Self-Assembly Behavior of Collagen and Its Composite Materials: Preparation, Characterizations, and Biomedical Engineering and Allied Applications
Abstract
1. Introduction
2. Structures and Properties of Collagen
2.1. Biosynthesis and Extraction of Collagen
2.2. Structure of Collagen
2.3. Properties of Collagen
3. Self-Assembly Behavior of Collagen
3.1. Self-Assembly Mechanism of Collagen
3.2. Factors Affecting Collagen Self-Assembly
3.2.1. Collagen Concentration
3.2.2. Temperature
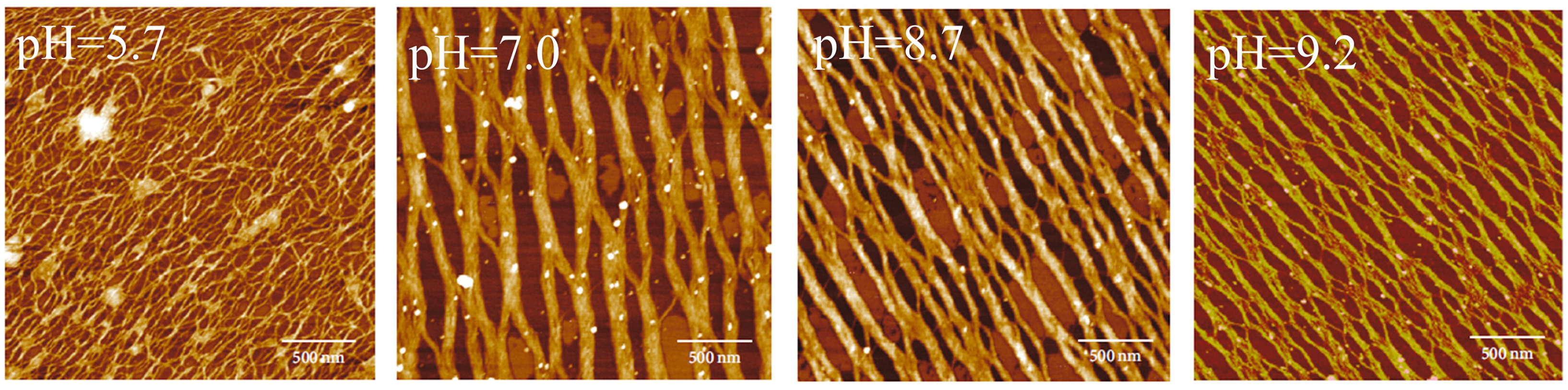
3.2.3. pH
3.2.4. Substrates
3.3. Characterization Methods of Collagen Self-Assembly
4. Collagen-Based Composite Materials
4.1. Preparation of Collagen-Based Composite Materials
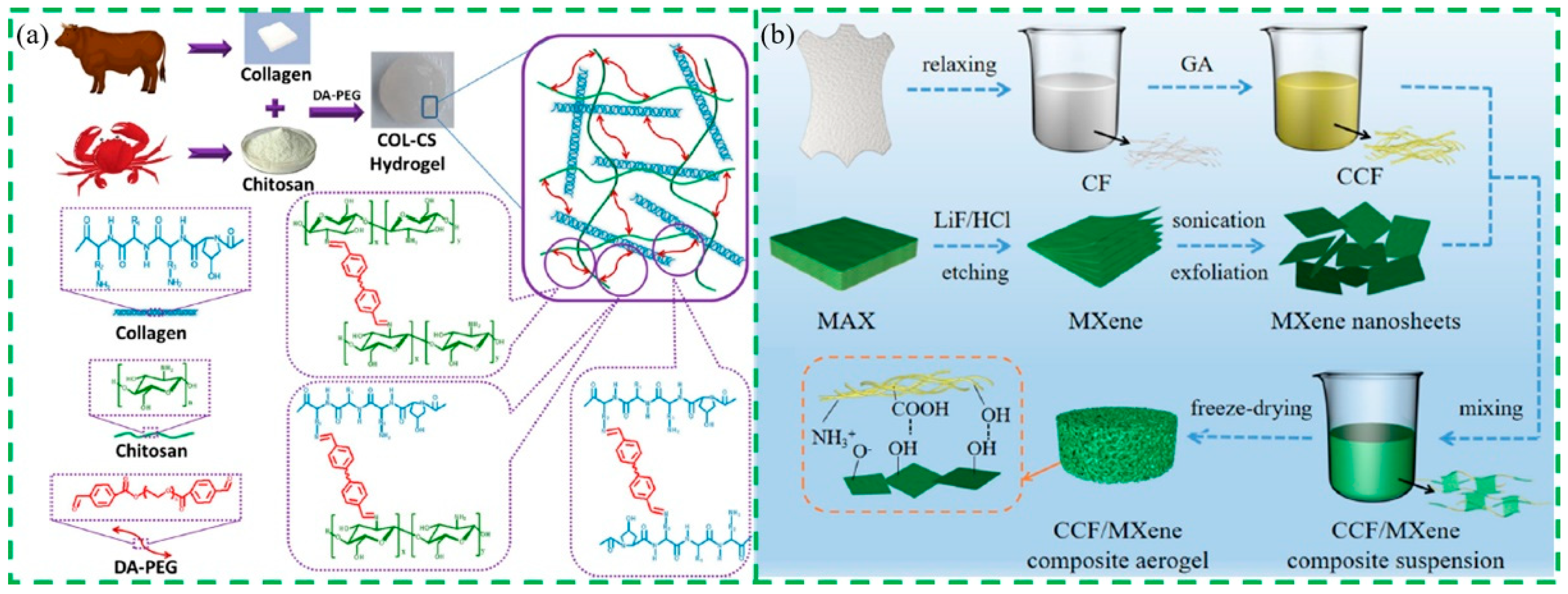
4.1.1. Collagen-Based Fiber Materials
4.1.2. Collagen-Based Film Materials
4.1.3. Collagen-Based Scaffold Materials
4.2. Modification of Collagen-Based Composite Materials
4.3. Application of Collagen-Based Composite Materials
4.3.1. Biomedical Engineering
4.3.2. Food Engineering
4.3.3. Cosmetics
4.3.4. Others
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, G.N.; Kartha, G. Structure of collagen. Nature 1955, 176, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Sherman, V.R.; Yang, W.; Meyers, M.A. The materials science of collagen. J. Mech. Behav. Biomed. Mater. 2015, 52, 22–50. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2010, 339, 247–257. [Google Scholar] [CrossRef]
- Gross, J.; Schmitt, F.O. The structure of human skin collagen as studied with the electron microscope. J. Exp. Med. 1948, 88, 555. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Kavitha, O.; Thampan, R.V. Factors influencing collagen biosynthesis. J. Cell. Biochem. 2008, 104, 1150–1160. [Google Scholar] [CrossRef]
- Hulmes, D.J.S. Building collagen molecules, fibrils, and suprafibrillar structures. J. Struct. Biol. 2002, 137, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Gelse, K.; Poschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Kuwahara, J. Extraction of type I collagen from tilapia scales using acetic acid and ultrafine bubbles. Processes 2021, 9, 288. [Google Scholar] [CrossRef]
- Solazzo, C.; Courel, B.; Connan, J.; van Dongen, B.E.; Barden, H.; Penkman, K.; Taylor, S.; Demarchi, B.; Adam, P.; Schaeffer, P.; et al. Identification of the earliest collagen- and plant-based coatings from Neolithic artefacts (Nahal Hemar cave, Israel). Sci. Rep. 2016, 6, 31053. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish collagen: Extraction, characterization, and applications for biomaterials engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef]
- Noorzai, S.; Verbeek, C.J.R.; Lay, M.C.; Swan, J. Collagen extraction from various waste bovine hide sources. Waste Biomass Valorization 2020, 11, 5687–5698. [Google Scholar] [CrossRef]
- Rodriguez, F.; Moran, L.; Gonzalez, G.; Troncoso, E.; Zuniga, R.N. Collagen extraction from mussel byssus: A new marine collagen source with physicochemical properties of industrial interest. J. Food Sci. Technol.-Mysore 2017, 54, 1228–1238. [Google Scholar] [CrossRef]
- Ahmed, M.; Verma, A.K.; Patel, R. Collagen extraction and recent biological activities of collagen peptides derived from sea-food waste: A review. Sustain. Chem. Pharm. 2020, 18, 100315. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.J.; Xiao, Y.L.; Liu, Z.J.; Pei, Y.; Wang, G.Z.; Tang, K.Y. Dissolution of collagen fibers from tannery solid wastes in salt aqueous solutions: Hofmeister series evaluation. J. Chem. Technol. Biotechnol. 2020, 95, 1225–1233. [Google Scholar] [CrossRef]
- Okur, H.I.; Hladilkova, J.; Rembert, K.B.; Cho, Y.; Heyda, J.; Dzubiella, J.; Cremer, P.S.; Jungwirth, P. Beyond the hofmeister series: Ion-specific effects on proteins and their biological functions. J. Phys. Chem. B 2017, 121, 1997–2014. [Google Scholar] [CrossRef]
- Kunz, W. Specific ion effects in colloidal and biological systems. Curr. Opin. Colloid Interface Sci. 2010, 15, 34–39. [Google Scholar] [CrossRef]
- Russell, A.E. Effect of pH on thermal stability of collagen in the dispersed and aggregated states. Biochem. J. 1974, 137, 599–602. [Google Scholar] [CrossRef]
- Adibzadeh, N.; Aminzadeh, S.; Jamili, S.; Karkhane, A.A.; Farrokhi, N. Purification and characterization of pepsin-solubilized collagen from skin of sea cucumber holothuria parva. Appl. Biochem. Biotechnol. 2014, 173, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Olsen, D.; Jiang, J.; Chang, R.; Duffy, R.; Sakaguchi, M.; Leigh, S.; Lundgard, R.; Ju, J.; Buschman, F.; Truong-Le, V.; et al. Expression and characterization of a low molecular weight recombinant human gelatin: Development of a substitute for animal-derived gelatin with superior features. Protein Expr. Purif. 2005, 40, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.e.; Mu, T.; Fan, D. Preparation of a low-cost minimal medium for engineered Escherichia coli with high yield of human-like collagen II. Pak. J. Pharm. Sci. 2014, 27, 663–669. [Google Scholar] [PubMed]
- Liu, X.H.; Zheng, C.; Luo, X.M.; Wang, X.C.; Jiang, H. Recent advances of collagen-based biomaterials: Multi-hierarchical structure, modification and biomedical applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 99, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.Y.; Miller, A. Analysis of structural design features in collagen. J. Mol. Biol. 1991, 218, 209–219. [Google Scholar] [CrossRef]
- Zhu, S.; Yuan, Q.; Yin, T.; You, J.; Gu, Z.; Xiong, S.; Hu, Y. Self-assembly of collagen-based biomaterials: Preparation, characterizations and biomedical applications. J. Mater. Chem. B 2018, 6, 2650–2676. [Google Scholar] [CrossRef]
- Nomura, S.; Hiltner, A.; Lando, J.B.; Baer, E. Interaction of water with native collagen. Biopolymers 1977, 16, 231–246. [Google Scholar] [CrossRef]
- Znidarsic, W.J.; Chen, I.W.; Shastri, V.P. Zeta-potential characterization of collagen and bovine serum albumin modified silica nanoparticles: A comparative study. J. Mater. Sci. 2009, 44, 1374–1380. [Google Scholar] [CrossRef]
- Xu, J.; Liu, F.; Wang, T.; Goff, H.D.; Zhong, F. Fabrication of films with tailored properties by regulating the swelling of collagen fiber through pH adjustment. Food Hydrocoll. 2020, 108, 106016. [Google Scholar] [CrossRef]
- Bruckner, P.; Eikenberry, E.F.; Prockop, D.J. Formation of the Triple Helix of Type I Procollagen in cellulo. A Kinetic Model Based on cis-trans Isomerization of Peptide Bonds. Eur. J. Biochem. 1981, 118, 607–613. [Google Scholar] [CrossRef]
- Burjanadze, T.V.; Kisiriya, E.L. Dependence of thermal stability on the number of hydrogen bonds in water-bridged collagen structure. Biopolymers 1982, 21, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Douglas, E.P. Effects of various salts on structural polymorphism of reconstituted type I collagen fibrils. Colloids Surf. B 2013, 112, 42–50. [Google Scholar] [CrossRef]
- Rai, R.K.; Singh, C.; Sinha, N. Predominant role of water in native collagen assembly inside the bone matrix. J. Phys. Chem. B 2015, 119, 201–211. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, K.; Khan, I.J.; Nanda, V. Morphological diversity and polymorphism of self-assembling collagen peptides controlled by length of hydrophobic domains. ACS Nano 2014, 8, 12514–12523. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Comparative study on molecular characteristics of acid soluble collagens from skin and swim bladder of seabass (Lates calcarifer). Food Chem. 2013, 138, 2435–2441. [Google Scholar] [CrossRef]
- Liu, D.S.; Liang, L.; Regenstein, J.M.; Zhou, P. Extraction and characterisation of pepsin-solubilised collagen from fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalmichthys nobilis). Food Chem. 2012, 133, 1441–1448. [Google Scholar] [CrossRef]
- Jarvinen, T.A.H.; Jarvinen, T.L.N.; Kannus, B.B.; Jozsa, L.; Jarvinen, M. Collagen fibres of the spontaneously ruptured human tendons display decreased thickness and crimp angle. J. Orthop. Res. 2004, 22, 1303–1309. [Google Scholar] [CrossRef]
- Franchi, M.; Trire, A.; Quaranta, M.; Orsini, E.; Ottani, V. Collagen structure of tendon relates to function. Sci. World J. 2007, 7, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Reichenberger, E.; Olsen, B.R. Collagens as organizers of extracellular matrix during morphogenesis. Semin. Cell Dev. Biol. 1996, 7, 631–638. [Google Scholar] [CrossRef]
- Kurniawan, N.A.; Wong, L.H.; Rajagopalan, R. Early stiffening and softening of collagen: Interplay of deformation mechanisms in biopolymer networks. Biomacromolecules 2012, 13, 691–698. [Google Scholar] [CrossRef]
- Fratzl, P.; Misof, K.; Zizak, I.; Rapp, G.; Amenitsch, H.; Bernstorff, S. Fibrillar structure and mechanical properties of collagen. J. Struct. Biol. 1998, 122, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Pins, G.D.; Christiansen, D.L.; Patel, R.; Silver, F.H. Self-assembly of collagen fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophys. J. 1997, 73, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Wilks, B.T.; Evans, E.B.; Nakhla, M.N.; Morgan, J.R. Directing fibroblast self-assembly to fabricate highly-aligned, collagen-rich matrices. Acta Biomater. 2018, 81, 70–79. [Google Scholar] [CrossRef]
- Jiang, F.Z.; Horber, H.; Howard, J.; Muller, D.J. Assembly of collagen into microribbons: Effects of pH and electrolytes. J. Struct. Biol. 2004, 148, 268–278. [Google Scholar] [CrossRef]
- Elmi, F.; Elmi, M.M.; Amiri, F.N. Thermodynamic parameters and influence of kinetic factors on the self-assembly of acid-soluble collagen nanofibrils. Food Biophys. 2017, 12, 365–373. [Google Scholar] [CrossRef]
- Yue, C.; Ding, C.; Su, J.; Cheng, B. Effect of copper and zinc ions on type I collagen self-assembly. Int. J. Polym. Anal. Charact. 2022, 27, 394–408. [Google Scholar] [CrossRef]
- Li, Y.; Asadi, A.; Monroe, M.R.; Douglas, E.P. pH effects on collagen fibrillogenesis in vitro: Electrostatic interactions and phosphate binding. Mater. Sci. Eng. C 2009, 29, 1643–1649. [Google Scholar] [CrossRef]
- Yan, M.; Li, B.; Zhao, X.; Qin, S. Effect of concentration, pH and ionic strength on the kinetic self-assembly of acid-soluble collagen from walleye pollock (Theragra chalcogramma) skin. Food Hydrocoll. 2012, 29, 199–204. [Google Scholar] [CrossRef]
- Farber, S.; Garg, A.K.; Birk, D.E.; Silver, F.H. Collagen fibrillogenesis in vitro: Evidence for pre-nucleation and nucleation steps. Int. J. Biol. Macromol. 1986, 8, 37–42. [Google Scholar] [CrossRef]
- Wood, G.C.; Keech, M.K. The formation of fibrils from collagen solutions. 1. The effect of experimental conditions: Kinetic and electron-microscope studies. Biochem. J. 1960, 75, 588–598. [Google Scholar] [CrossRef]
- Wood, G.C. The formation of fibrils from collagen solutions. 2. A mechanism for collagen-fibril formation. Biochem. J. 1960, 75, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.C. The formation of fibrils from collagen solutions. 3. Effect of chondroitin sulphate and some other naturally occurring polyanions on the rate of formation. Biochem. J. 1960, 75, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Goh, M.C.; Paige, M.F.; Gale, M.A.; Yadegari, I.; Edirisinghe, M.; Strzelczyk, J. Fibril formation in collagen. Phys. A 1997, 239, 95–102. [Google Scholar] [CrossRef]
- Dan, W.H.; Chen, Y.N.; Dan, N.H.; Zheng, X.; Wang, L.; Yang, C.K.; Huang, Y.P.; Liu, X.H.; Hu, Y. Multi-level collagen aggregates and their applications in biomedical applications. Int. J. Polym. Anal. Charact. 2019, 24, 667–683. [Google Scholar] [CrossRef]
- Liu, X.H.; Zheng, M.H.; Wang, X.C.; Luo, X.M.; Hou, M.D.; Yue, O. Biofabrication and characterization of collagens with different hierarchical architectures. ACS Biomater. Sci. Eng. 2020, 6, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Goldstein, E.L.; Matich, E.K.; Orr, B.G.; Holl, M.M.B. Type I collagen self-assembly: The roles of substrate and concentration. Langmuir 2013, 29, 2330–2338. [Google Scholar] [CrossRef]
- Huelin, S.D.; Baker, H.R.; Poduska, K.M.; Erika, F.; Merschrod, S. Aggregation and adsorption of type I collagen near an electrified interface. Macromolecules 2007, 40, 8440–8444. [Google Scholar] [CrossRef]
- Noitup, P.; Morrissey, M.T.; Garnjanagoonchorn, W. In vitro self-assembly of silver-line grunt type I collagen: Effects of collagen concentrations, pH and temperatures on collagen self-assembly. J. Food Biochem. 2006, 30, 547–555. [Google Scholar] [CrossRef]
- Na, G.C.; Phillips, L.J.; Freire, E. In vitro collagen fibril assembly: Thermodynamic studies. Biochemistry 1989, 28, 7153–7161. [Google Scholar] [CrossRef]
- de Wild, M.; Pomp, W.; Koenderink, G.H. Thermal memory in self-assembled collagen fibril networks. Biophys. J. 2013, 105, 200–210. [Google Scholar] [CrossRef]
- Li, Y.L.; Qiao, C.D.; Shi, L.; Jiang, Q.W.; Li, T.D. Viscosity of collagen solutions: Influence of concentration, temperature, adsorption, and role of intermolecular interactions. J. Macromol. Sci. Part B Phys. 2014, 53, 893–901. [Google Scholar] [CrossRef]
- Meiners, F.; Ahlers, M.; Brand, I.; Wittstock, G. Impact of temperature and electrical potentials on the stability and structure of collagen adsorbed on the gold electrode. Surf. Sci. 2015, 631, 220–228. [Google Scholar] [CrossRef]
- Song, X.; Wang, Z.; Tao, S.; Li, G.; Zhu, J. Observing effects of calcium/magnesium ions and pH value on the self-assembly of extracted swine tendon collagen by atomic force microscopy. J. Food Qual. 2017, 2017, 925706. [Google Scholar] [CrossRef]
- Yadavalli, V.K.; Svintradze, D.V.; Pidaparti, R.M. Nanoscale measurements of the assembly of collagen to fibrils. Int. J. Biol. Macromol. 2010, 46, 458–464. [Google Scholar] [CrossRef]
- Dehsorkhi, A.; Castelletto, V.; Hamley, I.W.; Adamcik, J.; Mezzenga, R. The effect of pH on the self-assembly of a collagen derived peptide amphiphile. Soft Matter 2013, 9, 6033–6036. [Google Scholar] [CrossRef]
- Dupont-Gillain, C.C. Understanding and controlling type I collagen adsorption and assembly at interfaces, and application to cell engineering. Colloids Surf. B 2014, 124, 87–96. [Google Scholar] [CrossRef]
- Narayanan, B.; Gilmer, G.H.; Tao, J.; De Yoreo, J.J.; Ciobanu, C.V. Self-assembly of collagen on flat surfaces: The interplay of collagen-collagen and collagen-substrate interactions. Langmuir 2014, 30, 1343–1350. [Google Scholar] [CrossRef]
- Torbet, J.; Malbouyres, M.; Builles, N.; Justin, V.; Roulet, M.; Damour, O.; Oldberg, A.; Ruggieo, F.; Hulmes, D.J.S. Orthogonal scaffold of magnetically aligned collagen lamellae for corneal stroma reconstruction. Biomaterials 2007, 28, 4268–4276. [Google Scholar] [CrossRef]
- Lowack, K.; Helm, C.A. Molecular mechanisms controlling the self-assembly process of polyelectrolyte multilayers. Macromolecules 1998, 31, 823–833. [Google Scholar] [CrossRef]
- Chung, E.J.; Jakus, A.E.; Shah, R.N. In situ forming collagen-hyaluronic acid membrane structures: Mechanism of self-assembly and applications in regenerative medicine. Acta Biomater. 2013, 9, 5153–5161. [Google Scholar] [CrossRef]
- Friess, W.; Schlapp, M. Effects of processing conditions on the rheological behavior of collagen dispersions. Eur. J. Pharm. Biopharm. 2001, 51, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Ding, C.; Cheng, B.; Du, X.; Su, J. Preparation of collagen/aspartic acid nanocomposite fibers and their self-assembly behaviors. J. Nat. Fibers 2022, 19, 8830–8841. [Google Scholar] [CrossRef]
- Yue, C.; Ding, C.; Du, X.; Wang, Y.; Su, J.; Cheng, B. Self-assembly of collagen fibrils on graphene oxide and their hybrid nanocomposite films. Int. J. Biol. Macromol. 2021, 193, 173–182. [Google Scholar] [CrossRef]
- Stylianou, A. Assessing collagen D-band periodicity with atomic force microscopy. Materials 2022, 15, 1608. [Google Scholar] [CrossRef]
- Stamov, D.R.; Stock, E.; Franz, C.M.; Jaehnke, T.; Haschke, H. Imaging collagen type I fibrillogenesis with high spatiotemporal resolution. Ultmi 2015, 149, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, F.; Pieterse, K.; George, A.; Bomans, P.P.; Friedrich, H.; Brylka, L.J.; Hilbers, P.A.J.; Sommerdijk, N.A.J.M. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat. Mater. 2010, 9, 1004–1009. [Google Scholar] [CrossRef]
- Mesquita, J.P.d.; Patrício, P.S.d.O.; Donnici, C.L.; Petri, D.F.S.; Oliveira, L.C.A.; Pereira, F.V. Hybrid layer-by-layer assembly based on animal and vegetable structural materials: Multilayered films of collagen and cellulose nanowhiskers. Soft Matter 2011, 7, 4405–4413. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.; Selvakumar, R.; Sastry, T.P.; Mandal, A.B.; Doble, M. Effect of D-amino acids on collagen fibrillar assembly and stability: Experimental and modelling studies. Biochem. Eng. J. 2013, 75, 92–100. [Google Scholar] [CrossRef]
- Kezwon, A.; Wojciechowski, K. Collagen-surfactant mixtures at fluid/fluid interfaces. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 390–400. [Google Scholar] [CrossRef]
- Zeng, S.; Yin, J.; Yang, S.; Zhang, C.; Yang, P.; Wu, W. Structure and characteristics of acid and pepsin-solubilized collagens from the skin of cobia (Rachycentron canadum). Food Chem. 2012, 135, 1975–1984. [Google Scholar] [CrossRef]
- Pal, G.K.; Suresh, P.V. Comparative assessment of physico-chemical characteristics and fibril formation capacity of thermostable carp scales collagen. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 70, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Tonndorf, R.; Aibibu, D.; Cherif, C. Collagen multifilament spinning. Mater. Sci. Eng. C 2020, 106, 110105. [Google Scholar] [CrossRef] [PubMed]
- Bazrafshan, Z.; Stylios, G.K. Spinnability of collagen as a biomimetic material: A review. Int. J. Biol. Macromol. 2019, 129, 693–705. [Google Scholar] [CrossRef]
- DeFrates, K.G.; Moore, R.; Borgesi, J.; Lin, G.; Mulderig, T.; Beachley, V.; Hu, X. Protein-based fiber materials in medicine: A review. Nanomaterials 2018, 8, 457. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Qin, X.; Hu, M.; Zhang, R.; Cheng, B. Microfluidic wet spinning of antibacterial collagen composite fibers supported by chelate effect of tannic acid with silver ions. React. Funct. Polym. 2024, 194, 105798. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, X.Z.; Song, Z.Y.; Wang, L.; Gao, J. Electrospun collagen/chitosan composite fibrous membranes for accelerating wound healing. Biomed. Mater. 2024, 19, 055024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lu, Q.; Yue, X.; Zuo, B.; Qin, M.; Li, F.; Kaplan, D.L.; Zhang, X. Regeneration of high-quality silk fibroin fiber by wet spinning from CaCl2-formic acid solvent. Acta Biomater. 2015, 12, 139–145. [Google Scholar] [CrossRef]
- Green, E.C.; Zhang, Y.; Li, H.; Minus, M.L. Gel-spinning of mimetic collagen and collagen/nano-carbon fibers: Understanding multi-scale influences onmolecular ordering and fibril alignment. J. Mech. Behav. Biomed. Mater. 2017, 65, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Du, J.; Cao, Y.; Yue, C.; Cheng, B. Effects of the aspect ratio of multi-walled carbon nanotubes on the structure and properties of regenerated collagen fibers. Int. J. Biol. Macromol. 2019, 126, 595–602. [Google Scholar] [CrossRef]
- Yue, C.; Ding, C.; Du, X.; Cheng, B. Novel collagen/GO-MWNT hybrid fibers with improved strength and toughness by dry-jet wet spinning. Compos. Interfaces 2022, 29, 413–429. [Google Scholar] [CrossRef]
- Dems, D.; Rodrigues da Silva, J.; Hélary, C.; Wien, F.; Marchand, M.; Debons, N.; Muller, L.; Chen, Y.; Schanne-Klein, M.-C.; Laberty-Robert, C.; et al. Native collagen: Electrospinning of pure, cross-linker-free, self-supported membrane. ACS Appl. Bio Mater. 2020, 3, 2948–2957. [Google Scholar] [CrossRef] [PubMed]
- Zimba, B.L.; Wang, M.; Hao, J.; Yu, X.; Li, Y.; Chen, C.; Xiong, G.; Wu, Q. Preparation of collagen/carboxylated graphene oxide nanofibrous membranes by electrospinning and their hemocompatibilities. Mater. Res. Express 2019, 6, 105415. [Google Scholar] [CrossRef]
- Sun, J.; Chen, J.S.; Liu, K.; Zeng, H.B. Mechanically strong proteinaceous fibers: Engineered fabrication by microfluidics. Engineering 2021, 7, 615–623. [Google Scholar] [CrossRef]
- Kang, E.; Jeong, G.S.; Choi, Y.Y.; Lee, K.H.; Khademhosseini, A.; Lee, S.H. Digitally tunable physicochemical coding of material composition and topography in continuous microfibres. Nat. Mater. 2011, 10, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Rosen, T.; Hsiao, B.S.; Soderberg, L.D. Elucidating the opportunities and challenges for nanocellulose spinning. Adv. Mater. 2021, 33, 2001238. [Google Scholar] [CrossRef]
- Du, X.-Y.; Li, Q.; Wu, G.; Chen, S. Multifunctional micro/nanoscale fibers based on microfluidic spinning technology. Adv. Mater. 2019, 31, 1903733. [Google Scholar] [CrossRef]
- Koster, S.; Evans, H.M.; Wong, J.Y.; Pfohl, T. An in situ study of collagen self-assembly processes. Biomacromolecules 2008, 9, 199–207. [Google Scholar] [CrossRef]
- Haynl, C.; Hofmann, E.; Pawar, K.; Foerster, S.; Scheibel, T. Microfluidics-produced collagen fibers show extraordinary mechanical properties. Nano Lett. 2016, 16, 5917–5922. [Google Scholar] [CrossRef]
- Suurs, P.; Barbut, S. Collagen use for co-extruded sausage casings—A review. Trends Food Sci. Technol. 2020, 102, 91–101. [Google Scholar] [CrossRef]
- Voicu, G.; Geanaliu-Nicolae, R.-E.; Pirvan, A.-A.; Andronescu, E.; Iordache, F. Synthesis, characterization and bioevaluation of drug-collagen hybrid materials for biomedical applications. Int. J. Pharm. 2016, 510, 474–484. [Google Scholar] [CrossRef]
- Peng, X.; Cui, Y.; Chen, J.; Gao, C.; Yang, Y.; Yu, W.; Rai, K.; Zhang, M.; Nian, R.; Bao, Z.; et al. High-strength collagen-based composite films regulated by water-soluble recombinant spider silk proteins and water annealing. ACS Biomater. Sci. Eng. 2022, 8, 3341–3353. [Google Scholar] [CrossRef]
- Jing, X.; Li, X.; Jiang, Y.F.; Zhao, R.H.; Ding, Q.J.; Han, W.J. Excellent coating of collagen fiber/chitosan-based materials that is water-and oil-resistant and fluorine-free. Carbohydr. Polym. 2021, 266, 118173. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Ding, C.; Yang, N.; Luo, Y.; Su, J.; Cao, L.; Cheng, B. Strong and tough collagen/cellulose nanofibril composite films via the synergistic effect of hydrogen and metal–ligand bonds. Eur. Polym. J. 2022, 180, 111628. [Google Scholar] [CrossRef]
- Yang, S.; Shi, X.X.; Li, X.M.; Wang, J.F.; Wang, Y.L.; Luo, Y.F. Oriented collagen fiber membranes formed through counter-rotating extrusion and their application in tendon regeneration. Biomaterials 2019, 207, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Liu, Y.S.; Yang, Y.J.; Li, L. Preparation of poly (vinyl alcohol)/gelatin composites via in-situ thermal/mechanochemical degradation of collagen fibers during melt extrusion: Effect of extrusion temperature. J. Polym. Res. 2017, 24, 203. [Google Scholar] [CrossRef]
- Andonegi, M.; Heras, K.L.; Santos-Vizcaino, E.; Igartua, M.; Hernandez, R.M.; de la Caba, K.; Guerrero, P. Structure-properties relationship of chitosan/collagen films with potential for biomedical applications. Carbohydr. Polym. 2020, 237, 116159. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Romero, A.; Guerrero, A. Influence of collagen concentration and glutaraldehyde on collagen-based scaffold properties. J. Biomed. Mater. Res. Part A 2016, 104, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Moxon, S.R.; Corbett, N.J.; Fisher, K.; Potjewyd, G.; Domingos, M.; Hooper, N.M. Blended alginate/collagen hydrogels promote neurogenesis and neuronal maturation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 104, 109904. [Google Scholar] [CrossRef]
- Bai, Z.X.; Wang, T.Y.; Zheng, X.; Huang, Y.P.; Chen, Y.N.; Dan, W.H. High strength and bioactivity polyvinyl alcohol/collagen composite hydrogel with tannic acid as cross-linker. Polym. Eng. Sci. 2021, 61, 278–287. [Google Scholar] [CrossRef]
- Ding, C.; Tian, M.; Feng, R.; Dang, Y.; Zhang, M. Novel self-healing hydrogel with injectable, pH-responsive, strain-sensitive, promoting wound-healing, and hemostatic properties based on collagen and chitosan. ACS Biomater. Sci. Eng. 2020, 6, 3855–3867. [Google Scholar] [CrossRef]
- Yue, C.; Xu, M.; Zhong, L.; Tang, S.; Cai, G.; Zhang, R.; Cheng, B. pH-responsive release features of collagen/carboxylated cellulose nanofiber composite aerogels through the incorporation of cyclodextrin/5-fluorouracil inclusion complexes. Eur. Polym. J. 2024, 207, 112807. [Google Scholar] [CrossRef]
- Zhang, W.B.; Pan, Z.Y.; Ma, J.Z.; Wei, L.F.; Chen, Z.; Wang, O. Degradable cross-linked collagen fiber/MXene composite aerogels as a high-performing sensitive pressure sensor. ACS Sustain. Chem. Eng. 2021, 10, 1408–1418. [Google Scholar] [CrossRef]
- Pot, M.W.; Faraj, K.A.; Adawy, A.; van Enckevort, W.J.P.; van Moerkerk, H.T.B.; Vlieg, E.; Daamen, W.F.; van Kuppevelt, T.H. Versatile wedge-based system for the construction of unidirectional collagen scaffolds by directional freezing: Practical and theoretical considerations. ACS Appl. Mater. Interfaces 2015, 7, 8495–8505. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, B.T.; Ragothaman, M.; Kalirajan, C.; Palanisamy, T. Conducting collagen-polypyrrole hybrid aerogels made from animal skin waste. RSC Adv. 2016, 6, 63071–63077. [Google Scholar] [CrossRef]
- Adamiak, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Matsumoto, H.N.; Yamada, T.; Koyama, Y.; Takakuda, K.; Tanaka, J. Glutaraldehyde cross-linked hydroxyapatite/collagen self-organized nanocomposites. Biomaterials 2004, 25, 63–69. [Google Scholar] [CrossRef]
- Tian, Z.H.; Li, C.H.; Duan, L.; Li, G.Y. Physicochemical properties of collagen solutions cross-linked by glutaraldehyde. Connect. Tissue Res. 2014, 55, 239–247. [Google Scholar] [CrossRef]
- Sundararaghavan, H.G.; Monteiro, G.A.; Lapin, N.A.; Chabal, Y.J.; Miksan, J.R.; Shreiber, D.I. Genipin-induced changes in collagen gels: Correlation of mechanical properties to fluorescence. J. Biomed. Mater. Res. Part A 2008, 87A, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Usha, R.; Sreeram, K.J.; Rajaram, A. Stabilization of collagen with EDC/NHS in the presence of L-lysine: A comprehensive study. Colloids Surf. B 2012, 90, 83–90. [Google Scholar] [CrossRef]
- Mu, C.; Liu, F.; Cheng, Q.; Li, H.; Wu, B.; Zhang, G.; Lin, W. Collagen cryogel cross-linked by dialdehyde starch. Macromol. Mater. Eng. 2010, 295, 100–107. [Google Scholar] [CrossRef]
- Haugh, M.G.; Jaasma, M.J.; O’Brien, F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J. Biomed. Mater. Res. Part A 2009, 89A, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Weadock, K.S.; Miller, E.J.; Bellincampi, L.D.; Zawadsky, J.P.; Dunn, M.G. Physical crosslinking of collagen fibers: Comparison of ultraviolet irradiation and dehydrothermal treatment. J. Biomed. Mater. Res. 1995, 29, 1373–1379. [Google Scholar] [CrossRef]
- Drexler, J.W.; Powell, H.M. Dehydrothermal crosslinking of electrospun collagen. Tissue Eng. Part C Methods 2010, 17, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Dong, P.; Gravesande, J.; Cheng, B.; Xing, J. UV-mediated solid-state cross-linking of electrospinning nanofibers of modified collagen. Int. J. Biol. Macromol. 2018, 120, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Reyna-Urrutia, V.A.; Mata-Haro, V.; Cauich-Rodriguez, J.V.; Herrera-Kao, W.A.; Cervantes-Uc, J.M. Effect of two crosslinking methods on the physicochemical and biological properties of the collagen-chitosan scaffolds. Eur. Polym. J. 2019, 117, 424–433. [Google Scholar] [CrossRef]
- Jafari-Sabet, M.; Nasiri, H.; Ataee, R. The effect of cross-linking agents and collagen concentrations on properties of collagen scaffolds. J. Arch. Mil. Med. 2016, 4, e42367. [Google Scholar] [CrossRef]
- Luo, X.; Guo, Z.; He, P.; Chen, T.; Li, L.; Ding, S.; Li, H. Study on structure, mechanical property and cell cytocompatibility of electrospun collagen nanofibers crosslinked by common agents. Int. J. Biol. Macromol. 2018, 113, 476–486. [Google Scholar] [CrossRef]
- Weadock, K.S.; Miller, E.J.; Keuffel, E.L.; Dunn, M.G. Effect of physical crosslinking methods on collagen-fiber durability in proteolytic solutions. J. Biomed. Mater. Res. 1996, 32, 221–226. [Google Scholar] [CrossRef]
- Ming-Che, W.; Pins, G.D.; Silver, F.H. Collagen fibres with improved strength for the repair of soft tissue injuries. Biomaterials 1994, 15, 507–512. [Google Scholar] [CrossRef]
- Hu, T.Y.; Lo, A.C.Y. Collagen-alginate composite hydrogel: Application in tissue engineering and biomedical sciences. Polymers 2021, 13, 1852. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Fan, D.D.; Shang, L.J. Exploring the potential of the recombinant human collagens for biomedical and clinical applications: A short review. Biomed. Mater. 2021, 16, 012001. [Google Scholar] [CrossRef]
- Sionkowska, A.; Adamiak, K.; Musial, K.; Gadomska, M. Collagen based materials in cosmetic applications: A review. Materials 2020, 13, 4217. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.I.A.; Barroso, L.G.R.; Sanchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Dong, Y. Collagen-based biomaterials for tissue engineering. ACS Biomater. Sci. Eng. 2023, 9, 1132–1150. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wang, Y.; Hao, B.; Cao, Y.; Cui, Y.; Huang, X.; Shi, B. Collagen fiber-based advanced separation materials: Recent developments and future perspectives. Adv. Mater. 2022, 34, 2107891. [Google Scholar] [CrossRef]
- Zheng, M.H.; Wang, X.C.; Chen, Y.N.; Yue, O.Y.; Bai, Z.X.; Cui, B.Q.; Jiang, H.; Liu, X.H. A review of recent progress on collagen-based biomaterials. Adv. Healthc. Mater. 2022, 12, 2202042. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.S.; Ok, Y.J.; Hwang, S.Y.; Kwak, J.Y.; Yoon, S. Marine collagen as a promising biomaterial for biomedical applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef]
- Gu, L.S.; Shan, T.T.; Ma, Y.X.; Tay, F.R.; Niu, L.N. Novel biomedical applications of crosslinked collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef] [PubMed]
- Coradin, T.; Wang, K.; Law, T.; Trichet, L. Type I collagen-fibrin mixed hydrogels: Preparation, properties and biomedical applications. Gels 2020, 6, 36. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Fayzullin, A.L.; Vukolova, M.N.; Rudenko, T.G.; Osipycheva, V.D.; Litvitsky, P.F. Medical applications of collagen and collagen-based materials. Curr. Med. Chem. 2019, 26, 506–516. [Google Scholar] [CrossRef]
- Rahman, M.A. Collagen of extracellular matrix from marine invertebrates and its medical applications. Mar. Drugs 2019, 17, 118. [Google Scholar] [CrossRef] [PubMed]
- Law, J.X.; Liau, L.L.; Saim, A.; Yang, Y.; Idrus, R. Electrospun collagen nanofibers and their applications in skin tissue engineering. Tissue Eng. Regen. Med. 2017, 14, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, I.; Fathima, N.N.; Rao, J.R.; Nair, B.U. Influence of PCL on the material properties of collagen based biocomposites and in vitro evaluation of drug release. Mater. Sci. Eng. C-Mater. Biol. Appl. 2013, 33, 4651–4659. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef]
- Manon-Jensen, T.; Kjeld, N.G.; Karsdal, M.A. Collagen-mediated hemostasis. J. Thromb. Haemost. 2016, 14, 438–448. [Google Scholar] [CrossRef]
- Yan, X.; Chen, Y.; Dan, N.; Dan, W. A novel thermosensitive growth-promoting collagen fibers composite hemostatic gel. J. Mater. Chem. B 2022, 10, 4070–4082. [Google Scholar] [CrossRef]
- Chen, Q.-Z.; Harding, S.E.; Ali, N.N.; Lyon, A.R.; Boccaccini, A.R. Biomaterials in cardiac tissue engineering: Ten years of research survey. Mater. Sci. Eng. R Rep. 2008, 59, 1–37. [Google Scholar] [CrossRef]
- Subhan, F.; Hussain, Z.; Tauseef, I.; Shehzad, A.; Wahid, F. A review on recent advances and applications of fish collagen. Crit. Rev. Food Sci. Nutr. 2021, 61, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yan, H.; Zhang, J.; Tian, B.; Li, W.; Xiao, J. Agarose-collagen composite microsphere implants: A biocompatible and robust approach for skin tissue regeneration. Int. J. Biol. Macromol. 2024, 277, 134510. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, X.; Liu, H.; Wang, L.; Liu, S.; Zhang, H. Type-I collagen polypeptide-based composite nanofiber membranes for fast and efficient bone regeneration. ACS Biomater. Sci. Eng. 2024, 10, 5632–5640. [Google Scholar] [CrossRef] [PubMed]
- Vijayalekha, A.; Anandasadagopan, S.K.; Pandurangan, A.K. An overview of collagen-based composite scaffold for bone tissue engineering. Appl. Biochem. Biotechnol. 2023, 195, 4617–4636. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, G. A biomimetic mineralized collagen hydrogel containing uniformly distributed and highly abundant dopamine-modified hydroxyapatite particles for bone tissue engineering. J. Appl. Polym. Sci. 2024, 141, e55567. [Google Scholar] [CrossRef]
- Balachandran Megha, K.; Syama, S.; Padmalayathil Sangeetha, V.; Vandana, U.; Oyane, A.; Valappil Mohanan, P. Development of a 3D multifunctional collagen scaffold impregnated with peptide LL-37 for vascularised bone tissue regeneration. Int. J. Pharm. 2024, 652, 123797. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.Y.; Hu, Y.; Liu, Z.; Ding, X.; Tian, J.; Xiao, J.X. Luminescent lanthanide-collagen peptide framework for pH-controlled drug delivery. Mol. Pharm. 2019, 16, 846–855. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, S.J.; Kang, J.H.; Shin, U.S. Positively and negatively charged collagen nanohydrogels: pH-responsive drug-releasing characteristics. Bull. Korean Chem. Soc. 2018, 39, 477–482. [Google Scholar] [CrossRef]
- Yue, C.; Ding, C.; Hu, M.; Zhang, R.; Cheng, B. Collagen/functionalized cellulose nanofibril composite aerogels with pH-responsive characteristics for drug delivery system. Int. J. Biol. Macromol. 2024, 261, 129650. [Google Scholar] [CrossRef]
- Padekan, B.; Dadvand Koohi, A.; Akbari Dogolsar, M. Collagen-based hydrogel with incorporated nano-hydroxyapatite for the delivery of a poorly water-soluble drug. J. Macromol. Sci. Part B 2024, 1–20. [Google Scholar] [CrossRef]
- Princy; Kaur, D.; Kaur, A. Engineering of electrospun polycaprolactone/polyvinyl alcohol-collagen based 3D nano scaffolds and their drug release kinetics using cetirizine as a model drug. Int. J. Biol. Macromol. 2024, 268, 131847. [Google Scholar] [CrossRef]
- Leon-Lopez, A.; Morales-Penaloza, A.; Martinez-Juarez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Alvarez, G. Hydrolyzed collagen-sources and applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Heimann, K.; Zhang, W. Protein recovery from underutilised marine bioresources for product development with nutraceutical and pharmaceutical bioactivities. Mar. Drugs 2020, 18, 391. [Google Scholar] [CrossRef] [PubMed]
- Irastorza, A.; Zarandona, I.; Andonegi, M.; Guerrero, P.; de la Caba, K. The versatility of collagen and chitosan: From food to biomedical applications. Food Hydrocoll. 2021, 116, 106633. [Google Scholar] [CrossRef]
- Mao, Q.; Zhuo, Y.; Luo, S.; Li, J.; Hu, F.; Zhao, Q. Preparation and characterisation of fish skin collagen–chitosan–cinnamon essential oil composite film. Int. J. Food Sci. Technol. 2024, 59, 6087–6101. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Shao, R.; Liu, M.; Zan, Y.; Dou, M.; Liu, J.; Zhang, Z.; Huang, Y.; Wang, F. Porous carbons derived from collagen-enriched biomass: Tailored design, synthesis, and application in electrochemical energy storage and conversion. Adv. Funct. Mater. 2019, 29, 1905095. [Google Scholar] [CrossRef]
- Liu, H.; Cao, Y.; Wang, F.; Huang, Y. Nitrogen-Doped Hierarchical Lamellar Porous Carbon Synthesized from the Fish Scale As Support Material for Platinum Nanoparticle Electrocatalyst toward the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2014, 6, 819–825. [Google Scholar] [CrossRef]
- Hooshvar, M.; Bagheri Marandi, G.; Taghvay Nakhjiri, M. Collagen-based hydrogel nanocomposite as adsorbent for methylene blue and crystal violet removal from aqueous solution: Isotherm, kinetic, and thermodynamic studies. Water Air Soil Pollut. 2024, 235, 161. [Google Scholar] [CrossRef]
- Zhou, J.; Du, X.; Lu, K.; Xiao, A. MXene@polydopamine/oxidized sodium alginate modified collagen composite aerogel as sustainable bio-adsorbent for heavy metal ion adsorption and solar driven water evaporation. Sep. Purif. Technol. 2025, 354, 129045. [Google Scholar] [CrossRef]
- Andonegi, M.; Correia, D.M.; Pereira, N.; Fernandes, M.M.; Costa, C.M.; Lanceros-Mendez, S.; de la Caba, K.; Guerrero, P. Sustainable antibacterial collagen composites with silver nanowires for resistive pressure sensor applications. Eur. Polym. J. 2023, 200, 112494. [Google Scholar] [CrossRef]
- Andonegi, M.; Correia, D.M.; Pereira, N.; Costa, C.M.; Lanceros-Mendez, S.; de la Caba, K.; Guerrero, P. Sustainable collagen composites with graphene oxide for bending resistive sensing. Polymers 2023, 15, 3855. [Google Scholar] [CrossRef]



| Classification | Possible Mechanism | Ref. | |
|---|---|---|---|
| Chemical modification | GA | GA–protein cross-links are formed through reaction of ε-amine groups of lysine or hydroxylysine residues with the aldehyde group of GA. | [107,116,117] |
| Genipin | (i) Fast nucleophilic attack of amine groups on lysine and arginine on the C3 atom of genipin, which leads to the formation of a heterocyclic compound of genipin connected with basic residues of proteins; (ii) Slow nucleophilic substitution of the ester groups in genipin which produces a secondary amide connection. | [118] | |
| EDC/NHS | The cross-linking reaction of collagen with the use of EDC/NHS induces the formation of a covalent bond between carboxylic acid groups from aspartic and glutamic acid. | [119] | |
| Dialdehyde starch | Dialdehyde starch aldehyde groups interact with the free amino group of collagen during the cross-linking reaction. | [120] | |
| Physical modification | Dehydrothermal | Under vacuum conditions, water molecules are removed which causes the formation of intramolecular links (amide bonds) between collagen. | [121,122,123] |
| UV light | This process involves the formation of free radicals on aromatic groups of tyrosine and phenylalanine, and radicals interact with each other and form chemical bonds form. | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, C.; Ding, C.; Xu, M.; Hu, M.; Zhang, R. Self-Assembly Behavior of Collagen and Its Composite Materials: Preparation, Characterizations, and Biomedical Engineering and Allied Applications. Gels 2024, 10, 642. https://doi.org/10.3390/gels10100642
Yue C, Ding C, Xu M, Hu M, Zhang R. Self-Assembly Behavior of Collagen and Its Composite Materials: Preparation, Characterizations, and Biomedical Engineering and Allied Applications. Gels. 2024; 10(10):642. https://doi.org/10.3390/gels10100642
Chicago/Turabian StyleYue, Chengfei, Changkun Ding, Minjie Xu, Min Hu, and Ruquan Zhang. 2024. "Self-Assembly Behavior of Collagen and Its Composite Materials: Preparation, Characterizations, and Biomedical Engineering and Allied Applications" Gels 10, no. 10: 642. https://doi.org/10.3390/gels10100642
APA StyleYue, C., Ding, C., Xu, M., Hu, M., & Zhang, R. (2024). Self-Assembly Behavior of Collagen and Its Composite Materials: Preparation, Characterizations, and Biomedical Engineering and Allied Applications. Gels, 10(10), 642. https://doi.org/10.3390/gels10100642