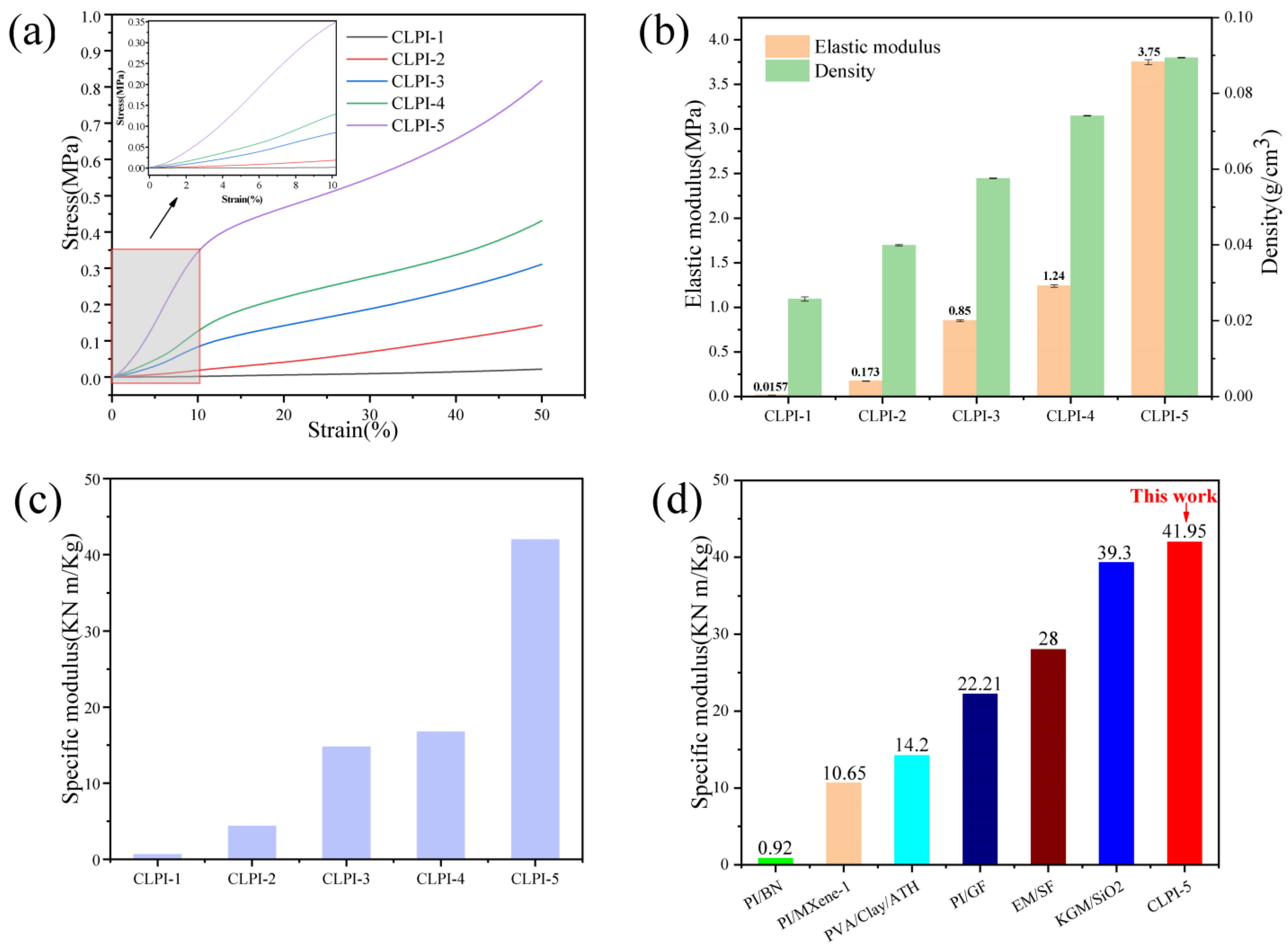
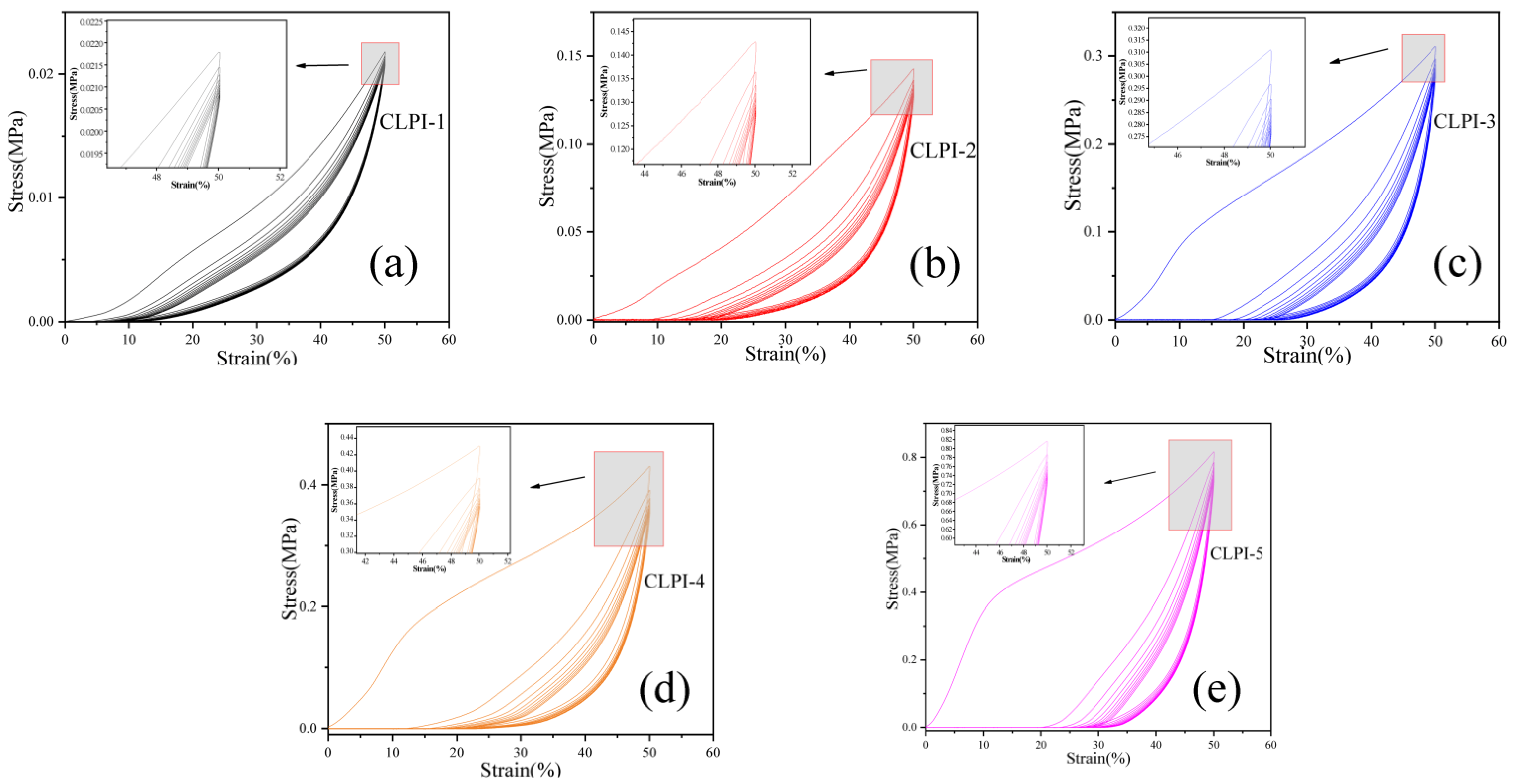
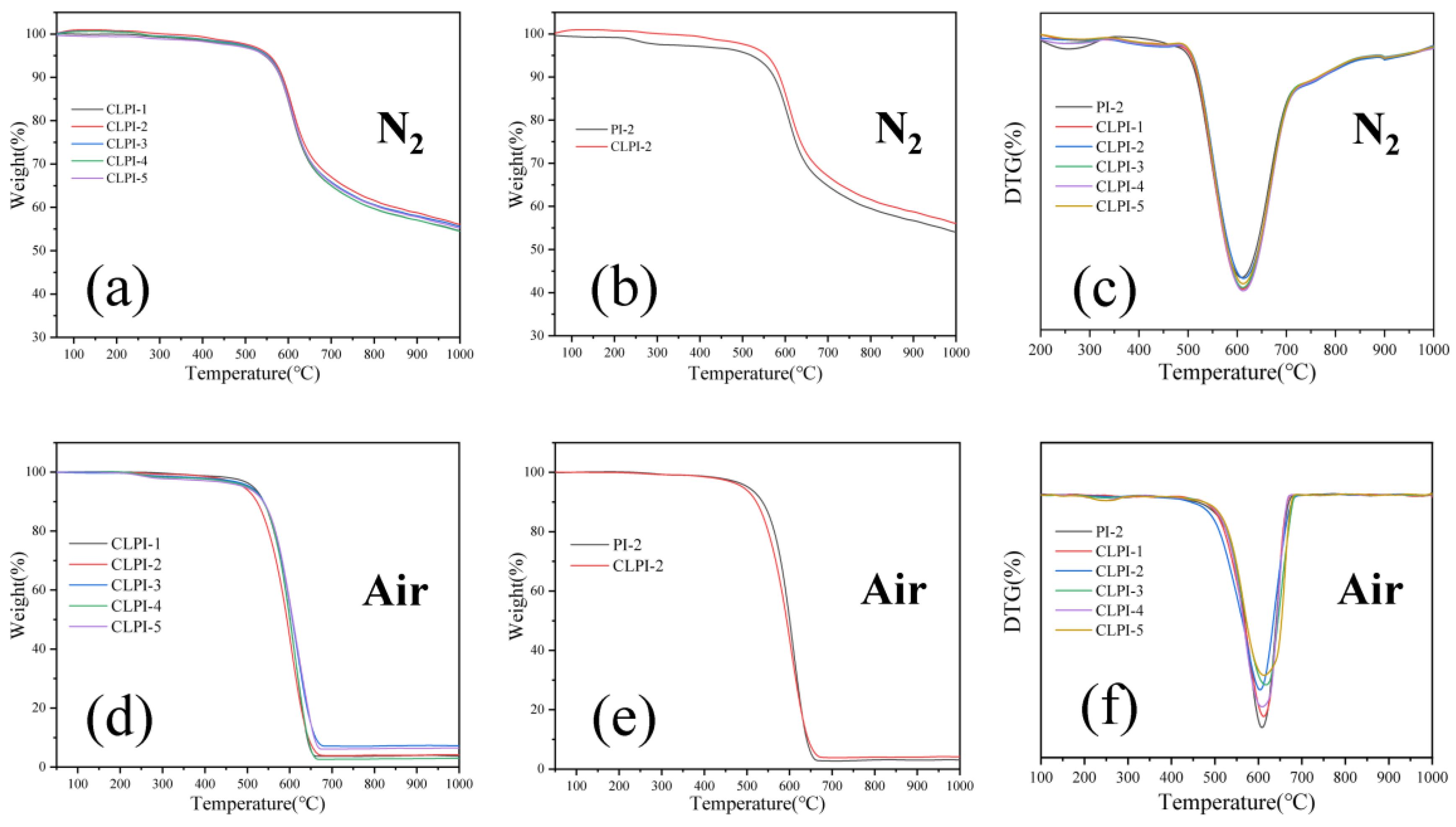
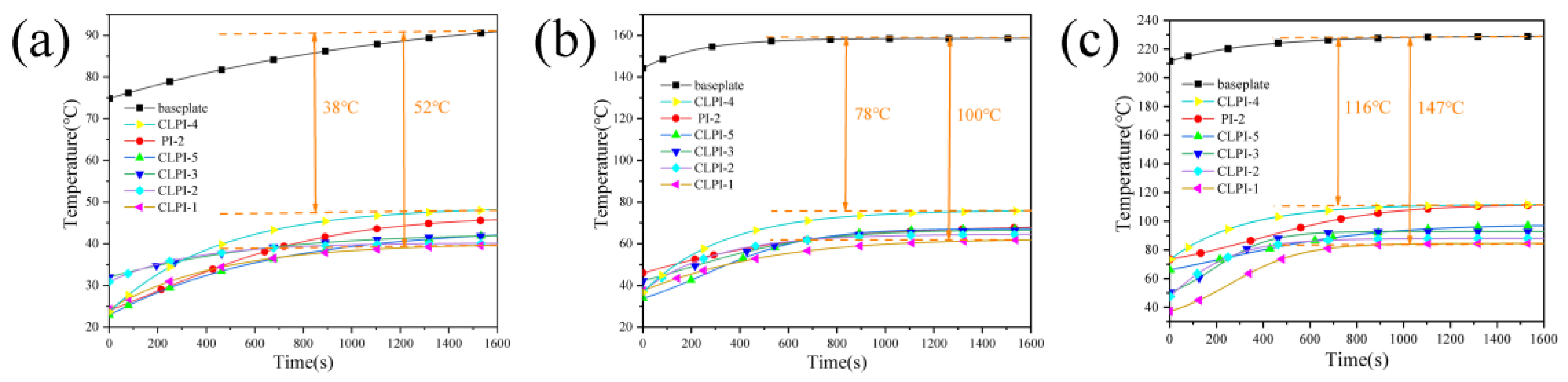
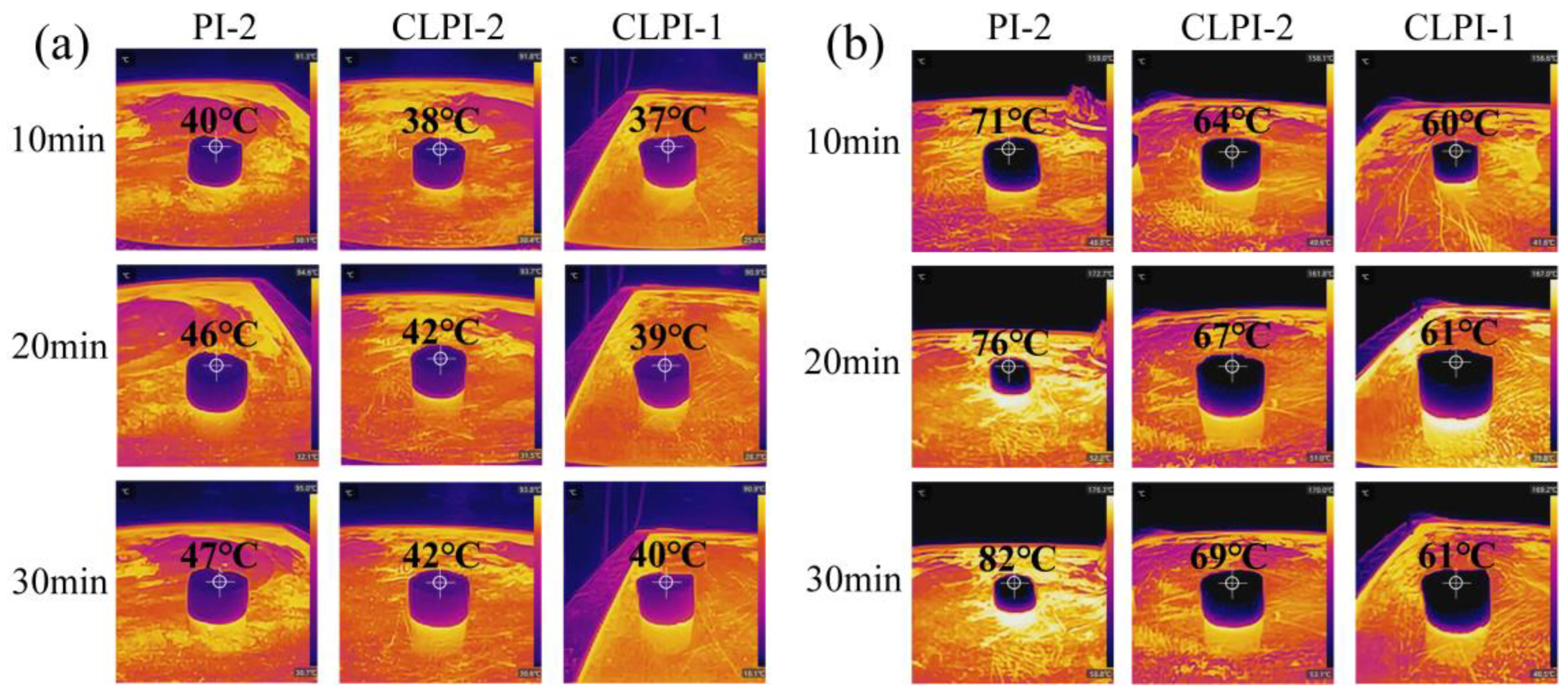
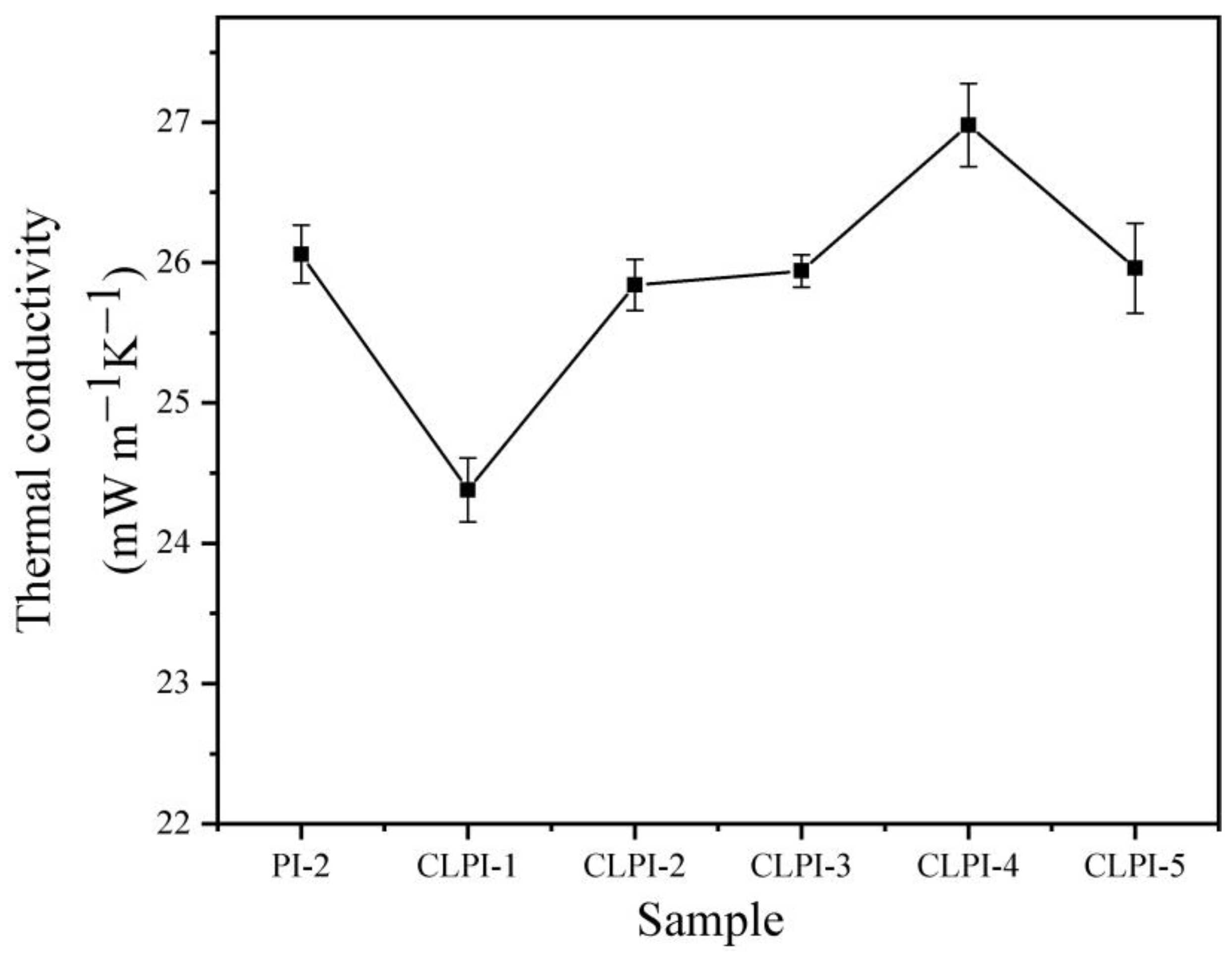
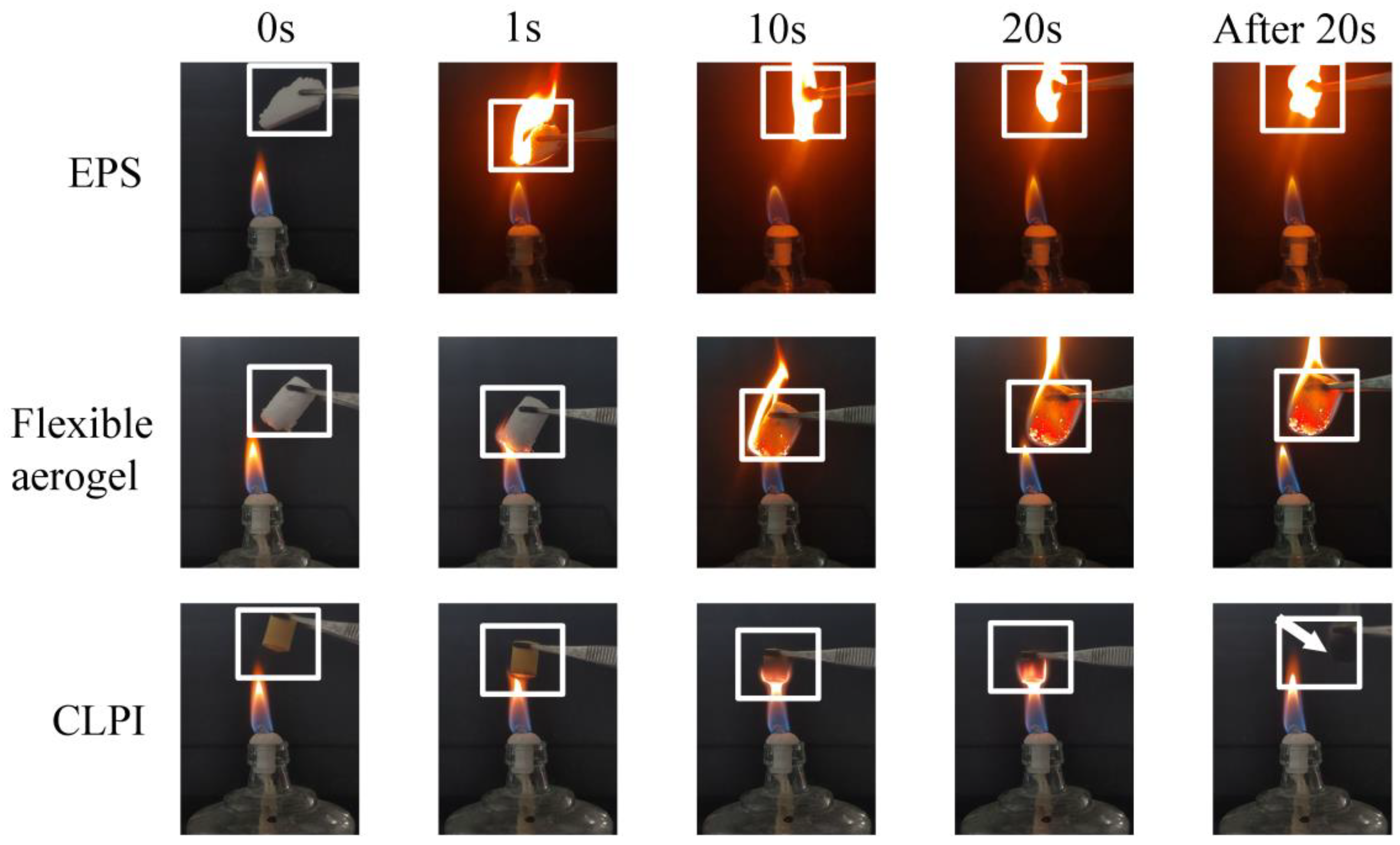
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Cross-Linked Water-Soluble Polyamides
4.3. Preparation of Aerogel Precursors
4.4. Preparation of Cross-Linking PI Aerogel
4.5. Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaughan, A. The first global energy crisis. New Sci. 2022, 253, 18–21. [Google Scholar] [CrossRef]
- Ngoune, N.F.; Djousse, B.M.K.; Djoukeng, G.H.; Nguimeya, C.G.F.; Tangka, K.J.; Tchoffo, M. Contribution of the mix renewable energy potentials in delivering parts of the electric energy needs in the west region of Cameroon. Heliyon 2023, 9, e14554. [Google Scholar] [CrossRef]
- Ebadollahi, M.; Amidpour, M.; Pourali, O.; Ghaebi, H. Development of a novel flexible multigeneration energy system for meeting the energy needs of remote areas. Renew. Energy 2022, 198, 1224–1242. [Google Scholar] [CrossRef]
- Sánchez-Tabernero, G.; Hidalgo-Muñoz, A.R.; Galán, J.I.; Tabernero, C. The energy crossroads: Exploring the moderating role of the energy crisis on the acceptance of the development of a uranium mine. J. Environ. Manag. 2024, 358, 120900. [Google Scholar] [CrossRef]
- Bagdadee, A.H.; Zhang, L. Electrical power crisis solution by the developing renewable energy based power generation expansion. Energy Rep. 2020, 6, 480–490. [Google Scholar] [CrossRef]
- Frilingou, N.; Xexakis, G.; Koasidis, K.; Nikas, A.; Campagnolo, L.; Delpiazzo, E.; Chiodi, A.; Gargiulo, M.; McWilliams, B.; Koutsellis, T.; et al. Navigating through an energy crisis: Challenges and progress towards electricity decarbonisation, reliability, and affordability in Italy. Energy Res. Soc. Sci. 2023, 96, 102934. [Google Scholar] [CrossRef]
- Lv, Q.; Zhu, X.; Zhou, T.; Tian, L.; Liu, Y.; Wang, Y.; Zhang, C. Multifunctional and recyclable aerogel/fiber building insulation composites with sandwich structure. Constr. Build. Mater. 2024, 423, 135902. [Google Scholar] [CrossRef]
- Song, Z.; Lei, Y.; Ran, W.; Yuan, M.; Shang, S.; Cui, S. Structural properties and barrier performance of low-cost aerogel composites for building insulation. J. Build. Eng. 2024, 90, 109485. [Google Scholar] [CrossRef]
- Wang, L.; Lian, W.; Yin, B.; Liu, X.; Tang, S. Silica nanowires-reinforced silica aerogels with outstanding thermal insulation, thermal stability and mechanical properties. Ceram. Int. 2024, 50, 6693–6702. [Google Scholar] [CrossRef]
- Xue, R.; Zhuge, Y.; Liu, G.; Liu, F. Flexible fabrication of a novel SiO2/AF/ZIF-L composite embedded with MOF structure and its thermal insulation properties. Ceram. Int. 2023, 49, 36619–36627. [Google Scholar] [CrossRef]
- Huang, Y.; He, S.; Chen, G.; Dai, H.; Yuan, B.; Chen, X.; Yang, X. Fast preparation of glass fiber/silica aerogel blanket in ethanol & water solvent system. J. Non-Cryst. Solids 2019, 505, 286–291. [Google Scholar] [CrossRef]
- Chang, M.-J.; Zhu, W.-Y.; Liu, J.; Bai, G.; Li, X.; Lu, X.-Q.; Lei, Y.-H. Fabrication of elastic SiO2 aerogels with prominent mechanical strength and stability reinforced by SiO2 nanofibers and polyurethane for oil adsorption. Sep. Purif. Technol. 2024, 341, 126914. [Google Scholar] [CrossRef]
- Long, X.; Wei, X.; Hu, M.; Yu, J.; Wang, S.; Zhou, L.; Liao, J. Anisotropic and high-strength SiO2/cellulose nanofiber composite aerogel with thermal superinsulation and superhydrophobicity. Ceram. Int. 2023, 49, 28621–28628. [Google Scholar] [CrossRef]
- Shi, B.; Zhou, Z.; Chen, Y.; Wang, X.; Xu, B. Preparation and properties of hydrophobic and highly transparent SiO2 aerogels. Ceram. Int. 2023, 49, 27597–27603. [Google Scholar] [CrossRef]
- Ruan, C.; Ma, Y.; Shi, G.; He, C.; Du, C.; Jin, X.; Liu, X.; He, S.; Huang, Y. Self-assembly cellulose nanocrystals/SiO2 composite aerogel under freeze-drying: Adsorption towards dye contaminant. Appl. Surf. Sci. 2022, 592, 153280. [Google Scholar] [CrossRef]
- Huang, Y.; He, S.; Feng, M.; Dai, H.; Pan, Y.; Cheng, X. Organic solvent-saving preparation of water glass based aerogel granules under ambient pressure drying. J. Non-Cryst. Solids 2019, 521, 119507. [Google Scholar] [CrossRef]
- Mosanenzadeh, S.G.; Saadatnia, Z.; Shi, F.; Park, C.B.; Naguib, H.E. Structure to properties relations of BPDA and PMDA backbone hybrid diamine polyimide aerogels. Polymer 2019, 176, 213–226. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Ma, X.; Wang, K. The effect of poor solvent on the microstructures and thermal insulation performance of polyimide aerogels. Mater. Lett. 2021, 300, 130151. [Google Scholar] [CrossRef]
- Miao, Y.; Pudukudy, M.; Zhi, Y.; Miao, Y.; Shan, S.; Jia, Q.; Ni, Y. A facile method for in situ fabrication of silica/cellulose aerogels and their application in CO2 capture. Carbohydr. Polym. 2020, 236, 116079. [Google Scholar] [CrossRef]
- Niu, F.; Wu, N.; Yu, J.; Ma, X. Gelation, flame retardancy, and physical properties of phosphorylated microcrystalline cellulose aerogels. Carbohydr. Polym. 2020, 242, 116422. [Google Scholar] [CrossRef]
- Zaman, A.; Huang, F.; Jiang, M.; Wei, W.; Zhou, Z. Preparation, Properties, and Applications of Natural Cellulosic Aerogels: A Review. Energy Built Environ. 2020, 1, 60–76. [Google Scholar] [CrossRef]
- Jia, T.; Chen, H.; Fan, Z.; Xu, H.; Huang, J.; Wang, P.; Xing, H.; Jia, H.; Fan, X.; Zhou, H.; et al. High-strength and high-temperature-resistant multilayer interconnected polyimide paper derived from anisotropic aerogel via a hot-extrusion strategy for aerospace applications. Appl. Surf. Sci. 2023, 611, 155592. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, L.; Xue, T.; Tian, J.; Fan, W.; Liu, T. Flame-retardant and thermal-protective polyimide-hydroxyapatite aerogel fiber-based composite textile for firefighting clothing. Compos. Part B Eng. 2023, 248, 110377. [Google Scholar] [CrossRef]
- Wei, H.; Hu, X.; Deng, Y.; Wei, X.; Yang, Z.; Han, G. Distributed activation energy treatment of polyimide aerogel and its blocking effect on thermal runaway propagation of ternary battery. J. Energy Storage 2024, 90, 111744. [Google Scholar] [CrossRef]
- Guo, H.; Meador, M.A.B.; McCorkle, L.; Quade, D.J.; Guo, J.; Hamilton, B.; Cakmak, M.; Sprowl, G. Polyimide aerogels cross-linked through amine functionalized polyoligomeric silsesquioxane. ACS Appl. Mater. Interfaces 2011, 3, 546–552. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Wang, J.; Xiao, Y.; Yang, Z.; Ji, H.; Xu, G.; Xiong, S.; Li, Z.; Ding, F. Polyimide Aerogels with Excellent Thermal Insulation, Hydrophobicity, Machinability, and Strength Evolution at Extreme Conditions. ACS Appl. Polym. Mater. 2022, 4, 8227–8237. [Google Scholar] [CrossRef]
- Zheng, R.; Hu, J.; Lin, Z.; Wu, G.; Lin, Y. Anisotropic Polyimide/Cellulose Nanofibril Composite Aerogels for Thermal Insulation and Flame Retardancy. ACS Appl. Polym. Mater. 2023, 5, 4180–4189. [Google Scholar] [CrossRef]
- Liu, T.; Liang, F.; Chen, S.; Zhang, P.; Qian, K.; Xu, Y.; Guo, W. Aramid reinforced polyimide aerogel composites with high-mechanical strength for thermal insulation material. Polym. Adv. Technol. 2023, 34, 1769–1776. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, X.; Zhang, Y.; Zhang, Y.; Liu, T. Lightweight, strong, and super-thermal insulating polyimide composite aerogels under high temperature. Compos. Sci. Technol. 2019, 173, 47–52. [Google Scholar] [CrossRef]
- Yin, S.; Du, Y.; Liang, X.; Xie, Y.; Xie, D.; Mei, Y. Surface coating of biomass-modified black phosphorus enhances flame retardancy of rigid polyurethane foam and its synergistic mechanism. Appl. Surf. Sci. 2023, 637, 157961. [Google Scholar] [CrossRef]
- Yuan, P.; Xue, R.; Wang, Y.; Su, Y.; Zhao, B.; Wu, C.; An, W.; Zhao, W.; Ma, R.; Hu, D. Horizontally-oriented barium titanate@polydomine/polyimide nanocomposite films for high-temperature energy storage. J. Colloid Interface Sci. 2024, 662, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Cashman, J.L.; Nguyen, B.N.; Dosa, B.; Meador, M.A.B. Flexible Polyimide Aerogels Derived from the Use of a Neopentyl Spacer in the Backbone. ACS Appl. Polym. Mater. 2020, 2, 2179–2189. [Google Scholar] [CrossRef]
- Zheng, S.; Jiang, L.; Chang, F.; Zhang, C.; Ma, N.; Liu, X. Mechanically Strong and Thermally Stable Chemical Cross-Linked Polyimide Aerogels for Thermal Insulator. ACS Appl. Mater. Interfaces 2022, 14, 50129–50141. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Xue, T.; Yang, F.; Fan, W.; Liu, T. Bidirectional anisotropic polyimide/bacterial cellulose aerogels by freeze-drying for super-thermal insulation. Chem. Eng. J. 2020, 385, 123963. [Google Scholar] [CrossRef]
- Shi, B.; Ma, B.; Wang, C.; He, H.; Qu, L.; Xu, B.; Chen, Y. Fabrication and applications of polyimide nano-aerogels. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106283. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Song, P.; You, B.; Sun, G. Double-cross-linking strategy for preparing flexible, robust, and multifunctional polyimide aerogel. Chem. Eng. J. 2020, 381, 122784. [Google Scholar] [CrossRef]
- Li, J.; Pang, Y.; Wang, X.; Bai, B.; Zhang, W.; Luo, C.; Liu, W.; Zhang, L. Polyimide composite aerogels towards highly efficient microwave absorption and thermal insulation. Compos. Part A Appl. Sci. Manuf. 2022, 161, 107112. [Google Scholar] [CrossRef]
- Zhao, F.; Zhu, J.; Peng, T.; Liu, H.; Ge, S.; Xie, H.; Xie, L.; Jiang, C. Preparation of functionalized halloysite reinforced polyimide composite aerogels with excellent thermal insulation properties. Appl. Clay Sci. 2021, 211, 106200. [Google Scholar] [CrossRef]
- Ma, S.; Wang, C.; Cong, B.; Zhou, H.; Zhao, X.; Chen, C.; Wang, D.; Liu, C.; Qu, C. Anisotropic all-aromatic polyimide aerogels with robust and high-temperature stable properties for flexible thermal protection. Chem. Eng. J. 2022, 431, 134047. [Google Scholar] [CrossRef]
- Zhang, X.; Ni, X.; Li, C.; You, B.; Sun, G. Co-gel strategy for preparing hierarchically porous silica/polyimide nanocomposite aerogel with thermal insulation and flame retardancy. J. Mater. Chem. A 2020, 8, 9701–9712. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Li, Q.; Chen, C.; Chen, Z.; Song, P.; Hao, J.; Li, Y.; Fakhrhoseini, S.; Naebe, M.; et al. Lightweight, Superelastic Yet Thermoconductive Boron Nitride Nanocomposite Aerogel for Thermal Energy Regulation. ACS Nano 2019, 13, 7860–7870. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.-N.; Wang, H.; Wang, Y.-Y.; Wei, Y.-H.; Si, J.-Y.; Yuen, A.C.Y.; Xie, J.-S.; Yu, B.; Zhu, S.-E.; Lu, H.-D.; et al. Robust, Lightweight, Hydrophobic, and Fire-Retarded Polyimide/MXene Aerogels for Effective Oil/Water Separation. ACS Appl. Mater. Interfaces 2019, 11, 40512–40523. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sánchez-Soto, M.; Maspoch, M.L. Polymer/clay aerogel composites with flame retardant agents: Mechanical, thermal and fire behavior. Mater. Des. (1980–2015) 2013, 52, 609–614. [Google Scholar] [CrossRef]
- Zhu, Z.; Yao, H.; Wang, F.; Dong, J.; Wu, K.; Cao, J.; Long, D. Fiber Reinforced Polyimide Aerogel Composites with High Mechanical Strength for High Temperature Insulation. Macromol. Mater. Eng. 2019, 304, 1800676. [Google Scholar] [CrossRef]
- Maleki, H.; Whitmore, L.; Hüsing, N. Novel multifunctional polymethylsilsesquioxane–silk fibroin aerogel hybrids for environmental and thermal insulation applications. J. Mater. Chem. A 2018, 6, 12598–12612. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, J.; Jiang, C.; Liu, S.; Li, Y. Ultralight, hydrophobic, monolithic konjac glucomannan-silica composite aerogel with thermal insulation and mechanical properties. Carbohydr. Polym. 2019, 207, 246–255. [Google Scholar] [CrossRef]
- Xue, T.; Fan, W.; Zhang, X.; Zhao, X.; Yang, F.; Liu, T. Layered double hydroxide/graphene oxide synergistically enhanced polyimide aerogels for thermal insulation and fire-retardancy. Compos. Part B Eng. 2021, 219, 108963. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Li, X.; Ma, X.; Li, S.; Sun, M.; Liu, H.; Wang, K. Ultralight and heat-insulating mesoporous polyimide aerogels cross-linked with aminated SiO2 nanoparticles. Microporous Mesoporous Mater. 2021, 319, 111074. [Google Scholar] [CrossRef]
- Fan, W.; Zuo, L.; Zhang, Y.; Chen, Y.; Liu, T. Mechanically strong polyimide / carbon nanotube composite aerogels with controllable porous structure. Compos. Sci. Technol. 2018, 156, 186–191. [Google Scholar] [CrossRef]
- Sun, Q.; Tian, K.; Liu, S.; Zhu, Q.; Zheng, S.; Chen, J.; Wang, L.; Cheng, S.; Fan, Z.; Fan, X.; et al. A combination of “Inner—Outer skeleton” strategy to improve the mechanical properties and heat resistance of polyimide composite aerogels as composite sandwich structures for space vehicles. Compos. Sci. Technol. 2024, 252, 110620. [Google Scholar] [CrossRef]
- Sun, J.; Yao, K.; Zhang, R. Robust-flexible and Highly-thermostable Polyimide-silica Aerogels with Adjustable Fiber Skeleton Structure for Efficient Aerospace Thermal Protection. Mater. Today Commun. 2024, 39, 109409. [Google Scholar] [CrossRef]
- Pan, Y.; Zheng, J.; Xu, Y.; Chen, X.; Yan, M.; Li, J.; Zhao, X.; Feng, Y.; Ma, Y.; Ding, M.; et al. Ultralight, highly flexible in situ thermally crosslinked polyimide aerogels with superior mechanical and thermal protection properties via nanofiber reinforcement. J. Colloid Interface Sci. 2022, 628, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ke, Z.; Xu, K.; Dai, F.; Wang, M.; Chen, C.; Qian, G.; Yu, Y. Mechanically strong polyimide aerogels containing benzimidazole groups with excellent flame-retardant, thermal insulation and high service temperature. Chem. Eng. J. 2023, 461, 141722. [Google Scholar] [CrossRef]
- Wu, T.; Dong, J.; Xu, G.; Gan, F.; Zhao, X.; Zhang, Q. Attapulgite-reinforced polyimide hybrid aerogels with high dimensional stability and excellent thermal insulation property. Polymer 2019, 176, 196–205. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, F.; Wang, Z.; Ma, P.; Dong, W.; Hou, H.; Fan, W.; Liu, T. Mechanically strong and thermally insulating polyimide aerogels by homogeneity reinforcement of electrospun nanofibers. Compos. Part B Eng. 2020, 182, 107624. [Google Scholar] [CrossRef]
- Wang, Y.; He, T.; Cheng, Z.; Liu, M.; Ji, J.; Chang, X.; Xu, Q.; Liu, Y.; Liu, X.; Qin, J. Mechanically strong and tough polyimide aerogels cross-linked with amine functionalized carbon nanotubes synthesized by fluorine displacement reaction. Compos. Sci. Technol. 2020, 195, 108204. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, F.; Peng, T.; Liu, H.; Xie, L.; Jiang, C. Highly elastic and robust hydroxyapatite nanowires/polyimide composite aerogel with anisotropic structure for thermal insulation. Compos. Part B Eng. 2021, 223, 109081. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, M.; Chen, Z.; Lu, L.; Ma, Z.; Ding, Y.; Yin, L.; Liu, T.; Li, M.; Yang, L.; et al. A layered aerogel composite with silica fibers, SiC nanowires, and silica aerogels ternary networks for thermal insulation at high-temperature. J. Mater. Sci. Technol. 2025, 204, 71–80. [Google Scholar] [CrossRef]
- He, S.; Wu, X.; Zhang, X.; Sun, J.; Tian, F.; Guo, S.; Du, H.; Li, P.; Huang, Y. Preparation and properties of thermal insulation coating based on silica aerogel. Energy Build. 2023, 298, 113556. [Google Scholar] [CrossRef]
- He, S.; Ruan, C.; Shi, Y.; Chen, G.; Ma, Y.; Dai, H.; Chen, X.; Yang, X. Insight to hydrophobic SiO2 encapsulated SiO2 gel: Preparation and application in fire extinguishing. J. Hazard. Mater. 2021, 405, 124216. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, F.; Xiong, R.; Peng, T.; Ma, Y.; Hu, J.; Xie, L.; Jiang, C. Thermal insulation and flame retardancy of attapulgite reinforced gelatin-based composite aerogel with enhanced strength properties. Compos. Part A Appl. Sci. Manuf. 2020, 138, 106040. [Google Scholar] [CrossRef]
- Zhu, J.; Xiong, R.; Zhao, F.; Peng, T.; Hu, J.; Xie, L.; Xie, H.; Wang, K.; Jiang, C. Lightweight, High-Strength, and Anisotropic Structure Composite Aerogel Based on Hydroxyapatite Nanocrystal and Chitosan with Thermal Insulation and Flame Retardant Properties. ACS Sustain. Chem. Eng. 2020, 8, 71–83. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, H.; Li, Z.; He, S. Cross-Linked Polyimide Aerogels with Excellent Thermal and Mechanical Properties. Gels 2024, 10, 667. https://doi.org/10.3390/gels10100667
Qian H, Li Z, He S. Cross-Linked Polyimide Aerogels with Excellent Thermal and Mechanical Properties. Gels. 2024; 10(10):667. https://doi.org/10.3390/gels10100667
Chicago/Turabian StyleQian, Haoran, Zhiqi Li, and Song He. 2024. "Cross-Linked Polyimide Aerogels with Excellent Thermal and Mechanical Properties" Gels 10, no. 10: 667. https://doi.org/10.3390/gels10100667
APA StyleQian, H., Li, Z., & He, S. (2024). Cross-Linked Polyimide Aerogels with Excellent Thermal and Mechanical Properties. Gels, 10(10), 667. https://doi.org/10.3390/gels10100667







