Exploring Calcium Alginate-Based Gels for Encapsulation of Lacticaseibacillus paracasei to Enhance Stability in Functional Breadmaking
Abstract
1. Introduction
2. Results and Discussion
2.1. The Encapsulation Efficiency and Morphology of the Alginate-Based Gel Microcapsules
2.1.1. Encapsulation Efficiency
2.1.2. The Shape and Size of Wet Alginate Gel Microcapsules
2.1.3. Microstructure and Size Distribution of Freeze-Dried Alginate-Based Gel Capsules
2.2. Survivability of L. paracasei Cells Microencapsulated in Alginate Gel Matrix during Storage
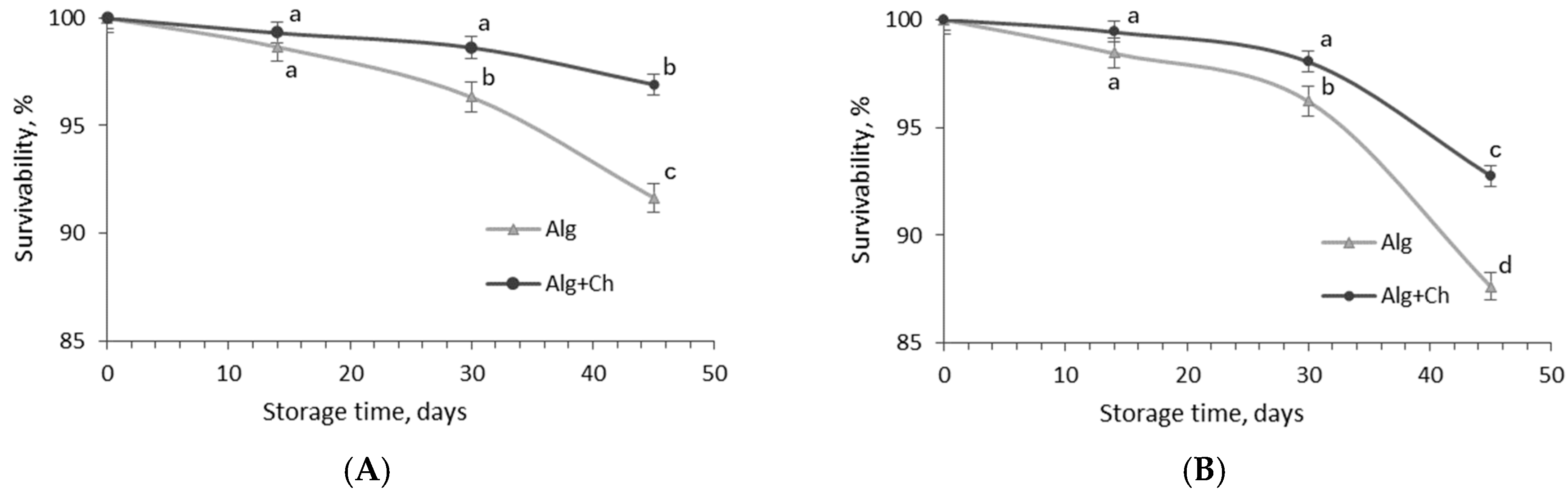
2.2.1. Bacterial Cell Stability during Storage of Wet Capsules
2.2.2. The Survivability of Bacteria during Storage of Freeze-Dried Alginate-Based Microcapsules
2.3. Fermentation Ability of Free and Microencapsulated L. paracasei Bacteria
2.4. The Influence of Sourdough and Bacteria Microencapsulated in Alginate Gel Matrix on the Quality and Acceptability of Baked Goods
2.5. The Influence of Sourdoughs and Microencapsulated Bacteria Additives on Bread Shelf Life during Storage
3. Conclusions
4. Materials and Methods
4.1. Bacterial Strain
4.2. Materials
4.3. Encapsulation of Bacterial Cells
4.3.1. Preparation of the Encapsulation Solutions
4.3.2. Encapsulation Procedure
4.4. Freeze-Drying
4.5. Microcapsule Morphology and Particle Size Analysis
4.6. Evaluation of the Survivability of the Microencapsulated Bacteria under Simulated Processing Conditions
4.6.1. Sourdough Fermentation
4.6.2. Storage of Microcapsules
4.7. Determination of pH and Total Titratable Acidity
4.8. Breadmaking Procedure
4.9. Determination of the Total Number of Lactic Acid Bacteria
4.10. Determination of Bread Quality
4.10.1. The Viability of Lactic Acid Bacteria in Baked Goods
4.10.2. Evaluation of Bread Molding during Storage
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ballini, A.; Charitos, I.A.; Cantore, S.; Topi, S.; Bottalico, L.; Santacroce, L. About functional foods: The probiotics and prebiotics state of art. Antibiotics 2023, 12, 635. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef] [PubMed]
- Akamine, I.T.; Mansoldo, F.R.P.; Vermelho, A.B. Probiotics in the sourdough bread fermentation: Current status. Fermentation 2023, 9, 90. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Gandomi, H.; Misaghi, A.; Bokaei, S.; Noori, N. The effect of alginate and chitosan concentrations on some properties of chitosan-coated alginate beads and survivability of encapsulated Lactobacillus rhamnosus in simulated gastrointestinal conditions and during heat processing. J. Sci. Food Agric. 2014, 94, 2210–2216. [Google Scholar] [CrossRef]
- Martín, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Microencapsulation of bacteria: A review of different technologies and their impact on the probiotic effects. Inn. Food Sci. Emerg. Technol. 2015, 27, 15–25. [Google Scholar] [CrossRef]
- Ramos, P.E.; Cerqueira, M.A.; Teixeira, J.A.; Vicente, A.A. Physiological protection of probiotic microcapsules by coatings. Crit. Rev. Food Sci. Nutr. 2018, 58, 1864–1877. [Google Scholar] [CrossRef]
- Oberoi, K.; Tolun, A.; Sharma, K.; Sharma, S. Microencapsulation: An overview for the survival of probiotic bacteria. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 280–287. [Google Scholar] [CrossRef]
- Leon, A.M.; Medina, W.T.; Park, D.J.; Aguilera, J.M. Properties of microparticles from a whey protein isolate/alginate emulsion gel. Food Sci. Technol. Int. 2018, 24, 414–423. [Google Scholar] [CrossRef]
- Tchabo, W.; Kaptso, G.K.; Ngea, G.L.N.; Wang, K.; Bao, G.; Ma, Y.; Wang, X.; Mbofung, C.M. In vitro assessment of the effect of microencapsulation techniques on the stability, bioaccessibility and bioavailability of mulberry leaf bioactive compounds. Food Biosci. 2022, 47, 101461. [Google Scholar] [CrossRef]
- Weng, Y.; Yang, G.; Li, Y.; Xu, L.; Chen, X.; Song, H.; Zhao, C.-X. Alginate-based materials for enzyme encapsulation. Adv. Colloid Interface Sci. 2023, 318, 102957. [Google Scholar] [CrossRef]
- Dong, Q.-Y.; Chen, M.-Y.; Xin, Y.; Qin, X.-Y.; Cheng, Z.; Shi, L.-E.; Tang, Z.-X. Alginate-based and protein-based materials for probiotics encapsulation: A review. Int. J. Food Sci. Technol. 2013, 48, 1339–1351. [Google Scholar] [CrossRef]
- Trabelsi, I.; Bejar, W.; Ayadi, D.; Chouayekh, H.; Kammoun, R.; Bejar, S.; Ben Salah, R. Encapsulation in alginate and alginate coated-chitosan improved the survival of a newly probiotic in oxgall and gastric juice. Int. J. Biol. Macromol. 2013, 61, 36–42. [Google Scholar] [CrossRef]
- Mahmoud, M.; Abdallah, N.A.; El-Shafei, K.; Tawfik, N.F.; El-Sayed, H.S. Survivability of alginate-microencapsulated Lactobacillus plantarum during storage, simulated food processing and gastrointestinal conditions. Heliyon 2020, 6, e03541. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, L.; Nouri, L.; Nafchi, A.M.; Al-Hassan, A.A. The effects of encapsulated probiotic bacteria on the physicochemical properties, staling, and viability of probiotic bacteria in gluten-free bread. J. Food Process. Preserv. 2022, 46, 16359. [Google Scholar] [CrossRef]
- Denkova, R.; Georgieva, L.; Denkova, Z. Freeze-dried sourdough starters. J. Food Packag. Sci. Techn. Technol. 2014, 3, 74–80. [Google Scholar]
- Zhang, L.; Taal, M.A.; Boom, R.M.; Chen, X.D.; Schutyser, M.A.I. Effect of baking conditions and storage on the viability of Lactobacillus plantarum supplemented to bread. LWT 2018, 87, 318–325. [Google Scholar] [CrossRef]
- Caglar, N.; Ermis, E.; Durak, M.Z. Spray-dried and freeze-dried sourdough powders: Properties and evaluation of their use in breadmaking. J. Food Eng. 2021, 292, 110355. [Google Scholar] [CrossRef]
- Tafti, A.G.; Peighambardoust, S.H.; Hesari, J.; Bahrami, A.; Bonab, E.S. Physicochemical and functional properties of spray-dried sourdough in breadmaking. Food Sci. Technol. Int. 2013, 19, 271–278. [Google Scholar] [CrossRef]
- Ilha, E.C.; da Silva, T.; Lorenz, J.G.; de Oliveira Rocha, G.; Sant’Anna, E.S. Lactobacillus paracasei isolated from grape sourdough: Acid, bile, salt, and heat tolerance after spray drying with skim milk and cheese whey. Eur. Food Res. Technol. 2015, 240, 977–984. [Google Scholar] [CrossRef]
- Zubair, W.; Afzaal, M.; Saeed, F.; Ahmed, A.; Ateeq, H.; Akram, N.; Abbas Shah, Y.; Asghar, A.; Manoharad, S.; Nawaz, A.; et al. Survivability of Lactobacillus rhamnosus under stressed conditions as affected by taro starch (Colocasia esculenta) encapsulation. Int. J. Food Prop. 2023, 26, 3465–3476. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. Int. Dairy J. 2004, 14, 737–743. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Hussain, M.; Ismail, Z.; Siddeeg, A.; Ammar, A.F.; Aljobair, M.O. Influence of encapsulation on the survival of probiotics in food matrix under simulated stress conditions. Saudi J. Biol. Sci. 2022, 29, 103394. [Google Scholar] [CrossRef] [PubMed]
- Etchepare, M.A.; Barin, J.S.; Cichoski, A.J.; Jacob-Lopes, E.; Wagner, R.; Fries, L.L.M.; Menezes, C.R. Microencapsulation of probiotics using sodium alginate. Ciênc. Rural 2015, 45, 1319–1326. [Google Scholar] [CrossRef]
- Gandomi, H.; Abbaszadeh, S.; Misaghi, A.; Bokaie, S.; Noori, N. Effect of chitosan-alginate encapsulation with inulin on survival of Lactobacillus rhamnosus GG during apple juice storage and under simulated gastrointestinal conditions. LWT 2016, 69, 365–371. [Google Scholar] [CrossRef]
- Lotfipour, F.; Mirzaeei, S.; Maghsoodi, M. Preparation and characterization of alginate and psyllium beads containing Lactobacillus acidophilus. Sci. World J. 2015, 2012, 680108. [Google Scholar]
- Parsana, Y.; Yadav, M.; Kumar, S. Microencapsulation in the chitosan-coated alginate-inulin matrix of Limosilactobacillus reuteri SW23 and Lactobacillus salivarius RBL50 and their characterization. Carbohydr. Polym. Technol. Appl. 2023, 5, 100285. [Google Scholar] [CrossRef]
- Heidebach, T.; Först, P.; Kulozik, U. Microencapsulation of probiotic cells for food applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 291–311. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Watcharapoka, S. Effect of addition of inulin and galactooligosaccharide on the survival of microencapsulated probiotics in alginate beads coated with chitosan in simulated digestive system, yogurt, and fruit juice. LWT Food Sci. Technol. 2014, 57, 761–766. [Google Scholar] [CrossRef]
- Corona-Hernandez, R.I.; Álvarez-Parrilla, E.; Lizardi-Mendoza, J.; Islas-Rubio, A.R.; de la Rosa, L.A.; Wall-Medrano, A. Structural Stability and Viability of Microencapsulated Probiotic Bacteria: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 614–628. [Google Scholar] [CrossRef]
- Fujiwara, G.M.; Campos, R.; Costa, C.K.; de Dias, J.F.G.; Miguel, O.G.; Miguel, M.D.; de Marques, F.A.; Zanin, S.M.W. Production and characterization of alginate-starch-chitosan microparticles containing stigmasterol through the external ionic gelation technique. Braz. J. Pharm. Sci. 2013, 49, 537–547. [Google Scholar] [CrossRef]
- Ji, R.; Wu, J.; Zhang, J.; Wang, T.; Zhang, X.; Shao, L.; Chen, D.; Wang, J. Extending viability of bifidobacterium longumin chitosan-coated alginate microcapsules using emulsification and internal gelation encapsulation technology. Front. Microbiol. 2019, 10, 1389. [Google Scholar] [CrossRef] [PubMed]
- Albadran, H.A.; Chatzifragkou, A.; Khutoryanskiy, V.V.; Charalampopoulos, D. Stability of probiotic Lactobacillus plantarum in dry microcapsules under accelerated storage conditions. Food Res. Int. 2015, 74, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Fareez, I.M.; Lim, S.M.; Mishra, R.K.; Ramasamy, K. Chitosan coated alginate-xanthan gum bead enhanced pH and thermotolerance of Lactobacillus plantarum LAB12. Int. J. Biol. Macromol. 2015, 72, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Chávarri, M.; Marañón, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; Villarán, C. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef]
- Kavitake, D.; Kandasamy, S.; Devi, P.B.; Shetty, P.H. Recent developments on encapsulation of lactic acid bacteria as potential starter culture in fermented foods—A review. Food Biosci. 2018, 21, 34–44. [Google Scholar] [CrossRef]
- Feng, C.; Zong, X.; Cui, B.; Guo, H.; Zhang, W.; Zhu, J. Application of carrier materials in self-healing cement-based materials based on microbial-induced mineralization. Crystals 2022, 12, 797. [Google Scholar] [CrossRef]
- Martin, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Effect of unmodified starch on viability of alginate-encapsulated Lactobacillus fermentum CECT5716. LWT 2013, 53, 480–486. [Google Scholar] [CrossRef]
- Vesterlund, S.; Salminen, K.; Salminen, S. Water activity in dry foods containing live probiotic bacteria should be carefully considered: A case study with Lactobacillus rhamnosus GG in flaxseed. Int. J. Food Microbiol. 2012, 157, 319–321. [Google Scholar] [CrossRef]
- Zanjani, M.A.K.; Tarzi, B.G.; Sharifan, A.; Mohammadi, N. Microencapsulation of probiotics by calcium alginate gelatinized starch with chitosan coating and evaluation of survival in simulated human gastrointestinal condition. Iran. J. Pharm. Res. 2014, 13, 843–852. [Google Scholar]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-induced gelation of alginate: Mechanisms and applications. Int. J. Biolog. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Hassan, A.A.M.; Sakr, S.S.; Ali, A.A.; Ahmed, I.A.M.; Elkashef, H. Isolation, identification, and biochemical characterization of five Lacticaseibacillus strains from Oggtt: A traditional fermented and dried buttermilk. Food Sci. Nutr. 2023, 11, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Jukonyte, R.; Zadeike, D.; Bartkiene, E.; Lele, V.; Cernauskas, D.; Suproniene, S.; Juodeikiene, G. A potential of brown rice polish as a substrate for the lactic acid and bioactive compounds production by the lactic acid bacteria newly isolated from cereal-based fermented products. LWT 2018, 97, 323–331. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Lele, V.; Pugajeva, I.; Zavistanaviciute, P.; Mickiene, R.; Zadeike, D.; Juodeikiene, G. A concept of mould spoilage prevention and acrylamide reduction in wheat bread: Application of lactobacilli in combination with a cranberry coating. Food Control 2018, 91, 284–293. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles–A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Contr. Release 2006, 114, 1–14. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent advances of chitosan and its derivatives for novel applications in food science. J. Food Proces. Preserv. 2014, 38, 314–328. [Google Scholar]
- Tharmaraj, J.S.; Hsieh, P.S. Biopolymers for the encapsulation of probiotics: A review. J. Food Sci. 2018, 83, 2011–2020. [Google Scholar]
- Katina, K.; Heiniö, R.-L.; Autio, K.; Poutanen, K. Optimization of sourdough process for improved sensory profile and texture of wheat bread. LWT 2006, 39, 1189–1202. [Google Scholar] [CrossRef]
- Temkov, M.; Rocha, J.M.; Rannou, C.; Ducasse, M.; Prost, C. Influence of baking time and formulation of part-baked wheat sourdough bread on the physical characteristics, sensory quality, glycaemic index and appetite sensations. Front. Nutr. 2024, 11, 1370086. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.D.; Boom, R.M.; Schutyser, M.A.I. Survival of encapsulated Lactobacillus plantarum during isothermal heating and bread baking. LWT 2018, 93, 396–404. [Google Scholar] [CrossRef]
- Seyedain-Ardabili, M.; Sharifan, A.; Ghiassitarzi, B. The production of synbiotic bread by microencapsulation. Food Technol. Biotechnol. 2016, 54, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Thang, T.D.; Hang, H.T.T.; Luan, N.T.; KimThuy, D.T.; Lieu, D.M. Survival survey of Lactobacillus acidophilus in additional probiotic bread. Turk. J. Agric. Food Sci. Technol. 2019, 7, 588–592. [Google Scholar]
- Denkova, R.; Ilieva, S.; Denkova, Z.; Georgieva, L.; Krastanov, A. Examination of the technological properties of newly isolated strains of the genus Lactobacillus and possibilities for their application in the composition of starters. Biotechnol. Biotechnol. Equip. 2014, 28, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Axel, C.; Zannini, E.; Arendt, E.K. Mould spoilage of bread and its biopreservation: A review of current strategies for bread shelf life extension. Crit. Rev. Food Sci. Nutr. 2017, 57, 3528–3542. [Google Scholar] [CrossRef]
- Tan, L.L.; Sampathkumar, K.; Wong, J.H.; Loo, S.C.J. Divalent cations are antagonistic to survivability of freeze-dried probiotics encapsulated in cross-linked alginate. Food Bioprod. Process. 2020, 124, 369–377. [Google Scholar] [CrossRef]
- AACC. Guidelines for Measurement of Volume by Rapeseed Displacement; Methods 10-05.01, 02-31.01; AACC International Approved Methods: St. Paul, MN, USA, 2003. [Google Scholar]
- ISO 15214; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Emumeration of Mesophilic Lactic Acid Bacteria—Colony Count Technique at 30 °C. International Organization for Standardization: Geneva, Switzerland, 1998.
- LST 1442; Bread and Baked Goods. Determination of Porosity. Lithuanian Standards Board (LST): Vilnius, Lithuania, 1996.
- ISO 8586-1; Sensory Analysis. General Guidance for Selection, Training and Monitoring of Assessors. Part 1: Selected Assessors. International Organization for Standardization: Geneva, Switzerland, 2000.
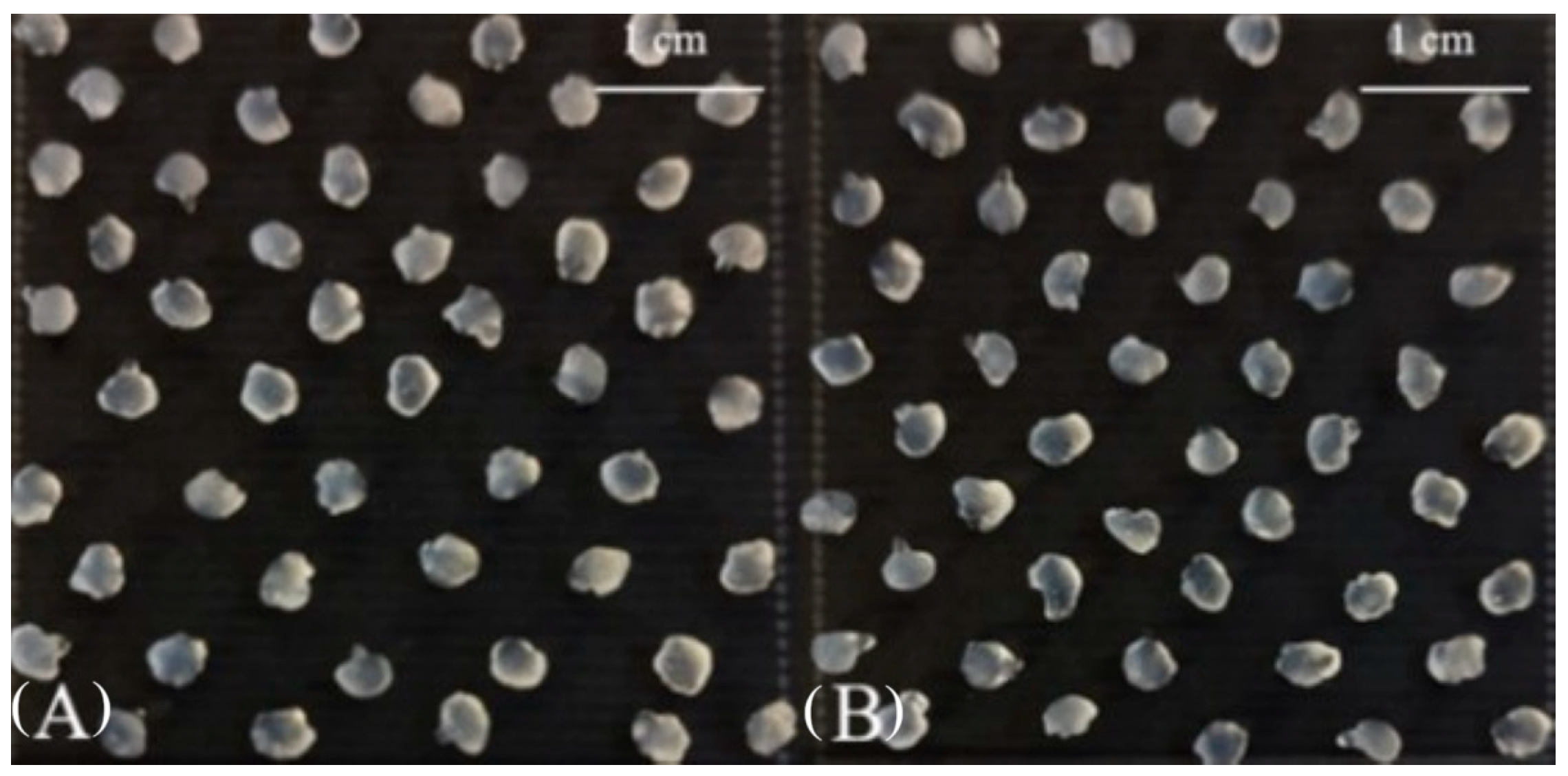
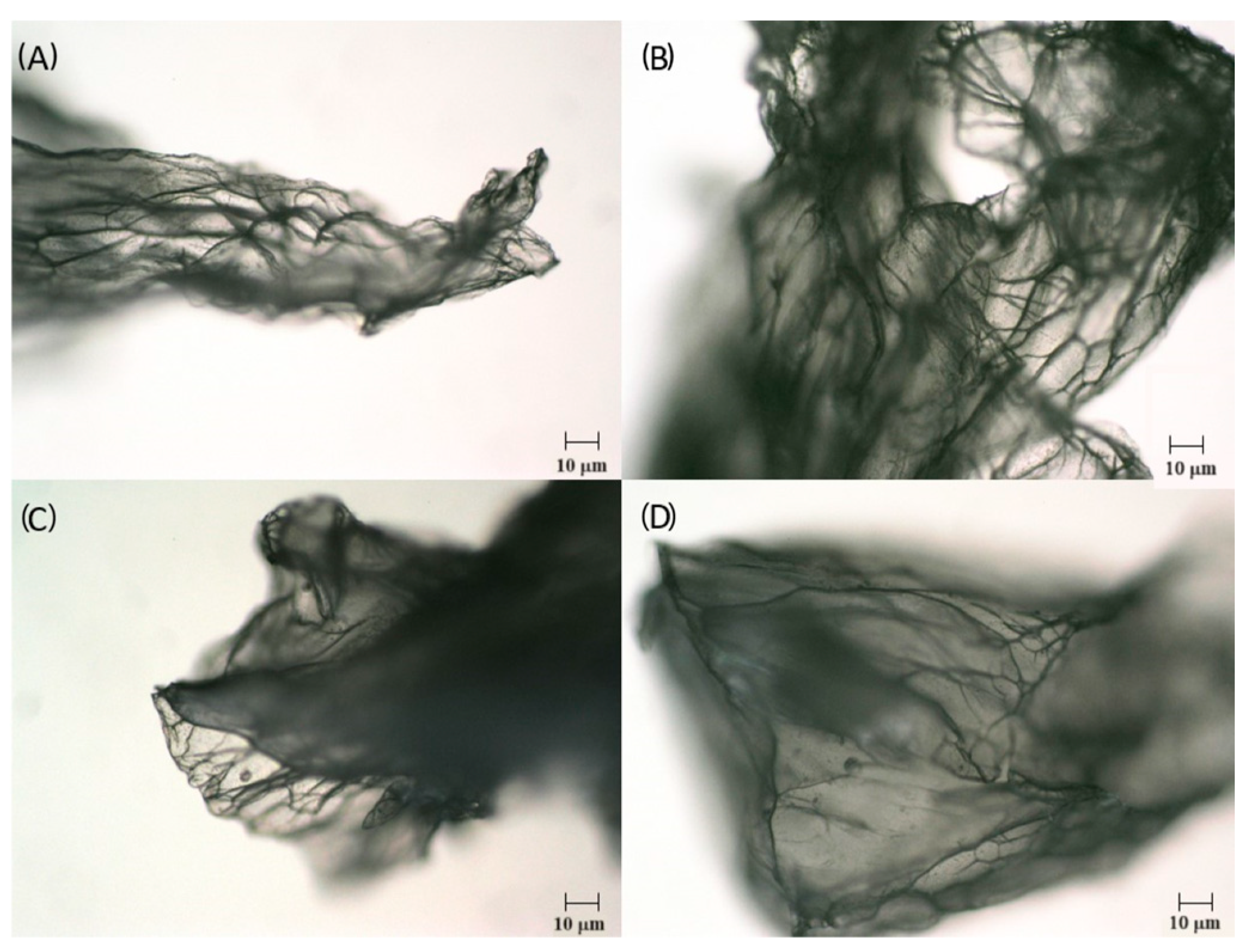


| Coating | LAB Count in Wet Microcapsules, log10 CFU/g | EE *, % | Microcapsule Size, mm | LAB Count in Freeze-Dried Microcapsules, log10 CFU/g |
|---|---|---|---|---|
| Alginate | 10.14 ± 0.01 a | 97.97 a | 2.8 ± 0.2 b | 9.50 ± 0.05 a |
| Alginate–chitosan | 10.01 ± 0.03 a | 96.71 a | 3.7 ± 0.3 a | 9.19 ± 0.03 a |
| Fermentation Time, h | pH | TTA | LAB Count, log10 CFU/g | |||
|---|---|---|---|---|---|---|
| 30 °C | 35 °C | 30 °C | 35 °C | 30 °C | 35 °C | |
| 0 | 5.70 ± 0.01 a | 5.71 ± 0.01 a | 1.13 ± 0.01 h | 1.12 ± 0.02 h | 7.15 ± 0.11 b | 7.15 ± 0.11 b |
| 6 | 5.23 ± 0.01 b | 5.12 ± 0.00 b | 1.53 ± 0.01 g | 1.65 ± 0.02 f | 7.21 ± 0.21 b | 7.24 ± 0.33 b |
| 12 | 4.33 ± 0.01 c | 4.21 ± 0.02 d | 2.93 ± 0.02 e | 3.28 ± 0.01 e | 7.36 ± 0.17 b | 7.46 ± 0.15 b |
| 24 | 3.78 ± 0.02 e | 3.56 ± 0.02 f | 4.76 ± 0.01 d | 5.48 ± 0.02 b | 8.48 ± 0.05 a | 8.69 ± 0.22 a |
| 36 | 3.60 ± 0.0 e,f | 3.38 ± 0.01 g | 5.25 ± 0.02 c | 6.19 ± 0.02 a | 8.86 ± 0.13 a | 9.06 ± 0.14 a |
| Fermentation Time, h | 30 °C | 35 °C | ||
|---|---|---|---|---|
| Alg | Alg+Ch | Alg | Alg+Ch | |
| pH | ||||
| 0 | 5.88 ± 0.01 a | 5.87 ± 0.01 a | 5.88 ± 0.01 a | 5.87 ± 0.01 a |
| 6 | 5.70 ± 0.02 a | 5.76 ± 0.02 a | 5.43 ± 0.01 b | 5.50 ± 0.01 b |
| 12 | 5.12 ± 0.01 d | 5.21 ± 0.01 c | 4.77 ± 0.01 f | 4.86 ± 0.02 e |
| 24 | 4.54 ± 0.01 h | 4.68 ± 0.01 g | 4.12 ± 0.01 m | 4.23 ± 0.01 k |
| 36 | 4.36 ± 0.01 i | 4.52 ± 0.01 h | 3.96 ± 0.01 n | 4.07 ± 0.01 m |
| TTA, °N | ||||
| 0 | 0.45 ± 0.01 p | 0.44 ± 0.01 p | 0.45 ± 0.01 p | 0.44 ± 0.01 p |
| 6 | 0.68 ± 0.01 m | 0.63 ± 0.01 n | 0.70 ± 0.01 m | 0.65 ± 0.01 n |
| 12 | 1.05 ± 0.01 k | 0.96 ± 0.01 l | 1.13 ± 0.02 h | 1.02 ± 0.01 k |
| 24 | 3.51 ± 0.02 e | 3.21 ± 0.01 g | 3.78 ± 0.01 d | 3.38 ± 0.01 f |
| 36 | 5.85 ± 0.01 b | 5.32 ± 0.02 c | 5.98 ± 0.01 a | 5.45 ± 0.02 b |
| Bread Samples | Bread Quality Attributes | LAB Count, log10 CFU/g | ||||||
|---|---|---|---|---|---|---|---|---|
| Porosity, % | Specific Volume, cm3/g | Ih | TTA, °N | Acceptability | Dough | Crumb | Reduction | |
| K | 67.64 ± 0.11 e | 1.91 ± 0.02 e | 0.64 ± 0.01 b | 0.90 ± 0.01 e | 4.2 ± 0.3 c | - | - | - |
| SR | 84.36 ± 0.12 a | 3.35 ± 0.03 a | 0.72 ± 0.03 a | 3.40 ± 0.01 a | 7.8 ± 0.4 a | 7.73 ± 0.21 a | 5.27 ± 0.11 b | 2.46 |
| LSR | 78.67 ± 0.06 b | 3.25 ± 0.02 b | 0.66 ± 0.02 b | 1.68 ± 0.01 b | 6.8 ± 0.2 b | 6.32 ± 0.14 b | 4.98 ± 0.05 b | 1.34 |
| LAlg | 74.42 ± 0.05 c | 3.19 ± 0.02 c | 0.58 ± 0.01 c | 1.20 ± 0.01 c | 6.5 ± 0.3 b | 7.59 ± 0.17 a | 6.36 ± 0.05 a | 1.23 |
| LAlg+Ch | 70.78 ± 0.02 d | 2.71 ± 0.04 d | 0.55 ± 0.01 d | 1.10 ± 0.01 d | 6.4 ± 0.5 b | 7.57 ± 0.23 a | 6.51 ± 0.21 a | 1.06 |
| Samples | 4 Days | 5 Days | 6 Days | 7 Days | 8 Days | 9 Days | 10 Days |
|---|---|---|---|---|---|---|---|
| K | + | ++ | ++ | +++ | +++ | +++ | +++ |
| SR | − | − | + | ++ | ++ | +++ | +++ |
| LSR | − | − | + | + | ++ | ++ | +++ |
| LAlg | − | − | − | + | + | ++ | +++ |
| LAlg+Ch | − | − | − | + | ++ | ++ | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zadeike, D.; Gaizauskaite, Z.; Basinskiene, L.; Zvirdauskiene, R.; Cizeikiene, D. Exploring Calcium Alginate-Based Gels for Encapsulation of Lacticaseibacillus paracasei to Enhance Stability in Functional Breadmaking. Gels 2024, 10, 641. https://doi.org/10.3390/gels10100641
Zadeike D, Gaizauskaite Z, Basinskiene L, Zvirdauskiene R, Cizeikiene D. Exploring Calcium Alginate-Based Gels for Encapsulation of Lacticaseibacillus paracasei to Enhance Stability in Functional Breadmaking. Gels. 2024; 10(10):641. https://doi.org/10.3390/gels10100641
Chicago/Turabian StyleZadeike, Daiva, Zydrune Gaizauskaite, Loreta Basinskiene, Renata Zvirdauskiene, and Dalia Cizeikiene. 2024. "Exploring Calcium Alginate-Based Gels for Encapsulation of Lacticaseibacillus paracasei to Enhance Stability in Functional Breadmaking" Gels 10, no. 10: 641. https://doi.org/10.3390/gels10100641
APA StyleZadeike, D., Gaizauskaite, Z., Basinskiene, L., Zvirdauskiene, R., & Cizeikiene, D. (2024). Exploring Calcium Alginate-Based Gels for Encapsulation of Lacticaseibacillus paracasei to Enhance Stability in Functional Breadmaking. Gels, 10(10), 641. https://doi.org/10.3390/gels10100641











