Tuning Antioxidant Function through Dynamic Design of Chitosan-Based Hydrogels
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Characterization
2.2. Morphology of the Hydrogels
2.3. Swelling Studies of 5-Methoxysalicyl-Imine-Chitosan Hydrogels (Sx)
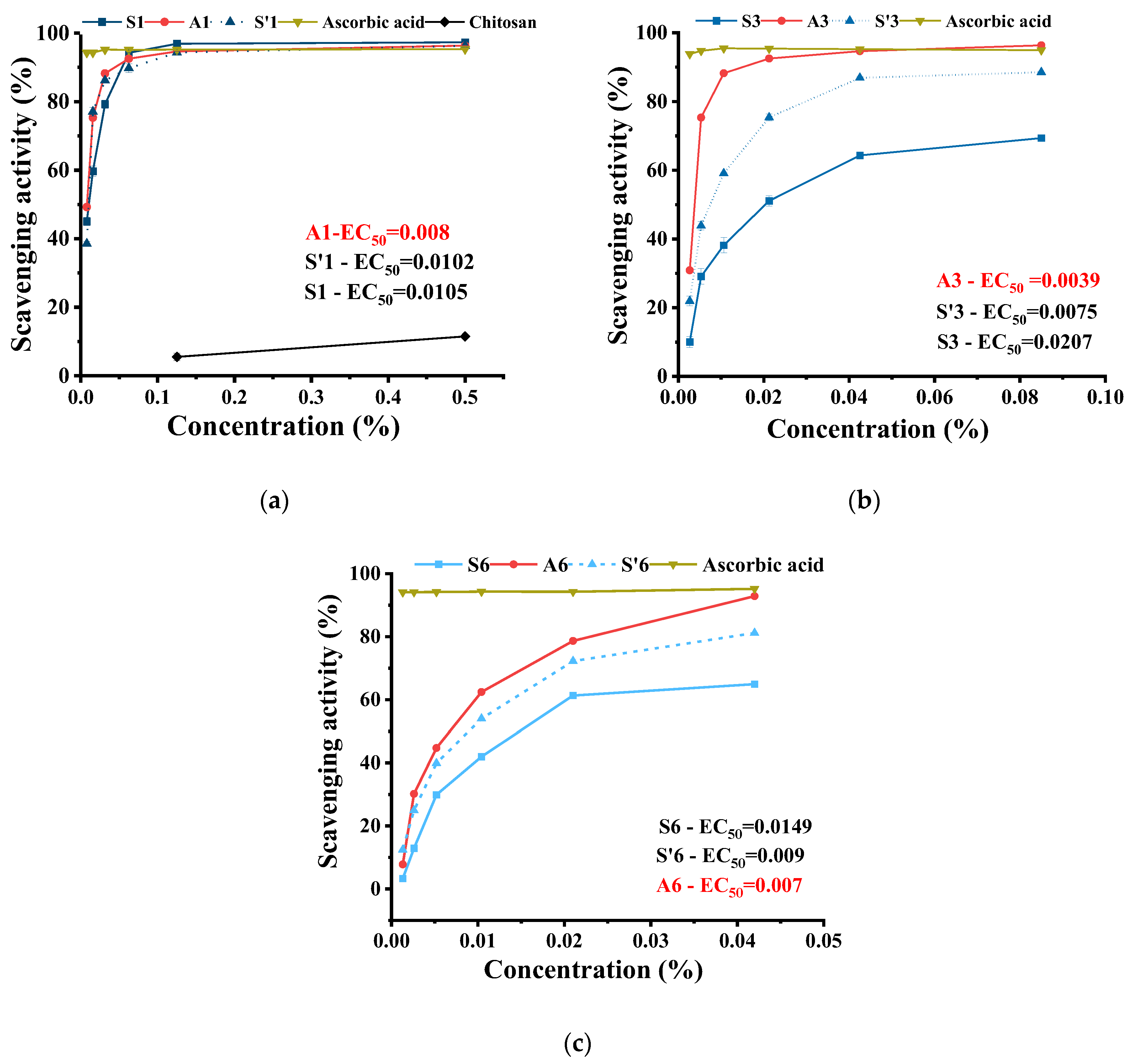
2.4. The Antioxidant Activity of the Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of 5-Methoxysalicyl-Imine-Chitosan Hydrogels (Sx)
4.3. Characterization
4.3.1. Xerogels
4.3.2. NMR Spectroscopy
4.3.3. Fourier–Transform Infrared (FTIR) Spectroscopy
4.3.4. The Scanning Electron Microscopy (SEM)
4.3.5. The Polarized Light Microscopy (PLM)
4.3.6. The Cumulative Aldehyde Releases
4.3.7. Swelling Behavior and Stability of the Xerogels in Media with Different pH
4.3.8. The Antioxidant Activity
4.3.9. Gel Fraction
4.3.10. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Ghany, N.A.; Abdel Aziz, M.S.; Abdel-Aziz, M.M.; Mahmoud, Z.M. Reinforcement of antimicrobial activity and swelling ability of starch-g-poly 4-acrylamidobenzoic acid using chitosan nanoparticles. Cellul. Chem. Technol. 2023, 57, 803–813. [Google Scholar] [CrossRef]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Sezgin-Bayindir, Z.; Losada-Barreiro, S.; Fernández-Bravo, S.; Bravo-Díaz, C. Innovative Delivery and Release Systems for Antioxidants and Other Active Substances in the Treatment of Cancer. Pharmaceuticals 2023, 16, 1038. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mu, Y.; Xu, Q.; Jin, L.; Fu, Y. Injectable, Rapid Self-Healing, Antioxidant and Antibacterial Nanocellulose-Tannin Hydrogels Formed via Metal-Ligand Coordination for Drug Delivery and Wound Dressing. Ind. Crop. Prod. 2024, 208, 117876. [Google Scholar] [CrossRef]
- Maeso, L.; Antezana, P.E.; Hvozda Arana, A.G.; Evelson, P.A.; Orive, G.; Desimone, M.F. Progress in the Use of Hydrogels for Antioxidant Delivery in Skin Wounds. Pharmaceutics 2024, 16, 524. [Google Scholar] [CrossRef]
- Hameed, H.; Faheem, S.; Paiva-Santos, A.C.; Sarwar, H.S.; Jamshaid, M. A Comprehensive Review of Hydrogel-Based Drug Delivery Systems: Classification, Properties, Recent Trends, and Applications. AAPS PharmSciTech 2024, 25, 64. [Google Scholar] [CrossRef]
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. [Google Scholar] [CrossRef]
- Solanki, R.; Dhanka, M.; Thareja, P.; Bhatia, D. Self-Healing, Injectable Chitosan-Based Hydrogels: Structure, Properties and Biological Applications. Mater. Adv. 2024, 5, 5365–5393. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Chen, Q.; Fu, L.; Tao, L.; Wei, Y. Injectable and Self-Healing Chitosan Hydrogel Based on Imine Bonds: Design and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 2198. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y.; Zhang, Z.; Li, C.; Mei, L.; Hou, R.; Liu, X.; Jiang, H. Effect of Chitosan and Its Water-Soluble Derivatives on Antioxidant Activity. Polymers 2024, 16, 867. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, K.; Raorane, C.J.; Ramkumar, V.; Ulagesan, S.; Santhamoorthy, M.; Raj, V.; Krishnakumar, G.S.; Phan, T.T.V.; Kim, S.-C. Update on Chitosan-Based Hydrogels: Preparation, Characterization, and Its Antimicrobial and Antibiofilm Applications. Gels 2022, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Qiu, P.; Wang, Y.; Ren, P.; Liu, J.; Zhao, J.; Gou, D. Chitosan-Based Hydrogels: From Preparation to Applications, a Review. Food Chem. X 2024, 21, 101095. [Google Scholar] [CrossRef] [PubMed]
- Domalik-Pyzik, P.; Chłopek, J.; Pielichowska, K. Chitosan-Based Hydrogels: Preparation, Properties, and Applications. In Cellulose-Based Superabsorbent Hydrogels; Springer: Cham, Switzerland, 2019; pp. 1665–1693. [Google Scholar]
- Yu, J.; Gaedke, M.; Schaufelberger, F. Dynamic Covalent Chemistry for Synthesis and Co-conformational Control of Mechanically Interlocked Molecules. Eur. J. Org. Chem. 2023, 26, e202201130. [Google Scholar] [CrossRef]
- Guo, Q.; Long, R. Mechanics of Polymer Networks with Dynamic Bonds. In Self-Healing and Self-Recovering Hydrogels; Springer: Cham, Switzerland, 2020; pp. 127–164. [Google Scholar]
- Yu, R.; Li, S. Synthesis, Properties, and Applications of Carboxymethyl Chitosan-Based Hydrogels. In Multifaceted Carboxymethyl Chitosan Derivatives: Properties and Biomedical Applications; Springer: Cham, Switzerland, 2023; pp. 59–87. [Google Scholar]
- Xianyu, B.; Xu, H. Dynamic Covalent Bond-Based Materials: From Construction to Biomedical Applications. Supramol. Mater. 2024, 3, 100070. [Google Scholar] [CrossRef]
- Sharma, M.; Thakur, A. Exploring Chitosan Hydrogels: Electrochemical Detection to Biomedical Applications. In Electrocatalytic Materials; Springer Nature: Cham, Switzerland, 2024; pp. 561–573. [Google Scholar]
- Xu, J.; Liu, Y.; Hsu, S.H. Hydrogels Based on Schiff Base Linkages for Biomedical Applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef] [PubMed]
- Marin, L.; Ailincai, D.; Mares, M.; Paslaru, E.; Cristea, M.; Nica, V.; Simionescu, B.C. Imino-Chitosan Biopolymeric Films. Obtaining, Self-Assembling, Surface and Antimicrobial Properties. Carbohydr. Polym. 2015, 117, 762–770. [Google Scholar] [CrossRef]
- Olaru, A.-M.; Marin, L.; Morariu, S.; Pricope, G.; Pinteala, M.; Tartau-Mititelu, L. Biocompatible Chitosan Based Hydrogels for Potential Application in Local Tumour Therapy. Carbohydr. Polym. 2018, 179, 59–70. [Google Scholar] [CrossRef]
- Marin, L.; Ailincai, D.; Morariu, S.; Tartau-Mititelu, L. Development of Biocompatible Glycodynameric Hydrogels Joining Two Natural Motifs by Dynamic Constitutional Chemistry. Carbohydr. Polym. 2017, 170, 60–71. [Google Scholar] [CrossRef]
- Ailincai, D.; Marin, L.; Morariu, S.; Mares, M.; Bostanaru, A.-C.; Pinteala, M.; Simionescu, B.C.; Barboiu, M. Dual Crosslinked Iminoboronate-Chitosan Hydrogels with Strong Antifungal Activity against Candida Planktonic Yeasts and Biofilms. Carbohydr. Polym. 2016, 152, 306–316. [Google Scholar] [CrossRef]
- Ailincai, D.; Turin Moleavin, I.-A.; Sarghi, A.; Fifere, A.; Dumbrava, O.; Pinteala, M.; Balan, G.G.; Rosca, I. New Hydrogels Nanocomposites Based on Chitosan, 2-Formylphenylboronic Acid, and ZnO Nanoparticles as Promising Disinfectants for Duodenoscopes Reprocessing. Polymers 2023, 15, 2669. [Google Scholar] [CrossRef] [PubMed]
- Iftime, M.-M.; Morariu, S.; Marin, L. Salicyl-Imine-Chitosan Hydrogels: Supramolecular Architecturing as a Crosslinking Method toward Multifunctional Hydrogels. Carbohydr. Polym. 2017, 165, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Iftime, M.-M.; Morariu, S. Rheological properties of salicyl-imine-chitosan hydrogels: Effect of crosslinking density. Cellul. Chem. Technol. 2022, 56, 757–765. [Google Scholar] [CrossRef]
- Iftime, M.-M.; Rosca, I.; Sandu, A.-I.; Marin, L. Chitosan Crosslinking with a Vanillin Isomer toward Self-Healing Hydrogels with Antifungal Activity. Int. J. Biol. Macromol. 2022, 205, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, H.; Attjioui, M.; Ferreira, A.; Dockal, E.; El Gueddari, N.; Moerschbacher, B.; Cavalheiro, É. Synthesis, Characterization and Biological Activities of Biopolymeric Schiff Bases Prepared with Chitosan and Salicylaldehydes and Their Pd(II) and Pt(II) Complexes. Molecules 2017, 22, 1987. [Google Scholar] [CrossRef]
- Caro, C.A.; Cabello, G.; Landaeta, E.; Pérez, J.; Zagal, J.H.; Lillo, L. Synthesis and Spectroscopic and Electrochemical Studies of Chitosan Schiff Base Derivatives. Russ. J. Appl. Chem. 2013, 86, 1791–1797. [Google Scholar] [CrossRef]
- dos Santos, J.E.; Dockal, E.R.; Cavalheiro, É.T.G. Synthesis and Characterization of Schiff Bases from Chitosan and Salicylaldehyde Derivatives. Carbohydr. Polym. 2005, 60, 277–282. [Google Scholar] [CrossRef]
- Damiri, F.; Bachra, Y.; Bounacir, C.; Laaraibi, A.; Berrada, M. Synthesis and Characterization of Lyophilized Chitosan-Based Hydrogels Cross-Linked with Benzaldehyde for Controlled Drug Release. J. Chem. 2020, 2020, 8747639. [Google Scholar] [CrossRef]
- Yadav, P.; Afgan, S.; Singh, V.; Pal, K.; Jaiswal, S.; Kumar, R.; Koch, B. Generation of Multicellular Tumor Spheroids via 3D Cell Culture Utilizing a Hydrogel Comprising Chitosan and Allylthiourea. New J. Chem. 2023, 47, 14684–14698. [Google Scholar] [CrossRef]
- Cibotaru, S.; Ailincai, D.; Andreica, B.-I.; Cheng, X.; Marin, L. TEGylated Phenothiazine-Imine-Chitosan Materials as a Promising Framework for Mercury Recovery. Gels 2022, 8, 692. [Google Scholar] [CrossRef]
- Chu, G.; Li, C. Convenient and Clean Synthesis of Imines from Primary Benzylamines. Org. Biomol. Chem. 2010, 8, 4716. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Wang, X.; Chen, S.; Chen, H.; Ouyang, J.; Yang, L.; Wang, X.; You, L.; Utz, M.; Tian, Z.; et al. Quantification and Prediction of Imine Formation Kinetics in Aqueous Solution by Microfluidic NMR Spectroscopy. Chem.—A Eur. J. 2021, 27, 9508–9513. [Google Scholar] [CrossRef] [PubMed]
- Saggiomo, V.; Lüning, U. On the Formation of Imines in Water—A Comparison. Tetrahedron Lett. 2009, 50, 4663–4665. [Google Scholar] [CrossRef]
- Zhang, Z.; He, C.; Chen, X. Hydrogels Based on PH-Responsive Reversible Carbon–Nitrogen Double-Bond Linkages for Biomedical Applications. Mater. Chem. Front. 2018, 2, 1765–1778. [Google Scholar] [CrossRef]
- Romo-Uribe, A. On the Banded Texture and Nematic Elasticity in a Thermotropic Liquid Crystalline Polymer. In Situ Optical Microscopy and Light Scattering. Polym. Adv. Technol. 2020, 31, 3008–3019. [Google Scholar] [CrossRef]
- Marin, L.; Destri, S.; Porzio, W.; Bertini, F. Synthesis and Characterization of New Azomethine Derivatives Exhibiting Liquid Crystalline Properties. Liq. Cryst. 2009, 36, 21–32. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, S.; Zhang, Y.; Chi, Z.; Xu, J. A PH-Responsive Polymer Based on Dynamic Imine Bonds as a Drug Delivery Material with Pseudo Target Release Behavior. Polym. Chem. 2018, 9, 878–884. [Google Scholar] [CrossRef]
- Qu, X.; Yang, Z. Benzoic-Imine-Based Physiological-pH-Responsive Materials for Biomedical Applications. Chem. Asian J. 2016, 11, 2633–2641. [Google Scholar] [CrossRef]
- Dash, A.C.; Dash, B.; Praharaj, S. Hydrolysis of Imines: Kinetics and Mechanism of Spontaneous Acid-, Base-, and Metal Ion-Induced Hydrolysis of N-Salicylidene-2-Aminothiazole. J. Chem. Soc. Dalton Trans. 1981, 1, 2063. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. PH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Peters, J.T.; Wechsler, M.E.; Peppas, N.A. Advanced Biomedical Hydrogels: Molecular Architecture and Its Impact on Medical Applications. Regen. Biomater. 2021, 8, rbab060. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Günter, E.A.; Melekhin, A.K.; Belozerov, V.S.; Martinson, E.A.; Litvinets, S.G. Preparation, Physicochemical Characterization and Swelling Properties of Composite Hydrogel Microparticles Based on Gelatin and Pectins with Different Structure. Int. J. Biol. Macromol. 2024, 258, 128935. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.H.; Ahmad, M.B.; Ibrahim, N.A.; Zainuddin, N. Effect of Crosslinking Concentration on Properties of 3-(Trimethoxysilyl) Propyl Methacrylate/N-Vinyl Pyrrolidone Gels. Chem. Cent. J. 2018, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Belowich, M.E.; Stoddart, J.F. Dynamic Imine Chemistry. Chem. Soc. Rev. 2012, 41, 2003. [Google Scholar] [CrossRef]
- Liu, F.; Anton, N.; Niko, Y.; Klymchenko, A.S. Controlled Release and Capture of Aldehydes by Dynamic Imine Chemistry in Nanoemulsions: From Delivery to Detoxification. ACS Appl. Bio Mater. 2023, 6, 246–256. [Google Scholar] [CrossRef]
- Marin, L.; Popa, M.; Anisiei, A.; Irimiciuc, S.-A.; Agop, M.; Petrescu, T.-C.; Vasincu, D.; Himiniuc, L. A Theoretical Model for Release Dynamics of an Antifungal Agent Covalently Bonded to the Chitosan. Molecules 2021, 26, 2089. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical Modeling of Drug Delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef]
- Treenate, P.; Monvisade, P. In Vitro Drug Release Profiles of PH-Sensitive Hydroxyethylacryl Chitosan/Sodium Alginate Hydrogels Using Paracetamol as a Soluble Model Drug. Int. J. Biol. Macromol. 2017, 99, 71–78. [Google Scholar] [CrossRef]
- Paul, D.R. Elaborations on the Higuchi Model for Drug Delivery. Int. J. Pharm. 2011, 418, 13–17. [Google Scholar] [CrossRef]
- Iftime, M.-M.; Mititelu Tartau, L.; Marin, L. New Formulations Based on Salicyl-Imine-Chitosan Hydrogels for Prolonged Drug Release. Int. J. Biol. Macromol. 2020, 160, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Rehim, H.A.; El-Sawy, N.M.; Hegazy, E.-S.A.; Soliman, E.-S.A.; Elbarbary, A.M. Improvement of Antioxidant Activity of Chitosan by Chemical Treatment and Ionizing Radiation. Int. J. Biol. Macromol. 2012, 50, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, C.; Wu, T.; Yuan, C.; Hu, Y. Antioxidant and Antibacterial Properties of Coating with Chitosan–Citrus Essential Oil and Effect on the Quality of Pacific Mackerel during Chilled Storage. Food Sci. Nutr. 2019, 7, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Zhao, J.; Gao, H.; Zhou, L.; Liu, Z.; Chen, Y.; Sui, P. Antimicrobial and Antioxidant Activities of the Root Bark Essential Oil of Periploca Sepium and Its Main Component 2-Hydroxy-4-Methoxybenzaldehyde. Molecules 2010, 15, 5807–5817. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Gull, N.; Khan, S.M.; Zahid Butt, M.T.; Khalid, S.; Shafiq, M.; Islam, A.; Asim, S.; Hafeez, S.; Khan, R.U. In Vitro Study of Chitosan-Based Multi-Responsive Hydrogels as Drug Release Vehicles: A Preclinical Study. RSC Adv. 2019, 9, 31078–31091. [Google Scholar] [CrossRef]












| Model | Zero Order | First Order | Higuchi | Korsmeyer–Peppas | Hixson–Crowell | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | ||||||||||||
| First stage | R2 | K0 | R2 | K | R2 | KH | R2 | K | n | R2 | K | |
| (a) H2O | ||||||||||||
| S1 | 0.98 | 0.21 | 0.98 | −0.002 | 0.99 | 0.76 | 0.99 | 0.004 | 0.82 | 0.98 | −0.003 | |
| S3 | 0.92 | 0.38 | 0.93 | −0.004 | 0.99 | 1.39 | 0.98 | 0.01 | 0.68 | 0.93 | −0.006 | |
| S6 | 0.81 | 0.69 | 0.82 | −0.007 | 0.92 | 2.63 | 0.96 | 0.04 | 0.39 | 0.82 | −0.01 | |
| (b) PBS (pH = 7.4) | ||||||||||||
| S1 | 0.96 | 0.49 | 0.98 | −0.002 | 0.99 | 1.79 | 0.98 | 0.011 | 0.69 | 0.96 | −0.008 | |
| S3 | 0.95 | 0.56 | 0.95 | −0.006 | 0.99 | 2.07 | 0.99 | 0.016 | 0.61 | 0.95 | −0.009 | |
| S6 | 0.96 | 1.1 | 0.96 | −0.012 | 0.99 | 4.03 | 0.99 | 0.05 | 0.45 | 0.96 | −0.02 | |
| (c) Acetate buffer (pH = 5.5) | ||||||||||||
| S1 | 0.97 | 0.33 | 0.98 | −0.003 | 0.99 | 1.2 | 0.99 | 0.006 | 0.80 | 0.98 | −0.005 | |
| S3 | 0.93 | 0.99 | 0.94 | −0.01 | 0.99 | 3.7 | 0.98 | 0.026 | 0.62 | 0.94 | −0.009 | |
| S6 | 0.85 | 2.1 | 0.87 | −0.03 | 0.96 | 8.08 | 0.97 | 0.11 | 0.44 | 0.87 | −0.02 | |
| Model Name | Equation | Description |
|---|---|---|
| Zero Order | Qt = ko·t | Qt: amount of aldehyde dissolved, t: time, k0: Zero order release constant |
| First Order | logQt = logQo + k·t/2.303 | Qo: initial amount of aldehyde, Qt: amount of aldehyde released, t: time, k: first order release constant |
| Korsmeyer–Peppas | Mt/M∞ = K·tn | Mt/M∞: fraction of aldehyde released, t: time, K: rate constant, n: release exponent |
| Higuchi | Qt = kH·t1/2 | Qt: amount of aldehyde released, t: time, kH: Higuchi dissolution constant |
| Hixson–Crowell | Wo1/3 − Wt1/3 = k·t | Wo: initial amount of aldehyde, Wt: remaining amount of aldehyde in formulation, t: time, k: constant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iftime, M.M.; Ailiesei, G.L.; Ailincai, D. Tuning Antioxidant Function through Dynamic Design of Chitosan-Based Hydrogels. Gels 2024, 10, 655. https://doi.org/10.3390/gels10100655
Iftime MM, Ailiesei GL, Ailincai D. Tuning Antioxidant Function through Dynamic Design of Chitosan-Based Hydrogels. Gels. 2024; 10(10):655. https://doi.org/10.3390/gels10100655
Chicago/Turabian StyleIftime, Manuela Maria, Gabriela Liliana Ailiesei, and Daniela Ailincai. 2024. "Tuning Antioxidant Function through Dynamic Design of Chitosan-Based Hydrogels" Gels 10, no. 10: 655. https://doi.org/10.3390/gels10100655
APA StyleIftime, M. M., Ailiesei, G. L., & Ailincai, D. (2024). Tuning Antioxidant Function through Dynamic Design of Chitosan-Based Hydrogels. Gels, 10(10), 655. https://doi.org/10.3390/gels10100655






