Recent Progress of Three-Dimensional Graphene-Based Composites for Photocatalysis
Abstract
1. Introduction
| Classification | Photocatalyst | Synthetic Method | Morphology | Application | Ref. |
|---|---|---|---|---|---|
| TiO2 | Anodic oxidation | Nanotube arrays | Degradation RhB and BPA | [17] | |
| ZnO | Calcination/Solvothermal | Nanorods | Degradation MB | [18] | |
| γ-Fe2O3 | Precipitation/Calcination | Nanosheets | Oxygen evolution | [19] | |
| Metal oxide | CuO | Ultrasound/Microwave | Flower-like | Cr (VI) reduction | [20] |
| WO3 | Precipitation/Calcination | Nanoflakes | Degradation CR and MB | [21] | |
| α-Bi2O3 | Calcination | Nanoflowers | Degradation CV | [22] | |
| CeO2 | Hydrothermal | Nanorods/Nanowires Octahedrons/Nanocubes | NO oxidation and CO2 conversion | [23] | |
| CdS | Etching/Sulfuration | Double-shelled nanocages | CO2 reduction | [24] | |
| MoS2 | Hydrothermal | Flower-like | N2 fixation | [25] | |
| Metal sulfide | FeS2 | Hydrothermal | Nanorods | Degradation MB | [26] |
| ZnS | Hydrothermal/Calcination | Nanosheets | H2 evolution | [27] | |
| CuS | Solvothermal | Microspheres | Degradation RhB, MB, MO | [28] | |
| Bi2S3 | Solvothermal | Nanorods | N2 fixation | [29] | |
| BiVO4 | Hydrothermal | Hollow nanotubes | Degradation CR | [30] | |
| FeVO4 | Hydrothermal | 3D nanowall-like | CO2 reduction | [31] | |
| Vanadates | Ag3VO4 | Hydrothermal | Mesoporous | Desulfurization | [32] |
| InVO4 | Microwave Hydrothermal | Nanocrystals Microspheres | H2 evolution | [33] | |
| Carbonitride | g-C3N4 | Molten salt | Nanorods | H2O2 generation | [34] |
| C3N5 | Molten salt | Nanosheets | H2 evolution | [35] | |
| Ti-MOF | Condensation | Needle-like | CO2 reduction | [36] | |
| MOFs | Ni-MOF | Hydrothermal | Nanosheets | CO2 reduction | [37] |
| Fe-MOF | Solvothermal | Hexagonal bipyramid | H2 evolution | [38] | |
| Co-MOF | Solvothermal | Nanosheets | Degradation dyes | [39] |
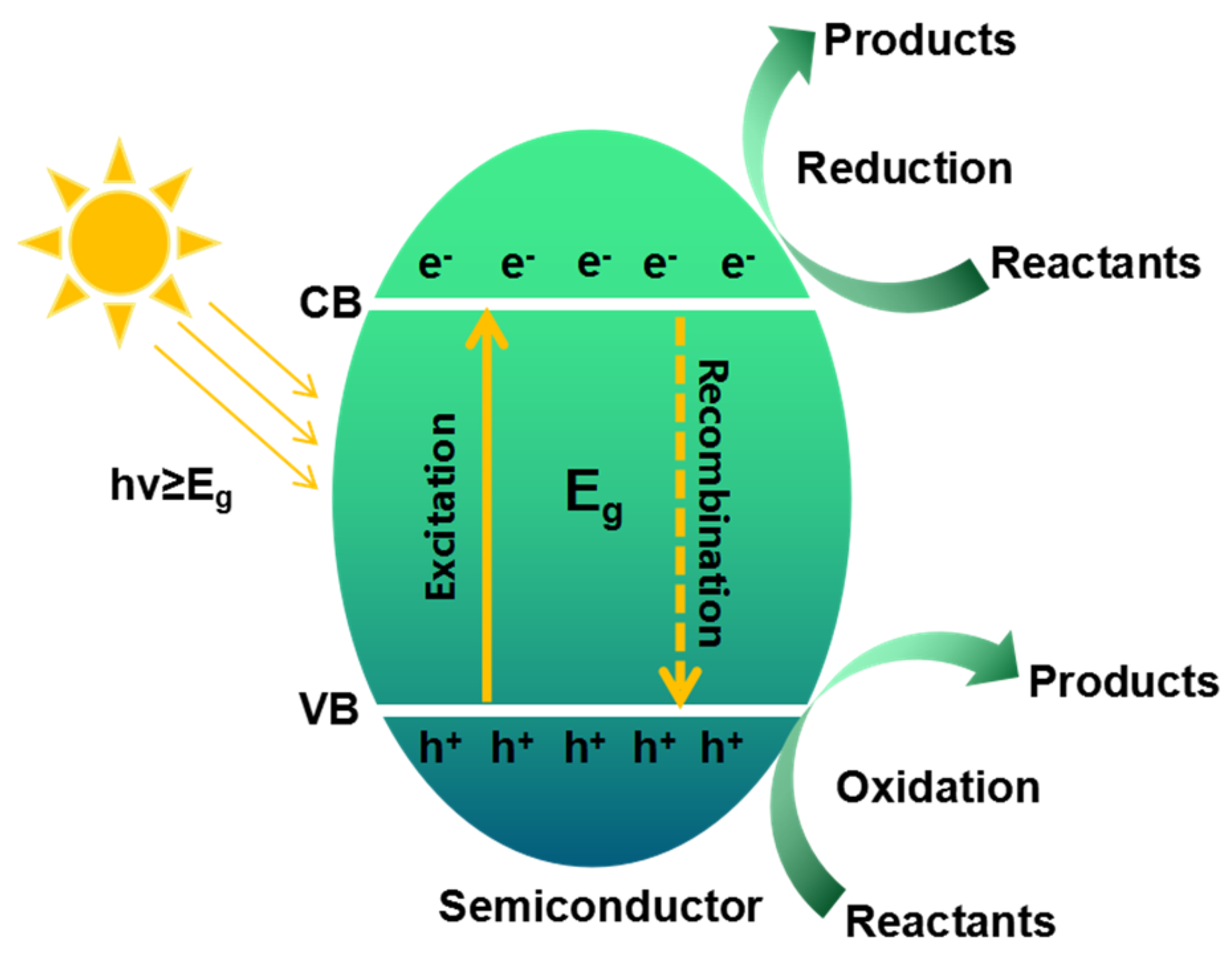
2. Basic Features
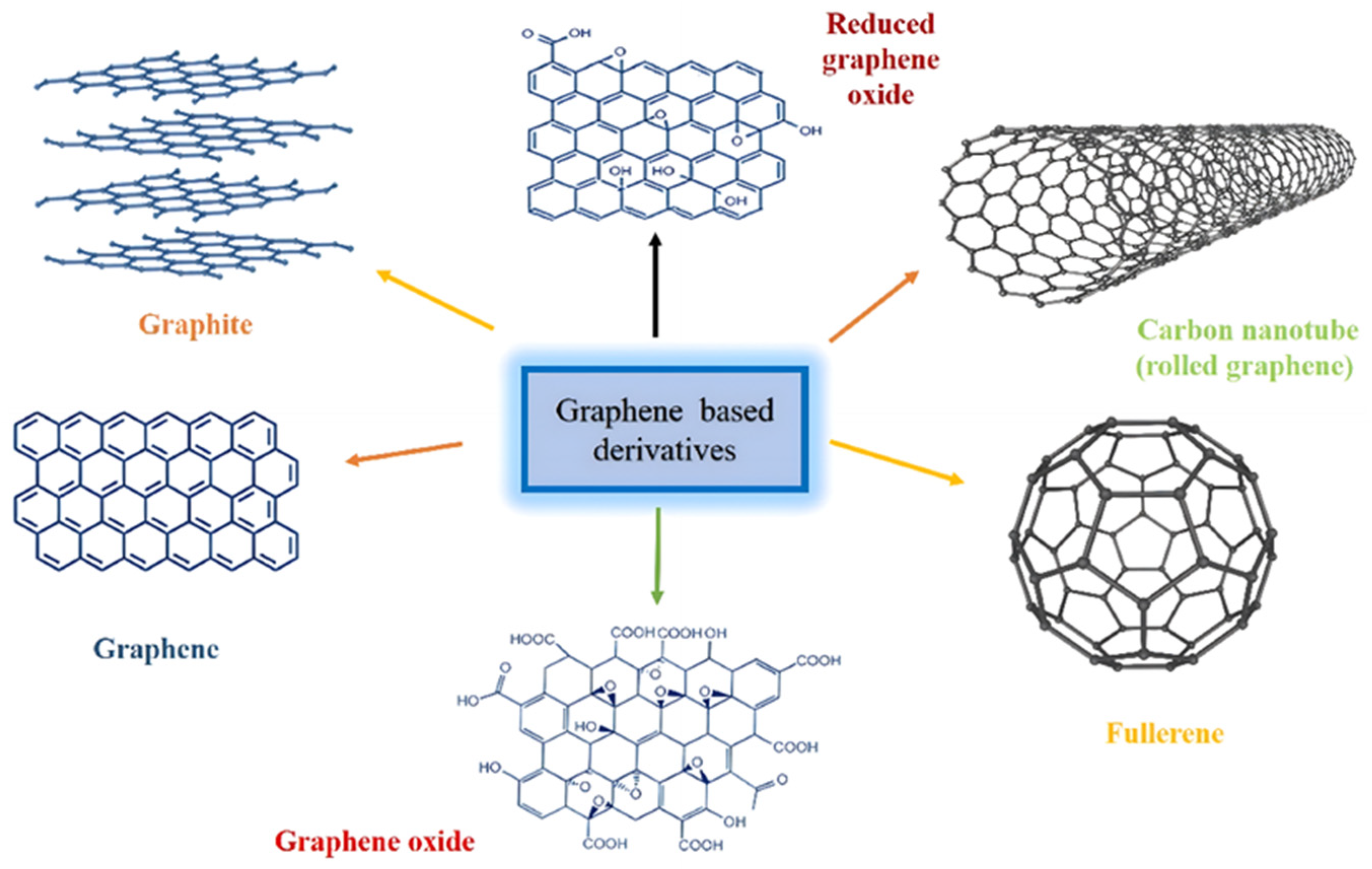
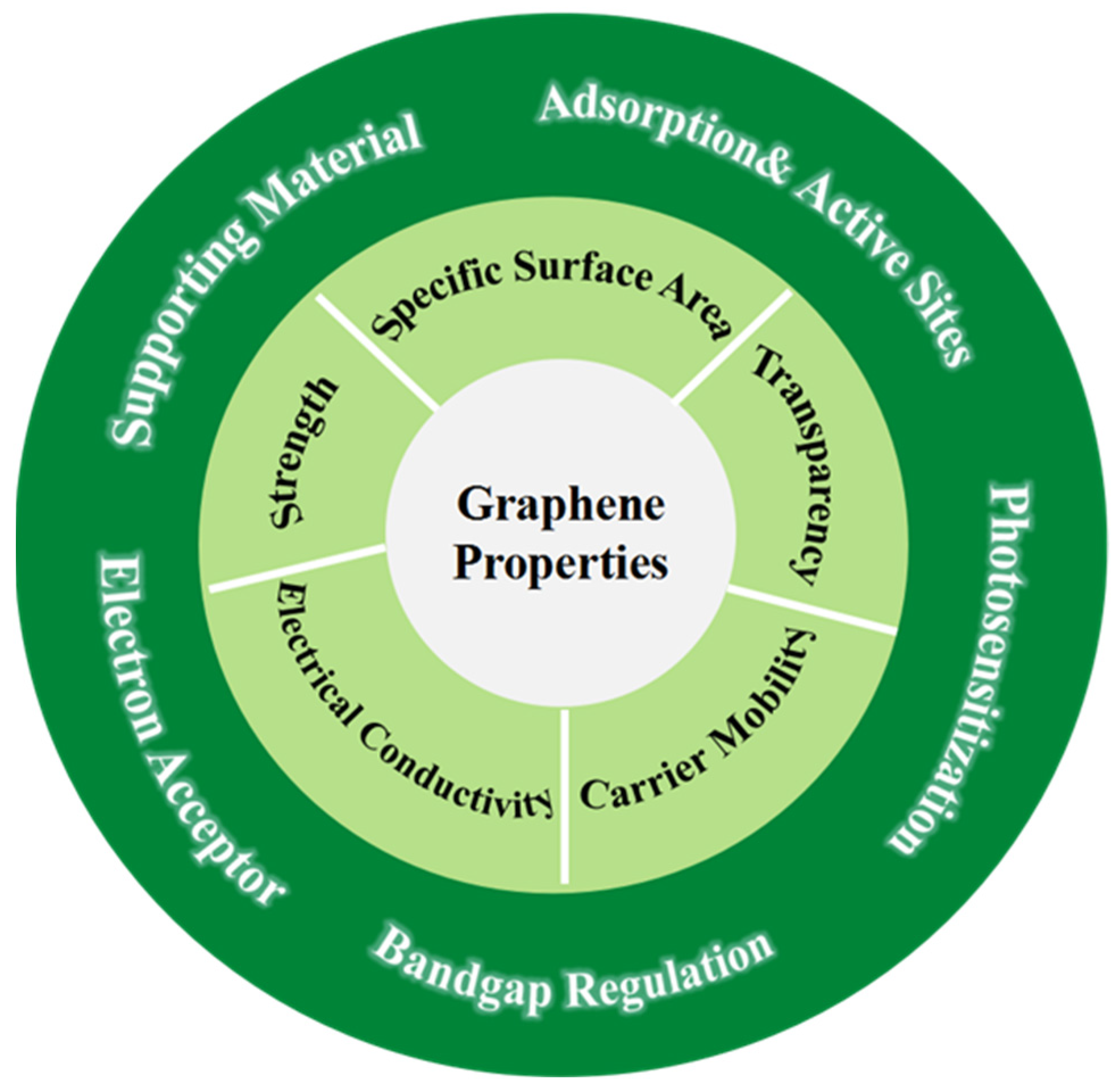
2.1. General Properties of Graphene and Its Derivatives
2.2. Functionalities of 3D Graphene for Photocatalysis
3. Synthetic Strategies of 3D Graphene Architectures
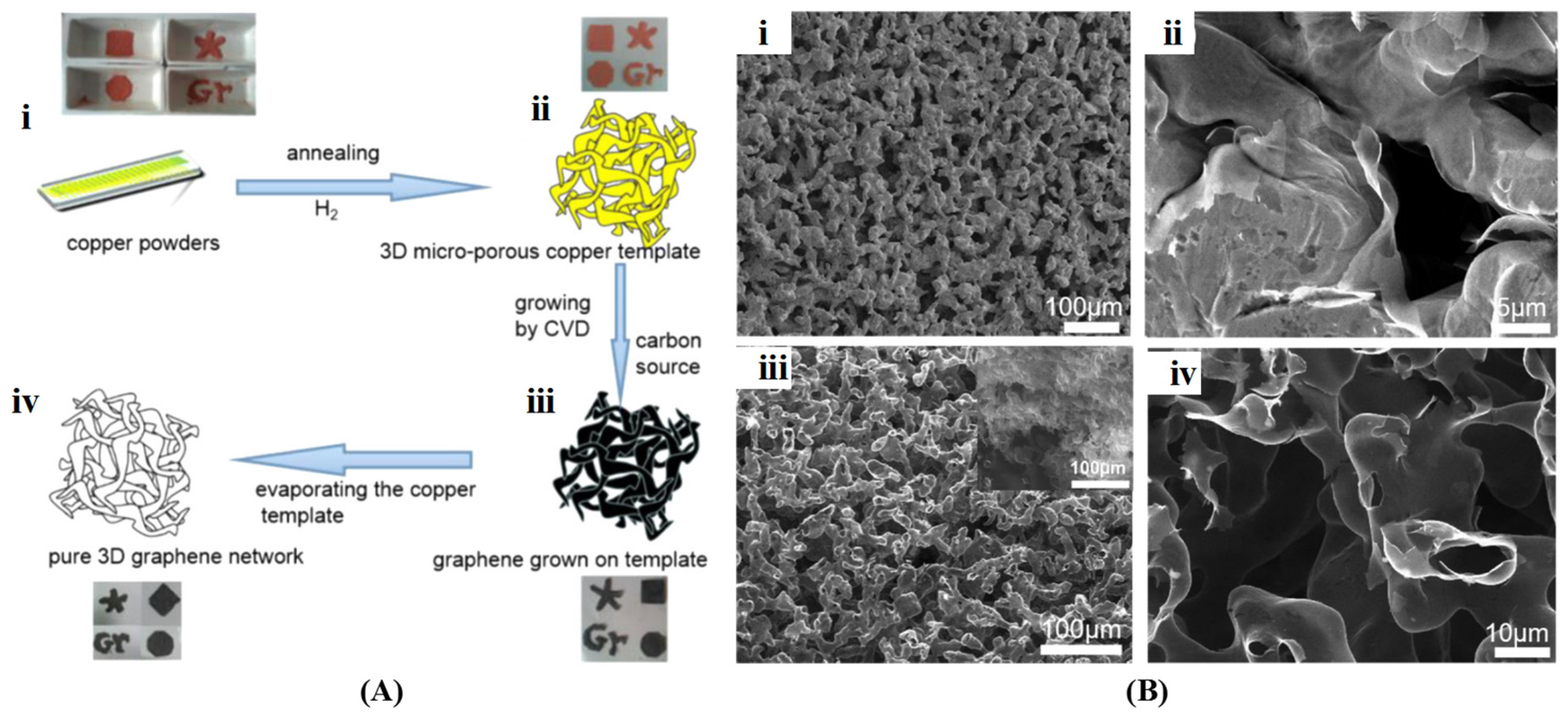
3.1. Template Method
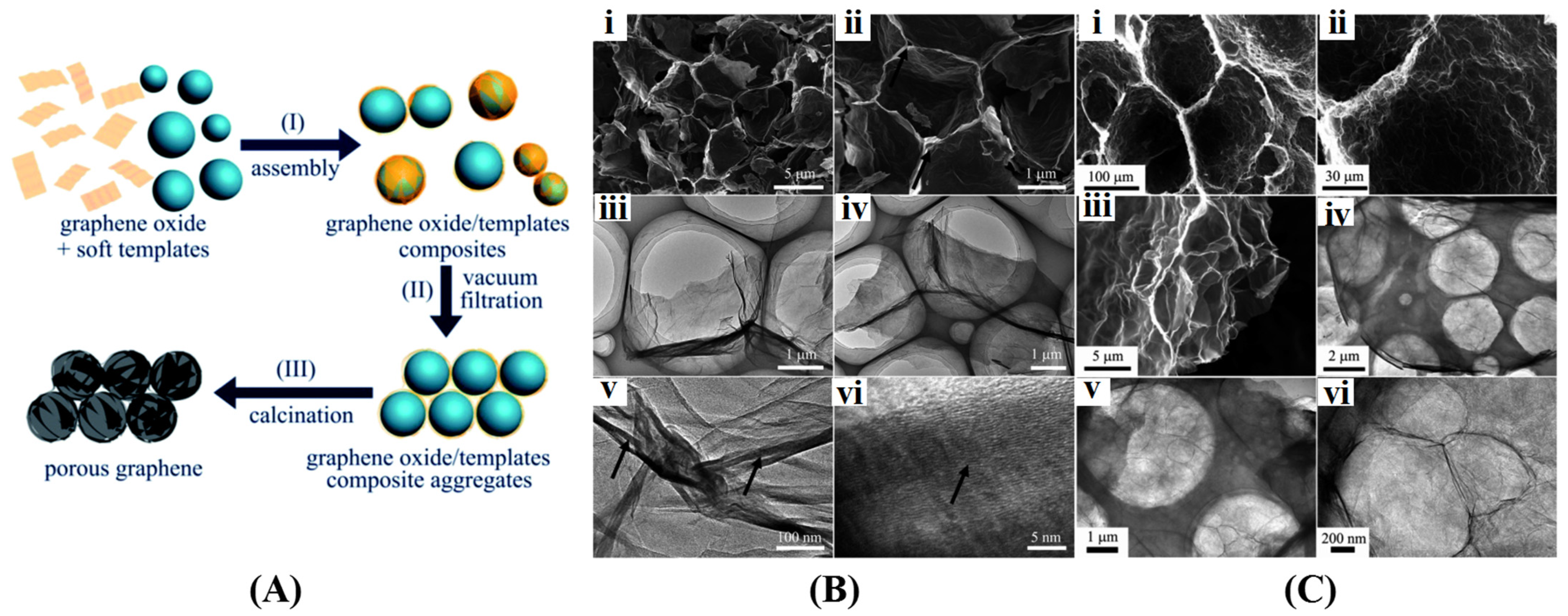
3.1.1. Hard Template Method
Template-Directed CVD Method
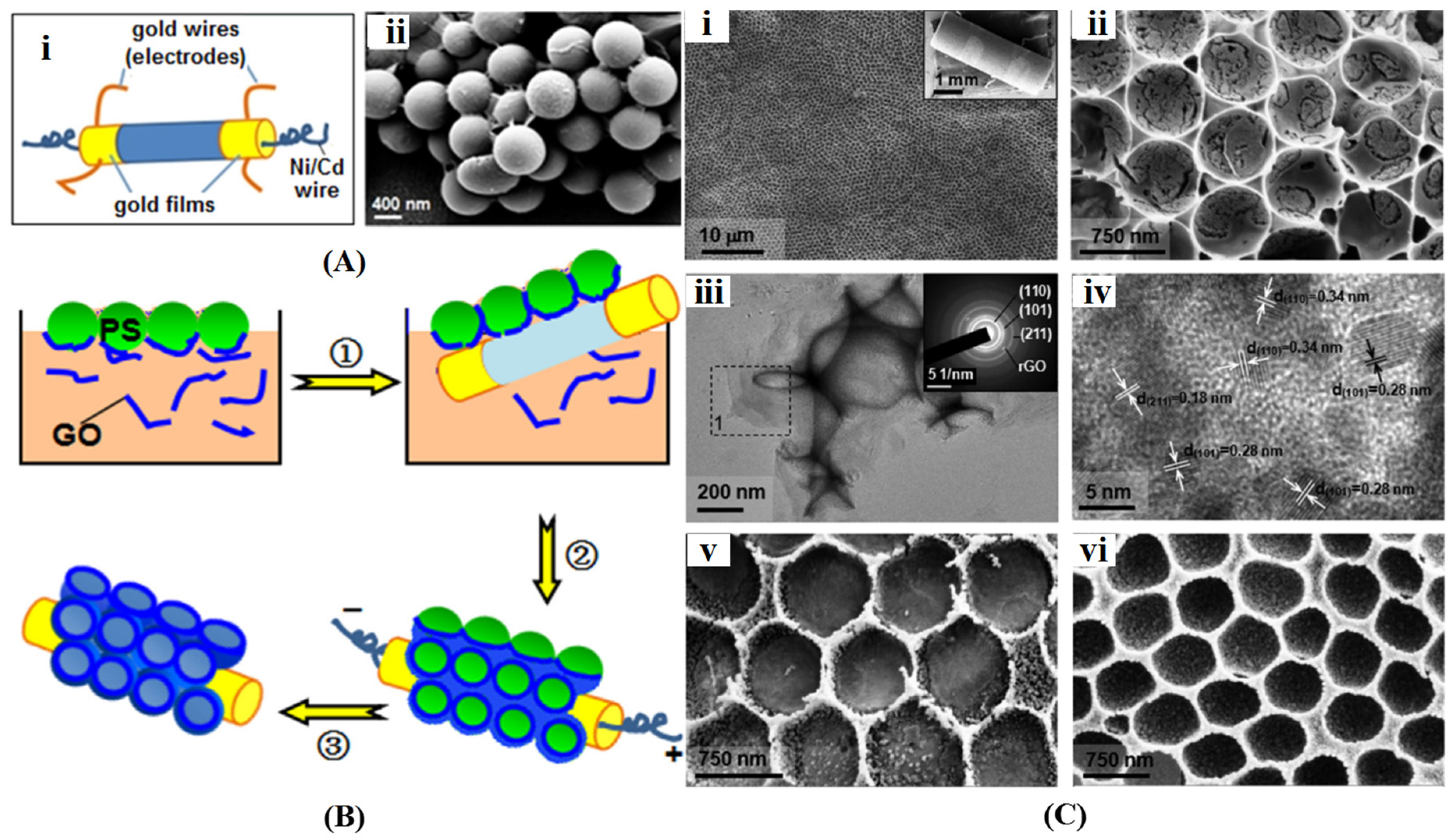
Microsphere Template-Assisted Method
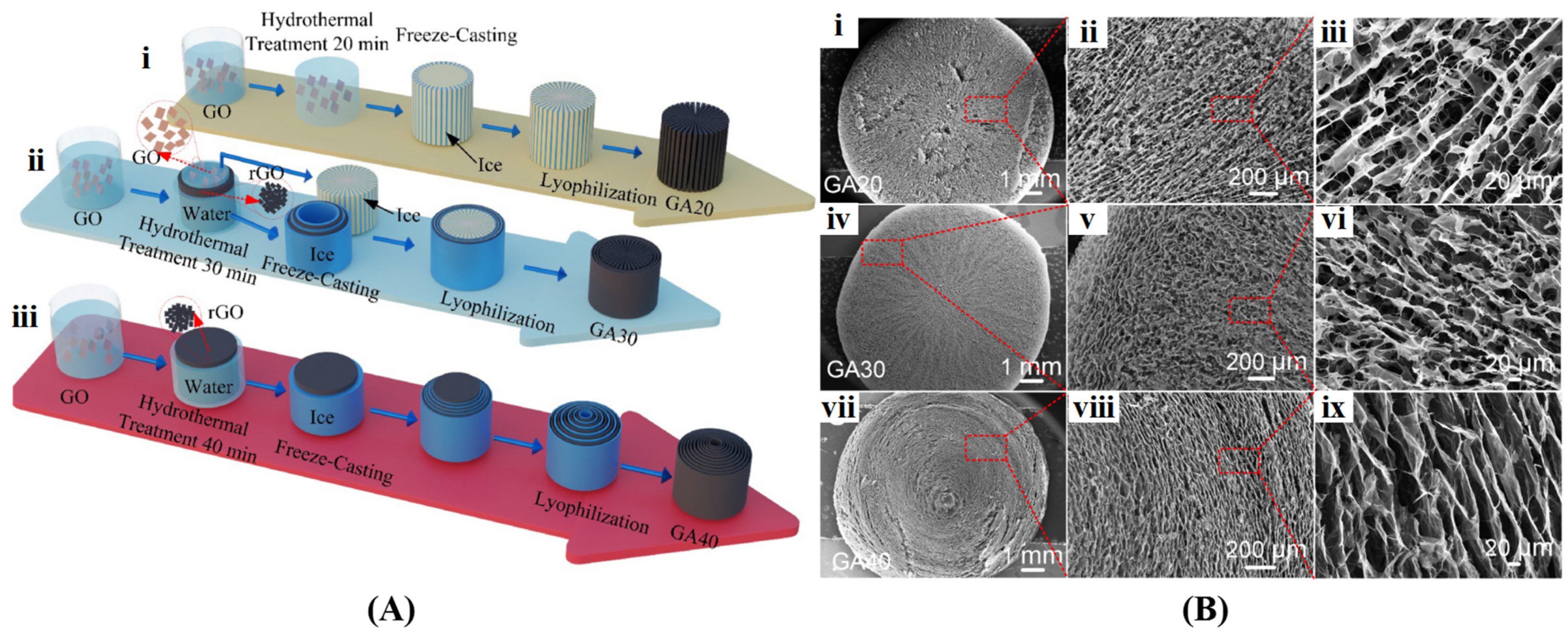
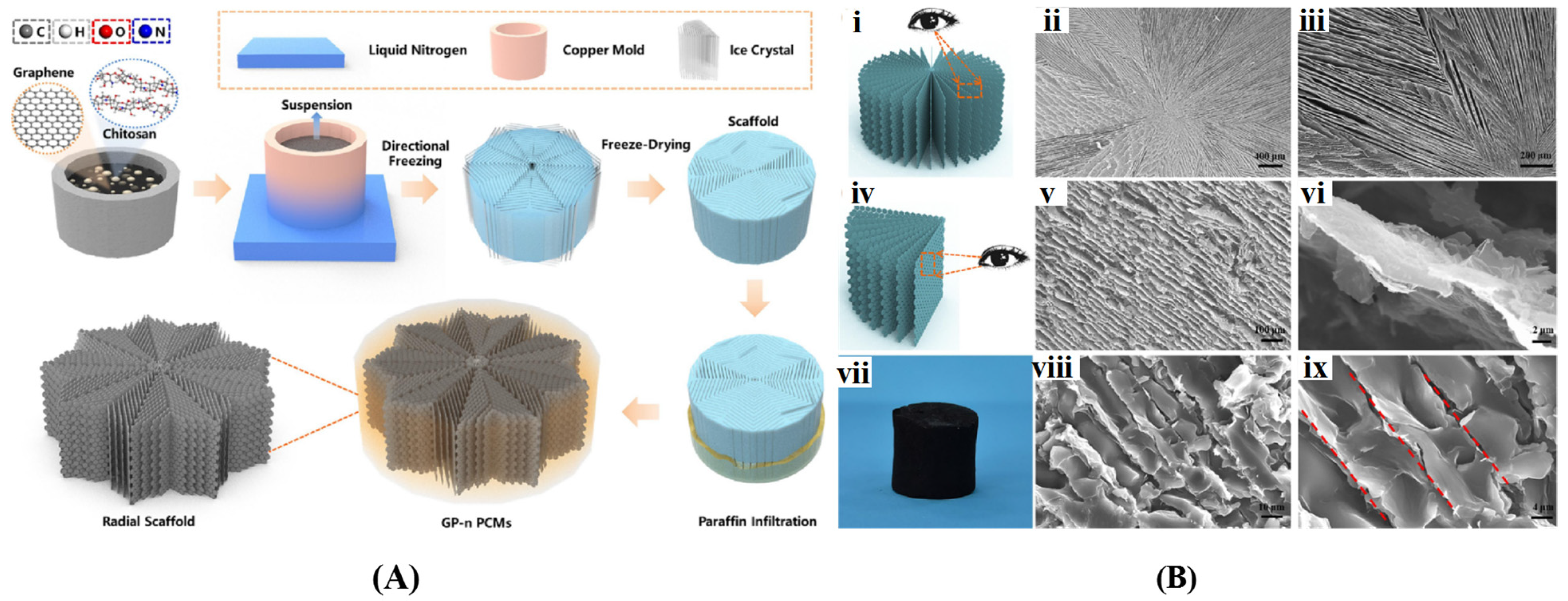
Ice Template-Assisted Method
3.1.2. Soft Template Method
3.2. Non-Template Method
3.2.1. Self-Assembly Method
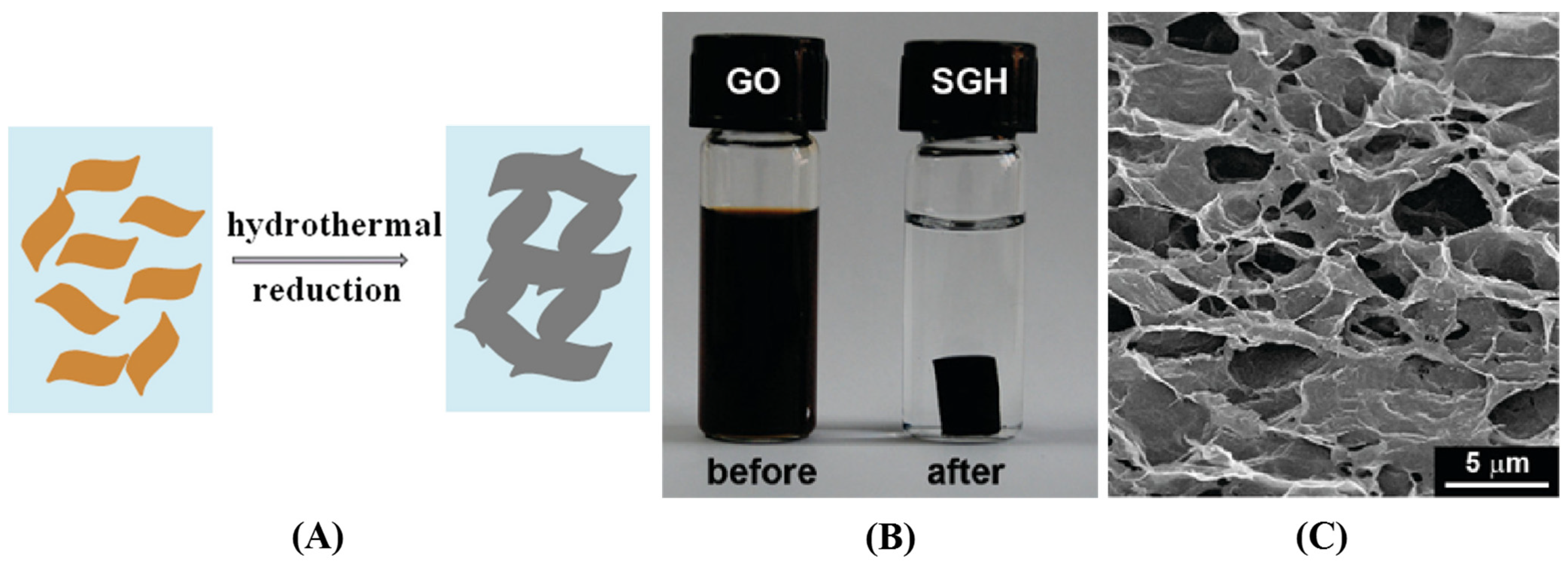
Hydrothermal/Solvothermal Reduction
Chemical Reduction
3.2.2. 3D Printing Method
4. Synthesis of 3D Graphene-Based Composite Photocatalysts
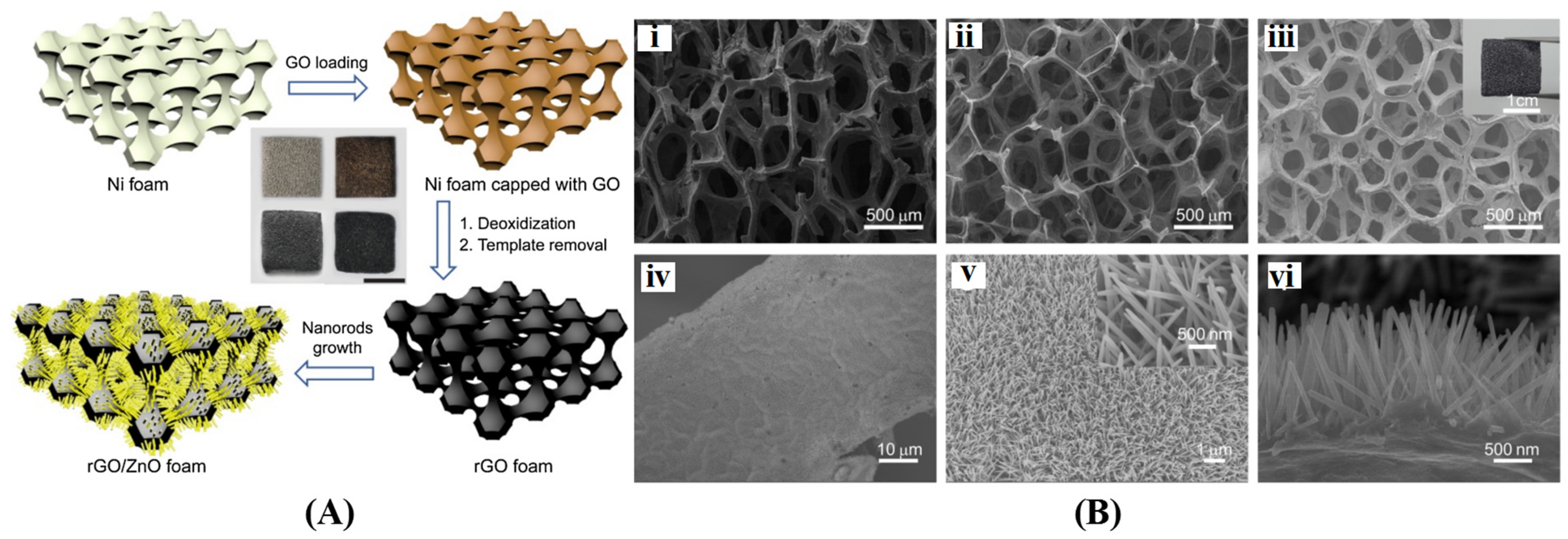
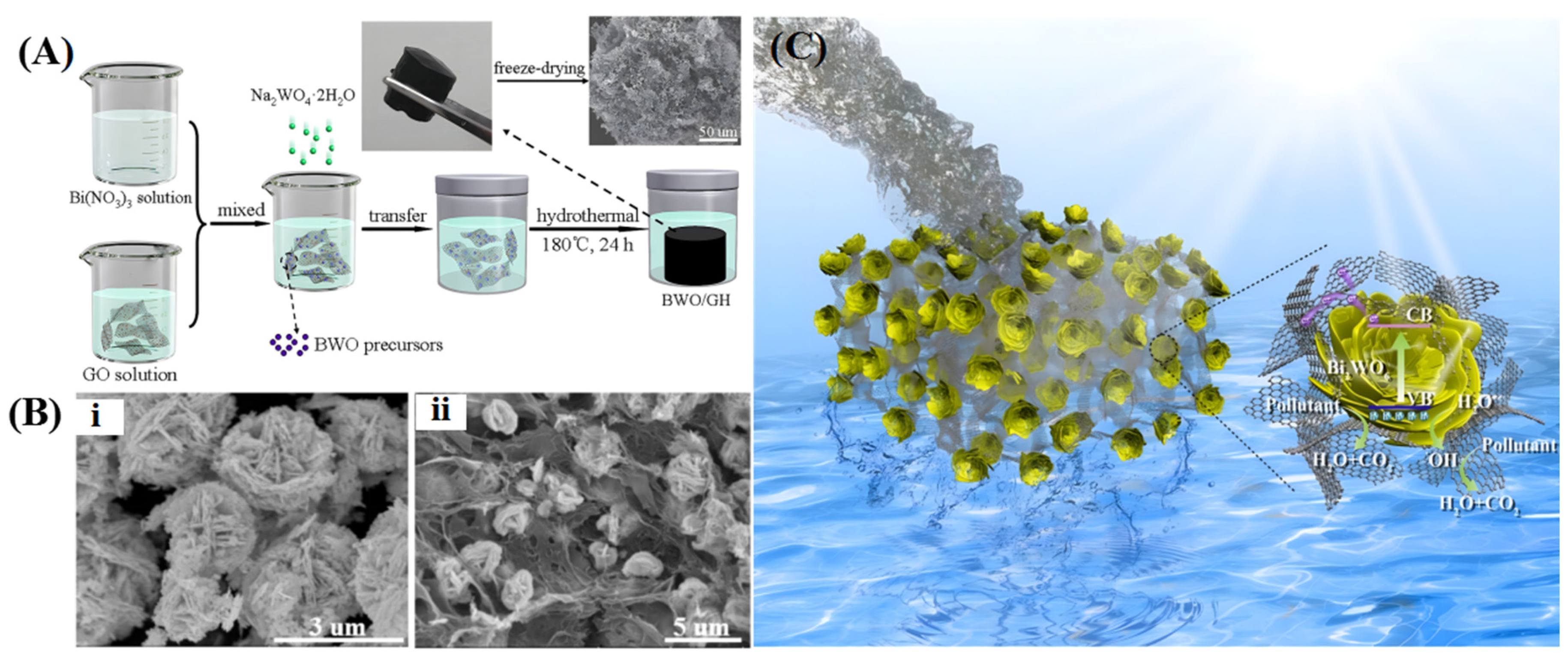
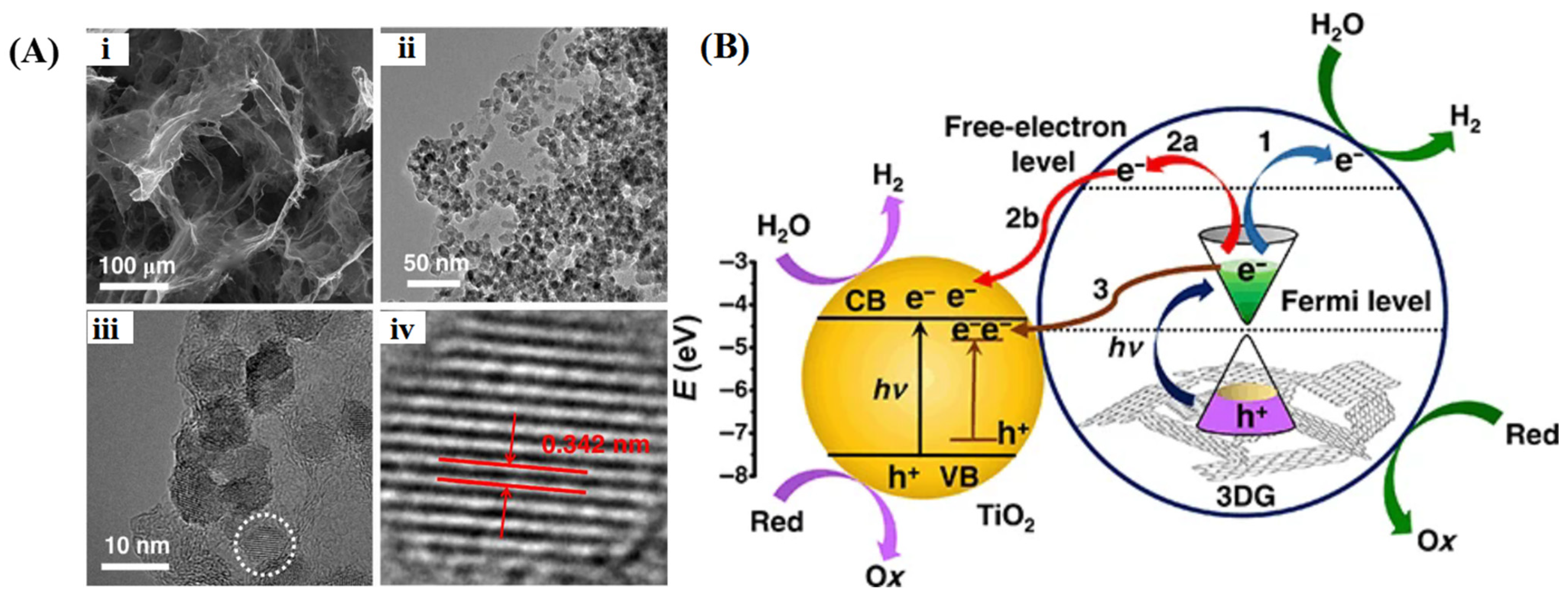
4.1. 3D Graphene/Metal Oxides
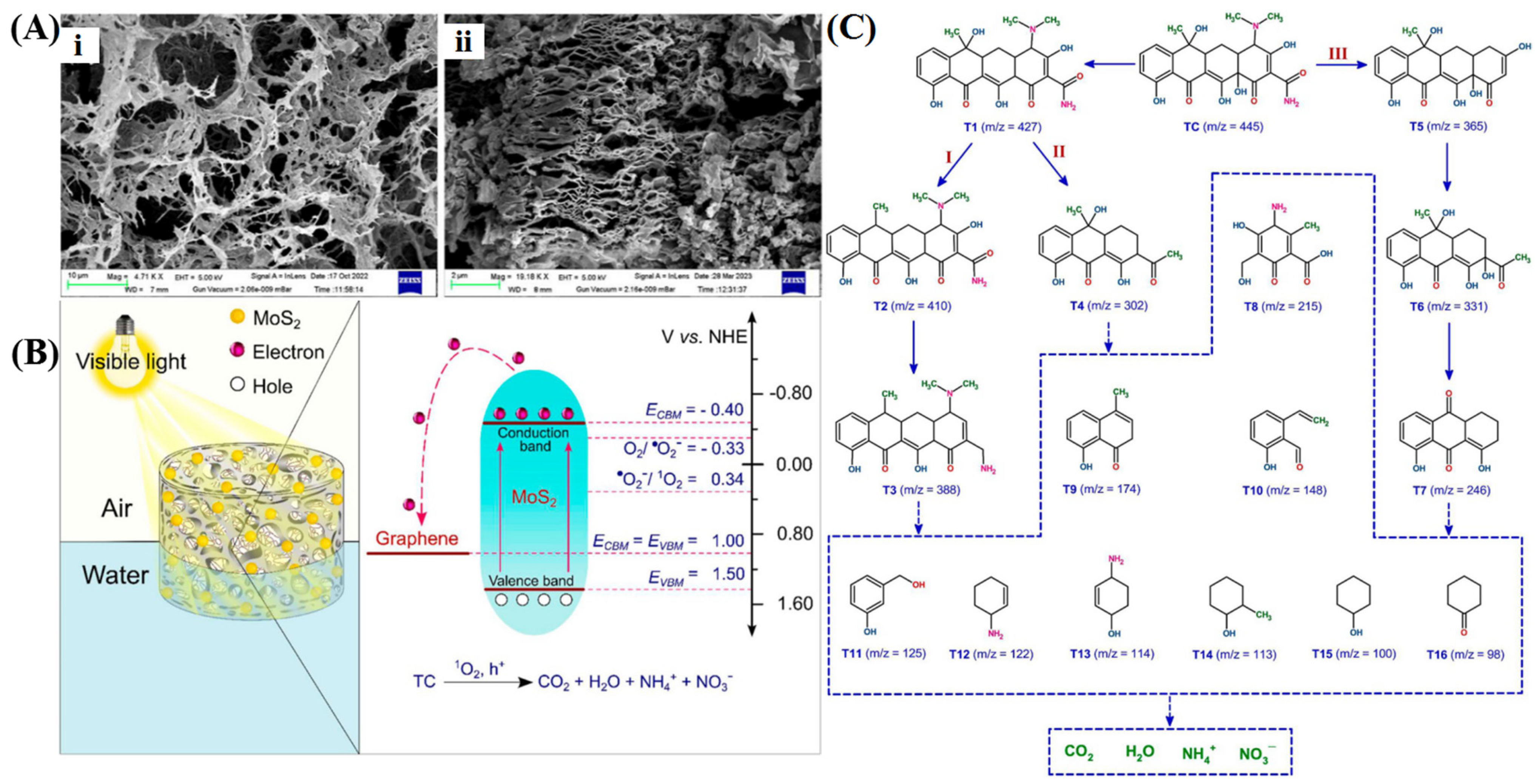
4.2. 3D Graphene/Metal Sulfides
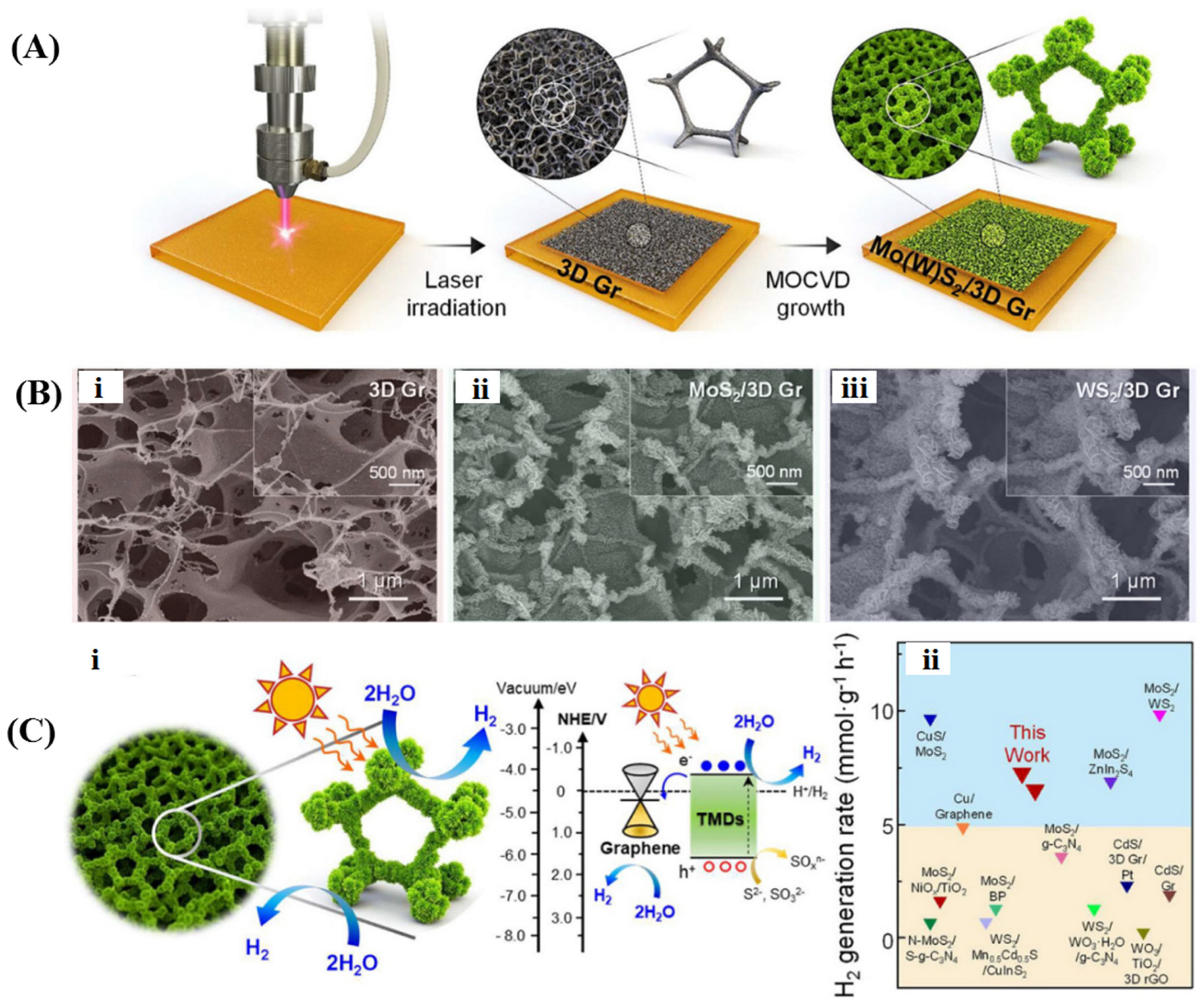
4.3. Other 3D Graphene-Based Photocatalysts
5. Applications of 3D Graphene-Based Photocatalysts
5.1. Photocatalytic Degradation of Organic Pollutants
5.2. Photocatalytic Hydrogen Evolution
5.3. Photocatalytic CO2 Reduction
| Photocatalyst | Light Source | Reaction Type | Reaction Conditions | Performance | Ref |
|---|---|---|---|---|---|
| Bi2WO6/graphene | Xenon lamp (500 W) >420 nm | Degradation | 40 ppm MB, 50 mL 5 ppm 2,4-CDP, 50 mL | Static system: 50.6% Dynamic system: 28.1% (MB), 17% (2,4-CDP) | [178] |
| TiO2/graphene | UV lamp (24 W) VUV lamp (1.2 W) | Degradation | 500 ppb ethenzamide, 150 mL | 98.5%, 60 min 99.8%, 3 min | [179] |
| ZnSnO3/graphene | Xenon lamp | Degradation | 100 mg/L CIP, 100 mL | almost 100%, 120 min | [180] |
| CeVO4/graphene/BiVO4 | Xenon lamp (500 W) | Degradation | 20 mg/L TC, 100 mL | 100%, 60 min | [181] |
| MoS2/graphene | Mercury lamp (250 W, with UV cutoff filter) | Degradation | 5 mg/L TC, 100 mL | 97%, 120 min | [110] |
| TiO2/graphene | Xenon lamp (200 W) 320~780 nm | H2 evolution | methanol (10 vol%) | 1205 μmol·h−1·g−1 | [66] |
| Na0.5Bi0.5TiO3/graphene | Xenon lamp (250 W) | H2 evolution | methanol (25 vol%) | 100 mmol·h−1·g−1 | [184] |
| CdS/graphene | Xenon lamp (500 W) 320~780 nm | H2 evolution | 0.1 M Na2S·9H2O, 0.5 M Na2SO3 | 213.358 μmol·h−1·g−1 | [185] |
| CdS/g-C3N4/graphene | Xenon lamp (300 W) >420 nm | H2 evolution | TEOA (5 vol%) | 86.38 μmol·h−1·g−1 | [186] |
| MoS2/graphene WS2/graphene | Xenon lamp (150 W) | H2 evolution | 0.35 M Na2S, 0.25 M Na2SO3 | 6.51 mmol·h−1·g−1 7.26 mmol·h−1·g−1 | [187] |
| TiO2/graphene | Mercury lamp (200 W) | CO2 reduction | CO2, triethylamine vapor | CO conversion: 1.26 μmol·mg−1 | [194] |
| ZnO/N-graphene | Xenon lamp (300 W) | CO2 reduction | 84 mg NaHCO3, 2M H2SO4 | CH3OH conversion: 1.51 μmol·h−1·g−1 | [195] |
| MOF-808/graphene | Xenon lamp | CO2 reduction | CO2, H2O | CO conversion: 14.35 μmol·g−1 | [196] |
| ZnIn2S4/N-graphene | Xenon lamp (300 W) | CO2 reduction | 84 mg NaHCO3, 2M H2SO4 | CH4: 1.01 μmol·h−1·g−1 CO: 2.45 μmol·h−1·g−1 CH3OH: 1.37 μmol·h−1·g−1 | [197] |
6. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mei, W.; Lu, H.; Dong, R.; Tang, S.; Xu, J. Ce-Doped Brookite TiO2 Quasi-Nanocubes for Efficient Visible Light Catalytic Degradation of Tetracycline. ACS Appl. Nano Mater. 2024, 7, 1825–1834. [Google Scholar] [CrossRef]
- He, J.; Hu, L.; Shao, C.; Jiang, S.; Sun, C.; Song, S. Photocatalytic H2O Overall Splitting into H2 Bubbles by Single Atomic Sulfur Vacancy CdS with Spin Polarization Electric Field. ACS Nano 2021, 15, 18006–18013. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, K.; Pan, Q.; Xu, Y.; Liu, Q.; Cui, G.; Guo, X.; Sun, X. Boron-Doped TiO2 for Efficient Electrocatalytic N2 Fixation to NH3 at Ambient Conditions. ACS Sustain. Chem. Eng. 2018, 7, 117–122. [Google Scholar] [CrossRef]
- Basyach, P.; Prajapati, P.K.; Rohman, S.S.; Sonowal, K.; Kalita, L.; Malik, A.; Guha, A.K.; Jain, S.L.; Saikia, L. Visible Light-Active Ternary Heterojunction Photocatalyst for Efficient CO2 Reduction with Simultaneous Amine Oxidation and Sustainable H2O2 Production. ACS Appl. Mater. Interfaces 2023, 15, 914–931. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Lin, Z.; Jiang, X.; Xu, W.; Li, F.; Chen, X.; Wang, H.; Liu, S.; Lu, X. The effects of water, substrate, and intermediate adsorption on the photocatalytic decomposition of air pollutants over nano-TiO2 photocatalysts. Phys. Chem. Chem. Phys. 2024, 26, 662–678. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, G.; Zheng, S.; Gao, X.; Xiang, Z.; Gao, M.; Wang, C.; Liu, M.; Zhong, J. Advanced photocatalytic disinfection mechanisms and their challenges. J. Environ. Manag. 2024, 366, 121875. [Google Scholar] [CrossRef]
- Batool, S.S.; Saleem, R.; Khan, R.R.M.; Saeed, Z.; Pervaiz, M.; Summer, M. Enhancing photocatalytic performance of zirconia-based nanoparticles: A comprehensive review of factors, doping strategies, and mechanisms. Mater. Sci. Semicond. Process. 2024, 178, 108419. [Google Scholar] [CrossRef]
- Han, H.; Khan, M.R.; Ahmad, I.; Al-Qattan, A.; Ali, I.; Karim, M.R.; Bayahia, H.; Khan, F.S.; Ahmad, Z.; Ullah, S. Hierarchical S-scheme heterojunction systems: A comprehensive review on outstanding photocatalysts. J. Water Process Eng. 2024, 61, 105346. [Google Scholar] [CrossRef]
- Wang, S.; Teramura, K.; Hisatomi, T.; Domen, K.; Asakura, H.; Hosokawa, S.; Tanaka, T. Effective Driving of Ag-Loaded and Al-Doped SrTiO3 under Irradiation at λ > 300 nm for the Photocatalytic Conversion of CO2 by H2O. ACS Appl. Energy Mater. 2020, 3, 1468–1475. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, P.; Deng, M.; Shen, H.; Feng, Z.; Li, H. Hybrid Density Functional Theory Study of Native Defects and Nonmetal (C, N, S, and P) Doping in a Bi2WO6 Photocatalyst. ACS Omega 2020, 5, 29081–29091. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dai, K.; Pan, C.; Zhang, J. Diethylenetriamine-Functionalized CdS Nanoparticles Decorated on Cu2S Snowflake Microparticles for Photocatalytic Hydrogen Production. ACS Appl. Nano Mater. 2020, 3, 11517–11526. [Google Scholar] [CrossRef]
- Liu, B.; Bie, C.; Zhang, Y.; Wang, L.; Li, Y.; Yu, J. Hierarchically Porous ZnO/g-C3N4 S-Scheme Heterojunction Photocatalyst for Efficient H(2)O(2) Production. Langmuir 2021, 37, 14114–14124. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, J.; Ming, T.; Wang, X.; Yu, H.; Yu, J.; Wang, Y.; Lei, M. Dye-sensitization-induced visible-light reduction of graphene oxide for the enhanced TiO2 photocatalytic performance. ACS Appl. Mater. Interfaces 2013, 5, 2924–2929. [Google Scholar] [CrossRef] [PubMed]
- Chibac-Scutaru, A.L.; Podasca, V.-E.; Dascalu, I.A.; Rusu, D.; Melinte, V. ZnO nanostructures with controlled morphological and optical properties for applications as efficient photocatalyst for malachite green degradation. Ceram. Int. 2024, 50, 34291–34303. [Google Scholar] [CrossRef]
- Dufour, F.; Pigeot-Remy, S.; Durupthy, O.; Cassaignon, S.; Ruaux, V.; Torelli, S.; Mariey, L.; Maugé, F.; Chanéac, C. Morphological control of TiO2 anatase nanoparticles: What is the good surface property to obtain efficient photocatalysts? Appl. Catal. B Environ. 2015, 174–175, 350–360. [Google Scholar] [CrossRef]
- Petrisková, P.; Monfort, O.; Satrapinskyy, L.; Dobročka, E.; Plecenik, T.; Plesch, G.; Papšík, R.; Bermejo, R.; Lenčéš, Z. Preparation and photocatalytic activity of TiO2 nanotube arrays prepared on transparent spinel substrate. Ceram. Int. 2021, 47, 12970–12980. [Google Scholar] [CrossRef]
- Kapusuz Yavuz, D.; El Accen, M.; Bedir, M. Unlocking the potential of ZnO nanorods: Structural insights for enhanced photocatalytic activity. J. Phys. Chem. Solids 2024, 193, 112120. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Zhao, X.; Chen, Y.; Wei, D.; Zhang, L.; Su, X. Facile synthesis of ultrathin γ-Fe2O3 magnetic nanosheets rich in oxygen vacancies and their photocatalytic activity for water oxidation. Appl. Surf. Sci. 2022, 578, 151999. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, Y.; Fang, D.; Liang, J.; Zhou, L. Simulated solarlight catalytic reduction of Cr(VI) on microwave–ultrasonication synthesized flower-like CuO in the presence of tartaric acid. Mater. Chem. Phys. 2016, 171, 386–393. [Google Scholar] [CrossRef]
- Hkiri, K.; Mohamed, H.E.A.; Harrisankar, N.; Gibaud, A.; van Steen, E.; Maaza, M. Environmental water treatment with green synthesized WO3 nanoflakes for cationic and anionic dyes removal: Photocatalytic studies. Catal. Commun. 2024, 187, 106851. [Google Scholar] [CrossRef]
- Ravi, A.; Annamalai, P.; Sankar, V.; Achutharaman, K.R.; Valdes, H.; SaravanaVadivu, A.; MuthaiahPillai, V.; Alharbi, S.A. Sustainable synthesis, superior performance: Nanoflower-like α-Bi2O3 from solvent-free solid state for photocatalytic crystal violet degradation. J. Taiwan Inst. Chem. E 2024, 157, 105413. [Google Scholar] [CrossRef]
- Tran, D.P.H.; You, S.J.; Wang, Y.F. Morphological optimization of CeO2 via low-temperature hydrothermal synthesis for enhanced photocatalytic performance in NO oxidation and CO2 conversion. J. Environ. Chem. Eng. 2024, 12, 112667. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, H.; Yang, L.; Pan, Y.; Zhao, X.; Wang, F.; Fang, R.; Li, Y. Photocatalytic CO2 reduction coupled with biomass-based amines oxidation over double-shelled CdS nanocages. Catal. Commun. 2024, 187, 106884. [Google Scholar] [CrossRef]
- Hu, B.; Wang, B.H.; Bai, Z.J.; Chen, L.; Guo, J.K.; Shen, S.; Xie, T.L.; Au, C.T.; Jiang, L.L.; Yin, S.F. Regulating MoS2 edge site for photocatalytic nitrogen fixation: A theoretical and experimental study. Chem. Eng. J. 2022, 442, 136211. [Google Scholar] [CrossRef]
- Morales Gallardo, M.V.; Ayala, A.M.; Pal, M.; Cortes Jacome, M.A.; Toledo Antonio, J.A.; Mathews, N.R. Synthesis of pyrite FeS2 nanorods by simple hydrothermal method and its photocatalytic activity. Chem. Phys. Lett. 2016, 660, 93–98. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Pan, Y.; Song, H.; Zhu, B.; Wu, Y. Two-dimensional ZnS (propylamine) photocatalyst for efficient visible light photocatalytic H2 production. Catal. Today 2021, 374, 4–11. [Google Scholar] [CrossRef]
- Yoo, J.-H.; Ji, M.; Kim, J.H.; Ryu, C.-H.; Lee, Y.-I. Facile synthesis of hierarchical CuS microspheres with high visible-light-driven photocatalytic activity. JPPA 2020, 401, 112782. [Google Scholar] [CrossRef]
- Lan, M.; Wang, Y.; Dong, X.; Yang, F.; Zheng, N.; Wang, Y.; Ma, H.; Zhang, X. Controllable fabrication of sulfur-vacancy-rich Bi2S3 nanorods with efficient near-infrared light photocatalytic for nitrogen fixation. Appl. Surf. Sci. 2022, 591, 153205. [Google Scholar] [CrossRef]
- Liu, K.; Shen, X.; Shen, H.; Jiang, K.; Sun, T.; Wang, M. One-step fabrication of novel BiVO4 hollow nanotubes with improved photocatalytic activity. Mater. Lett. 2023, 349, 134792. [Google Scholar] [CrossRef]
- Pawar, R.C.; Khan, H.; Charles, H.; Lee, C.S. Growth of 3D nanowall-like structures of FeVO4 by controlling reaction rate for effective CO2 reduction using UV-visible light. J. Environ. Chem. Eng. 2023, 11, 110236. [Google Scholar] [CrossRef]
- Mahboob, I.; Shafiq, I.; Shafique, S.; Akhter, P.; Amjad, U.-e.-S.; Hussain, M.; Park, Y.-K. Effect of active species scavengers in photocatalytic desulfurization of hydrocracker diesel using mesoporous Ag3VO4. Chem. Eng. J. 2022, 441, 136063. [Google Scholar] [CrossRef]
- Yan, Y.; Cai, F.; Song, Y.; Shi, W. InVO4 nanocrystal photocatalysts: Microwave-assisted synthesis and size-dependent activities of hydrogen production from water splitting under visible light. Chem. Eng. J. 2013, 233, 1–7. [Google Scholar] [CrossRef]
- Yang, T.; Tang, Y.; Yang, F.; Qu, J.; Yang, X.; Cai, Y.; Du, F.; Li, C.M.; Hu, J. Molten salt-assisted anti-defect engineering to tailor ordered, highly crystalline g-C3N4 nanorods for efficient photocatalytic H2O2 production. Chem. Eng. J. 2023, 475, 146497. [Google Scholar] [CrossRef]
- Shen, Y.; Du, X.; Shi, Y.; Nguetsa Kuate, L.J.; Chen, Z.; Zhu, C.; Tan, L.; Guo, F.; Li, S.; Shi, W. Bound-state electrons synergy over photochromic high-crystalline C3N5 nanosheets in enhancing charge separation for photocatalytic H2 production. APM 2024, 3, 100202. [Google Scholar] [CrossRef]
- Wei, Z.; Xu, W.; Peng, P.; Sun, Q.; Li, Y.; Ding, N.; Zhao, C.; Li, S.; Pang, S. Covalent synthesis of Ti-MOF for enhanced photocatalytic CO2 reduction. Mol. Catal. 2024, 558, 114042. [Google Scholar] [CrossRef]
- Song, K.; Liang, S.; Zhong, X.; Wang, M.; Mo, X.; Lei, X.; Lin, Z. Tailoring the crystal forms of the Ni-MOF catalysts for enhanced photocatalytic CO2-to-CO performance. Appl. Catal. B Environ. 2022, 309, 121232. [Google Scholar] [CrossRef]
- Li, S.; Wu, F.; Lin, R.; Wang, J.; Li, C.; Li, Z.; Jiang, J.; Xiong, Y. Enabling photocatalytic hydrogen production over Fe-based MOFs by refining band structure with dye sensitization. Chem. Eng. J. 2022, 429, 132217. [Google Scholar] [CrossRef]
- Lu, X.Y.; Zhang, M.L.; Ren, Y.X.; Wang, J.J.; Yang, X.G. Design of Co-MOF nanosheets for efficient adsorption and photocatalytic degradation of organic dyes. J. Mol. Struct. 2023, 1288, 135796. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, L.; Cheng, H.; Zhu, Y. Significantly enhanced photocatalytic performance of ZnO via graphene hybridization and the mechanism study. Appl. Catal. B Environ. 2011, 101, 382–387. [Google Scholar] [CrossRef]
- Yue, G.; Lin, J.Y.; Tai, S.Y.; Xiao, Y.; Wu, J. A catalytic composite film of MoS2/graphene flake as a counter electrode for Pt-free dye-sensitized solar cells. Electrochim. Acta 2012, 85, 162–168. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.; Chen, T.; Wang, H.; Lu, W. Graphene TiO2 nanocomposites with high photocatalytic activity for the degradation of sodium pentachlorophenol. J. Environ. Sci. 2014, 26, 2114–2122. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhan, S.; Xia, Y.; Ma, S.; Zhou, Q.; Li, Y. The fundamental role and mechanism of reduced graphene oxide in rGO/Pt-TiO2 nanocomposite for high-performance photocatalytic water splitting. Appl. Catal. B Environ. 2017, 207, 335–346. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, Y.M.; Saji, S.E.; Yin, X.; Wibowo, A.; Tang, C.S.; Xi, S.; Cao, P.; Tebyetekerwa, M.; Liu, B.; et al. All room-temperature synthesis, N2 photofixation and reactivation over 2D cobalt oxides. Appl. Catal. B Environ. 2022, 304, 121001. [Google Scholar] [CrossRef]
- Otgonbayar, Z.; Liu, Y.; Oh, W.C. Design of type-II heterojunction MoS2/gC3N4/graphene ternary nanocomposite for photocatalytic CO2 reduction to hydrocarbon fuels: In aqueous solvents with a sacrificial donor. J. Environ. Chem. Eng. 2023, 11, 109884. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, F.; Zhou, L.; Li, H.; Meng, Q.; Jing, L.; Tian, Z.; Hou, C. 2D N-doped graphene/CoFe MOFs heterostructure functionalized CNF aerogels impart highly efficient photocatalytic oxidation of gaseous VOCs. J. Environ. Chem. Eng. 2024, 12, 112225. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, W.; Wang, T.; Wang, X.; Li, C.; He, W. Investigation on the dual roles of pollutants adsorption on the catalyst surface during photocatalytic process. J. Water Process Eng. 2024, 63, 105558. [Google Scholar] [CrossRef]
- He, Y.; Yin, L.; Yuan, N.; Zhang, G. Adsorption and activation, active site and reaction pathway of photocatalytic CO2 reduction: A review. Chem. Eng. J. 2024, 481, 148754. [Google Scholar] [CrossRef]
- Xiong, T.; Ye, Y.; Luo, B.; Shen, L.; Wang, D.; Fan, M.; Gong, Z. Facile fabrication of 3D TiO2-graphene aerogel composite with enhanced adsorption and solar light-driven photocatalytic activity. Ceram. Int. 2021, 47, 14290–14300. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Zheng, S.; Zeng, W.; Zhao, N.; Shen, M. One-pot synthesis of 3D TiO2-reduced graphene oxide aerogels with superior adsorption capacity and enhanced visible-light photocatalytic performance. Ceram. Int. 2016, 42, 19091–19096. [Google Scholar] [CrossRef]
- Li, Q.; Sun, Z.; Yin, C.; Chen, Y.; Pan, D.; Yu, B.; Zhang, Y.; He, T.; Chen, S. Template-assisted synthesis of ultrathin graphene aerogels as bifunctional oxygen electrocatalysts for water splitting and alkaline/neutral zinc-air batteries. Chem. Eng. J. 2023, 458, 141492. [Google Scholar] [CrossRef]
- Elsehsah, K.A.A.A.; Noorden, Z.A.; Saman, N.M. Graphene aerogel electrodes: A review of synthesis methods for high-performance supercapacitors. J. Energy Storage 2024, 97, 112788. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, H.; Xu, Z.; Li, N.; Chen, C.; Li, C.; Li, J.; Lv, H.; Kuang, L.; Tian, X. A review on manifold synthetic and reprocessing methods of 3D porous graphene-based architecture for Li-ion anode. Chem. Eng. J. 2018, 335, 954–969. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, J.; Huang, L. A review of three-dimensional graphene-based materials: Synthesis and applications to energy conversion/storage and environment. Carbon 2019, 143, 610–640. [Google Scholar] [CrossRef]
- Thakur, A. Graphene aerogel based energy storage materials—A review. Mater. Today 2022, 65, 3369–3376. [Google Scholar] [CrossRef]
- Sharma, R.; Jaiswal, S.; Chauhan, R.; Bhardwaj, M.; Verma, K.; Dwivedi, J.; Sharma, S. Applications of graphene-based photocatalysts for efficient functionalized degradation of some common antibiotics. Inorg. Chem. Commun. 2024, 168, 112941. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, X.; Zhang, X.; Wang, L.; Wang, B.; Zeng, X.; Ren, B. Graphene-based aerogel for efficient oil sorption and water pollution remediation. Colloids Surf. A 2024, 699, 134693. [Google Scholar] [CrossRef]
- Gao, B.; Feng, X.; Zhang, Y.; Zhou, Z.; Wei, J.; Qiao, R.; Bi, F.; Liu, N.; Zhang, X. Graphene-based aerogels in water and air treatment: A review. Chem. Eng. J. 2024, 484, 149604. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Mohamed, A.A. Recent progress in semiconductor/graphene photocatalysts: Synthesis, photocatalytic applications, and challenges. RSC Adv. 2022, 13, 421–439. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.; Nayak, V.; Singh, K.R.; Jain, B.; Baid, M.; Alexis, F.; Singh, A.K. Potentialities of graphene and its allied derivatives to combat against SARS-CoV-2 infection. Mater. Today Adv. 2022, 13, 100208. [Google Scholar] [CrossRef] [PubMed]
- Samriti; Manisha; Chen, Z.; Sun, S.; Prakash, J. Design and engineering of graphene nanostructures as independent solar-driven photocatalysts for emerging applications in the field of energy and environment. Mol. Syst. Des. Eng. 2022, 7, 213–238. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, S.; Wang, G.; Cui, J.; Lu, Y.; Rong, X.; Gao, C. A review on mechanism, applications and influencing factors of carbon quantum dots based photocatalysis. Ceram. Int. 2022, 48, 35986–35999. [Google Scholar] [CrossRef]
- Shyamala, R.; Gomathi Devi, L. Reduced graphene oxide/SnO2 nanocomposites for the photocatalytic degradation of rhodamine B: Preparation, characterization, photosensitization, vectorial charge transfer mechanism and identification of reaction intermediates. Chem. Phys. Lett. 2020, 748, 137385. [Google Scholar] [CrossRef]
- Bramhaiah, K.; Bhattacharyya, S. Challenges and future prospects of graphene-based hybrids for solar fuel generation: Moving towards next generation photocatalysts. Mater. Adv. 2022, 3, 142–172. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, B.; Yang, Y.; Huang, E.; Ge, Z.; Zhang, T.; Zhang, S.; Li, L.; Guan, N.; Ma, Y.; et al. High activity of hot electrons from bulk 3D graphene materials for efficient photocatalytic hydrogen production. Nano Res. 2017, 10, 1662–1672. [Google Scholar] [CrossRef]
- Patnaik, S.; Behera, A.; Parida, K. A review on g-C3N4/graphene nanocomposites: Multifunctional roles of graphene in the nanohybrid photocatalyst toward photocatalytic applications. Catal. Sci. Technol. 2021, 11, 6018–6040. [Google Scholar] [CrossRef]
- Cheng, G.; Wang, X.; He, Y. 3D graphene paraffin composites based on sponge skeleton for photo thermal conversion and energy storage. Appl. Therm. Eng. 2020, 178, 115560. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, Z.; Ba, Z.; Li, X.; Dong, J.; Fang, Y.; Zhang, Q.; Zhao, X. 3D MXene-holey graphene hydrogel for supercapacitor with superior energy storage. J. Energy Storage 2022, 47, 103911. [Google Scholar] [CrossRef]
- Singh, M.; Dawsey, T.; Gupta, R.K. Recent advancement in 3D graphene for metal-sulfur batteries. J. Energy Storage 2023, 73, 109059. [Google Scholar] [CrossRef]
- Liu, H.; Sun, F.; Shao, H.; Yin, D.; Li, X.; Ma, Q.; Liu, G.; Yu, H.; Yu, W.; Dong, X. One-pot synthesis of ZnIn2S4 graphene aerogels with S vacancies for efficient photocatalytic reduction of Cr(VI) and hydrogen evolution. J. Environ. Chem. Eng. 2023, 11, 110181. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Z.; Li, M.; Zhou, L.; Liu, M.; Yang, D.; Zeng, J.; Xie, R.; Hu, W.; Dong, F. S defect-rich MoS2 aerogel with hierarchical porous structure: Efficient photocatalysis and convenient reuse for removal of organic dyes. Chemosphere 2024, 354, 141649. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, H.; Lei, Z.; Huang, L.; Wu, T.; Liu, S.; Ye, G.; Lu, Y.; Wang, X. Graphene aerogel for photocatalysis-assist uranium elimination under visible light and air atmosphere. Chem. Eng. J. 2020, 402, 126256. [Google Scholar] [CrossRef]
- Noer Addina, S.; Idrus Alhamid, M.; Usman, A.; Adi Prasetyanto, E.; Sufyan, M.; Kusrini, E. Economic analysis of graphene aerogel polyurethane sponge production as adsorbent for oil-water separation. Mater. Today 2022, 51, 1896–1907. [Google Scholar] [CrossRef]
- Chen, L.; Liu, S.; Guo, X.; Wang, S.; He, Z.; Xu, Q.; Zhang, Q.; Zhu, J.; Zhao, P.; Yang, S.; et al. Ultralight, superhydrophobic and fire-resistant nitrogen-doped graphene aerogel for oil/water separation. Sep. Purif. Technol. 2024, 330, 125192. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, B.; Zhao, P.; Fu, H.; Kamali, A.R. Magnetic luffa/graphene/CuFe2O4 sponge for efficient oil/water separation. Sep. Purif. Technol. 2024, 337, 126347. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Santos, A.R.; Cabral, D.C.O.; Oliveira, D.M.; Assis, L.K.C.S.; França, E.L.T.; Quirino, F.R.S.; Castro-Lopes, S.; da Costa, O.M.M.M.; Padrón-Hernández, E. Binder-free ultrathin pellets of nanocomposites based on Fe3O4@nitrogen-doped reduced graphene oxide aerogel for electromagnetic interference shielding. J. Alloys Compd. 2024, 978, 173329. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Yang, K.; Han, D.; Yong, H.; Yang, Q. Alternating current assisted preparation of three-dimensional graphene aerogels and the application in electromagnetic interference shielding. Mater. Today Phys. 2024, 40, 101308. [Google Scholar] [CrossRef]
- Cao, Y.; Cheng, Z.; Wang, R.; Liu, X.; Zhang, T.; Fan, F.; Huang, Y. Multifunctional graphene/carbon fiber aerogels toward compatible electromagnetic wave absorption and shielding in gigahertz and terahertz bands with optimized radar cross section. Carbon 2022, 199, 333–346. [Google Scholar] [CrossRef]
- González, M.; Baselga, J.; Pozuelo, J. Modulating the electromagnetic shielding mechanisms by thermal treatment of high porosity graphene aerogels. Carbon 2019, 147, 27–34. [Google Scholar] [CrossRef]
- Mao, R.; Yao, W.; Qadir, A.; Chen, W.; Gao, W.; Xu, Y.; Hu, H. 3-D graphene aerogel sphere-based flexible sensors for healthcare applications. Sens. Actuat. A Phys. 2020, 312, 112144. [Google Scholar] [CrossRef]
- Xie, M.; Qian, G.; Yu, Y.; Chen, C.; Li, H.; Li, D. High-performance flexible reduced graphene oxide/polyimide nanocomposite aerogels fabricated by double crosslinking strategy for piezoresistive sensor application. Chem. Eng. J. 2024, 480, 148203. [Google Scholar] [CrossRef]
- Deng, Z.; Gao, C.; Feng, S.; Zhang, H.; Liu, Y.; Zhu, Y.; Wang, J.; Xiang, X.; Xie, H. Highly Compressible, Light-Weight and robust Nitrogen-Doped graphene composite aerogel for sensitive pressure sensors. Chem. Eng. J. 2023, 471, 144790. [Google Scholar] [CrossRef]
- Yao, W.; Mao, R.; Gao, W.; Chen, W.; Xu, Z.; Gao, C. Piezoresistive effect of superelastic graphene aerogel spheres. Carbon 2020, 158, 418–425. [Google Scholar] [CrossRef]
- Chen, Y.; Pang, K.; Liu, X.; Li, K.; Lu, J.; Cai, S.; Liu, Y.; Xu, Z.; Gao, C. Environment-adaptive, anti-fatigue thermal interface graphene foam. Carbon 2023, 212, 118142. [Google Scholar] [CrossRef]
- Lu, Z.; Zhao, Z.; Gong, W.; Zhang, H.; Pang, M.; Tang, X.-Z. The impact of reduction mode on the microwave absorption characteristics of reduced graphene oxide aerogels. J. Alloys Compd. 2024, 991, 174451. [Google Scholar] [CrossRef]
- Xi, J.; Li, Y.; Zhou, E.; Liu, Y.; Gao, W.; Guo, Y.; Ying, J.; Chen, Z.; Chen, G.; Gao, C. Graphene aerogel films with expansion enhancement effect of high-performance electromagnetic interference shielding. Carbon 2018, 135, 44–51. [Google Scholar] [CrossRef]
- Li, G.; Fang, D.; Hong, G.; Eychmüller, A.; Zhang, X.; Song, W. Assembling graphene aerogel hollow fibres for solar steam generation. Compos. Commun. 2022, 35, 101302. [Google Scholar] [CrossRef]
- Sunahiro, S.; Pirabul, K.; Pan, Z.; Yoshii, T.; Hayasaka, Y.; Zhao, Q.; Crespo-Otero, R.; Di Tommaso, D.; Kyotani, T.; Nishihara, H. Synthesis of graphene mesosponge using CaO nanoparticles formed from CaCO3. Catal. Today 2024, 437, 114763. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef]
- Xia, D.; Yi, K.; Zheng, B.; Li, M.; Qi, G.; Cai, Z.; Cao, M.; Liu, D.; Peng, L.; Wei, D.; et al. Solvent-Free Process to Produce Three Dimensional Graphene Network with High Electrochemical Stability. J. Phys. Chem. C 2017, 121, 3062–3069. [Google Scholar] [CrossRef]
- Jia, J.; Sun, X.; Lin, X.; Shen, X.; Mai, Y.; Kim, J. Exceptional Electrical Conductivity and Fracture Resistance of 3D Interconnected Graphene Foam/Epoxy Composites. ACS Nano 2014, 8, 5774–5783. [Google Scholar] [CrossRef]
- Choi, B.; Yang, M.; Hong, W.; Choi, J.; Huh, Y. 3D Macroporous Graphene Frameworks for Supercapacitors with High Energy and Power Densities. ACS Nano 2012, 6, 4020–4028. [Google Scholar] [CrossRef]
- Xu, S.; Sun, F.; Pan, Z.; Huang, C.; Yang, S.; Long, J.; Chen, Y. Reduced Graphene Oxide-Based Ordered Macroporous Films on a Curved Surface: General Fabrication and Application in Gas Sensors. ACS Appl. Mater. Interfaces 2016, 8, 3428–3437. [Google Scholar] [CrossRef]
- Salazar-Aguilar, A.D.; Rodriguez Rodriguez, J.I.; Piñeiro-García, A.; Tristan, F.; Labrada Delgado, G.J.; Meneses-Rodríguez, D.; Vega Díaz, S.M. Layer-by-Layer Method to Prepare Three-Dimensional Reduced Graphene Materials with Controlled Architectures Using SiO2 as a Sacrificial Template. Ind. Eng. Chem. Res. 2021, 60, 11063–11069. [Google Scholar] [CrossRef]
- Lai, K.C.; Lee, L.Y.; Hiew, B.Y.Z.; Thangalazhy-Gopakumar, S.; Gan, S. Environmental application of three-dimensional graphene materials as adsorbents for dyes and heavy metals: Review on ice-templating method and adsorption mechanisms. J. Environ. Sci. 2019, 79, 174–199. [Google Scholar] [CrossRef]
- Zong, Y.; Fan, Y.; Chen, Z.; Ouyang, X.; Liu, Y.; Zhou, S.; Mei, J.; Niu, K. Control of microstructure to prepare compressible graphene aerogel via ice template. Carbon 2024, 227, 119269. [Google Scholar] [CrossRef]
- Qiu, L.; Han, W.; Yu, Q.; Yin, Y.; Yi, L.; Ji, X.; Yu, Y. Radial assembly of graphene nanoplates via ice-template method to construct 3D thermally conductive phase change materials. Fuel 2024, 369, 131738. [Google Scholar] [CrossRef]
- Kota, M.; Yu, X.; Yeon, S.H.; Cheong, H.W.; Park, H.S. Ice-templated three dimensional nitrogen doped graphene for enhanced supercapacitor performance. J. Power Sources 2016, 303, 372–378. [Google Scholar] [CrossRef]
- Peng, L.; Domenech Carbo, A.; Primo, A.; Garcia, H. 3D defective graphenes with subnanometric porosity obtained by soft-templating following zeolite procedures. Nanoscale Adv. 2019, 1, 4827–4833. [Google Scholar] [CrossRef]
- Huang, X.; Sun, B.; Su, D.; Zhao, D.; Wang, G. Soft-template synthesis of 3D porous graphene foams with tunable architectures for lithium-O2 batteries and oil adsorption applications. J. Mater. Chem. A 2014, 2, 7973–7979. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Huang, J.; Zhou, Y.; Xu, K.; Zhao, N.; Cheng, X. Soft template-assisted method for synthesis of nitrogen and sulfur co-doped three-dimensional reduced graphene oxide as an efficient metal free catalyst for oxygen reduction reaction. Carbon 2017, 122, 237–246. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, G.; Duan, X. Self-Assembled Three-Dimensional Graphene Macrostructures: Synthesis and Applications in Supercapacitors. Acc. Chem. Res. 2015, 48, 1666–1675. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, Q.; Shi, G. Supercapacitors based on self-assembled graphene organogel. Phys. Chem. Chem. Phys. 2011, 13, 17249–17254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Z.-B.; Li, J.-L.; Zhang, J.-J.; Sui, X.-L.; Zhang, L.-M. Hybrid of carbon-supported Pt nanoparticles and three dimensional graphene aerogel as high stable electrocatalyst for methanol electrooxidation. Electrochim. Acta 2016, 189, 175–183. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, J.; Wang, Y.; Guo, W.; Lv, L.; Li, B.; Zheng, K.; Bo, T.; Peng, D.; Zhou, Z.; et al. Thermally stable Pd/reduced graphene oxide aerogel catalysts for solvent-free oxidation of benzyl alcohol. Phys. Chem. Chem. Phys. 2011, 13, 17249–17254. [Google Scholar] [CrossRef]
- Kwok, Y.H.; Tsang, A.C.H.; Wang, Y.; Leung, D.Y.C. Ultra-fine Pt nanoparticles on graphene aerogel as a porous electrode with high stability for microfluidic methanol fuel cell. J. Power Sources 2017, 349, 75–83. [Google Scholar] [CrossRef]
- Liu, W.; Cai, J.; Li, Z. Self-Assembly of Semiconductor Nanoparticles/Reduced Graphene Oxide (RGO) Composite Aerogels for Enhanced Photocatalytic Performance and Facile Recycling in Aqueous Photocatalysis. ACS Sustain. Chem. Eng. 2015, 3, 277–282. [Google Scholar] [CrossRef]
- Das, C.; Shafi, T.; Thulluru, L.P.; Naushad, M.; Dubey, B.K.; Chowdhury, S. Three-Dimensional Highly Porous MoS2/Graphene Aerogel for Visible-Light-Driven Photocatalytic Degradation of Tetracycline. ACS EST Water 2024, 4, 2144–2158. [Google Scholar] [CrossRef]
- Zhu, K.; Yan, W.; Liu, S.; Wu, X.; Cui, S.; Shen, X. One-step hydrothermal synthesis of MnOx-CeO2/reduced graphene oxide composite aerogels for low temperature selective catalytic reduction of NOx. Appl. Surf. Sci. 2020, 508, 145024. [Google Scholar] [CrossRef]
- Liu, Y.; He, D.; Wu, H.; Duan, J.; Zhang, Y. Hydrothermal Self-assembly of Manganese Dioxide/Manganese Carbonate/Reduced Graphene Oxide Aerogel for Asymmetric Supercapacitors. Electrochim. Acta 2015, 164, 154–162. [Google Scholar] [CrossRef]
- Kim, M.Y.; Seo, K.D.; Park, H.; Mahmudunnabi, R.G.; Hwan Lee, K.; Shim, Y.B. Graphene-anchored conductive polymer aerogel composite for the electrocatalytic detection of hydrogen peroxide and bisphenol A. Appl. Surf. Sci. 2022, 604, 154430. [Google Scholar] [CrossRef]
- Li, C.; Yang, Z.; Tang, Z.; Guo, B.; Tian, M.; Zhang, L. A scalable strategy for constructing three-dimensional segregated graphene network in polymer via hydrothermal self-assembly. Chem. Eng. J. 2019, 363, 300–308. [Google Scholar] [CrossRef]
- Narukulla, R.; Ojha, U.; Sharma, T. Facile one pot green synthesis of -NH2 surface functionalized graphene-polymer nanocomposite: Subsequent utilization as stabilizer in pickering emulsions. Colloids Surf. A 2022, 641, 128594. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, R.; Gu, J.; Liu, H.; Liu, C.; Luo, C.; Kong, J.; Shao, Q.; Wang, N.; Guo, Z.; et al. Ultralight, highly compressible and fire-retardant graphene aerogel with self-adjustable electromagnetic wave absorption. Carbon 2018, 139, 1126–1135. [Google Scholar] [CrossRef]
- Chen, W.; Yan, L. In situ self-assembly of mild chemical reduction graphene for three-dimensional architectures. Nanoscale 2011, 3, 3132–3137. [Google Scholar] [CrossRef]
- Wu, X.; Mu, F.; Lin, Z. Three-dimensional printing of graphene-based materials and the application in energy storage. Mater. Today Adv. 2021, 11, 100157. [Google Scholar] [CrossRef]
- Li, D.; Zhang, H.; Zhang, L.; Wang, P.; Xu, H.; Xuan, J. Rapid Synthesis of Porous Graphene Microspheres through a Three-Dimensionally Printed Inkjet Nozzle for Selective Pollutant Removal from Water. ACS Omega 2019, 4, 20509–20518. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, Y.; Jia, H.; Bi, H.; Chen, L.; Que, M.; Xiong, Y.; Han, L.; Sun, L. A novel pre-deposition assisted strategy for inkjet printing graphene-based flexible pressure sensor with enhanced performance. Carbon 2022, 196, 85–91. [Google Scholar] [CrossRef]
- You, X.; Yang, J.; Feng, Q.; Huang, K.; Zhou, H.; Hu, J.; Dong, S. Three-dimensional graphene-based materials by direct ink writing method for lightweight application. IJLLM 2018, 1, 96–101. [Google Scholar] [CrossRef]
- Li, Q.; Dong, Q.; Wang, J.; Xue, Z.; Li, J.; Yu, M.; Zhang, T.; Wan, Y.; Sun, H. Direct ink writing (DIW) of graphene aerogel composite electrode for vanadium redox flow battery. J. Power Sources 2022, 542, 231810. [Google Scholar] [CrossRef]
- van Hazendonk, L.S.; Vonk, C.F.; van Grondelle, W.; Vonk, N.H.; Friedrich, H. Towards a predictive understanding of direct ink writing of graphene-based inks. Appl. Mater. Today 2024, 36, 102014. [Google Scholar] [CrossRef]
- Wajahat, M.; Lee, S.; Kim, J.H.; Ahn, J.; Sim, H.H.; Kim, J.H.; Bae, J.; Kim, S.H.; Pyo, J.; Seol, S.K. Three-dimensional printing of silver nanoparticle-decorated graphene microarchitectures. Addit. Manuf. 2022, 60, 103249. [Google Scholar] [CrossRef]
- Chi, H.; Lin, Z.; Chen, Y.; Zheng, R.; Qiu, H.; Hu, X.; Bai, H. Three-Dimensional Printing and Recycling of Multifunctional Composite Material Based on Commercial Epoxy Resin and Graphene Nanoplatelet. ACS Appl. Mater. Interfaces 2022, 14, 13758–13767. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chao, B.; Wu, H.; Liu, S.; Fu, W.; Jiang, J.; Deng, K.; Li, Y. Preparation of GR-TiN/PLA/TPU composite materials and study on their microwave absorption properties. Mater. Sci. Eng. B 2024, 299, 116964. [Google Scholar] [CrossRef]
- Jing, J.; Xiong, Y.; Shi, S.; Pei, H.; Chen, Y.; Lambin, P. Facile fabrication of lightweight porous FDM-Printed polyethylene/graphene nanocomposites with enhanced interfacial strength for electromagnetic interference shielding. Compos. Sci. Technol. 2021, 207, 108732. [Google Scholar] [CrossRef]
- Jing, J.; Chen, Y.; Shi, S.; Yang, L.; Lambin, P. Facile and scalable fabrication of highly thermal conductive polyethylene/graphene nanocomposites by combining solid-state shear milling and FDM 3D-printing aligning methods. Chem. Eng. J. 2020, 402, 126218. [Google Scholar] [CrossRef]
- Markandan, K.; Lai, C.Q. Enhanced mechanical properties of 3D printed graphene-polymer composite lattices at very low graphene concentrations. Compos. Part A-Appl. Sci. Manuf. 2020, 129, 105726. [Google Scholar] [CrossRef]
- Korhonen, H.; Sinh, L.H.; Luong, N.D.; Lehtinen, P.; Verho, T.; Partanen, J.; Seppälä, J. Fabrication of graphene-based 3D structures by stereolithography. Phys. Status Solidi (A) 2016, 213, 982–985. [Google Scholar] [CrossRef]
- Hanon, M.M.; Ghaly, A.; Zsidai, L.; Klébert, S. Tribological characteristics of digital light processing (DLP) 3D printed graphene/resin composite: Influence of graphene presence and process settings. Mater. Design 2022, 218, 110718. [Google Scholar] [CrossRef]
- Peng, M.; Wen, Z.; Xie, L.; Cheng, J.; Jia, Z.; Shi, D.; Zeng, H.; Zhao, B.; Liang, Z.; Li, T.; et al. 3D Printing of Ultralight Biomimetic Hierarchical Graphene Materials with Exceptional Stiffness and Resilience. Adv. Mater. 2019, 31, 1902930. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Z.; Tang, L.; Liu, J.; Yang, J.; Lai, W.-F.; Wu, T.; Liao, S.; Zhang, X.; Pan, H.; et al. A universally dispersible graphene-based ink modifier facilitates 3D printing of multi-functional tissue-engineered scaffolds. Mater. Design 2022, 216, 110551. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, H.; Cai, Z.; Cheng, D.; He, P.; Xie, P.; Zhang, D.; Fan, T. Generalized 3D Printing of Graphene-Based Mixed-Dimensional Hybrid Aerogels. ACS Nano 2018, 12, 3502–3511. [Google Scholar] [CrossRef]
- Li, Y.H.; Tang, Z.R.; Xu, Y.J. Multifunctional graphene-based composite photocatalysts oriented by multifaced roles of graphene in photocatalysis. Chin. J. Catal. 2022, 43, 708–730. [Google Scholar] [CrossRef]
- Lu, K.Q.; Xin, X.; Zhang, N.; Tang, Z.R.; Xu, Y.J. Photoredox catalysis over graphene aerogel-supported composites. J. Mater. Chem. A 2018, 6, 4590–4604. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, T.; Fan, M.; Tan, G.; Cui, S.; Shen, X. Nanostructure rod-like TiO2-reduced graphene oxide composite aerogels for highly-efficient visible-light photocatalytic CO2 reduction. J. Alloys Compd. 2021, 861, 158598. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, J.; Lu, S.; Xue, H.; Chen, Q.; Yang, M.Q.; Qian, Q. Insight into the Real Efficacy of Graphene for Enhancing Photocatalytic Efficiency: A Case Study on CVD Graphene-TiO2 Composites. ACS Appl. Energy Mater. 2021, 4, 8755–8764. [Google Scholar] [CrossRef]
- Nawaz, M.; Miran, W.; Jang, J.; Lee, D.S. One-step hydrothermal synthesis of porous 3D reduced graphene oxide/TiO2 aerogel for carbamazepine photodegradation in aqueous solution. Appl. Catal. B Environ. 2017, 203, 85–95. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Ma, L.; Sun, F.; Yue, B.; Ma, Q.; Wang, J.; Liu, G.; Yu, H.; Yu, W.; et al. A three-dimensional TiO2/C/BiOBr graphene aerogel for enhancing photocatalysis and bidirectional sulfur conversion reactions in lithium-sulfur batteries. J. Environ. Chem. Eng. 2022, 10, 108606. [Google Scholar] [CrossRef]
- Jung, H.; Cho, K.M.; Kim, K.H.; Yoo, H.-W.; Al-Saggaf, A.; Gereige, I.; Jung, H.-T. Highly Efficient and Stable CO2 Reduction Photocatalyst with a Hierarchical Structure of Mesoporous TiO2 on 3D Graphene with Few-Layered MoS2. ACS Sustain. Chem. Eng. 2018, 6, 5718–5724. [Google Scholar] [CrossRef]
- Men, X.; Chen, H.; Chang, K.; Fang, X.; Wu, C.; Qin, W.; Yin, S. Three-dimensional free-standing ZnO/graphene composite foam for photocurrent generation and photocatalytic activity. Appl. Catal. B Environ. 2016, 187, 367–374. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.M. α-Fe2O3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, S.; Guan, Y.; Zhang, Y. A 3D printed synergistic aerogel microreactor toward stable and high-efficiency photocatalytic degradation. Mater. Today Chem. 2021, 22, 100566. [Google Scholar] [CrossRef]
- Singha, A.; Kaishyop, J.; Khan, T.S.; Chowdhury, B. Visible-Light-Driven Toluene Oxidation to Benzaldehyde over WO3 Nanostructures. ACS Appl. Nano Mater. 2023, 6, 21818–21828. [Google Scholar] [CrossRef]
- Lei, Y.; Deng, Y.; Luo, L.; Bao, S.; Tang, Z.; Chen, J.; Wang, Y.; Li, C.; Du, B. In Situ Synthesis of Mixed-Phase WO3 on Nitrogen-Doped Graphene with Enhanced Photoelectric Properties. ACS Appl. Nano Mater. 2023, 6, 16805–16814. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, C.; Ni, Z.; Hojo, H.; Einaga, H. Enhanced Photocatalytic Benzene Oxidation to Phenol over Monoclinic WO3 Nanorods under Visible Light. ACS Catal. 2022, 12, 14976–14989. [Google Scholar] [CrossRef]
- Azimirad, R.; Safa, S. Preparation of three dimensional graphene foam-WO3 nanocomposite with enhanced visible light photocatalytic activity. Mater. Chem. Phys. 2015, 162, 686–691. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Z.; Zhang, J.; Zhang, Z. Reduced graphene oxide three-dimensionally wrapped WO3 hierarchical nanostructures as high-performance solar photocatalytic materials. Appl. Catal. A Gen. 2016, 522, 90–100. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, B.; Guan, D.; Luo, Z. Essential roles of defects in pure graphene/Cu2O photocatalyst. Catal. Commun. 2016, 76, 7–12. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Bai, X.; Zhao, W.; Zhao, S.; Chang, C.; Wang, S.; Duan, E. Facet-Engineered Cu2O Microparticles on Graphitic Carbon Nitride Nanosheets as Z-Scheme Heterojunction Photocatalysts for Degradation of Tetracycline. ACS Appl. Nano Mater. 2024, 7, 16127–16140. [Google Scholar] [CrossRef]
- Ding, S.; Bai, X.; Cui, L.; Shen, Q.; Zhang, X.; Jia, H.; Xue, J. Ag as an Electron Mediator in Porous Cu2O Nanostructures for Photocatalytic CO2 Reduction to CO. ACS Appl. Nano Mater. 2023, 6, 10539–10550. [Google Scholar] [CrossRef]
- Cai, J.; Liu, W.; Li, Z. One-pot self-assembly of Cu2O/RGO composite aerogel for aqueous photocatalysis. Appl. Surf. Sci. 2015, 358, 146–151. [Google Scholar] [CrossRef]
- Zhang, S.; Ou, X.; Xiang, Q.; Carabineiro, S.A.C.; Fan, J.; Lv, K. Research progress in metal sulfides for photocatalysis: From activity to stability. Chemosphere 2022, 303 Pt 2, 135085. [Google Scholar] [CrossRef]
- Di, J.; Jiang, W. Recent progress of low-dimensional metal sulfides photocatalysts for energy and environmental applications. Mater. Today Catal. 2023, 1, 100001. [Google Scholar] [CrossRef]
- Parihar, A.; Sharma, P.; Choudhary, N.K.; Khan, R.; Gupta, A.; Sen, R.K.; Prasad, H.C.; Ashiq, M. Green Synthesis of CdS and CdS/rGO Nanocomposites: Evaluation of Electrochemical, Antimicrobial, and Photocatalytic Properties. ACS Appl. Bio Mater. 2023, 6, 3706–3716. [Google Scholar] [CrossRef] [PubMed]
- Das, C.; Shafi, T.; Pan, S.; Naushad, M.; Dubey, B.K.; Chowdhury, S. MoS2 Nanoflowers Decorated on Graphene Aerogels for Visible-Light-Driven Photocatalytic Degradation of Tetracycline. ACS Appl. Nano Mater. 2023, 6, 12991–13000. [Google Scholar] [CrossRef]
- Wei, X.N.; Ou, C.L.; Fang, S.S.; Zheng, X.C.; Zheng, G.P.; Guan, X.X. One-pot self-assembly of 3D CdS-graphene aerogels with superior adsorption capacity and photocatalytic activity for water purification. Powder Technol. 2019, 345, 213–222. [Google Scholar] [CrossRef]
- Bano, Z.; Saeed, R.M.Y.; Zhu, S.; Xia, M.; Mao, S.; Lei, W.; Wang, F. Mesoporous CuS nanospheres decorated rGO aerogel for high photocatalytic activity towards Cr(VI) and organic pollutants. Chemosphere 2020, 246, 125846. [Google Scholar] [CrossRef]
- Jiang, Y.; Chowdhury, S.; Balasubramanian, R. Nitrogen-doped graphene hydrogels as potential adsorbents and photocatalysts for environmental remediation. Chem. Eng. J. 2017, 327, 751–763. [Google Scholar] [CrossRef]
- Shafi, T.; Das, C.; Dubey, B.K.; Chowdhury, S. Aerogels Made of Few-Layer WS2 Nanosheets and Nitrogen-Doped Graphene for Photocatalytic Degradation of Caffeine. ACS Appl. Nano Mater. 2024, 7, 1723–1737. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, L.; Feng, Z.; Meng, Q.; Li, Y.; Duan, T. Adopting sulfur-atom sharing strategy to construct CoS2/MoS2 heterostructure on three-dimensional nitrogen-doped graphene aerogels: A novel photocatalyst for wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 104771. [Google Scholar] [CrossRef]
- Wang, L.; Wang, K.; He, T.; Zhao, Y.; Song, H.; Wang, H. Graphitic Carbon Nitride-Based Photocatalytic Materials: Preparation Strategy and Application. ACS Sustain. Chem. Eng. 2020, 8, 16048–16085. [Google Scholar] [CrossRef]
- El Messaoudi, N.; Ciğeroğlu, Z.; Şenol, Z.M.; Elhajam, M.; Noureen, L. A comparative review of the adsorption and photocatalytic degradation of tetracycline in aquatic environment by g-C3N4-based materials. J. Water Process Eng. 2023, 55, 104150. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhang, S.H.; Li, J.; Zheng, X.C.; Guan, X.X. Constructing of 3D graphene aerogel-g-C3N4 metal-free heterojunctions with superior purification efficiency for organic dyes. J. Mol. Liq. 2020, 310, 113242. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, Z.; Li, C.; Zuo, Y.; Zhou, Y. Monolithic g-C3N4/reduced graphene oxide aerogel with in situ embedding of Pd nanoparticles for hydrogenation of CO2 to CH4. Appl. Surf. Sci. 2019, 475, 953–960. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, S.; Hu, Y.; Lv, F.; Zhang, Y. Preparation of Bi-based porous and magnetic electrospun fibers and their photocatalytic properties in weak polar medium. Colloids Surf. A 2021, 610, 125718. [Google Scholar] [CrossRef]
- Zuarez Chamba, M.; Rajendran, S.; Herrera Robledo, M.; Priya, A.K.; Navas Cardenas, C. Bi-based photocatalysts for bacterial inactivation in water: Inactivation mechanisms, challenges, and strategies to improve the photocatalytic activity. Environ. Res. 2022, 209, 112834. [Google Scholar] [CrossRef]
- Yu, X.; Shi, J.; Feng, L.; Li, C.; Wang, L. A three-dimensional BiOBr/RGO heterostructural aerogel with enhanced and selective photocatalytic properties under visible light. Appl. Surf. Sci. 2017, 396, 1775–1782. [Google Scholar] [CrossRef]
- Yang, J.; Shi, Q.; Zhang, R.; Xie, M.; Jiang, X.; Wang, F.; Cheng, X.; Han, W. BiVO4 quantum tubes loaded on reduced graphene oxide aerogel as efficient photocatalyst for gaseous formaldehyde degradation. Carbon 2018, 138, 118–124. [Google Scholar] [CrossRef]
- Jafarzadeh, M. Recent Progress in the Development of MOF-Based Photocatalysts for the Photoreduction of Cr((VI)). ACS Appl. Mater. Interfaces 2022, 14, 24993–25024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, D.; Li, J.; Zhi, C.; Zhang, Y.; Liang, L.; Mao, S.; Shi, J.W. Recent research advances of metal organic frameworks (MOFs) based composites for photocatalytic H2 evolution. Coord. Chem. Rev. 2024, 517, 215955. [Google Scholar] [CrossRef]
- Sandhu, Z.A.; Farwa, U.; Danish, M.; Raza, M.A.; Talib, A.; Amjad, H.; Riaz, R.; Al-Sehemi, A.G. Sustainability and photocatalytic performance of MOFs: Synthesis strategies and structural insights. J. Clean. Prod. 2024, 470, 143263. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, D.; Wang, C.; You, B.; Li, B.; Han, J.; Jiang, S.; Zhang, C.; He, S. Zeolitic imidazolate framework-67 and its derivatives for photocatalytic applications. Coord. Chem. Rev. 2024, 502, 215612. [Google Scholar] [CrossRef]
- Nazir, M.A.; Javed, M.S.; Islam, M.; Assiri, M.A.; Hassan, A.M.; Jamshaid, M.; Najam, T.; Shah, S.S.A.; Rehman, A.U. MOF@graphene nanocomposites for energy and environment applications. Compos. Commun. 2024, 45, 101783. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, M.; Song, X.; Zhou, W.; Liu, X.; Wang, H.; Huo, P. Integration of 3D macroscopic reduced graphene oxide aerogel with DUT-67 for selective CO2 photoreduction to CO in Gas-Solid reaction. Chem. Eng. J. 2022, 446, 137034. [Google Scholar] [CrossRef]
- Shah, S.J.; Luan, X.; Yu, X.; Su, W.; Wang, Y.; Zhao, Z.; Zhao, Z. Construction of 3D-graphene/NH(2)-MIL-125 nanohybrids via amino-ionic liquid dual-mode bonding for advanced acetaldehyde photodegradation under high humidity. J. Colloid Interface Sci. 2024, 663, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, D.; Zhu, Y.; Zhang, Y.; Zhu, Y. 3D-3D porous Bi2WO6/graphene hydrogel composite with excellent synergistic effect of adsorption-enrichment and photocatalytic degradation. Appl. Catal. B Environ. 2017, 205, 228–237. [Google Scholar] [CrossRef]
- Jin, S.; Yang, Y.; Zhang, J.; Zheng, H. Preparation of a novel TiO2-graphene 3D framework material for efficient adsorption-photocatalytic removal of micro-organic contaminants from water. Mater. Chem. Phys. 2021, 263, 124339. [Google Scholar] [CrossRef]
- Dong, S.; Xia, L.; Chen, X.; Cui, L.; Zhu, W.; Lu, Z.; Sun, J.; Fan, M. Interfacial and electronic band structure optimization for the adsorption and visible-light photocatalytic activity of macroscopic ZnSnO3/graphene aerogel. Compos. Part B Eng. 2021, 215, 108765. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, J.; Yang, X.; Zhang, T.; Tang, H. 3D reduced graphene oxide aerogel-mediated Z-scheme photocatalytic system for highly efficient solar-driven water oxidation and removal of antibiotics. Appl. Catal. B Environ. 2018, 232, 562–573. [Google Scholar] [CrossRef]
- Shen, Z.K.; Yuan, Y.J.; Pei, L.; Yu, Z.T.; Zou, Z. Black phosphorus photocatalysts for photocatalytic H2 generation: A review. Chem. Eng. J. 2020, 386, 123997. [Google Scholar] [CrossRef]
- Ganguly, P.; Harb, M.; Cao, Z.; Cavallo, L.; Breen, A.; Dervin, S.; Dionysiou, D.D.; Pillai, S.C. 2D Nanomaterials for Photocatalytic Hydrogen Production. ACS Energy Lett. 2019, 4, 1687–1709. [Google Scholar] [CrossRef]
- Samajdar, S.; Ghosh, S.; Thandavarayan, M.; Medda, S.K.; Manna, S.; Mohapatra, M. Construction of a 3D/2D Z-Scheme Heterojunction for Promoting Charge Separation and Augmented Photocatalytic Hydrogen Evolution. Energy Fuels 2023, 37, 14290–14302. [Google Scholar] [CrossRef]
- Zhang, X.; An, W.; Li, Y.; Hu, J.; Gao, H.; Cui, W. Efficient photo-catalytic hydrogen production performance and stability of a three-dimensional porous CdS NPs-graphene hydrogel. Int. J. Hydrogen Energy 2018, 43, 9902–9913. [Google Scholar] [CrossRef]
- Liu, J.; Wei, X.; Sun, W.; Guan, X.; Zheng, X.; Li, J. Fabrication of S-scheme CdS-g-C3N4-graphene aerogel heterojunction for enhanced visible light driven photocatalysis. Environ. Res. 2021, 197, 111136. [Google Scholar] [CrossRef]
- Seo, D.B.; Kwon, Y.M.; Kim, J.; Kang, S.; Yim, S.; Lee, S.S.; Kim, E.T.; Song, W.; An, K.S. Edge-Rich 3D Structuring of Metal Chalcogenide/Graphene with Vertical Nanosheets for Efficient Photocatalytic Hydrogen Production. ACS Appl. Mater. Interfaces 2024, 16, 28613–28624. [Google Scholar] [CrossRef]
- Guo, K.; Hussain, I.; Jie, G.A.; Fu, Y.; Zhang, F.; Zhu, W. Strategies for improving the photocatalytic performance of metal-organic frameworks for CO(2) reduction: A review. J. Environ. Sci. 2023, 125, 290–308. [Google Scholar] [CrossRef]
- Alkhatib, I.I.; Garlisi, C.; Pagliaro, M.; Al-Ali, K.; Palmisano, G. Metal-organic frameworks for photocatalytic CO2 reduction under visible radiation: A review of strategies and applications. Catal. Today 2020, 340, 209–224. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Hussain, S.; Wang, Y.; Guo, L.; He, T. Theoretical insights into the mechanism of photocatalytic reduction of CO2 over semiconductor catalysts. J. Photoch. Photobio. C 2022, 52, 100538. [Google Scholar] [CrossRef]
- Hussain, S.; Yang, X.; Yang, J.; Li, Q. Theoretical insights into the mechanism of photocatalytic reduction of CO2 and water splitting over II-VI zinc chalcogenide semiconductor. Mater. Today Sustain. 2024, 25, 100686. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhu, X.; Gao, X.; An, C.; Wang, Z.; Zuo, C.; Dionysiou, D.D.; He, H.; Jiang, Z. Enhancing photocatalytic CO2 reduction with TiO(2)-based materials: Strategies, mechanisms, challenges, and perspectives. Environ. Sci. Ecotechnol. 2024, 20, 100368. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jin, T.; Liu, C.; Li, G.; Yan, M. Three-Dimensional Graphene-TiO2 Nanocomposite Photocatalyst Synthesized by Covalent Attachment. ACS Omega 2016, 1, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shao, S.; Zhou, D.; Xu, Q.; Wang, T. ZnO nanowire arrays decorated 3D N-doped reduced graphene oxide nanotube framework for enhanced photocatalytic CO2 reduction performance. J. CO2 Util. 2021, 50, 101584. [Google Scholar] [CrossRef]
- Song, M.; Li, J.; Xu, M.; Xu, Z.; Song, X.; Liu, X.; Zhang, J.; Yang, Y.; Xie, X.; Zhou, W.; et al. Facile synthesis of MOF-808/RGO-based 3D macroscopic aerogel for enhanced photoreduction CO2. J. Colloid Interface Sci. 2024, 668, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Cheng, B.; Fan, J.; Yu, J.; Liu, G. Near-infrared absorbing 2D/3D ZnIn2S4/N-doped graphene photocatalyst for highly efficient CO2 capture and photocatalytic reduction. Sci. China Mater. 2020, 63, 552–565. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Liu, J.; Hu, L.; Guo, C. Recent Progress of Three-Dimensional Graphene-Based Composites for Photocatalysis. Gels 2024, 10, 626. https://doi.org/10.3390/gels10100626
Zhang F, Liu J, Hu L, Guo C. Recent Progress of Three-Dimensional Graphene-Based Composites for Photocatalysis. Gels. 2024; 10(10):626. https://doi.org/10.3390/gels10100626
Chicago/Turabian StyleZhang, Fengling, Jianxing Liu, Liang Hu, and Cean Guo. 2024. "Recent Progress of Three-Dimensional Graphene-Based Composites for Photocatalysis" Gels 10, no. 10: 626. https://doi.org/10.3390/gels10100626
APA StyleZhang, F., Liu, J., Hu, L., & Guo, C. (2024). Recent Progress of Three-Dimensional Graphene-Based Composites for Photocatalysis. Gels, 10(10), 626. https://doi.org/10.3390/gels10100626






