The Sol–Gel Process, a Green Method Used to Obtain Hybrid Materials Containing Phosphorus and Zirconium
Abstract
1. Introduction
2. Sol–Gel Method in Phosphorus Chemistry
2.1. Gels and Gel-like Hybrid Materials
2.2. Organic–Inorganic Hybrid Networks
- –
- Photocatalysis [29];
- –
- Catalysis of aerobic oxidation by a vanadium phosphonate hybrid material [26];
- –
- Catalysis for the synthesis of benzimidazoles [45];
- –
- Catalysis for the hydrogenation of ketones [40];
- –
- Dye removal materials from wastewater [36];
- –
- –
- –
- –
- Synthesis of biocompatible materials for medicine [27].
2.3. The Use of Surfactants in the Sol–Gel Process
3. Conclusions
4. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mar Gómez-Alcántara, M.; Cabeza, A.; Martínez-Lara, M.; Aranda, M.A.G.; Suau, R.; Bhuvanesh, N.; Clearfield, A. Layered microporous tin(iv) bisphosphonates. Inorg. Chem. 2004, 43, 5283–5293. [Google Scholar] [CrossRef] [PubMed]
- Alberti, G.; Costantino, U. Inclusion Compounds; University Press: New York, NY, USA, 1982. [Google Scholar]
- Jang, M.Y.; Yamazaki, Y. Preparation, characterization and proton conductivity of membrane based on zirconium tricarboxybutylphosphonate and polybenzimidazole for fuel cells. Solid. State Ion. 2004, 167, 107–112. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Donnadio, A.; Piaggio, P.; Pica, M.; Sisani, N. Preparation and characterisation of α-layered zirconium phosphate sulfophenylenphosphonates with variable concentration of sulfonic groups. Solid. State Ion. 2005, 176, 2893–2898. [Google Scholar] [CrossRef]
- Hogarth, W.H.G.; Diniz da Costa, J.C.; Max Lu, G.Q. Solid acid membranes for high temperature (140 °C) proton exchange membrane fuel cells. J. Power Sources 2005, 142, 223–237. [Google Scholar] [CrossRef]
- Clearfield, A. Inorganic ion exchangers, past, present, and future. Solvent Extr. Ion. Exch. 2000, 18, 655–678. [Google Scholar] [CrossRef]
- Xu, Z.P.; Jin, Y.; Diniz da Costa, J.C.; Max Lu, G.Q. Zr(HPO4)2 based organic/inorganic nanohybrids as new proton conductors. Solid. State Ion. 2008, 178, 1654–1659. [Google Scholar] [CrossRef]
- Simulescu, V.; Crasmareanu, E.; Ilia, G. Synthesis, Properties and Structures of Phosphorus–Nitrogen Heterocycles. Heterocycles 2011, 83, 275–291. [Google Scholar] [CrossRef]
- Mutin, P.H.; Guerrero, G.; Vioux, A. Organic-inorganic hybrid materials based on organophosphorus coupling molecules: From metal phosphonates to surface modification of oxides. Comptes Rendus Chim. 2003, 6, 1153–1164. [Google Scholar] [CrossRef]
- Mar Gómez-Alcántara, M.; Cabeza, A.; Olivera-Pastor, P.; Fernandez-Moreno, F.; Sobrados, I.; Sanz, J.; Morris, R.E.; Clearfield, A.; Aranda, M.A.G. Layered microporous tin(iv) bisphosphonates. Dalton Trans. 2007, 23, 2394–2404. [Google Scholar] [CrossRef]
- Mutin, P.H.; Guerrero, G.; Alauzun, J.G. Sol-gel processing of phosphonate-based organic-inorganic hybrid materials. J. Ceram. Soc. Jpn. 2015, 123, 709–713. [Google Scholar] [CrossRef]
- Simulescu, V.; Ilia, G. Macrocycles and cavitands containing phosphorus. J. Incl. Phenom. Macrocycl. Chem. 2010, 66, 3–14. [Google Scholar] [CrossRef]
- Sanchez, C.; Julian, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Mutin, P.H.; Guerrero, G.; Vioux, A. Hybrid materials from organophosphorus coupling molecules. J. Mater. Chem. 2005, 15, 3761–3768. [Google Scholar] [CrossRef]
- Gheonea, R.; Crasmareanu, E.; Plesu, N.; Sauca, S.; Simulescu, V.; Ilia, G. New hybrid materials synthesized with different dyes by sol-gel method, Hindawi Publishing Corporation. Adv. Mater. Sci. Eng. 2017, 4537039. [Google Scholar] [CrossRef]
- Guerrero, G.; Mutin, P.H.; Vioux, A. Mixed Nonhydrolytic/Hydrolytic Sol-Gel Routes to Novel Metal Oxide/Phosphonate Hybrids. Chem. Mater. 2000, 12, 1268–1272. [Google Scholar] [CrossRef]
- Mutin, H.; Delenne, C.; Medoukali, D.; Corriu, R.; Vioux, A. Introduction of Organic Moieties into Transition-Metal Oxide Matrices via Phosphonate Groups. Mat. Res. Soc. Symp. Proc. 1998, 519, 345–350. [Google Scholar] [CrossRef]
- Guerrero, G.; Mutin, P.H.; Vioux, A. Organically modified aluminas by grafting and sol-gel processes involving phosphonate derivatives. J. Mater. Chem. 2001, 11, 3161–3165. [Google Scholar] [CrossRef]
- Patel, H.; Chudasama, U. A comparative study of proton transport properties of zirconium (IV) phosphonates. Bull. Mater. Sci. 2006, 29, 665–671. [Google Scholar]
- Judeinstein, P.; Sanchez, C. Hybrid organic-inorganic materials: A land of multidisciplinarity. J. Mater. Chem. 1996, 6, 511–525. [Google Scholar] [CrossRef]
- Sanchez, C.; Lebeau, B.; Ribot, F.; In, M. Molecular Design of Sol-Gel Derived Hybrid Organic-Inorganic Nanocomposites. J. Sol-Gel Sci. Technol. 2000, 19, 31–38. [Google Scholar] [CrossRef]
- Wight, A.P.; Davis, M.E. Design and Preparation of Organic-Inorganic Hybrid Catalysts. Chem. Rev. 2002, 102, 3589–3614. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.-Y.; Lin, X.-Z.; Yuan, Z.-Y. Periodic mesoporous titanium phosphonate hybrid materials. J. Mater. Chem. 2010, 20, 7406–7415. [Google Scholar] [CrossRef]
- Clearfield, A. Metal Phosphonate Chemistry: From Synthesis to Applications; Clearfield, A., Demadis, K., Eds.; Royal Society of Chemistry: London, UK, 2012. [Google Scholar]
- Gagnon, K.J.; Perry, H.P.; Clearfield, A. Conventional and Unconventional Metal-Organic Frameworks Based on Phosphonate Ligands: MOFs and UMOFs. Chem. Rev. 2012, 112, 1034–1054. [Google Scholar] [CrossRef] [PubMed]
- Vasylyev, M.; Neumann, R. Preparation, Characterizaton, and Catalytic Aerobic Oxidation by a Vanadium Phosphonate Mesoporous Material Constructed from a Dendritic Tetraphosphonate. Chem. Mater. 2006, 18, 2781–2783. [Google Scholar] [CrossRef]
- Khaled, Z.; Ilia, G.; Watz, C.; Macașoi, I.; Drăghici, G.; Simulescu, V.; Merghes, P.E.; Varan, N.I.; Dehelean, C.A.; Vlaia, L.; et al. The Biological Impact of Some Phosphonic and Phosphinic Acid Derivatives on Human Osteosarcoma. Curr. Issues Mol. Biol. 2024, 46, 4815–4831. [Google Scholar] [CrossRef]
- Ma, T.-Y.; Liu, L.; Deng, Q.-F.; Lin, X.-Z.; Yuan, Z.-Y. Increasing the H+ exchange capacity of porous titanium phosphonate materials by protecting defective P-OH groups. Chem. Commun. 2011, 47, 6015–6017. [Google Scholar] [CrossRef]
- Ma, T.-Y.; Zhang, X.-J.; Shao, G.-S.; Cao, J.-L.; Yuan, Z.-Y. Ordered macroporous titanium phosphonate materials: Synthesis, photocatalytic activity, and heavy metal ion adsorption. J. Phys. Chem. C 2008, 112, 3090–3096. [Google Scholar] [CrossRef]
- Lin, X.-Z.; Ren, T.-Z.; Yuan, Z.-Y. Mesoporous zirconium phosphonate materials as efficient water-tolerable solid acid catalysts. Catal. Sci. Technol. 2015, 5, 1485–1494. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Liu, Y.-L.; Ren, T.-Z.; Yuan, Z.-Y. Hollow manganese phosphonate microspheres with hierarchical porosity for efficient adsorption and separation. Nanoscale 2014, 6, 6627–6636. [Google Scholar] [CrossRef]
- Ma, T.-Y.; Lin, X.-Z.; Zhang, X.-J.; Yuan, Z.-Y. Hierarchical mesostructured titanium phosphonates with unusual uniform lines of macropores. Nanoscale 2011, 3, 1690–1696. [Google Scholar] [CrossRef]
- Ma, T.-Y.; Yuan, Z.-Y. Metal Phosphonate Hybrid Mesostructures: Environmentally Friendly Multifunctional Materials for Clean Energy and Other Applications. Chem. Sus. Chem. 2011, 4, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-P.; Ren, T.-Z.; Yuan, Z.-Y. Co2+-loaded periodic mesoporous aluminum phosphonates for efficient modified Fenton catalysis. RSC Adv. 2015, 5, 7628–7636. [Google Scholar] [CrossRef]
- Ma, T.-Y.; Yuan, Z.-Y. Functionalized periodic mesoporous titanium phosphonate monoliths with large ion exchange capacity. Chem. Commun. 2010, 46, 2325–2327. [Google Scholar] [CrossRef] [PubMed]
- Nistor, M.A.; Muntean, S.M.; Maranescu, B.; Visa, A. Phosphonate metal–organic frameworks used as dye removal materials from wastewaters. Appl. Organomet. Chem. 2020, 34, e5939. [Google Scholar] [CrossRef]
- Visa, A.; Maranescu, B.; Bucur, A.; Iliescu, S.; Demadis, K.S. Synthesis and Characterization of a Novel Phosphonate Metal Organic Framework Starting from Copper Salts. Phosph. Suflur Silicon Rel. Elem. 2013, 189, 630–639. [Google Scholar] [CrossRef]
- Guizard, C.; Bac, A.; Barboiu, M.; Hovnanian, N. Hybrid organic-inorganic membranes with specific transport properties, Applications in separation and sensors technologies. Sep. Purif. Tech. 2001, 25, 167–180. [Google Scholar] [CrossRef]
- Maillet, C.; Janvier, P.; Bertrand, M.-J.; Praveen, T.; Bujoli, B. Phosphonate-Based Hybrid Materials for Catalysis? Supported Rhodium/2,2′-Bipyridine Complexes as Reduction Catalysts Under Hydrogen Pressure. Eur. J. Org. Chem. 2002, 2002, 1685–1689. [Google Scholar] [CrossRef]
- Maillet, C.; Janvier, P.; Pipelier, M.; Praveen, T.; Andres, Y.; Bujoli, B. Hybrid Materials for Catalysis? Design of New Phosphonate-Based Supported Catalysts for the Hydrogenation of Ketones under Hydrogen Pressure. Chem. Mater. 2001, 13, 2879–2884. [Google Scholar] [CrossRef]
- Yazawa, T.; Shojo, T.; Mineshige, A.; Yusa, S.; Kobune, M.; Kuraoka, K. Solid electrolyte membranes based on polyvinyl phosphonic acid and alkoxysilane for intermediate-temperature fuel cells. Solid. State Ion. 2008, 178, 1958–1962. [Google Scholar] [CrossRef]
- Lin, X.-Z.; Yuan, Z.-Y. Synthesis of mesoporous zirconium organophosphonate solid-acid catalysts. Eur. J. Inorg. Chem. 2012, 2012, 2661–2664. [Google Scholar] [CrossRef]
- Ma, T.Y.; Qiao, S.Z. Acid-Base Bifunctional Periodic Mesoporous Metal Phosphonate Materials for Synergistically and Heterogeneously Catalyzing CO2 Conversion. Acs Catal. 2014, 4, 3847–3855. [Google Scholar] [CrossRef]
- Ma, T.-Y.; Lin, X.-Z.; Yuan, Z.-Y. Cubic Mesoporous Titanium Phosphonates with Multifunctionality. Chem. Eur. J. 2010, 16, 8487–8494. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, M.; Bhaumik, A. Self-Assembled Hybrid Molybdenum Phosphonate Porous Nanomaterials and Their Catalytic Activity for the Synthesis of Benzimidazoles. Chem. Cat. Chem. 2014, 6, 2577–2586. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Liu, Y.-L.; Ren, T.-Z.; Yuan, Z.-Y. Mesoporous nickel phosphate/phosphonate hybrid microspheres with excellent performance for adsorption and catalysis. RSC Adv. 2014, 4, 16018–16021. [Google Scholar] [CrossRef]
- Kato, M.; Katayama, S.; Sakamoto, W.; Yogo, T. Synthesis of organosiloxane-based inorganic/organic hybrid membranes with chemically bound phosphonic acid for proton-conductors. Electrochim. Acta 2007, 52, 5924–5931. [Google Scholar] [CrossRef]
- Kato, M.; Sakamoto, W.; Yogo, T. Proton-conductive sol-gel membranes from phenylvinylphosphonic acid and organoalkoxysilanes with different functionalities. J. Membr. Sci. 2008, 311, 182–191. [Google Scholar] [CrossRef]
- Schubert, U. Chemical modification of titanium alkoxides for sol-gel processing. J. Mater. Chem. 2005, 15, 3701–3715. [Google Scholar] [CrossRef]
- Alberti, G.; Costantino, U.; Allulli, S.; Tomassini, N. Crystalline Zr(R-PO3)2 and Zr(R-OPO3)2 compounds (R = organic radical): A new class of materials having layered structure of the zirconium phosphate type. J. Inorg. Nucl. Chem. 1978, 40, 1113–1117. [Google Scholar] [CrossRef]
- Vasylyev, M.V.; Wachtel, E.J.; Popovitz-Biro, R.; Neumann, R. Titanium Phosphonate Porous Materials Constructed from Dendritic Tetraphosphonates. Chem. Eur. J. 2006, 12, 3507–3514. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Ma, T.-Y.; Liu, Y.-L.; Ren, T.-Z.; Yuan, Z.-Y. Metal phosphonate hybrid materials: From densely layered to hierarchically nanoporous structures. Inorg. Chem. Front. 2014, 1, 360–383. [Google Scholar] [CrossRef]
- Dziuba, K.; Wnuczek, K.; Wojtachnio, P.; Sonnier, R.; Podkoscielna, B. New Polymer Composites with Aluminum Phosphates as Hybrid Flame Retardants. Materials 2023, 16, 426. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Mehring, M.; Lafond, V.; Mutin, P.H.; Vioux, A. New Sol-Gel Routes to Organic-Inorganic Hybrid Materials: Modification of Metal Alkoxide by Phosphonic or Phosphinic Acids. J. Sol-Gel Sci. Technol. 2003, 26, 99–102. [Google Scholar] [CrossRef]
- Ma, T.-Y.; Yuan, Z.-Y. Organic-Additive-Assisted Synthesis of Hierarchically Meso-/Macroporous Titanium Phosphonates. Eur. J. Inorg. Chem. 2010, 2010, 2941–2948. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ansary, J.; Reza Ghoreishi, S. Sol-gel process applications: A mini-review. Proc. Nat. Res. Soc. 2018, 2, 02008–02029. [Google Scholar] [CrossRef]
- Sedev, R.; Exerova, D. DLVO and non-DLVO surface forces in foam films from amphiphilic block copolymers. Adv. Coll. Interface Sci. 1999, 83, 111–136. [Google Scholar] [CrossRef]
- Evans, D.F.; Wennerström, H. The Colloidal Domain—Where Physics, Chemistry, Biology, and Technology Meet; VCH: New York, NY, USA, 1994. [Google Scholar]
- Derjaguin, B.V.; Churaev, N.V.; Muller, V.M. Surface Forces; Kitchener, J.A., Ed.; Consultants Bureau: New York, NY, USA, 1987. [Google Scholar]
- Derjaguin, B.V.; Landau, L. Theory of the stability of strongly charged lyophobic sols and the adhesion of strongly charged particles in solutions of electrolytes. Acta Phys. Chim. 1941, 14, 633–662. [Google Scholar] [CrossRef]
- Verwey, E.; Overbeek, J. Theory of the Stability of Lyophobic Colloids; Elsevier: Amsterdam, The Netherlands, 1948. [Google Scholar]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Farani, M.R.; Farsadrooh, M.; Zare, I.; Gholami, A.; Akhavan, O. Green Synthesis of Magnesium Oxide Nanoparticles and Nanocomposites for Photocatalytic Antimicrobial, Antibiofilm and Antifungal Applications. Catalysts 2023, 13, 642. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.-W. Metal–Organic Frameworks for Biomedical Applications. Nano-Micro-Small 2020, 16, 1906846. [Google Scholar] [CrossRef]



| Dispersion Medium | Dispersed Phase | ||
|---|---|---|---|
| Gas | Liquid | Solid | |
| Gas | - | Liquid aerosol (fog, clouds, steam, spray) | Solid aerosol (smoke, ice clouds) |
| Liquid | Foam | Emulsion, liquid crystals | Sol, sediment, precipitates, suspension, aggregates |
| Solid | Solid foam, aerogel | Gel, hydrogel, gelatine, jelly | Solid sol |
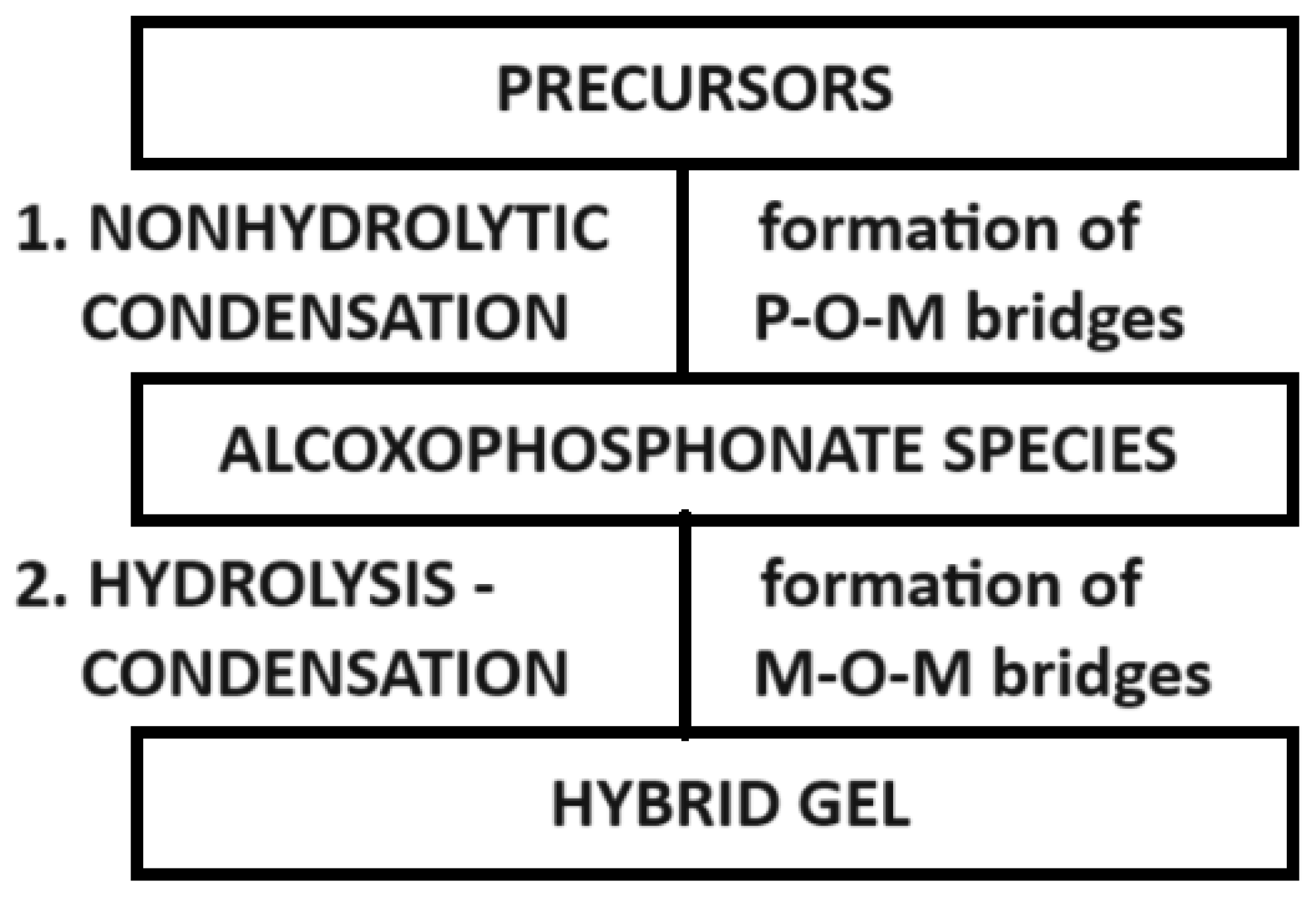
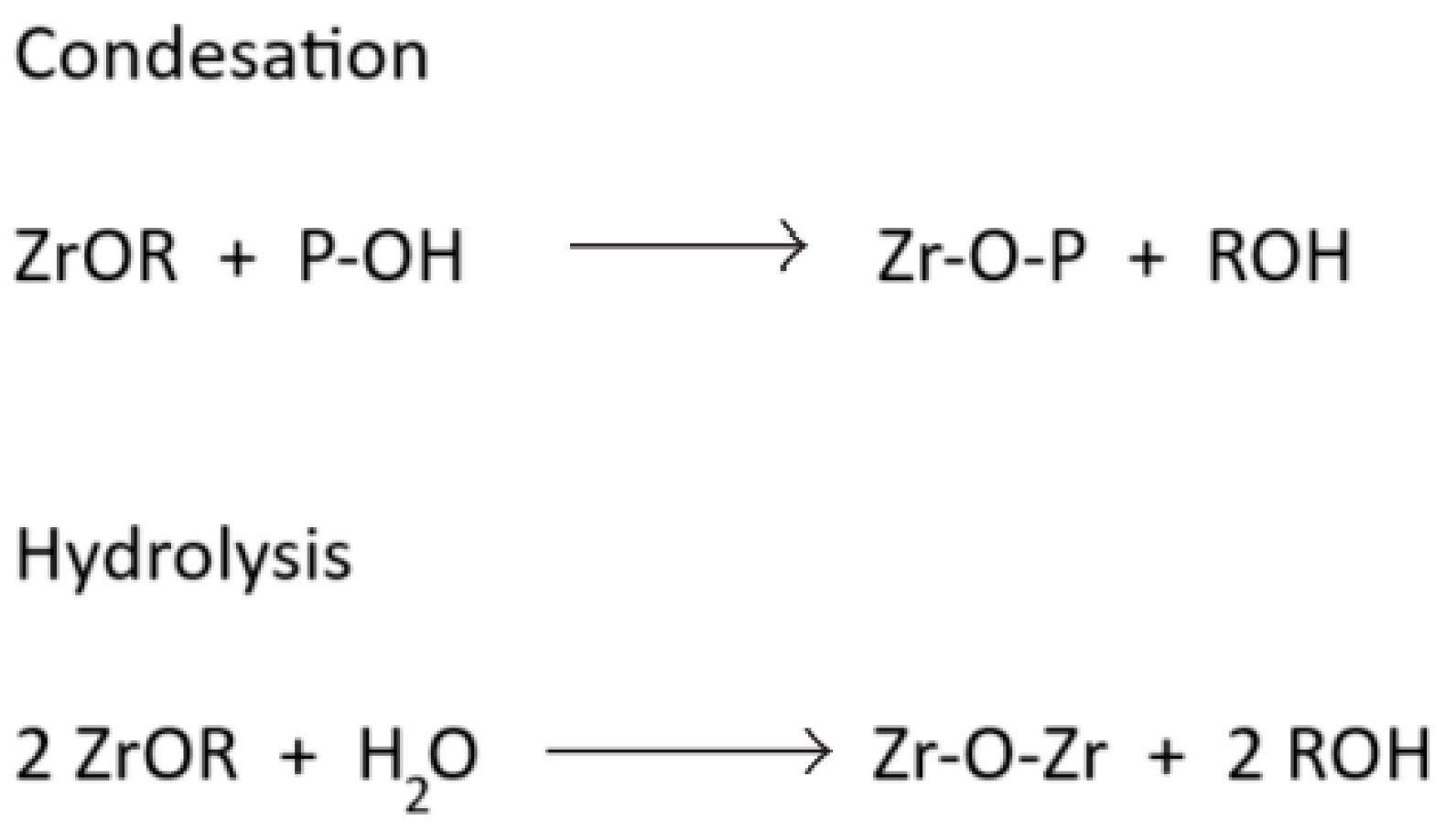
| Bridges | Sol–Gel Step | Chemical Reaction |
|---|---|---|
| Zr–O–P | During the first step | Condensation |
| Zr–O–Zr | During the second step | Hydrolysis |
| Zr–O–B | During the first step | Condensation |
| P–O–P | Not possible in mild conditions | |
| B–O–B | Not possible in mild conditions | |
| P–O–B | Not possible in mild conditions | |
| Sol–Gel | Surface Grafting | Polymerization by UV Curing |
|---|---|---|
| Not soluble | Not soluble (the synthesis could be conducted even in solid state) | Generally soluble |
| Mass loss up of 20–30% at 500 °C | Mass loss up to 50% at 500 °C | Mass loss around 60% at 500 °C |
| Mass loss up to 40% at 700 °C | Mass loss up to 60% at 700 °C | Mass loss up to 70% at 700 °C |
| No further mass loss observed above 700 °C until 900 °C | Mass loss still slowly increase until 900 °C | Mass increase significantly above 700 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merghes, P.; Ilia, G.; Maranescu, B.; Varan, N.; Simulescu, V. The Sol–Gel Process, a Green Method Used to Obtain Hybrid Materials Containing Phosphorus and Zirconium. Gels 2024, 10, 656. https://doi.org/10.3390/gels10100656
Merghes P, Ilia G, Maranescu B, Varan N, Simulescu V. The Sol–Gel Process, a Green Method Used to Obtain Hybrid Materials Containing Phosphorus and Zirconium. Gels. 2024; 10(10):656. https://doi.org/10.3390/gels10100656
Chicago/Turabian StyleMerghes, Petru, Gheorghe Ilia, Bianca Maranescu, Narcis Varan, and Vasile Simulescu. 2024. "The Sol–Gel Process, a Green Method Used to Obtain Hybrid Materials Containing Phosphorus and Zirconium" Gels 10, no. 10: 656. https://doi.org/10.3390/gels10100656
APA StyleMerghes, P., Ilia, G., Maranescu, B., Varan, N., & Simulescu, V. (2024). The Sol–Gel Process, a Green Method Used to Obtain Hybrid Materials Containing Phosphorus and Zirconium. Gels, 10(10), 656. https://doi.org/10.3390/gels10100656







