Hydroxypropyl Cellulose Hydrogel Containing Origanum vulgare ssp. hirtum Essential-Oil-Loaded Polymeric Micelles for Enhanced Treatment of Melanoma
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Origanum vulgare ssp. hirtum Essential Oil
2.2. Preparation and Characterization of OEO-Loaded Polymeric Micelles
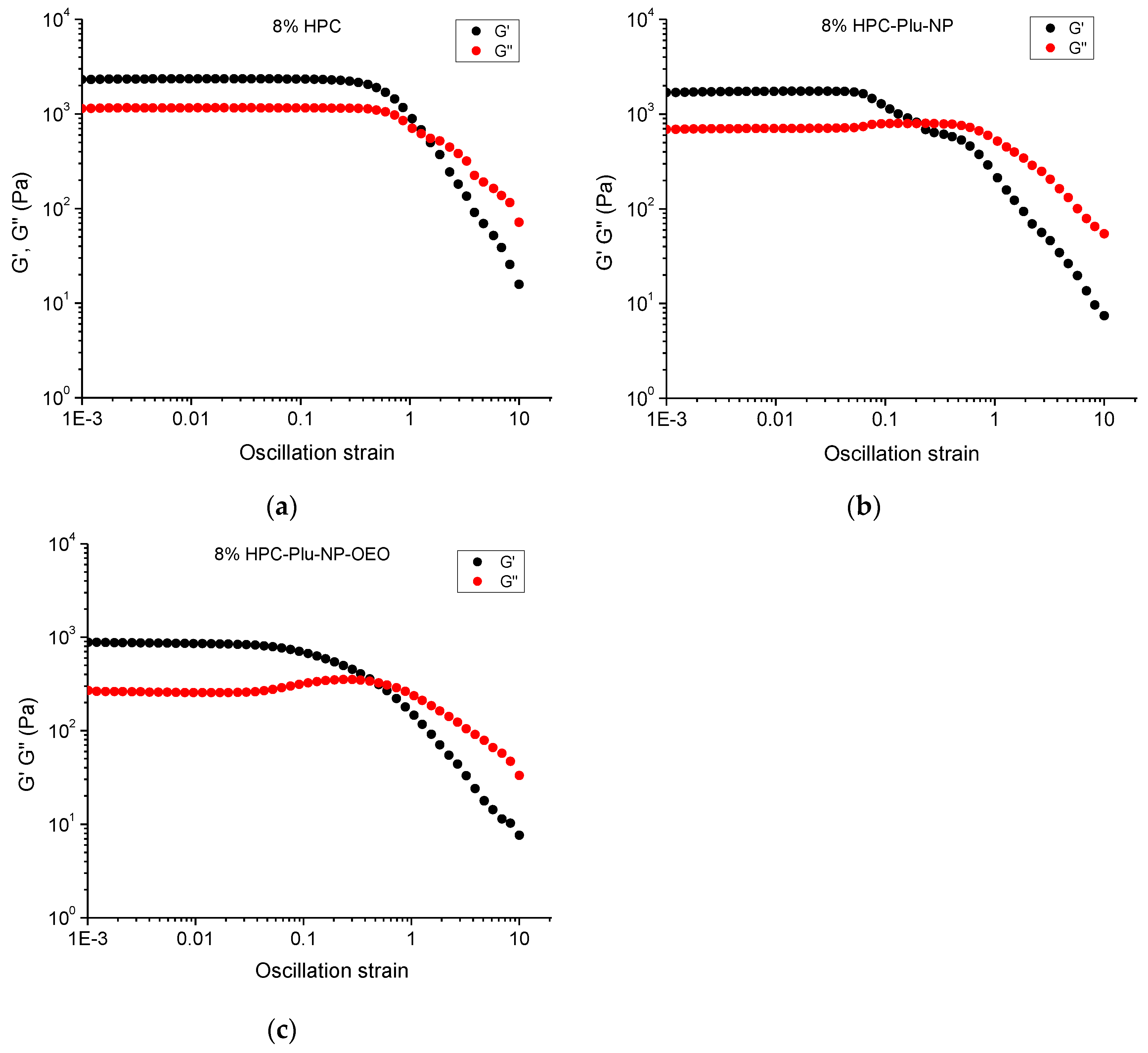
2.3. Preparation and Characterization of HPC Hydrogels, HPC-Plu-Nanocomposite Hydrogels and OEO-Loaded HPC-Plu-Nanocomposite Hydrogels
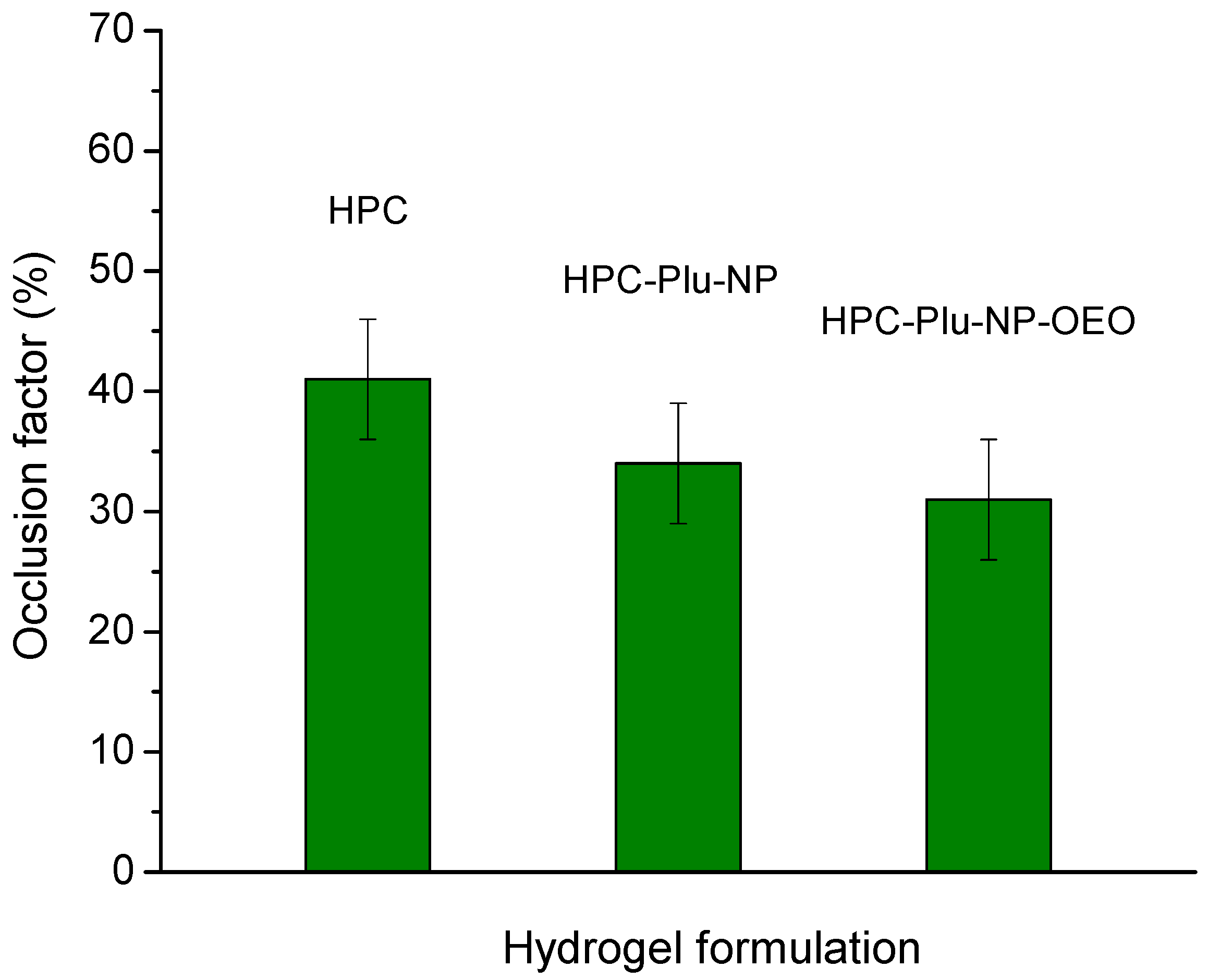
2.4. Occlusion Test
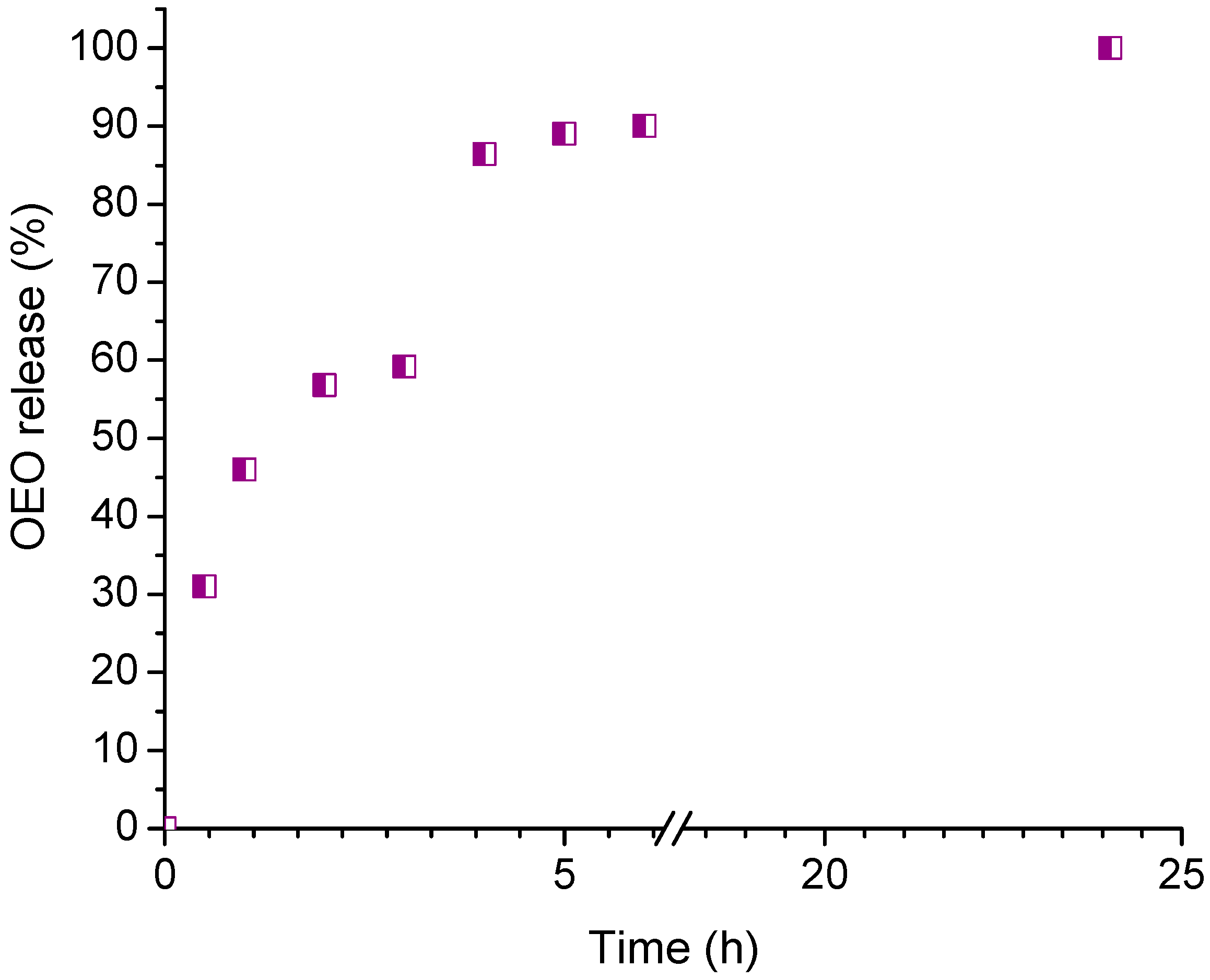
2.5. In Vitro Release of Oregano Essential Oil
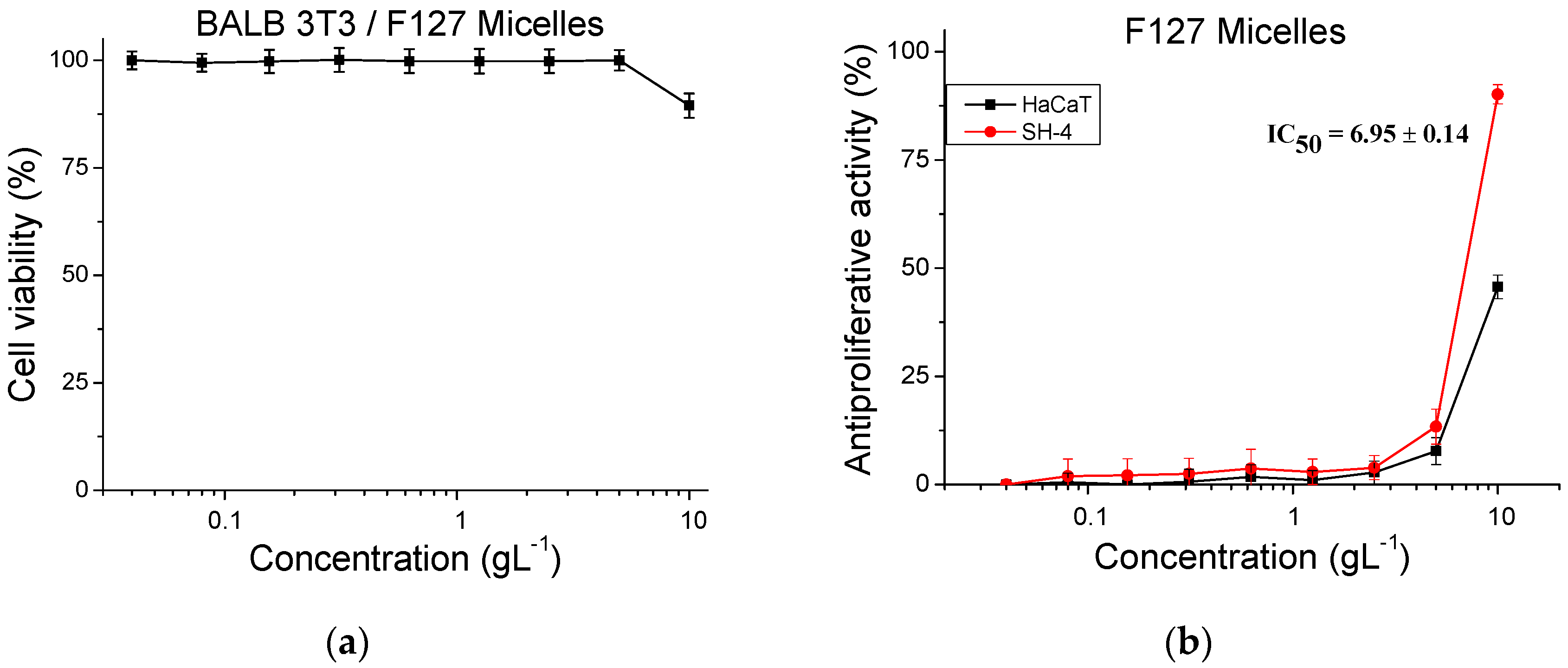
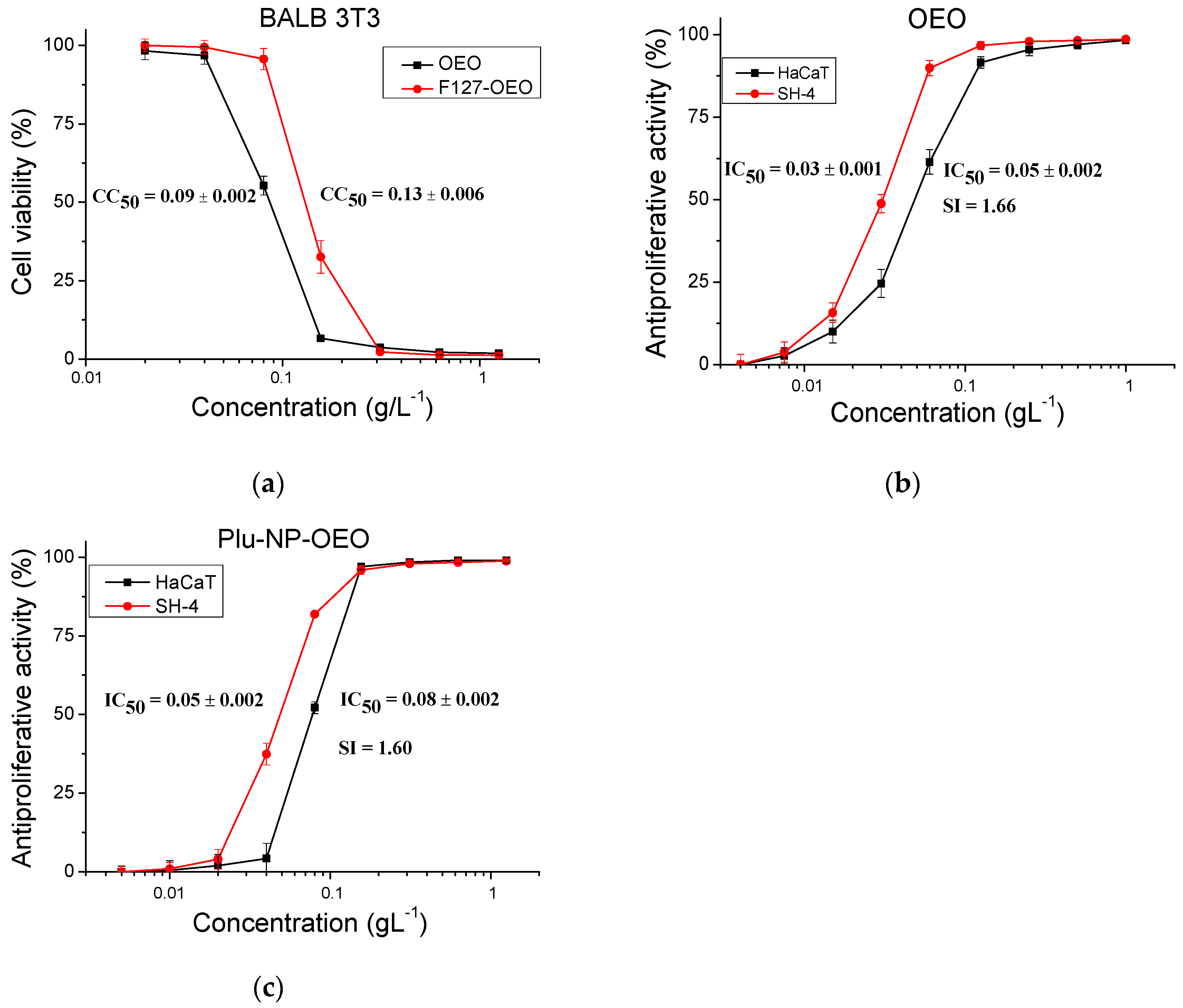
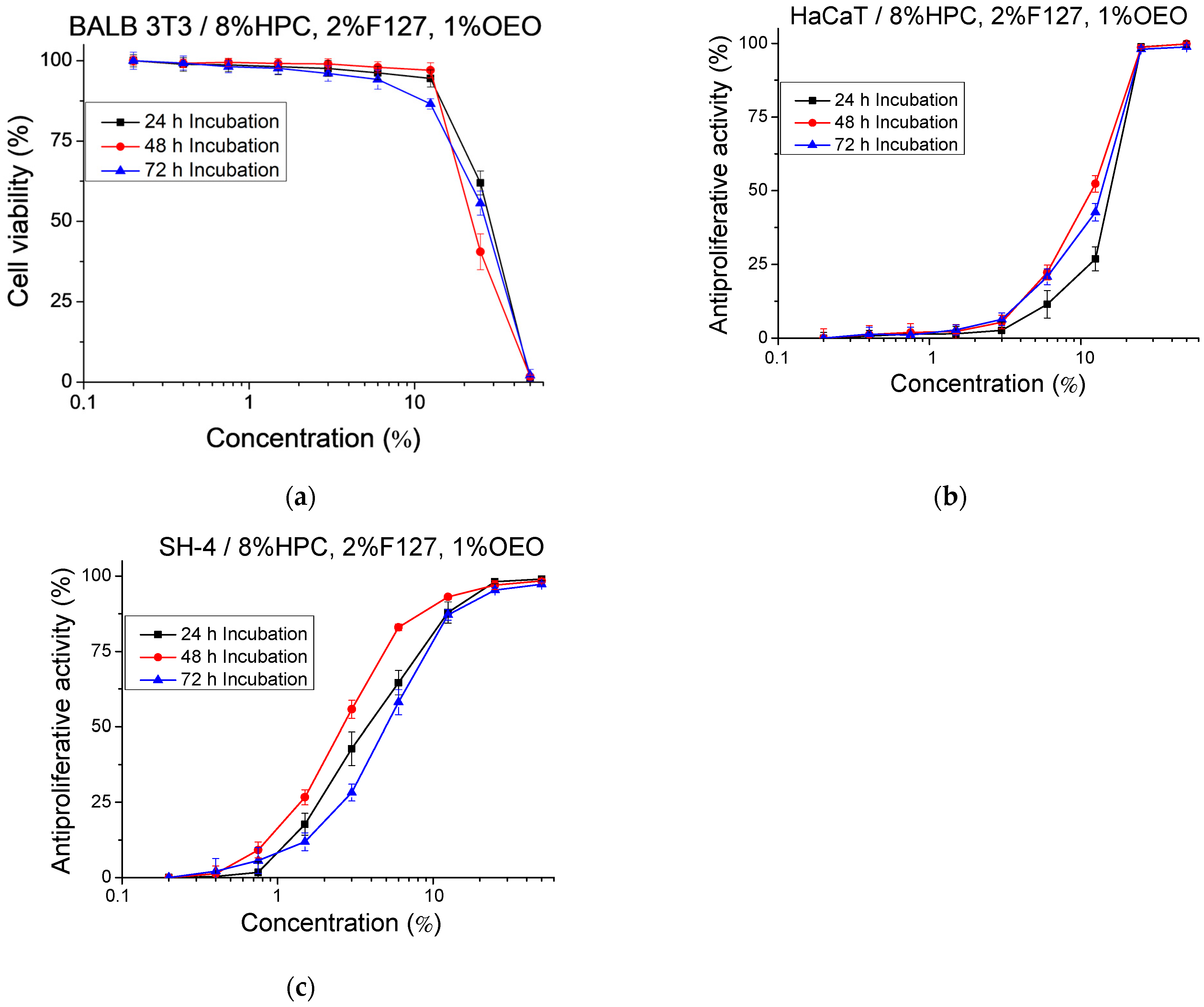
2.6. Cytotoxicity and Antiproliferative Activity
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Plant Material and Essential Oil Distillation
4.3. GC–MS/FID Analysis of Oregano Essential Oil
4.4. Preparation of Blank and OEO-Loaded Micelles
4.5. Preparation of Pure HPC Hydrogel and Nanocomposite HPC Hydrogels with OEO-Loaded Micelles
4.6. Analysis
4.7. Occlusion Factor Determination
4.8. In Vitro Release of Oregano Essential Oil
4.9. In Vitro Cytotoxicity and Antiproliferative Activity Studies
4.9.1. Cell Cultures
4.9.2. Cytotoxicity
4.9.3. Antiproliferative Activity
4.9.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adamus-Grabicka, A.A.; Hikisz, P.; Sikora, J. Nanotechnology as a Promising Method in the Treatment of Skin Cancer. Int. J. Mol. Sci. 2024, 25, 2165. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liu, C.; Chen, S.; Zhang, W. Nanocarrier-Based Drug Delivery for Melanoma Therapeutics. Int. J. Mol. Sci. 2021, 22, 1873. [Google Scholar] [CrossRef] [PubMed]
- Fateeva, A.; Eddy, K.; Chen, S. Current State of Melanoma Therapy and Next Steps: Battling Therapeutic Resistance. Cancers 2024, 16, 1571. [Google Scholar] [CrossRef] [PubMed]
- Vaienti, S.; Calzari, P.; Nazzaro, G. Topical Treatment of Melanoma In Situ, Lentigo Maligna, and Lentigo Maligna Melanoma with Imiquimod Cream: A Systematic Review of the Literature. Dermatol. Ther. 2023, 13, 2187–2215. [Google Scholar] [CrossRef] [PubMed]
- Della Vittoria Scarpati, G.; Fusciello, C.; Perri, F.; Sabbatino, F.; Ferrone, S.; Carlomagno, C.; Pepe, S. Ipilimumab in the treatment of metastatic melanoma: Management of adverse events. OncoTargets Ther. 2014, 7, 203–209. [Google Scholar] [CrossRef]
- Tanaka, R.; Fujisawa, Y.; Sae, I.; Maruyama, H.; Ito, S.; Hasegawa, N.; Sekine, I.; Fujimoto, M. Severe Hepatitis Arising from Ipilimumab Administration, Following Melanoma Treatment with Nivolumab. Jpn. J. Clin. Oncol. 2017, 47, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, F.L.; Bakshi, H.A.; Khan, S.; Nasef, M.; Farzand, R.; Sam, S.; Rashan, L.; Al-Baloshi, M.S.; Abdo Hasson, S.A.; Jabri, A.A.; et al. Frankincense essential oil suppresses melanoma cancer through down regulation of Bcl-2/Bax cascade signaling and ameliorates heptotoxicity via phase I and II drug metabolizing enzymes. Oncotarget 2019, 10, 3472–3490. [Google Scholar] [CrossRef] [PubMed]
- Nanni, V.; Di Marco, G.; Sacchetti, G.; Canini, A.; Gismondi, A. Oregano Phytocomplex Induces Programmed Cell Death in Melanoma Lines via Mitochondria and DNA Damage. Foods 2020, 9, 1486. [Google Scholar] [CrossRef]
- Diaz, M.J.; Natarelli, N.; Aflatooni, S.; Aleman, S.J.; Neelam, S.; Tran, J.T.; Taneja, K.; Lucke-Wold, B.; Forouzandeh, M. Nanoparticle-Based Treatment Approaches for Skin Cancer: A Systematic Review. Curr. Oncol. 2023, 30, 7112–7131. [Google Scholar] [CrossRef]
- Madawi, E.A.; Al Jayoush, A.R.; Rawas-Qalaji, M.; Thu, H.E.; Khan, S.; Sohail, M.; Mahmood, A.; Hussain, Z. Polymeric Nanoparticles as Tunable Nanocarriers for Targeted Delivery of Drugs to Skin Tissues for Treatment of Topical Skin Diseases. Pharmaceutics 2023, 15, 657. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Abdoul-Latif, F.; Ainane, A.; Houmed Aboubaker, I.; Mohamed, J.; Ainane, T. Exploring the Potent Anticancer Activity of Essential Oils and Their Bioactive Compounds: Mechanisms and Prospects for Future Cancer Therapy. Pharmaceuticals 2023, 16, 1086. [Google Scholar] [CrossRef] [PubMed]
- Fitsiou, E.; Pappa, A. Anticancer Activity of Essential Oils and Other Extracts from Aromatic Plants Grown in Greece. Antioxidants 2019, 8, 290. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, A.P.; Rusu, A.G.; Nita, L.E.; Chiriac, V.M.; Neamtu, I.; Sandu, A. Polymeric Carriers Designed for Encapsulation of Essential Oils with Biological Activity. Pharmaceutics 2021, 13, 631. [Google Scholar] [CrossRef] [PubMed]
- El-Shemy, H.A. (Ed.) Nashwa Fathy Sayed Morsy Chemical Structure, Quality Indices and Bioactivity of Essential Oil Constituents. In Active Ingredients from Aromatic and Medicinal Plants; IntechOpen: Rijeka, Croatia, 2017; p. Ch. 11. ISBN 978-953-51-2976-9. [Google Scholar]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, P.S.; Mehta, A.; Verma, R. Essential Oils: From Prevention to Treatment of Skin Cancer. Drug Discov. Today 2018, 24, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Lanzerstorfer, P.; Sandner, G.; Pitsch, J.; Mascher, B.; Aumiller, T.; Weghuber, J. Acute, Reproductive, and Developmental Toxicity of Essential Oils Assessed with Alternative In Vitro and In Vivo Systems. Arch. Toxicol. 2021, 95, 3. [Google Scholar] [CrossRef]
- Omeragic, E.; Dedic, M.; Elezovic, A.; Becic, E.; Imamovic, B.; Kladar, N.; Niksic, H. Application of Direct Peptide Reactivity Assay for Assessing the Skin Sensitization Potential of Essential Oils. Sci. Rep. 2022, 12, 7470. [Google Scholar] [CrossRef]
- Horky, P.; Skalickova, S.; Smerkova, K.; Skladanka, J. Essential Oils as a Feed Additives: Pharmacokinetics and Potential Toxicity in Monogastric Animals. Animals 2019, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Romero-Montero, A.; Melgoza-Ramírez, L.J.; Ruíz-Aguirre, J.A.; Chávez-Santoscoy, A.; Magaña, J.J.; Cortés, H.; Leyva-Gómez, G.; Del Prado-Audelo, M.L. Essential-Oils-Loaded Biopolymeric Nanoparticles as Strategies for Microbial and Biofilm Control: A Current Status. Int. J. Mol. Sci. 2024, 25, 82. [Google Scholar] [CrossRef] [PubMed]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential Oils: Pharmaceutical Applications and Encapsulation Strategies into Lipid-Based Delivery Systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Slavkova, M.; Tzankov, B.; Popova, T.; Voycheva, C. Gel Formulations for Topical Treatment of Skin Cancer: A Review. Gels 2023, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Bora, L.; Avram, S.; Pavel, I.Z.; Muntean, D.; Liga, S.; Buda, V.; Gurgus, D.; Danciu, C. An Up-To-Date Review Regarding Cutaneous Benefits of Origanum vulgare L. Essential Oil. Antibiotics 2022, 11, 549. [Google Scholar] [CrossRef] [PubMed]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Nair, A.B.; YT, K. Progress in Polymeric Micelles for Drug Delivery Applications. Pharmaceutics 2022, 14, 1636. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.; Hennink, W. Polymeric Micelles in Anticancer Therapy: Targeting, Imaging and Triggered Release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Jarak, I.; Santos, A.; Veiga, F.; Figueiras, A. Polymeric Micelles: A Promising Pathway for Dermal Drug Delivery. Materials 2021, 14, 7278. [Google Scholar] [CrossRef]
- Ataide, J.A.; Zanchetta, B.; Santos, É.M.; Fava, A.L.M.; Alves, T.F.R.; Cefali, L.C.; Chaud, M.V.; Oliveira-Nascimento, L.; Souto, E.B.; Mazzola, P.G. Nanotechnology-Based Dressings for Wound Management. Pharmaceuticals 2022, 15, 1286. [Google Scholar] [CrossRef]
- Khurana, B.; Arora, D.; Narang, R.K. QbD based exploration of resveratrol loaded polymeric micelles based carbomer gel for topical treatment of plaque psoriasis: In vitro, ex vivo and in vivo studies. J. Drug Deliv. Sci. Technol. 2020, 59, 101901. [Google Scholar] [CrossRef]
- An, J.; Yang, H.; Park, N.; Koo, T.-S.; Shin, B.; Lee, E.; Cho, S. Development of Polymeric Micelles of Oleanolic Acid and Evaluation of Their Clinical Efficacy. Nanoscale Res. Lett. 2020, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsalam, W.H.; El-Zahaby, S.A.; Al-Mahallawi, A.M. Formulation and in vivo assessment of terconazole-loaded polymeric mixed micelles enriched with Cremophor EL as dual functioning mediator for augmenting physical stability and skin delivery. Drug Deliv. 2018, 25, 484–492. [Google Scholar] [CrossRef]
- Bora, L.; Burkard, T.; Juan, M.H.S.; Radeke, H.H.; Muț, A.M.; Vlaia, L.L.; Magyari-Pavel, I.Z.; Diaconeasa, Z.; Socaci, S.; Borcan, F.; et al. Phytochemical Characterization and Biological Evaluation of Origanum vulgare L. Essential Oil Formulated as Polymeric Micelles Drug Delivery Systems. Pharmaceutics 2022, 14, 2413. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Bodratti, A.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Cellulose-Based Hydrogels as Sustained Drug-Delivery Systems. Materials 2020, 13, 5270. [Google Scholar] [CrossRef]
- Kamenova, K.; Momekova, D.; Grancharov, G.; Prancheva, A.; Toncheva-Moncheva, N.; Ivanov, E.; Konstantinov, S.; Petrov, P.D. In Situ Gelling Hydroxypropyl Cellulose Formulation Comprising Cannabidiol-Loaded Block Copolymer Micelles for Sustained Drug Delivery. Int. J. Mol. Sci. 2023, 24, 16534. [Google Scholar] [CrossRef]
- Nakas, A.; Giannarelli, G.; Fotopoulos, I.; Chainoglou, E.; Peperidou, A.; Kontogiannopoulos, K.N.; Tsiaprazi-Stamou, A.; Varsamis, V.; Gika, H.; Hadjipavlou-Litina, D.; et al. Optimizing the Distillation of Greek Oregano—Do Process Parameters Affect Bioactive Aroma Constituents and In Vitro Antioxidant Activity? Molecules 2023, 28, 971. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Shakeri, A.; Iranshahi, M.; Boozari, M. A Review of the Phytochemistry and Antimicrobial Properties of Origanum vulgare L. and Subspecies. Iran. J. Pharm. Res. (IJPR) 2021, 20, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Parker, T. Anti-Inflammatory, Tissue Remodeling, Immunomodulatory, and Anticancer Activities of Oregano (Origanum vulgare) Essential Oil in a Human Skin Disease Model. Biochim. Open 2017, 4, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Yılmaz, Y.B.; Antika, G.; Salehi, B.; Tumer, T.B.; Venil, C.K.; Das, G.; Patra, J.K.; Karazhan, N.; Akram, M.; et al. Phytochemical Constituents, Biological Activities, and Health-Promoting Effects of the Genus Origanum. Phytother. Res. PTR 2021, 35, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Alekseeva, M.; Zagorcheva, T.; Atanassov, I.; Rusanov, K. Origanum vulgare L.—A review on genetic diversity, cultivation, biological activities and perspectives for molecular breeding. Bulg. J. Agric. Sci. 2020, 26, 1183–1197. [Google Scholar]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.-M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.; Cervellieri, S.; Netti, T.; Lippolis, V.; Baruzzi, F. Antibacterial Activity of Oregano (Origanum vulgare L.) Essential Oil Vapors against Microbial Contaminants of Food-Contact Surfaces. Antibiotics 2024, 13, 371. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, J.; Song, Y. Antibacterial activity of oregano essential oils against Streptococcus mutans in vitro and analysis of active components. BMC Complement. Med. Ther. 2023, 23, 61. [Google Scholar] [CrossRef]
- Manohar, V.; Ingram, C.; Gray, J. Antifungal activities of origanum oil against Candida albicans. Mol. Cell. Biochem. 2011, 228, 111–117. [Google Scholar] [CrossRef]
- Chaftar, N.; Girardot, M.; Labanowski, J.; Ghrairi, T.; Hani, K.; Frère, J.; Imbert, C. Comparative Evaluation of the Antimicrobial Activity of 19 Essential Oils. Adv. Exp. Med. Biol. 2016, 901, 1–15. [Google Scholar] [CrossRef]
- Adame-Gallegos, J.R.; Andrade-Ochoa, S.; Nevarez-Moorillon, G.V. Potential Use of Mexican Oregano Essential Oil against Parasite, Fungal and Bacterial Pathogens. J. Essent. Oil Bear. Plants 2016, 19, 553–567. [Google Scholar] [CrossRef]
- Avola, R.; Granata, G.; Geraci, C.; Napoli, E.; Graziano, A.; Cardile, V. Oregano (Origanum Vulgare L.) Essential Oil Provides Anti-Inflammatory Activity and Facilitates Wound Healing in a Human Keratinocytes Cell Model. Food Chem. Toxicol. 2020, 144, 111586. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Abdelgaleil, S.A.M.; Mahmoud, E.A.; Yessoufou, K.; Elhindi, K.; El-Hendawy, S. Effective Antioxidant, Antimicrobial and Anticancer Activities of Essential Oils of Horticultural Aromatic Crops in Northern Egypt. BMC Complement. Altern. Med. 2018, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Emire, Z.; Yabalak, E. Can Origanum be a hope for cancer treatment? A review on the potential of Origanum species in preventing and treating cancers. Int. J. Environ. Health Res. 2023, 33, 894–910. [Google Scholar] [CrossRef] [PubMed]
- Grondona, E.; Gatti, G.; López, A.G.; Sánchez, L.R.; Rivero, V.; Pessah, O.; Zunino, M.P.; Ponce, A.A. Bio-efficacy of the essential oil of oregano (Origanum vulgare Lamiaceae. ssp. hirtum). Plant Foods Hum. Nutr. 2014, 69, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Balusamy, S.R.; Perumalsamy, H.; Huq, M.A.; Balasubramanian, B. Anti-Proliferative Activity of Origanum vulgare Inhibited Lipogenesis and Induced Mitochondrial Mediated Apoptosis in Human Stomach Cancer Cell Lines. Biomed. Pharmacother. 2018, 108, 1835–1844. [Google Scholar] [CrossRef]
- Pimentel, L.S.; Bastos, L.M.; Goulart, L.R.; Ribeiro, L.N.d.M. Therapeutic Effects of Essential Oils and Their Bioactive Compounds on Prostate Cancer Treatment. Pharmaceutics 2024, 16, 583. [Google Scholar] [CrossRef] [PubMed]
- Pontes-Quero, G.M.; Esteban-Rubio, S.; Pérez Cano, J.; Aguilar, M.R.; Vázquez-Lasa, B. Oregano Essential Oil Micro- and Nanoencapsulation with Bioactive Properties for Biotechnological and Biomedical Applications. Front. Bioeng. Biotechnol. 2021, 9, 703684. [Google Scholar] [CrossRef]
- Dupuis, V.; Cerbu, C.; Witkowski, L.; Potârniche, A.-V.; Timar, M.; Żychska, M.; Sabliov, C. Nanodelivery of Essential Oils as Efficient Tools against Antimicrobial Resistance: A Review of the Type and Physical-Chemical Properties of the Delivery Systems and Applications. Drug Deliv. 2022, 29, 1007–1024. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Mazlan, S.A.; Ubaidillah; Imaduddin, F. Rheological Properties of Plate-like Shape Carbonyl Iron Particles Compositions Based Magnetorheological Grease in Oscillatory Mode. IOP Conf. Ser. Mater. Sci. Eng. 2018, 333, 012001. [Google Scholar] [CrossRef]
- Gugleva, V.; Titeva, S.; Ermenlieva, N.; Tsibranska, S.; Tcholakova, S.; Rangelov, S.; Momekova, D. Development and Evaluation of Doxycycline Niosomal Thermoresponsive in Situ Gel for Ophthalmic Delivery. Int. J. Pharm. 2020, 591, 120010. [Google Scholar] [CrossRef]
- Zhai, H.; Maibach, H. Effects of Skin Occlusion on Percutaneous Absorption: An Overview. Ski. Pharmacol. Appl. Ski. Physiol. 2001, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Parenti, C.; Turnaturi, R.; Pasquinucci, L. Resveratrol-Loaded Lipid Nanocarriers: Correlation between In Vitro Occlusion Factor and In Vivo Skin Hydrating Effect. Pharmaceutics 2017, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Slavkova, M.; Lazov, C.; Spassova, I.; Kovacheva, D.; Tibi, I.P.-E.; Stefanova, D.; Tzankova, V.; Petrov, P.D.; Yoncheva, K. Formulation of Budesonide-Loaded Polymeric Nanoparticles into Hydrogels for Local Therapy of Atopic Dermatitis. Gels 2024, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Stolwijk, J.A.; Sauer, L.; Ackermann, K.; Nassios, A.; Aung, T.; Haerteis, S.; Bäumner, A.J.; Wegener, J.; Schreml, S. pH sensing in skin tumors: Methods to study the involvement of GPCRs, acid-sensing ion channels and transient receptor potential vanilloid channels. Exp. Dermatol. 2020, 29, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Martínez, O.M.; Narváez Ramos, M.A.; Soto Acevedo, A.A.; Colón Colón, C.C.; Malavé Ramos, D.; Castro Rivera, C.; Castro Rosario, M.E. pH-Selective Reactions to Selectively Reduce Cancer Cell Proliferation: Effect of CaS Nanostructures in Human Skin Melanoma and Benign Fibroblasts. BioChem 2023, 3, 15–30. [Google Scholar] [CrossRef]
- Avram, Ș.; Bora, L.; Vlaia, L.L.; Muț, A.M.; Olteanu, G.-E.; Olariu, I.; Magyari-Pavel, I.Z.; Minda, D.; Diaconeasa, Z.; Sfirloaga, P.; et al. Cutaneous Polymeric-Micelles-Based Hydrogel Containing Origanum vulgare L. Essential Oil: In Vitro Release and Permeation, Angiogenesis, and Safety Profile In Ovo. Pharmaceuticals 2023, 16, 940. [Google Scholar] [CrossRef] [PubMed]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Alekseeva, M.; Zagorcheva, T.; Rusanova, M.; Rusanov, K.; Atanassov, I. Genetic and Flower Volatile Diversity in Natural Populations of Origanum vulgare subsp. hirtum (Link) Ietsw. in Bulgaria: Toward the Development of a Core Collection. Front. Plant Sci. 2021, 15, 679063. [Google Scholar] [CrossRef]
- Spielmann, H.; Balls, M.; Dupuis, J.; Pape, W.J.; Pechovitch, G.; de Silva, O.; Holzhütter, H.G.; Clothier, R.; Desolle, P.; Gerberick, F.; et al. The International EU/COLIPA In Vitro Phototoxicity Validation Study: Results of Phase II (Blind Trial). Part 1: The 3T3 NRU Phototoxicity Test. Toxicol. Vitr. 1998, 12, 305–327. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]



| Compound | Rt (min) | Concentration before Loading (% of Total Peak Areas) | Concentration after Loading (% of Total Peak Areas) |
|---|---|---|---|
| Carvacrol | 58.66 | 90.31 | 90.28 |
| Thymol | 58.18 | 1.62 | 1.62 |
| Sample | Mass Ratio F127/OEO | Dh (nm) | ζ-Potential (mV) | Transmittance (%) |
|---|---|---|---|---|
| 1 | 2 | 34 ± 2 | −3.16 ± 0.55 | 85.35 |
| 2 | 1.8 | 36 ± 2 | −2.46 ± 0.24 | 56.52 |
| 3 | 1.7 | 67 ± 3 | −0.52 ± 0.14 | 1.76 |
| 4 | 1.5 | - | - | 0.26 |
| 5 | 1.2 | - | - | 0.03 |
| 6 | 1 | - | - | 0.01 |
| F127 | - | 25 ± 1 | −6.91 ± 1.32 | - |
| HPC Concentration | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1% | 2% | 3% | 4% | 5% | 6% | 7% | 8% | 9% | 10% | |
| G′ (Pa) | 1.3 | 18.7 | 53.3 | 201.6 | 773.8 | 897.4 | 1368.0 | 2341.2 | 2426.9 | 2817.4 |
| G″ (Pa) | 6.1 | 32.9 | 61.6 | 176.7 | 494.3 | 502.7 | 757.6 | 1140.3 | 1108.6 | 1133.1 |
| η* (Pa·s) | 0.9 | 6.0 | 12.9 | 42.6 | 146.1 | 163.7 | 248.9 | 414.3 | 424.4 | 483.2 |
| HPC/F127/OEO | Mean IC50 ± SD (%) | SI * | ||
|---|---|---|---|---|
| Cytotoxicity | Antiproliferative Activity | |||
| BALB 3T3 | HaCaT | SH-4 | ||
| 24 h | 28.63 ± 0.96 | 15.61 ± 0.45 | 3.78 ± 0.62 | 4.13 |
| 48 h | 22.36 ± 1.38 | 11.84 ± 0.73 | 2.63 ± 0.16 | 4.50 |
| 72 h | 26.83 ± 1.13 | 13.70 ± 0.46 | 5.02 ± 0.36 | 2.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamenova, K.; Iliev, I.; Prancheva, A.; Tuleshkov, P.; Rusanov, K.; Atanassov, I.; Petrov, P.D. Hydroxypropyl Cellulose Hydrogel Containing Origanum vulgare ssp. hirtum Essential-Oil-Loaded Polymeric Micelles for Enhanced Treatment of Melanoma. Gels 2024, 10, 627. https://doi.org/10.3390/gels10100627
Kamenova K, Iliev I, Prancheva A, Tuleshkov P, Rusanov K, Atanassov I, Petrov PD. Hydroxypropyl Cellulose Hydrogel Containing Origanum vulgare ssp. hirtum Essential-Oil-Loaded Polymeric Micelles for Enhanced Treatment of Melanoma. Gels. 2024; 10(10):627. https://doi.org/10.3390/gels10100627
Chicago/Turabian StyleKamenova, Katya, Ivan Iliev, Anna Prancheva, Pencho Tuleshkov, Krasimir Rusanov, Ivan Atanassov, and Petar D. Petrov. 2024. "Hydroxypropyl Cellulose Hydrogel Containing Origanum vulgare ssp. hirtum Essential-Oil-Loaded Polymeric Micelles for Enhanced Treatment of Melanoma" Gels 10, no. 10: 627. https://doi.org/10.3390/gels10100627
APA StyleKamenova, K., Iliev, I., Prancheva, A., Tuleshkov, P., Rusanov, K., Atanassov, I., & Petrov, P. D. (2024). Hydroxypropyl Cellulose Hydrogel Containing Origanum vulgare ssp. hirtum Essential-Oil-Loaded Polymeric Micelles for Enhanced Treatment of Melanoma. Gels, 10(10), 627. https://doi.org/10.3390/gels10100627










