A Paintable Small-Molecule Hydrogel with Antimicrobial and ROS Scavenging Activities for Burn Wound Healing
Abstract
1. Introduction
2. Results and Discussion
2.1. Self-Assembly and Characterization of Nap-F3K-CA Hydrogel
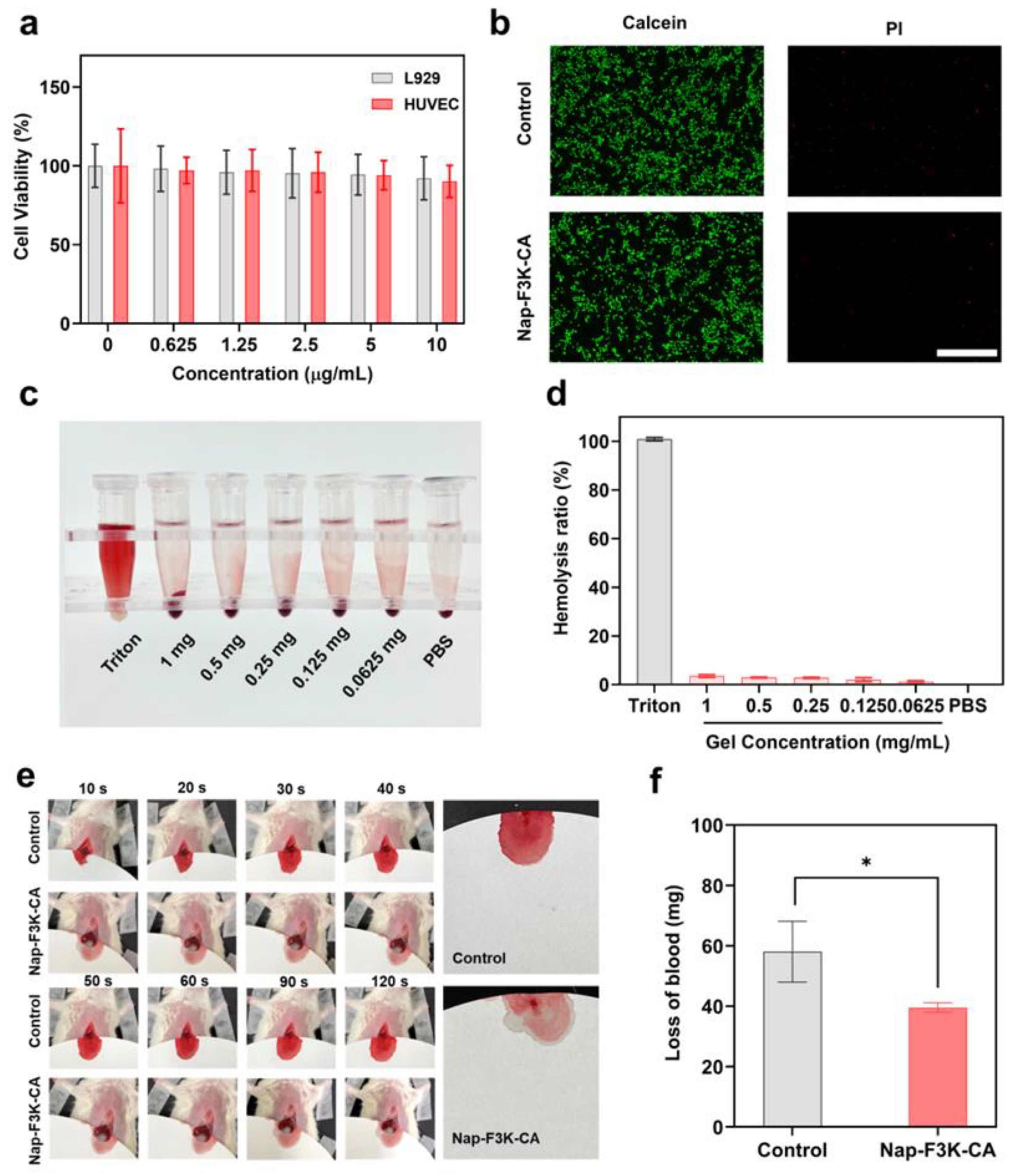
2.2. Biocompatibility Evaluation of Nap-F3K-CA In Vitro
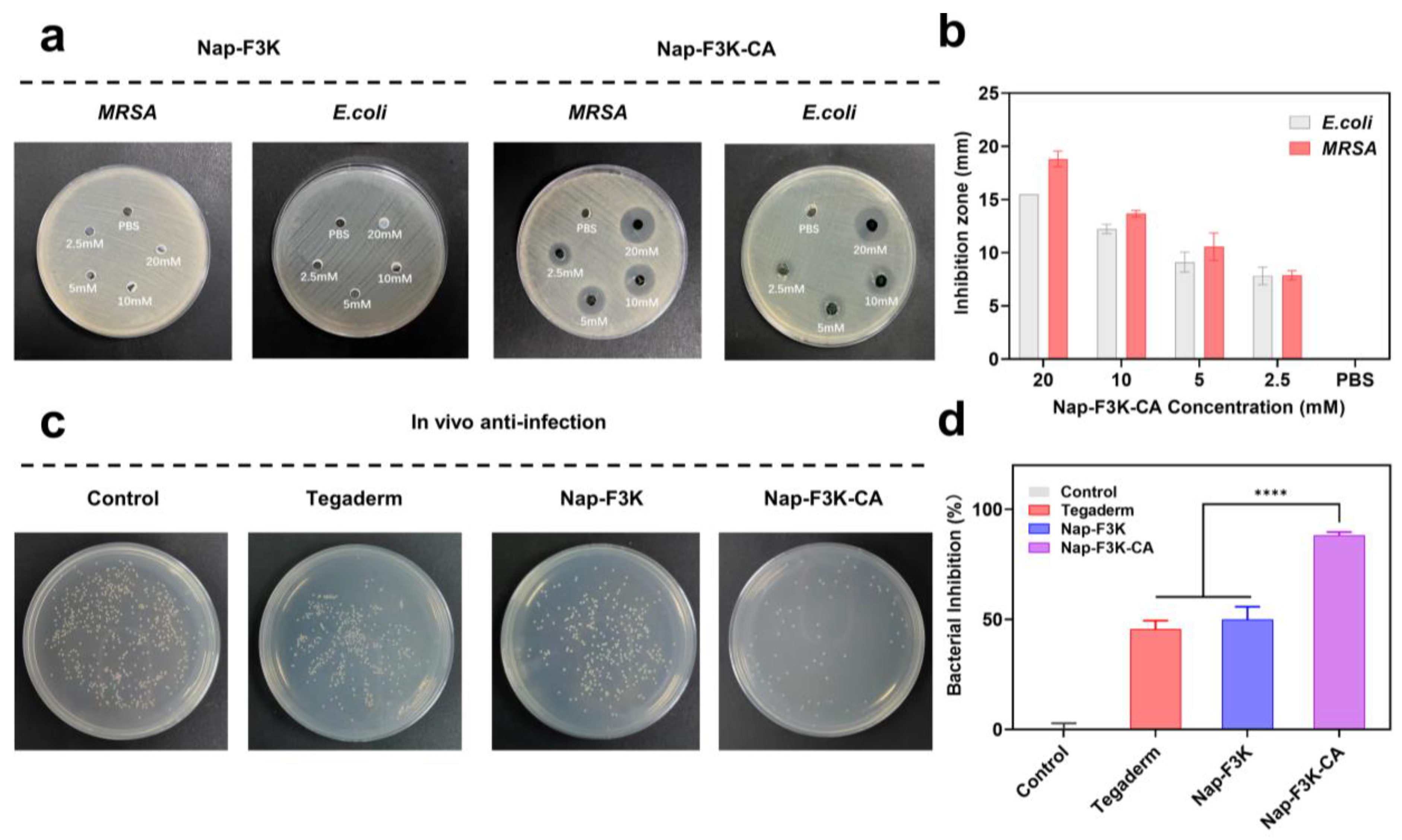
2.3. Anti-Infection Evaluation
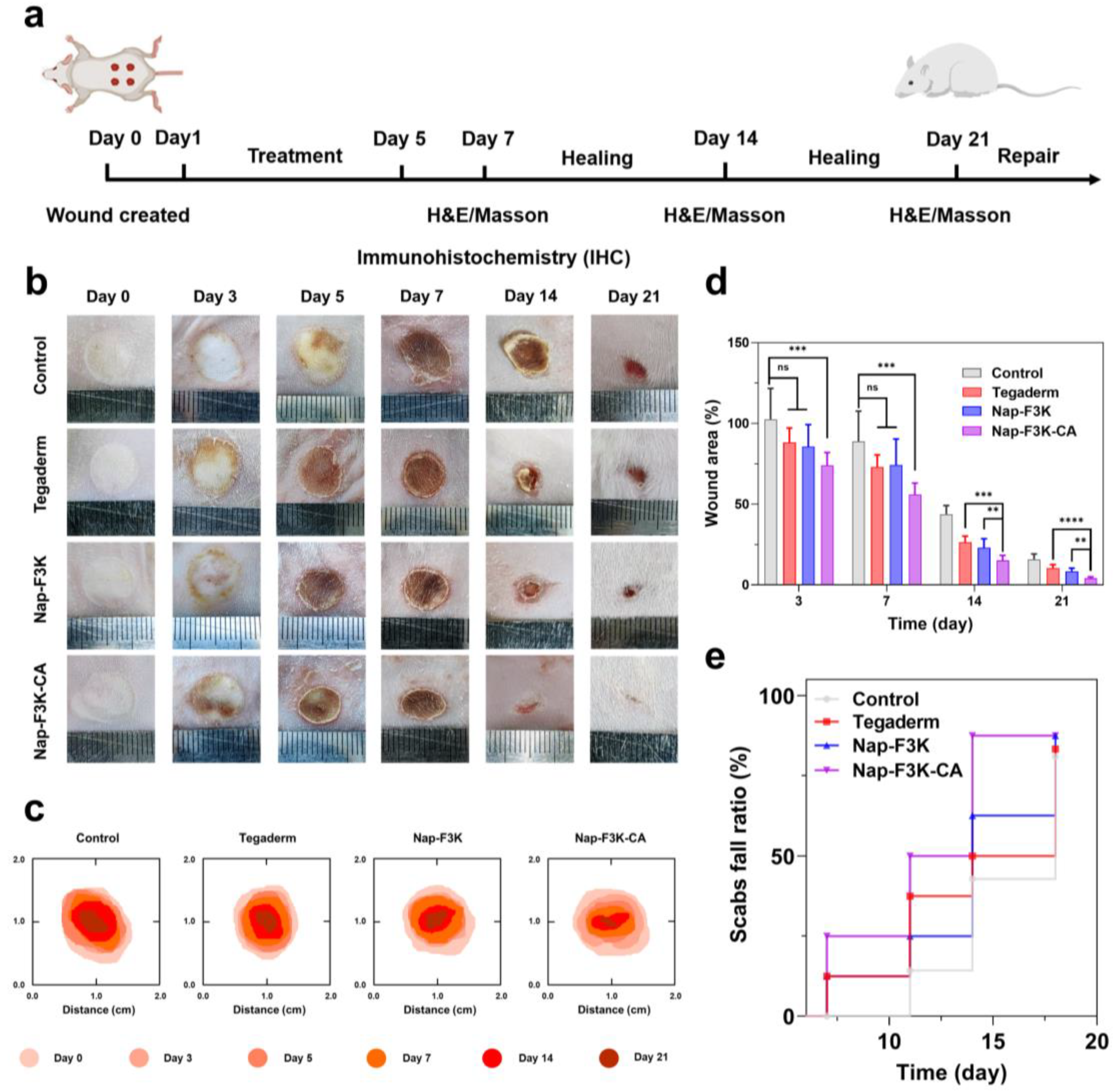
2.4. Effects of Nap-F3K-CA Hydrogel on Burn Wound Healing
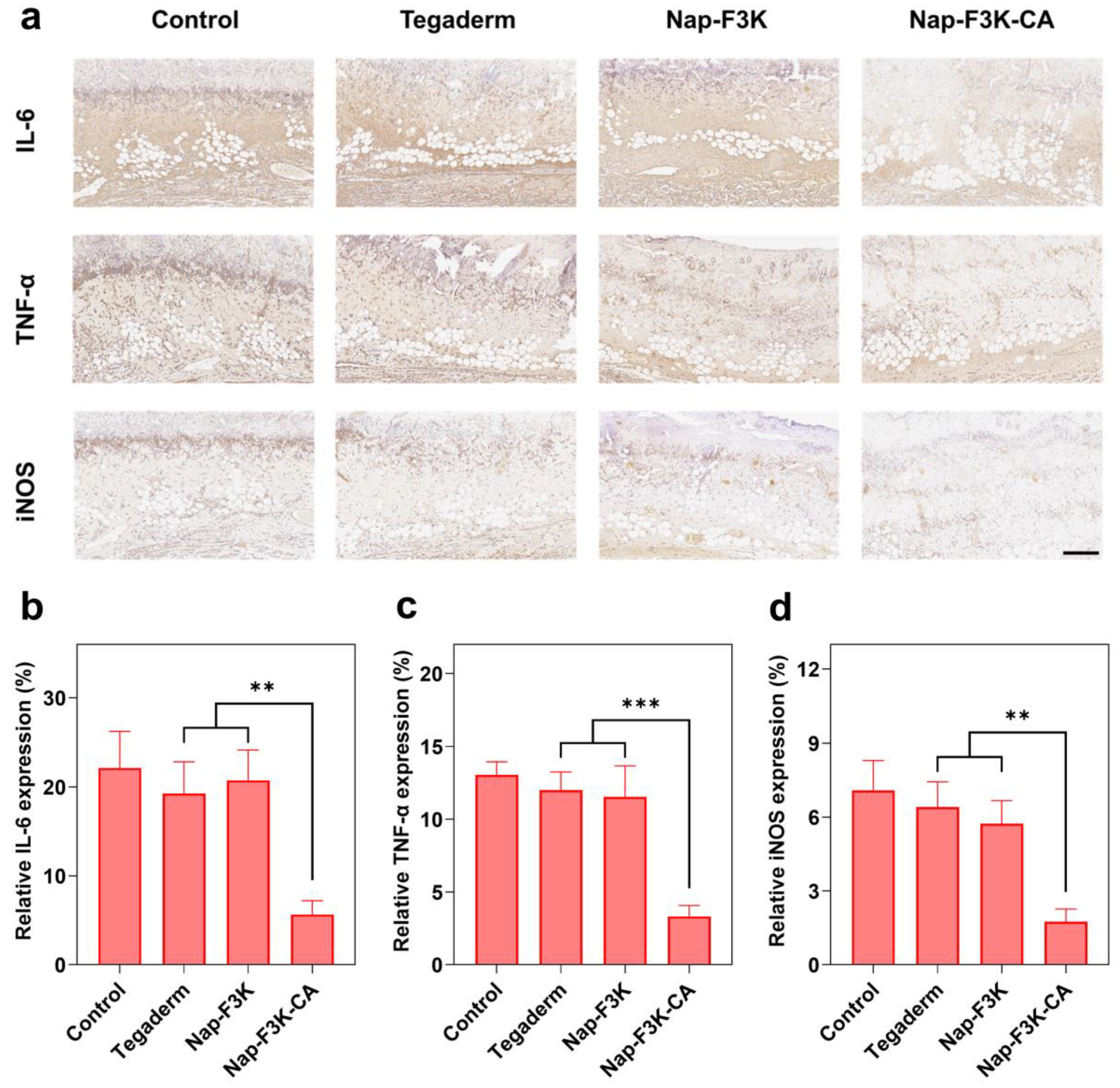
2.5. Histological and Immunochemical Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation and Characterisation of Nap-F3K and Nap-F3K-CA Hydrogels
4.3. CD Measurement
4.4. Rheological Analysis
4.5. Free Radical Scavenging Assay
4.6. MTT Assay
4.7. Calcein-AM/PI Live/Dead Staining Assay
4.8. Hemolysis Assay
4.9. Hemostasis Test
4.10. Antimicrobial Test
4.11. Burn Wound Model Establishment
4.12. In Vivo Antibacterial Activity
4.13. In Vivo Burn Wound Healing Evaluation
4.14. Histology Staining
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Holzer, J.; Tiffner, K.; Kainz, S.; Reisenegger, P.; Bernardelli, D.M.I.; Funk, M.; Lemarchand, T.; Laaff, H.; Bal, A.; Birngruber, T.; et al. A novel human ex-vivo burn model and the local cooling effect of a bacterial nanocellulose-based wound dressing. Burns 2020, 46, 1924–1932. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, B.; Fischer, S.; Harhaus, L.; Schmidt, V.J.; Horter, J.; Kremer, T.; Kneser, U.; Hirche, C. Aktuelle Standards in der Verbrennungsmedizin. Trauma Und Berufskrankh. 2016, 18, 300–307. [Google Scholar] [CrossRef]
- Burgess, M.; Valdera, F.; Varon, D.; Kankuri, E.; Nuutila, K. The Immune and Regenerative Response to Burn Injury. Cells 2022, 11, 3073. [Google Scholar] [CrossRef]
- Sierawska, O.; Malkowska, P.; Taskin, C.; Hrynkiewicz, R.; Mertowska, P.; Grywalska, E.; Korzeniowski, T.; Torres, K.; Surowiecka, A.; Niedzwiedzka-Rystwej, P.; et al. Innate Immune System Response to Burn Damage-Focus on Cytokine Alteration. Int. J. Mol. Sci. 2022, 23, 716. [Google Scholar] [CrossRef]
- Banerjee, J.; Seetharaman, S.; Wrice, N.L.; Christy, R.J.; Natesan, S. Delivery of silver sulfadiazine and adipose derived stem cells using fibrin hydrogel improves infected burn wound regeneration. PLoS ONE 2019, 14, e0217965. [Google Scholar] [CrossRef]
- Li, B.; Wang, W.; Zhao, L.; Yan, D.; Li, X.; Gao, Q.; Zheng, J.; Zhou, S.; Lai, S.; Feng, Y.; et al. Multifunctional AIE Nanosphere-Based “Nanobomb” for Trimodal Imaging-Guided Photothermal/Photodynamic/Pharmacological Therapy of Drug-Resistant Bacterial Infections. ACS Nano 2023, 17, 4601–4618. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Ribeiro, A.F.; Ferreira, M.I.; Teixeira, M.C.; Shimojo, A.; Soriano, J.L.; Naveros, B.C.; Durazzo, A.; Lucarini, M.; Souto, S.B.; et al. New Nanotechnologies for the Treatment and Repair of Skin Burns Infections. Int. J. Mol. Sci. 2020, 21, 393. [Google Scholar] [CrossRef]
- Edwards, R.; Harding, K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 2004, 17, 91–96. [Google Scholar] [CrossRef]
- Esa, N.; Ansari, M.; Razak, S.; Ismail, N.I.; Jusoh, N.; Zawawi, N.A.; Jamaludin, M.I.; Sagadevan, S.; Nayan, N. A Review on Recent Progress of Stingless Bee Honey and Its Hydrogel-Based Compound for Wound Care Management. Molecules 2022, 27, 3080. [Google Scholar] [CrossRef]
- Correa, S.; Grosskopf, A.K.; Lopez, H.H.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zheng, J.; Oh, X.Y.; Chan, C.Y.; Low, B.; Tor, J.Q.; Jiang, W.; Ye, E.; Loh, X.J.; Li, Z. Nanoarchitecture-Integrated Hydrogel Systems toward Therapeutic Applications. ACS Nano 2023, 17, 7953–7978. [Google Scholar] [CrossRef] [PubMed]
- Derkenne, C.; Ronchi, L.; Prunet, B. Management of Burns. N. Engl. J. Med. 2019, 381, 1188. [Google Scholar] [CrossRef]
- Chen, F.; Qin, J.; Wu, P.; Gao, W.; Sun, G. Glucose-Responsive Antioxidant Hydrogel Accelerates Diabetic Wound Healing. Adv. Healthc. Mater. 2023, 12, e2300074. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, S.; Yi, J.; Zhang, H.; Ameer, G.A. A Cooperative Copper Metal-Organic Framework-Hydrogel System Improves Wound Healing in Diabetes. Adv. Funct. Mater. 2017, 27, 1604872. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Wang, J.; Kang, Y.; Wang, Z.; Cao, W.; Ye, J.; Gao, C. A tough, antibacterial and antioxidant hydrogel dressing accelerates wound healing and suppresses hypertrophic scar formation in infected wounds. Bioact. Mater. 2024, 34, 269–281. [Google Scholar] [CrossRef]
- Shi, Y.; Wareham, D.W.; Yuan, Y.; Deng, X.; Mata, A.; Azevedo, H.S. Polymyxin B-Triggered Assembly of Peptide Hydrogels for Localized and Sustained Release of Combined Antimicrobial Therapy. Adv. Healthc. Mater. 2021, 10, e2101465. [Google Scholar] [CrossRef]
- Loo, Y.; Wong, Y.C.; Cai, E.Z.; Ang, C.H.; Raju, A.; Lakshmanan, A.; Koh, A.G.; Zhou, H.J.; Lim, T.C.; Moochhala, S.M.; et al. Ultrashort peptide nanofibrous hydrogels for the acceleration of healing of burn wounds. Biomaterials 2014, 35, 4805–4814. [Google Scholar] [CrossRef]
- Zhou, J.; Cha, R.; Wu, Z.; Zhang, C.; He, Y.; Zhang, H.; Liu, K.; Fareed, M.S.; Wang, Z.; Yang, C.; et al. An injectable, natural peptide hydrogel with potent antimicrobial activity and excellent wound healing-promoting effects. Nano Today 2023, 49, 101801. [Google Scholar] [CrossRef]
- Cai, C.; Meng, Z.; Zhao, L.; Wu, T.; Xu, X.; Zhu, Y. A self-assembled peptide hydrogel for wound repair. J. Mater. Sci. 2022, 57, 1345–1361. [Google Scholar] [CrossRef]
- Estroff, L.A.; Hamilton, A.D. Water gelation by small organic molecules. Chem. Rev. 2004, 104, 1201–1218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ai, S.; Yang, Z.; Li, X. Peptide-based supramolecular hydrogels for local drug delivery. Adv. Drug Deliv. Rev. 2021, 174, 482–503. [Google Scholar] [CrossRef] [PubMed]
- Espindola, K.; Ferreira, R.G.; Narvaez, L.; Silva, R.A.; Da, S.A.; Silva, A.; Vieira, A.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Pavlikova, N. Caffeic Acid and Diseases-Mechanisms of Action. Int. J. Mol. Sci. 2022, 24, 588. [Google Scholar] [CrossRef]
- Sguizzato, M.; Mariani, P.; Ferrara, F.; Drechsler, M.; Hallan, S.S.; Huang, N.; Simeliere, F.; Khunti, N.; Cortesi, R.; Marchetti, N.; et al. Nanoparticulate Gels for Cutaneous Administration of Caffeic Acid. Nanomaterials 2020, 10, 961. [Google Scholar] [CrossRef]
- Mori, H.; Iwahashi, H. Antioxidant Activity of Caffeic Acid through a Novel Mechanism under UVA Irradiation. J. Clin. Biochem. Nutr. 2009, 45, 49–55. [Google Scholar] [CrossRef][Green Version]
- Gulcin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Gamaro, G.D.; Suyenaga, E.; Borsoi, M.; Lermen, J.; Pereira, P.; Ardenghi, P. Effect of rosmarinic and caffeic acids on inflammatory and nociception process in rats. ISRN Pharmacol. 2011, 2011, 451682. [Google Scholar] [CrossRef]
- Almeida, A.A.; Farah, A.; Silva, D.A.; Nunan, E.A.; Gloria, M.B. Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. J. Agr. Food Chem. 2006, 54, 8738–8743. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Liu, K.; Wang, K.; Fang, L.; Weng, L.T.; Zhang, H.; Tang, Y.; Ren, F.; Zhao, C.; et al. Mussel-Inspired Adhesive and Tough Hydrogel Based on Nanoclay Confined Dopamine Polymerization. ACS Nano 2017, 11, 2561–2574. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, X.; Cai, P.; Huang, X.; Huang, Y.; Liu, R.; Zhang, M.; Song, J.; Chen, X.; Yang, H. A silk-based sealant with tough adhesion for instant hemostasis of bleeding tissues. Nanoscale Horiz. 2019, 4, 1333–1341. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Zhang, M.; Zhou, L.; Huang, Z.; Duan, Y.; Wang, C.; Gu, Z.; Zhang, P.; Wen, Y. Anti-Dehydration and Rapid Trigger-Detachable Multifunctional Hydrogels Promote Scarless Therapeutics of Deep Burn. Adv. Funct. Mater. 2023, 33, 2211182. [Google Scholar] [CrossRef]
- Lan, J.; Shi, L.; Xiao, W.; Zhang, X.; Wang, S. A Rapid Self-Pumping Organohydrogel Dressing with Hydrophilic Fractal Microchannels to Promote Burn Wound Healing. Adv. Mater. 2023, 35, 2301765. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez, D.G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Kharaziha, M.; Baidya, A.; Annabi, N. Rational Design of Immunomodulatory Hydrogels for Chronic Wound Healing. Adv. Mater. 2021, 33, e2100176. [Google Scholar] [CrossRef]
- Hu, C.; Long, L.; Cao, J.; Zhang, S.; Wang, Y. Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release. Chem. Eng. J. 2021, 411, 128564. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Q.; Chen, K.; Yi, H.; He, B.; Jiang, T. A Paintable Small-Molecule Hydrogel with Antimicrobial and ROS Scavenging Activities for Burn Wound Healing. Gels 2024, 10, 621. https://doi.org/10.3390/gels10100621
Ji Q, Chen K, Yi H, He B, Jiang T. A Paintable Small-Molecule Hydrogel with Antimicrobial and ROS Scavenging Activities for Burn Wound Healing. Gels. 2024; 10(10):621. https://doi.org/10.3390/gels10100621
Chicago/Turabian StyleJi, Qingchun, Kehan Chen, Han Yi, Bingfang He, and Tianyue Jiang. 2024. "A Paintable Small-Molecule Hydrogel with Antimicrobial and ROS Scavenging Activities for Burn Wound Healing" Gels 10, no. 10: 621. https://doi.org/10.3390/gels10100621
APA StyleJi, Q., Chen, K., Yi, H., He, B., & Jiang, T. (2024). A Paintable Small-Molecule Hydrogel with Antimicrobial and ROS Scavenging Activities for Burn Wound Healing. Gels, 10(10), 621. https://doi.org/10.3390/gels10100621








