Advances and Functional Integration of Hydrogel Composites as Drug Delivery Systems in Contemporary Dentistry
Abstract
1. Introduction
2. Historical Development of Hydrogels
3. Hydrogels’ Composition and Properties
4. Hydrogel Classification
4.1. Natural, Synthetic, and Semi-Synthetic
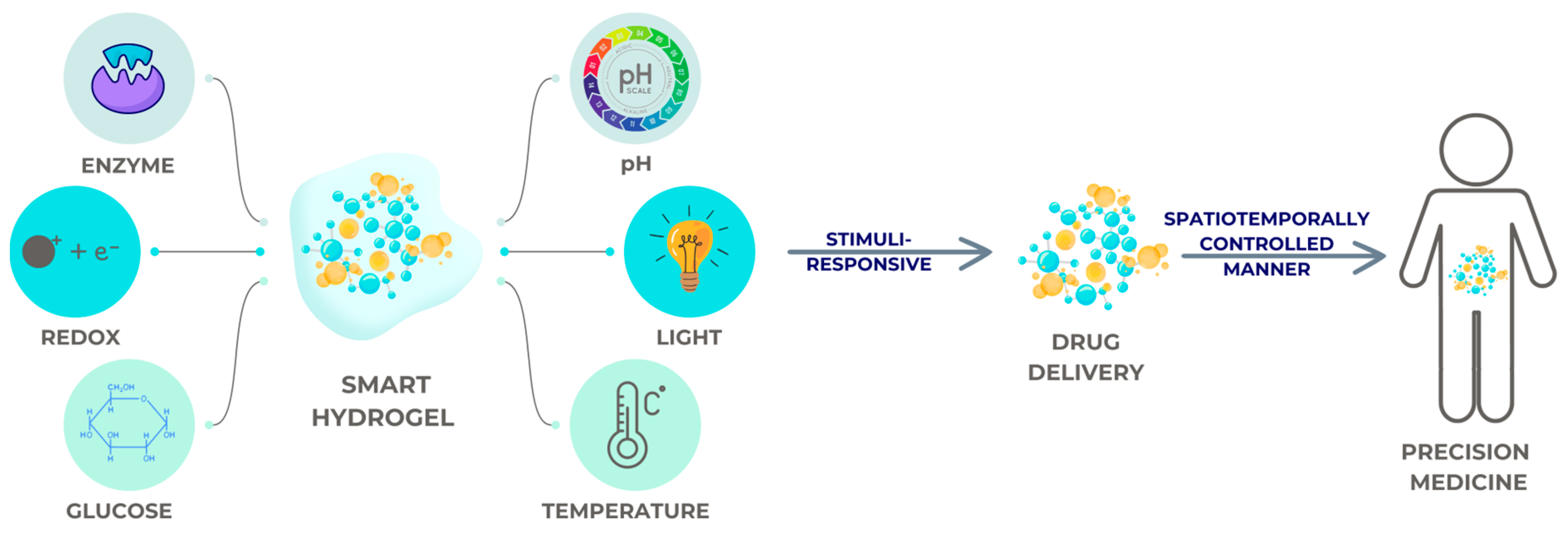
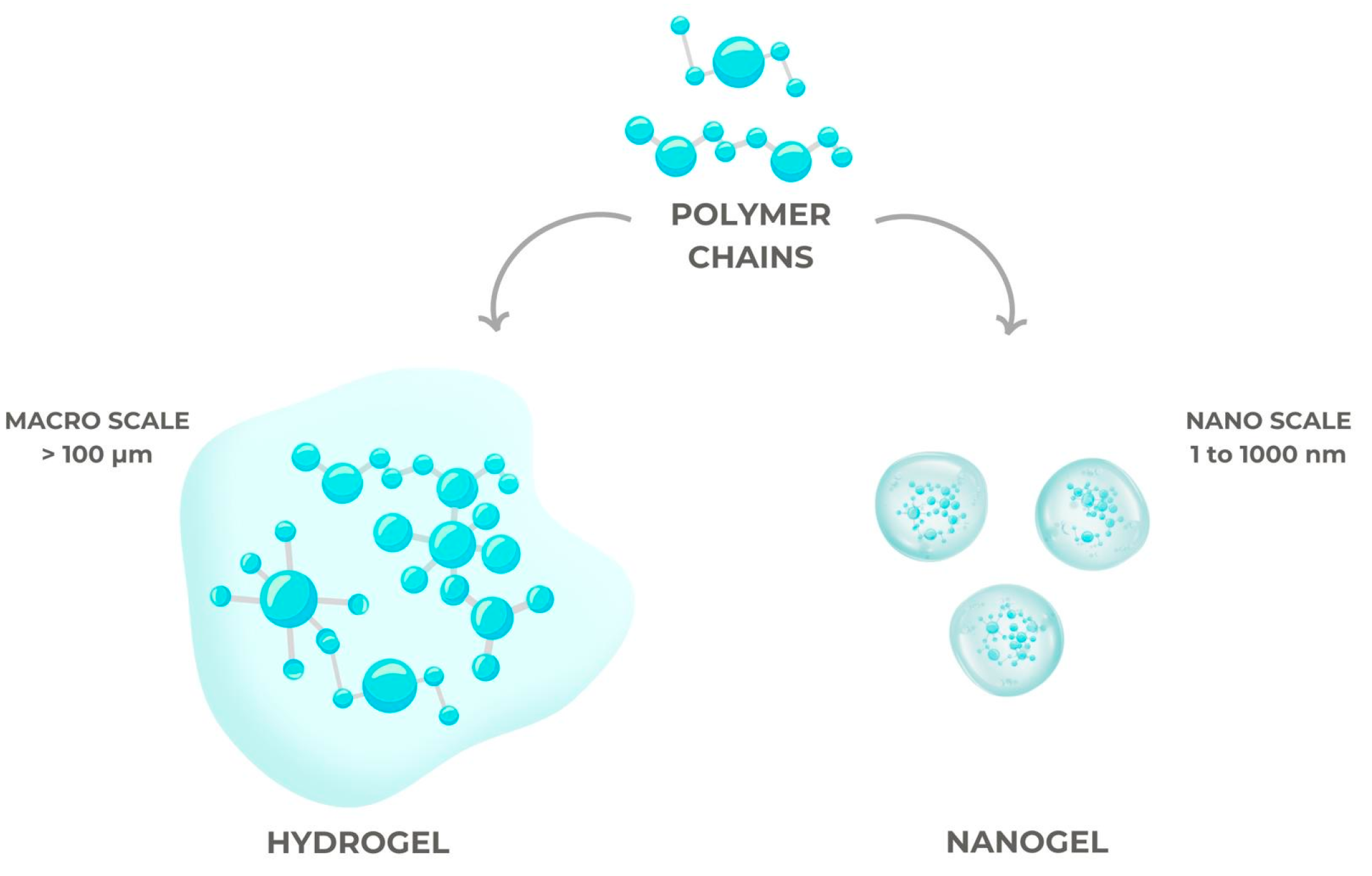
4.2. Conventional Hydrogels, Smart Hydrogels, and Nanogels
5. Innovative Systems for Drug Administration
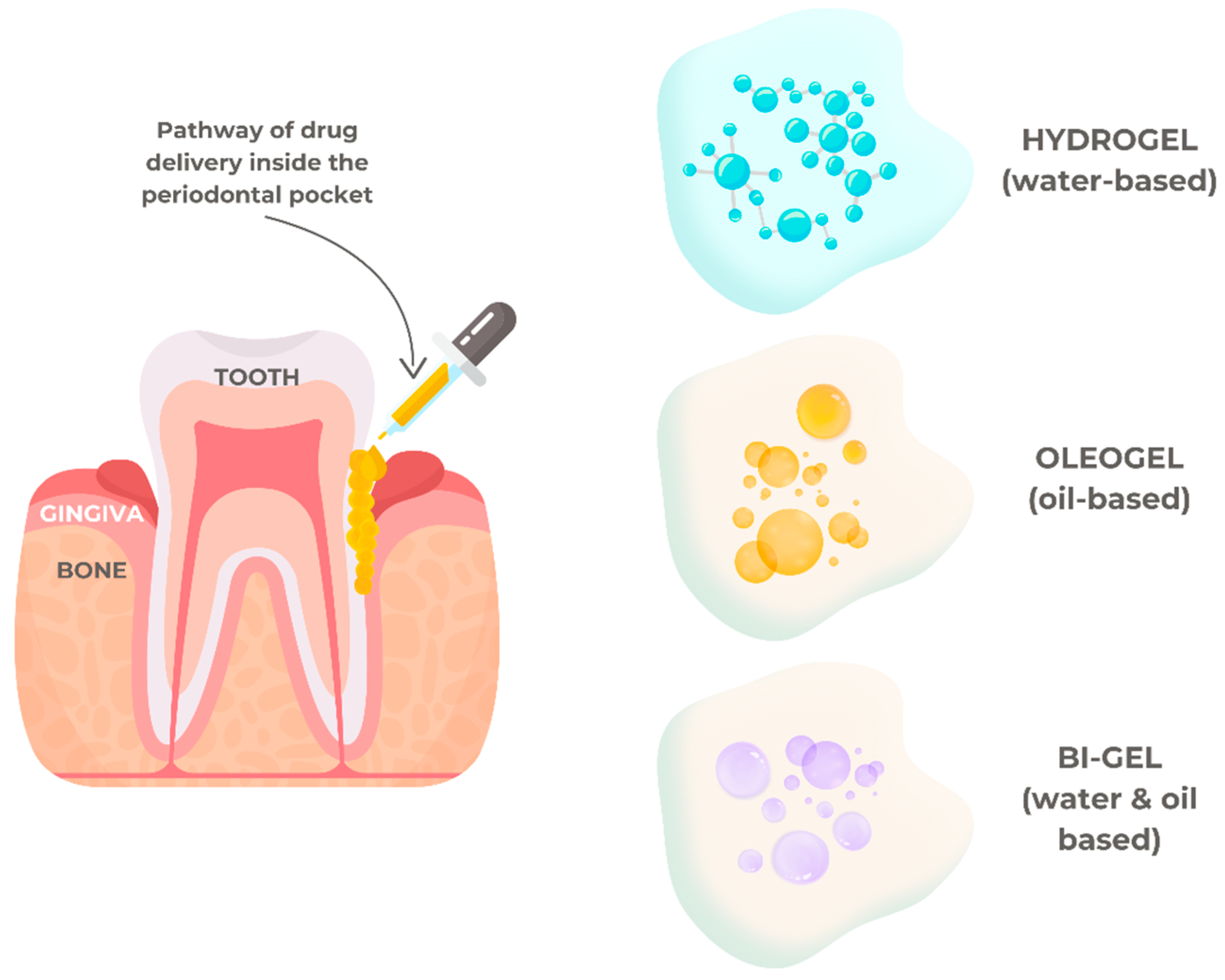
5.1. Drug Delivery in Periodontal Diseases
5.2. Drug Delivery in Dental Caries
5.3. Drug Delivery in Dental Pulp Regeneration
5.4. Drug Delivery in Maxillofacial Diseases
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oliva, N.; Conde, J.; Wang, K.; Artzi, N. Designing Hydrogels for On-Demand Therapy. Acc. Chem. Res. 2017, 50, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Bako, J.; Szepesi, M.; Marton, I.; Borbely, J.; Hegedus, C. Synthesis of nanoparticles for dental drug delivery systems. Fogorv. Szle. 2007, 100, 109–113. [Google Scholar]
- Chuang, C.H.; Lin, R.Z.; Melero-Martin, J.M.; Chen, Y.C. Comparison of covalently and physically cross-linked collagen hydrogels on mediating vascular network formation for engineering adipose tissue. Artif. Cells Nanomed. Biotechnol. 2018, 46, S434–S447. [Google Scholar] [CrossRef] [PubMed]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Nazari, B.; Miller, D.W. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov. Today 2016, 21, 1835–1849. [Google Scholar] [CrossRef]
- Shahini, A.; Yazdimamaghani, M.; Walker, K.J.; Eastman, M.A.; Hatami-Marbini, H.; Smith, B.J.; Ricci, J.L.; Madihally, S.V.; Vashaee, D.; Tayebi, L. 3D conductive nanocomposite scaffold for bone tissue engineering. Int. J. Nanomed. 2014, 9, 167–181. [Google Scholar]
- Kondiah, P.J.; Choonara, Y.E.; Kondiah, P.P.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Pillay, V. A Review of Injectable Polymeric Hydrogel Systems for Application in Bone Tissue Engineering. Molecules 2016, 21, 1580. [Google Scholar] [CrossRef]
- Xiao, Y.; Gu, Y.; Qin, L.; Chen, L.; Chen, X.; Cui, W.; Li, F.; Xiang, N.; He, X. Injectable thermosensitive hydrogel-based drug delivery system for local cancer therapy. Colloids Surf. B Biointerfaces 2021, 200, 111581. [Google Scholar] [CrossRef]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef]
- Arora, S.; Das, G.; Alqarni, M.; Grover, V.; Manzoor Baba, S.; Saluja, P.; Hassan, S.A.B.; Abdulla, A.M.; Bavabeedu, S.S.; Abullais, S.S.; et al. Role of Chitosan Hydrogels in Clinical Dentistry. Gels 2023, 9, 698. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.M. Current Advances in Stimuli-Responsive Hydrogels as Smart Drug Delivery Carriers. Gels 2023, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Zong, S.; Wen, H.; Lv, H.; Li, T.; Tang, R.; Liu, L.; Jiang, J.; Wang, S.; Duan, J. Intelligent hydrogel with both redox and thermo-response based on cellulose nanofiber for controlled drug delivery. Carbohydr. Polym. 2022, 278, 118943. [Google Scholar] [CrossRef] [PubMed]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart hydrogels in tissue engineering and regenerative medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Darvish, S.; Budala, D.-G.; Goriuc, A. Antibacterial Properties of an Experimental Dental Resin Loaded with Gold Nanoshells for Photothermal Therapy Applications. J. Funct. Biomater. 2024, 15, 100. [Google Scholar] [CrossRef]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Haldon, R.; Lee, B. Structure and permeability of porous films of poly (hydroxy ethyl methacrylate). Br. Polym. J. 1972, 4, 491–501. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Peppas, N.A. Effect of the morphology of hydrophilic polymeric matrices on the diffusion and release of water soluble drugs. J. Membr. Sci. 1981, 9, 211–227. [Google Scholar] [CrossRef]
- Song, S.; Cardinalx, J.; Kim, S.; Kim, S. Progestin permeation through polymer membranes V: Progesterone release from monolithic hydrogel devices. J. Pharm. Sci. 1981, 70, 216–219. [Google Scholar] [CrossRef]
- Yean, L.; Bunel, C.; Vairon, J.P. Reversible immobilization of drugs on a hydrogel matrix, 2. Diffusion of free chloramphenicol from poly (2-hydroxyethyl methacrylate) hydrogels. Makromol. Chem. 1990, 191, 1119–1129. [Google Scholar] [CrossRef]
- Roorda, W.; De Vries, M.; De Leede, L.; De Boer, A.; Breimer, D.; Junginger, H. Zero-order release of oxprenolol-HCl, a new approach. J. Control. Release 1988, 7, 45–52. [Google Scholar] [CrossRef]
- Yu, A.C.; Lopez Hernandez, H.; Kim, A.H.; Stapleton, L.M.; Brand, R.J.; Mellor, E.T.; Bauer, C.P.; McCurdy, G.D.; Wolff, A.J., 3rd. Wildfire prevention through prophylactic treatment of high-risk landscapes using viscoelastic retardant fluids. Proc. Natl. Acad. Sci. USA 2019, 116, 20820–20827. [Google Scholar] [CrossRef]
- Migliorini, L.; Santaniello, T.; Yan, Y.; Lenardi, C.; Milani, P. Low-voltage electrically driven homeostatic hydrogel-based actuators for underwater soft robotics. Sens. Actuators B Chem. 2016, 228, 758–766. [Google Scholar] [CrossRef]
- Surlari, Z.; Budală, D.G.; Lupu, C.I.; Stelea, C.G.; Butnaru, O.M.; Luchian, I. Current Progress and Challenges of Using Artificial Intelligence in Clinical Dentistry—A Narrative Review. J. Clin. Med. 2023, 12, 7378. [Google Scholar] [CrossRef]
- Miao, L.; Hu, J.; Lu, M.; Tu, Y.; Chen, X.; Li, Y.; Lin, S.; Li, F.; Hu, S. Alkynyl-functionalization of hydroxypropyl cellulose and thermoresponsive hydrogel thereof prepared with P(NIPAAm-co-HEMAPCL). Carbohydr. Polym. 2016, 137, 433–440. [Google Scholar] [CrossRef]
- Lin, C.Y.; Battistoni, C.M.; Liu, J.C. Redox-Responsive Hydrogels with Decoupled Initial Stiffness and Degradation. Biomacromolecules 2021, 22, 5270–5280. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, Y.; Sun, M.; Jin, M.; Xia, W.; Yang, H.; Wang, T. Effect of Freezing Process on the Microstructure of Gelatin Methacryloyl Hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 810155. [Google Scholar] [CrossRef]
- Antić, K.; Onjia, A.; Vasiljević-Radović, D.; Veličković, Z.; Tomić, S.L. Removal of Nickel Ions from Aqueous Solutions by 2-Hydroxyethyl Acrylate/Itaconic Acid Hydrogels Optimized with Response Surface Methodology. Gels 2021, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, K.; Omidian, H.; Zohuriaan-Mehr, M.J.; Doroudiani, S. Superabsorbent Hydrogel Composites and Nanocomposites: A Review. Polym. Compos. 2011, 32, 277–289. [Google Scholar] [CrossRef]
- Omidian, H.; Chowdhury, S.D. High-Performing Conductive Hydrogels for Wearable Applications. Gels 2023, 9, 549. [Google Scholar] [CrossRef]
- Omidian, H.; Chowdhury, S.D. Advancements and Applications of Injectable Hydrogel Composites in Biomedical Research and Therapy. Gels 2023, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Park, K. Introduction to Hydrogels. In Biomedical Applications of Hydrogels Handbook; Ottenbrite, R., Park, K., Okano, T., Eds.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Omidian, H.; Park, K. Hydrogels. In Fundamentals and Applications of Controlled Release Drug Delivery; Siepmann, J., Siegel, R., Rathbone, M., Eds.; Advances in Delivery Science and Technology; Springer: Boston, MA, USA, 2012. [Google Scholar]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef] [PubMed]
- Aghabegi Moghanjoughi, A.; Khoshnevis, D.; Zarrabi, A. A concise review on smart polymers for controlled drug release. Drug Deliv. Transl. Res. 2016, 6, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as drug delivery systems; pros and cons. Trends Pharm. Sci. 2019, 5, 7–24. [Google Scholar]
- Oluwatoyin, S.M. Application of Nanogel in Drug Delivery. In Hydrogels and Nanogels—Applications in Medicine; Umeyor, C.E., Uronnachi, E., Kakade, P., Eds.; IntechOpen: London, UK, 2023. [Google Scholar]
- Sharpe, L.A.; Daily, A.M.; Horava, S.D.; Peppas, N.A. Therapeutic applications of hydrogels in oral drug delivery. Expert Opin. Drug Deliv. 2014, 6, 901–915. [Google Scholar] [CrossRef]
- Sant, S.; Tao, S.L.; Fisher, O.Z.; Xu, Q.; Peppas, N.A.; Khademhosseini, A. Microfabrication technologies for oral drug delivery. Adv. Drug Deliv Rev. 2012, 64, 496–507. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, Z.; Sun, L.; Li, M.; Han, L.; Wang, J.; Wu, X.; Sang, S. Constructing epidermal rete ridges using a composite hydrogel to enhance multiple signaling pathways for the maintenance of epidermal stem cell niche. Acta Biomater. 2023, 169, 273–288. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, P.; Huang, J.; Yang, L.; Liu, Z.; Fu, D.; Hu, Z.; Huang, W.; Miao, Y. Scalable and high-throughput production of an injectable platelet-rich plasma (PRP)/cell-laden microcarrier/hydrogel composite system for hair follicle tissue engineering. J. Nanobiotechnol. 2022, 20, 465. [Google Scholar] [CrossRef]
- Xin, T.; Mao, J.; Liu, L.; Tang, J.; Wu, L.; Yu, X.; Gu, Y.; Cui, W.; Chen, L. Programmed Sustained Release of Recombinant Human Bone Morphogenetic Protein-2 and Inorganic Ion Composite Hydrogel as Artificial Periosteum. ACS Appl. Mater. Interfaces 2020, 12, 6840–6851. [Google Scholar] [CrossRef]
- Qiu, Y.; Tian, J.; Kong, S.; Feng, Y.; Lu, Y.; Su, L.; Cai, Y.; Li, M.; Chang, J.; Yang, C.; et al. SrCuSi(4) O(10)/GelMA Composite Hydrogel-Mediated Vital Pulp Therapy: Integrating Antibacterial Property and Enhanced Pulp Regeneration Activity. Adv. Healthc. Mater. 2023, 12, e2300546. [Google Scholar] [CrossRef]
- Dong, W.; Ma, W.; Zhao, S.; Wang, Y.; Yao, J.; Liu, Z.; Chen, Z.; Sun, D.; Jiang, Z.; Zhang, M. The surface modification of long carbon fiber reinforced polyether ether ketone with bioactive composite hydrogel for effective osteogenicity. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112451. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, D.; Jang, C.H.; Kim, G.H. Highly elastic 3D-printed gelatin/HA/placental-extract scaffolds for bone tissue engineering. Theranostics 2022, 12, 4051–4066. [Google Scholar] [CrossRef] [PubMed]
- Bushra, A.; Subhani, A.; Islam, N. A comprehensive review on biological and environmental applications of chitosanhydroxyapatite biocomposites. Compos. Pt. C-Open Access 2023, 12, 24. [Google Scholar]
- Budală, D.G.; Luchian, I.; Tatarciuc, M.; Butnaru, O.; Armencia, A.O.; Virvescu, D.I.; Scutariu, M.M.; Rusu, D. Are Local Drug Delivery Systems a Challenge in Clinical Periodontology? J. Clin. Med. 2023, 12, 4137. [Google Scholar] [CrossRef]
- Chen, Y.; Sheng, W.; Lin, J.; Fang, C.; Deng, J.; Zhang, P.; Zhou, M.; Liu, P.; Weng, J.; Yu, F.; et al. Magnesium Oxide Nanoparticle Coordinated Phosphate-Functionalized Chitosan Injectable Hydrogel for Osteogenesis and Angiogenesis in Bone Regeneration. ACS Appl. Mater. Interfaces 2022, 14, 7592–7608. [Google Scholar] [CrossRef]
- Sultana, F.; Imran-Ul-Haque, M.; Arafat, M.; Sharmin, S. An overview of nanogel drug delivery system. J. Appl. Pharm. Sci. 2013, 3, S95–S105. [Google Scholar] [CrossRef]
- Cuggino, J.C.; Blanco, E.R.O.; Gugliotta, L.M.; Igarzabal, C.I.A.; Calderón, M. Crossing biological barriers with nanogels to improve drug delivery performance. J. Control. Release 2019, 307, 221–246. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release Off. J. Control. Release Soc. 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Chen, X.; Song, Z.; Li, S.; Thang, N.T.; Gao, X.; Gong, X.; Guo, M. Facile one-pot synthesis of self-assembled nitrogen-doped carbon dots/cellulose nanofibril hydrogel with enhanced fluorescence and mechanical properties. Green Chem. 2020, 22, 3296–3308. [Google Scholar] [CrossRef]
- Mendoza, L.; Batchelor, W.; Tabor, R.F.; Garnier, G. Gelation mechanism of cellulose nanofibre gels: A colloids and interfacial perspective. J. Colloid Interface Sci. 2018, 509, 39–46. [Google Scholar] [CrossRef]
- Bertasa, M.; Dodero, A.; Alloisio, M.; Vicini, S.; Riedo, C.; Sansonetti, A.; Scalarone, D.; Castellano, M. Agar gel strength: A correlation study between chemical composition and rheological properties. Eur. Polym. J. 2020, 123, 109442. [Google Scholar] [CrossRef]
- Antoniuk, I.; Kaczmarek, D.; Kardos, A.; Varga, I.; Amiel, C. Supramolecular Hydrogel Based on pNIPAm Microgels Connected via Host–Guest Interactions. Polymers 2018, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, S.; Zhang, C.; Liang, K.; Li, J.; Yang, H.; Gu, S.; Bai, Z.; Ye, D.; Xu, W. Photopolymerized maleilated chitosan/thiolterminated poly (vinyl alcohol) hydrogels as potential tissue engineering scaffolds. Carbohydr. Polym. 2018, 184, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.E.; Carberry, B.J.; Worrell, B.T.; Dudaryeva, O.Y.; McBride, M.K.; Bowman, C.N.; Anseth, K.S. Photopolymerized dynamic hydrogels with tunable viscoelastic properties through thioester exchange. Biomaterials 2018, 178, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Wang, L.L.; Chung, J.J.; Kim, Y.-H.; Atluri, P.; Burdick, J.A. Methods to assess shear-thinning hydrogels for application as injectable biomaterials. ACS Biomater. Sci. Eng. 2017, 3, 3146–3160. [Google Scholar] [CrossRef]
- Ye, S.; Wei, B.; Zeng, L. Advances on Hydrogels for Oral Science Research. Gels 2022, 8, 302. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y. Rational Design of Smart Hydrogels for Biomedical Applications. Front. Chem. 2021, 8, 615665. [Google Scholar] [CrossRef]
- Benwood, C.; Chrenek, J.; Kirsch, R.L.; Masri, N.Z.; Richards, H.; Teetzen, K.; Willerth, S.M. Natural Biomaterials and Their Use as Bioinks for Printing Tissues. Bioengineering 2021, 8, 27. [Google Scholar] [CrossRef]
- Şenel, S.; Özdoğan, A.I.; Akca, G. Current Status and Future of Delivery Systems for Prevention and Treatment of Infections in the Oral Cavity. Drug Deliv. Transl. Res. 2021, 11, 1703–1734. [Google Scholar] [CrossRef]
- Seliktar, D. Designing Cell-Compatible Hydrogels for Biomedical Applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef]
- Tabatabaei, F.; Moharamzadeh, K.; Tayebi, L. Fibroblast Encapsulation in Gelatin Methacryloyl (GelMA) versus Collagen Hydrogel as Substrates for Oral Mucosa Tissue Engineering. J. Oral Biol. Craniofacial Res. 2020, 10, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Hu, B.; Yuan, X.; Cai, L.; Gao, H.; Yang, Q. Nanogel: A Versatile Nano-Delivery System for Biomedical Applications. Pharmaceutics 2020, 12, 290. [Google Scholar] [CrossRef]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.L.; Giannobile, W.V. Extracellular Matrix-Based Scaffolding Technologies for Periodontal and Peri-Implant Soft Tissue Regeneration. J. Periodontol. 2020, 91, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Dienes, J.; Browne, S.; Farjun, B.; Amaral Passipieri, J.; Mintz, E.L.; Killian, G.; Healy, K.E.; Christ, G.J. Semisynthetic Hyaluronic Acid-Based Hydrogel Promotes Recovery of the Injured Tibialis Anterior Skeletal Muscle Form and Function. ACS Biomater. Sci. Eng. 2021, 7, 1587–1599. [Google Scholar] [CrossRef]
- Berkovitch, Y.; Seliktar, D. Semi-synthetic hydrogel composition and stiffness regulate neuronal morphogenesis. Int. J. Pharm. 2017, 523, 545–555. [Google Scholar] [CrossRef]
- Park, S.; Park, K.M. Engineered Polymeric Hydrogels for 3D Tissue Models. Polymers 2016, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Ma, D.; Wang, J.; Zhang, S. Structure and properties of semi-interpenetrating network hydrogel based on starch. Carbohydr. Polym. 2015, 133, 448–455. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart hydrogels for advanced drug delivery systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef]
- Chao, Y.; Chen, Q.; Liu, Z. Smart injectable hydrogels for cancer immunotherapy. Adv. Funct. Mater. 2020, 30, 1902785. [Google Scholar] [CrossRef]
- Fuchs, S.; Ernst, A.U.; Wang, L.-H.; Shariati, K.; Wang, X.; Liu, Q.; Ma, M. Hydrogels in emerging technologies for type 1 diabetes. Chem. Rev. 2020, 121, 11458–11526. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Tang, M.; Peng, C.; Wang, G.; Wang, J.; Wang, X.; Chang, X.; Guo, J.; Gui, S. Smart stimuli-responsive hydrogels for drug delivery in periodontitis treatment. Biomed. Pharmacother. 2023, 162, 114688. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, G.; Zare, M.; Ramakrishna, S. Intelligent hydrogels and their biomedical applications. Mater. Adv. 2022, 3, 7757–7772. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J. Control. Release 2020, 328, 251–262. [Google Scholar] [CrossRef]
- Li, C.; Obireddy, S.R.; Lai, W.F. Preparation and use of nanogels as carriers of drugs. Drug Deliv. 2021, 28, 1594–1602. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem. Int. Ed. Engl. 2009, 48, 5418–5429. [Google Scholar] [CrossRef]
- Mauri, E.; Giannitelli, S.M.; Trombetta, M.; Rainer, A. Synthesis of nanogels: Current trends and future outlook. Gels 2021, 7, 36. [Google Scholar] [CrossRef]
- Ma, X.; Li, S.-J.; Liu, Y.; Zhang, T.; Xue, P.; Kang, Y.; Sun, Z.-J.; Xu, Z. Bioengineered nanogels for cancer immunotherapy. Chem. Soc. Rev. 2022, 51, 5136–5174. [Google Scholar] [CrossRef]
- Oishi, M.; Nagasaki, Y. Stimuli-responsive smart nanogels for cancer diagnostics and therapy. Nanomedicine 2010, 5, 451–468. [Google Scholar] [CrossRef]
- Mabrouk, M.; Chejara, D.R.; Mulla, J.A.; Badhe, R.V.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Pillay, V. Design of a novel crosslinked HEC-PAA porous hydrogel composite for dissolution rate and solubility enhancement of efavirenz. Int. J. Pharm. 2015, 490, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Eltahir, S.; Al Homsi, R.; Jagal, J.; Ahmed, I.S.; Haider, M. Graphene Oxide/Chitosan Injectable Composite Hydrogel for Controlled Release of Doxorubicin: An Approach for Enhanced Intratumoral Delivery. Nanomaterials 2022, 12, 4261. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Gupta, A.; Agrawal, A.K.; Jassal, M.; Dinda, A.K.; Koul, V. Bi-layer composite dressing of gelatin nanofibrous mat and poly vinyl alcohol hydrogel for drug delivery and wound healing application: In-vitro and in-vivo studies. J. Biomed. Nanotechnol. 2013, 9, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwari, H.; Dhamecha, D.; Jagwani, S.; Rao, M.; Jadhav, K.; Shaikh, S.; Puzhankara, L.; Jalalpure, S. Local drug delivery systems in the management of periodontitis: A scientific review. J. Control. Release 2019, 307, 393–409. [Google Scholar]
- Makvandi, P.; Josic, U.; Delfi, M.; Pinelli, F.; Jahed, V.; Kaya, E.; Ashrafizadeh, M.; Zarepour, A.; Rossi, F.; Zarrabi, A. Drug delivery (nano) platforms for oral and dental applications: Tissue regeneration, infection control, and cancer management. Adv. Sci. 2021, 8, 2004014. [Google Scholar] [CrossRef]
- Luchian, I.; Budală, D.G.; Baciu, E.-R.; Ursu, R.G.; Diaconu-Popa, D.; Butnaru, O.; Tatarciuc, M. The Involvement of Photobiology in Contemporary Dentistry—A Narrative Review. Int. J. Mol. Sci. 2023, 24, 3985. [Google Scholar] [CrossRef]
- Alavi, S.E.; Ebrahimi Shahmabadi, H. Anthelmintics for drug repurposing: Opportunities and challenges. Saudi Pharm. J. SPJ 2021, 29, 434. [Google Scholar] [CrossRef]
- Liang, J.; Peng, X.; Zhou, X.; Zou, J.; Cheng, L. Emerging Applications of Drug Delivery Systems in Oral Infectious Diseases Prevention and Treatment. Molecules 2020, 25, 516. [Google Scholar] [CrossRef]
- Ghaferi, M.; Raza, A.; Koohi, M.; Zahra, W.; Akbarzadeh, A.; Shahmabadi, H.E.; Alavi, S.E. Impact of PEGylated Liposomal Doxorubicin and Carboplatin Combination on Glioblastoma. Pharmaceutics 2022, 14, 2183. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Álvarez, A.L.; Espinar, F.O.; Méndez, J.B. The application of microencapsulation techniques in the treatment of endodontic and periodontal diseases. Pharmaceutics 2011, 3, 538–571. [Google Scholar] [CrossRef] [PubMed]
- Kmiec, M.; Pighinelli, L.; Tedesco, M.; Silva, M.; Reis, V. Chitosan-properties and applications in dentistry. Adv. Tissue Eng. Regen. Med. 2017, 2, 00035. [Google Scholar]
- Innocenzi, P.; Stagi, L. Carbon-based antiviral nanomaterials: Graphene, C-dots, and fullerenes. A perspective. Chem. Sci. 2020, 11, 6606–6622. [Google Scholar] [CrossRef]
- Maus, A.; Strait, L.; Zhu, D. Nanoparticles as delivery vehicles for antiviral therapeutic drugs. Eng. Regen. 2021, 2, 31–46. [Google Scholar] [CrossRef]
- Esfahani, M.K.M.; Alavi, S.E.; Cabot, P.J.; Islam, N.; Izake, E.L. Application of Mesoporous Silica Nanoparticles in Cancer Therapy and Delivery of Repurposed Anthelmintics for Cancer Therapy. Pharmaceutics 2022, 14, 1579. [Google Scholar] [CrossRef]
- Bernegossi, J.; Calixto, G.M.F.; Sanches, P.R.d.S.; Fontana, C.R.; Cilli, E.M.; Garrido, S.S.; Chorilli, M. Peptide KSL-W-loaded mucoadhesive liquid crystalline vehicle as an alternative treatment for multispecies oral biofilm. Molecules 2016, 21, 37. [Google Scholar] [CrossRef]
- Chang, P.C.; Chao, Y.C.; Hsiao, M.H.; Chou, H.S.; Jheng, Y.H.; Yu, X.H.; Lee, N.; Yang, C.; Liu, D.M. Inhibition of Periodontitis Induction Using a Stimuli-Responsive Hydrogel Carrying Naringin. J. Periodontol. 2017, 88, 190–196. [Google Scholar] [CrossRef]
- Tarawneh, O.; Hamadneh, I.; Huwaitat, R.; Al-Assi, A.R.; El Madani, A. Characterization of chlorhexidine-impregnated cellulosebased hydrogel films intended for the treatment of periodontitis. BioMed Res. Int. 2021, 2021, 9853977. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Bhavsar, C.; Sawarkar, S.; D’Souza, A. Current and novel approaches for control of dental biofilm. Int. J. Pharm. 2018, 536, 199–210. [Google Scholar] [CrossRef]
- Yadav, R.; Kanwar, I.L.; Haider, T.; Pandey, V.; Gour, V.; Soni, V. In Situ gel drug delivery system for periodontitis: An insight review. Future J. Pharm. Sci. 2020, 6, 1–13. [Google Scholar]
- Rehman, K.; Amin, M.C.I.M.; Zulfakar, M.H. Development and physical characterization of polymer-fish oil bigel (hydrogel/oleogel) system as a transdermal drug delivery vehicle. J. Oleo Sci. 2014, 63, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Juvekar, S.; Kathpalia, H. Solvent removal precipitation based in situ forming implant for controlled drug delivery in periodontitis. J. Control. Release 2017, 251, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Hamed, R.; AbuRezeq, A.; Tarawneh, O. Development of hydrogels, oleogels, and bigels as local drug delivery systems for periodontitis. Drug Dev. Ind. Pharm. 2018, 44, 1488–1497. [Google Scholar] [CrossRef]
- Gopalakrishna, P.K.; Jayaramu, R.A.; Boregowda, S.S.; Eshwar, S.; Suresh, N.V.; Abu Lila, A.S.; Moin, A.; Alotaibi, H.F.; Obaidullah, A.J.; Khafagy, E.-S. Piperine-Loaded In Situ Gel: Formulation, In Vitro Characterization, and Clinical Evaluation against Periodontitis. Gels 2023, 9, 577. [Google Scholar] [CrossRef]
- Su, K.; Wang, C. Recent Advances in the Use of Gelatin in Biomedical Research. Biotechnol. Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef]
- Budala, D.G.; Martu, M.-A.; Maftei, G.-A.; Diaconu-Popa, D.A.; Danila, V.; Luchian, I. The Role of Natural Compounds in Optimizing Contemporary Dental Treatment—Current Status and Future Trends. J. Funct. Biomater. 2023, 14, 273. [Google Scholar] [CrossRef]
- Huang, J.; Tian, Z.; Su, X.; Xie, W. Gelatin-Based Low-Temperature Injection Anti-Inflammatory Antibacterial Viscous Hydrogel as Well as Preparation Method and Application Thereof. CN Patent No. CN113230448B, 19 May 2021. [Google Scholar]
- Ruocco, N.; Costantini, S.; Guariniello, S.; Costantini, M. Polysaccharides from the marine environment with pharmacological, cosmeceutical and nutraceutical potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Bao, Z.; Bi, S.; Li, Y.; Yu, X.; Hu, S.; Tian, M.; Zhang, X.; Cheng, X.; Chen, X. Chitosan-based thermo/pH double sensitive hydrogel for controlled drug delivery. Macromol. Biosci. 2018, 18, 1700305. [Google Scholar] [CrossRef]
- Blanchemain, N.; Martel, B.; Flores, C.; Cazaux, F.; Chai, F.; Tabary, N.; Lopez, H.M. Method for the Production of Hydrogel Comprising Chitosan and Negatively Charged Polyelectrolytes, and Cellular, Porous Material Resulting from Said Hydrogel. EP Patent No. EP3317326B1, 2 July 2015. [Google Scholar]
- Swain, G.P.; Patel, S.; Gandhi, J.; Shah, P. Development of Moxifloxacin Hydrochloride loaded in-situ gel for the treatment of periodontitis: In-vitro drug release study and antibacterial activity. J. Oral Biol. Craniofacial Res. 2019, 9, 190–200. [Google Scholar] [CrossRef]
- Ranch, K.M.; Maulvi, F.A.; Koli, A.R.; Desai, D.T.; Parikh, R.K.; Shah, D.O. Tailored Doxycycline Hyclate Loaded In Situ Gel for the Treatment of Periodontitis: Optimization, In Vitro Characterization, and Antimicrobial Studies. AAPS PharmSciTech 2021, 22, 1–11. [Google Scholar] [CrossRef]
- de Santana, R.B.; de Santana, C.M.M. Human intrabony defect regeneration with rhFGF-2 and hyaluronic acid—A randomized controlled clinical trial. J. Clin. Periodontol. 2015, 42, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Olszewska-Czyz, I.; Kralik, K.; Prpic, J. Biomolecules in Dental Applications: Randomized, Controlled Clinical Trial Evaluating the Influence of Hyaluronic Acid Adjunctive Therapy on Clinical Parameters of Moderate Periodontitis. Biomolecules 2021, 11, 1491. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Yanagawa, F.; Sugiura, S.; Takagi, T.; Sumaru, K.; Kanamori, T. Click-crosslinkable and photodegradable gelatin hydrogels for cytocompatible optical cell manipulation in natural environment. Sci. Rep. 2015, 9, 15060. [Google Scholar] [CrossRef]
- Hussain, B.; Karaca, E.O.; Kuru, B.E.; Gursoy, H.; Haugen, H.J.; Wohlfahrt, J.C. Treatment of residual pockets using an oscillating chitosan device versus regular curettes alone—A randomized, feasibility parallel-arm clinical trial. J. Periodontol. 2021, 93, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Chen, M.; Long, L.; Ren, X. Antibacterial Keratin-Based Hydrogel and Preparation Method Thereof. CN Patent No. CN110511405B, 11 May 2019. [Google Scholar]
- Muntean, A.; Sarosi, C.; Petean, I.; Cuc, S.; Carpa, R.; Chis, I.A.; Ilea, A.; Delean, A.G.; Moldovan, M. Developing Bioactive Hydrogels with Peptides for Dental Application. Biomedicines 2024, 12, 694. [Google Scholar] [CrossRef]
- Ding, L.; Han, S.; Wang, K.; Zheng, S.; Zheng, W.; Peng, X.; Niu, Y.; Li, W.; Zhang, L. Remineralization of enamel caries by an amelogenin-derived peptide and fluoride in vitro. Regen. Biomater. 2020, 7, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Chakraborty, A.; Sandhu, G.; Naim, S.; Nowotny, E.B.; Moradian-Oldak, J. Amelogenin Peptide-Chitosan Hydrogel for Biomimetic Enamel Regrowth. Front. Dent. Med. 2021, 2, 697544. [Google Scholar] [CrossRef]
- Campodoni, E.; Dozio, S.M.; Panseri, S.; Montesi, M.; Tampieri, A.; Sandri, M. Mimicking Natural Microenvironments: Design of 3D-Aligned Hybrid Scaffold for Dentin Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 836. [Google Scholar] [CrossRef]
- Ren, Q.; Ding, L.; Li, Z.; Wang, X.; Wang, K.; Han, S.; Li, W.; Zhou, X.; Zhang, L. Chitosan hydrogel containing amelogenin-derived peptide: Inhibition of cariogenic bacteria and promotion of remineralization of initial caries lesions. Arch. Oral. Biol. 2019, 100, 42–48. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Elsalawy, R.; Ibrahim, N.; Gadalla, M.; Albargasy, H.; Zahra, N.; Mokhtar, S.; El Nahhas, N.; El Kaliouby, Y.; Dorfer, C.E. The Dental Pulp Stem/Progenitor Cells-Mediated Inflammatory-Regenerative Axis. Tissue Eng. Part B Rev. 2019, 25, 445–460. [Google Scholar] [CrossRef]
- Chang, B.; Ahuja, N.; Ma, C.; Liu, X. Injectable scaffolds: Preparation and application in dental and craniofacial regeneration. Mater. Sci. Eng. R Rep. 2017, 111, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xie, L.; Wu, H.; Yang, T.; Zhang, Q.; Tian, Y.; Liu, Y.; Han, X.; Guo, W.; He, M. Alginate/laponite hydrogel microspheres co-encapsulating dental pulp stem cells and VEGF for endodontic regeneration. Acta Biomater. 2020, 113, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Holiel, A.A.; Mahmoud, E.M.; Abdel-Fattah, W.M.; Kawana, K.Y. Histological evaluation of the regenerative potential of a novel treated dentin matrix hydrogel in direct pulp capping. Clin. Oral Investig. 2020, 25, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Osmond, M.J.; Krebs, M.D. Tunable chitosan-calcium phosphate composites as cell-instructive dental pulp capping agents. J. Biomater. Sci.-Polym. Ed. 2021, 32, 1450–1465. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.G.; Anovazzi, G.; Bordini, E.A.F.; Zuta, U.O.; Silva Leite, M.L.A.; Basso, F.G.; Hebling, J.; de Souza Costa, C.A. Biological Analysis of Simvastatin-releasing Chitosan Scaffold as a Cell-free System for Pulp-dentin Regeneration. J. Endod. 2018, 44, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Limjeerajarus, C.N.; Kreua-Ongarjnukool, N.; Seang, S.; Pavasant, P.; Niyomthai, S.T. Characterization of a Thermo-Sensitive Injectable Hydrogel as an Iloprost Delivery System for Dental Use. Key Eng. Mater. 2020, 856, 391–398. [Google Scholar] [CrossRef]
- Matoug-Elwerfelli, M.; Nazzal, H.; Duggal, M.; El-Gendy, R. What the future holds for regenerative endodontics: Novel antimicrobials and regenerative strategies. Eur. Cells Mater. 2021, 41, 811–833. [Google Scholar] [CrossRef]
- Samiei, M.; Fathi, M.; Barar, J.; Fathi, N.; Amiryaghoubi, N.; Omidi, Y. Bioactive hydrogel-based scaffolds for the regeneration of dental pulp tissue. J. Drug Deliv. Sci. Technol. 2021, 64, 102600. [Google Scholar] [CrossRef]
- Hayden, R.E.; Mullin, D.P.; Patel, A.K. Reconstruction of the segmental mandibular defect: Current state of the art. Curr. Opin. Otolaryngol. Head. Neck. Surg. 2012, 20, 231–236. [Google Scholar] [CrossRef]
- Gordon, M.; Hahn, R. Collagens. Cell Tissue Res. 2009, 339, 247–257. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annl. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S.; Huang, C.; Chen, Y.; Ding, Z.; Chen, L. Temperature-Sensitive Collagen-Based Hydrogel Loaded with Bioactive Polypeptides and Preparation Method of Temperature-Sensitive Collagen-Based Hydrogel. CN Patent No. CN111184917B, 27 February 2020. [Google Scholar]
- Lei, L.; Liu, Z.; Yuan, P.; Jin, R.; Wang, X.; Jiang, T.; Chen, X. Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of microRNA-222 and aspirin to achieve innervated bone regeneration in rat mandibular defects. J. Mater. Chem. B 2019, 7, 2722–2735. [Google Scholar] [CrossRef]
- Pan, H.; Han, J.J.; Park, Y.D.; Cho, T.H.; Hwang, S.J. Effect of sustained release of rhBMP-2 from dried and wet hyaluronic acid hydrogel carriers compared with direct dip coating of rhBMP-2 on peri-implant osteogenesis of dental implants in canine mandibles. J. Cranio-Maxill. Surg. 2016, 44, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Li, Z.; Zhang, Y.; Shen, K.; Zhang, X.; Xie, R.; Liu, F.; Fan, W. Injectable ultrasonication-induced silk fibroin hydrogel for cartilage repair and regeneration. Tissue Eng. Part A 2020, 27, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Liu, K. Injectable Silk Fibroin Porous Hydrogel and Preparation Method Thereof. CN Patent No. CN109851819B, 8 March 2019. [Google Scholar]
- Xu, W.; Sun, Y.; Wang, J.; Wang, B.; Xu, F.; Xie, Z.; Wang, Y. Controlled release of silibinin in GelMA hydrogels inhibits inflammation by inducing M2-type macrophage polarization and promotes vascularization in vitro. RSC Adv. 2020, 12, 13192–13202. [Google Scholar] [CrossRef]
- Davis-Searles, P.R.; Nakanishi, Y.; Kim, N.-C.; Graf, T.N.; Oberlies, N.H.; Wani, M.C.; Wall, M.E.; Agarwal, R.; Kroll, D.J. Milk thistle and prostate cancer: Differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005, 65, 4448–4457. [Google Scholar] [CrossRef]
- Espacenet Patent Search. Available online: https://worldwide.espacenet.com (accessed on 29 September 2024).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fratila, D.N.; Virvescu, D.I.; Luchian, I.; Hancianu, M.; Baciu, E.R.; Butnaru, O.; Budala, D.G. Advances and Functional Integration of Hydrogel Composites as Drug Delivery Systems in Contemporary Dentistry. Gels 2024, 10, 661. https://doi.org/10.3390/gels10100661
Fratila DN, Virvescu DI, Luchian I, Hancianu M, Baciu ER, Butnaru O, Budala DG. Advances and Functional Integration of Hydrogel Composites as Drug Delivery Systems in Contemporary Dentistry. Gels. 2024; 10(10):661. https://doi.org/10.3390/gels10100661
Chicago/Turabian StyleFratila, Dragos Nicolae, Dragos Ioan Virvescu, Ionut Luchian, Monica Hancianu, Elena Raluca Baciu, Oana Butnaru, and Dana Gabriela Budala. 2024. "Advances and Functional Integration of Hydrogel Composites as Drug Delivery Systems in Contemporary Dentistry" Gels 10, no. 10: 661. https://doi.org/10.3390/gels10100661
APA StyleFratila, D. N., Virvescu, D. I., Luchian, I., Hancianu, M., Baciu, E. R., Butnaru, O., & Budala, D. G. (2024). Advances and Functional Integration of Hydrogel Composites as Drug Delivery Systems in Contemporary Dentistry. Gels, 10(10), 661. https://doi.org/10.3390/gels10100661









