Engineering 3D Printed Gummies Loaded with Metformin for Paediatric Use
Abstract
1. Introduction
2. Results and Discussion
2.1. Design of Experiments (DoE)
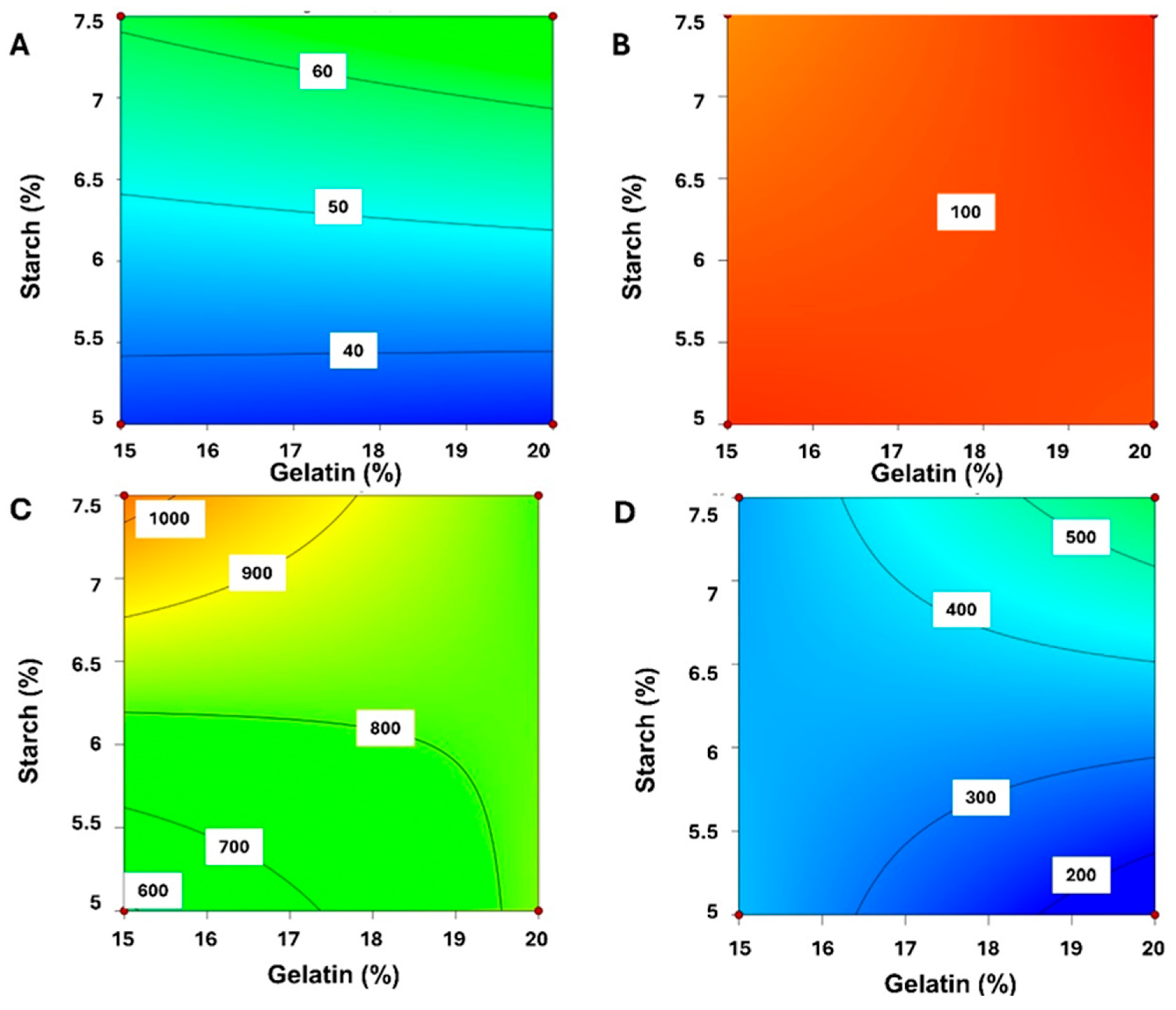
2.1.1. Quality by Desing (QbD)-Based Model Development and Response Surface Analysis
2.1.2. Optimal Formulation and Validation of QbD
2.2. Optimization of Gummies
2.2.1. Casting Method
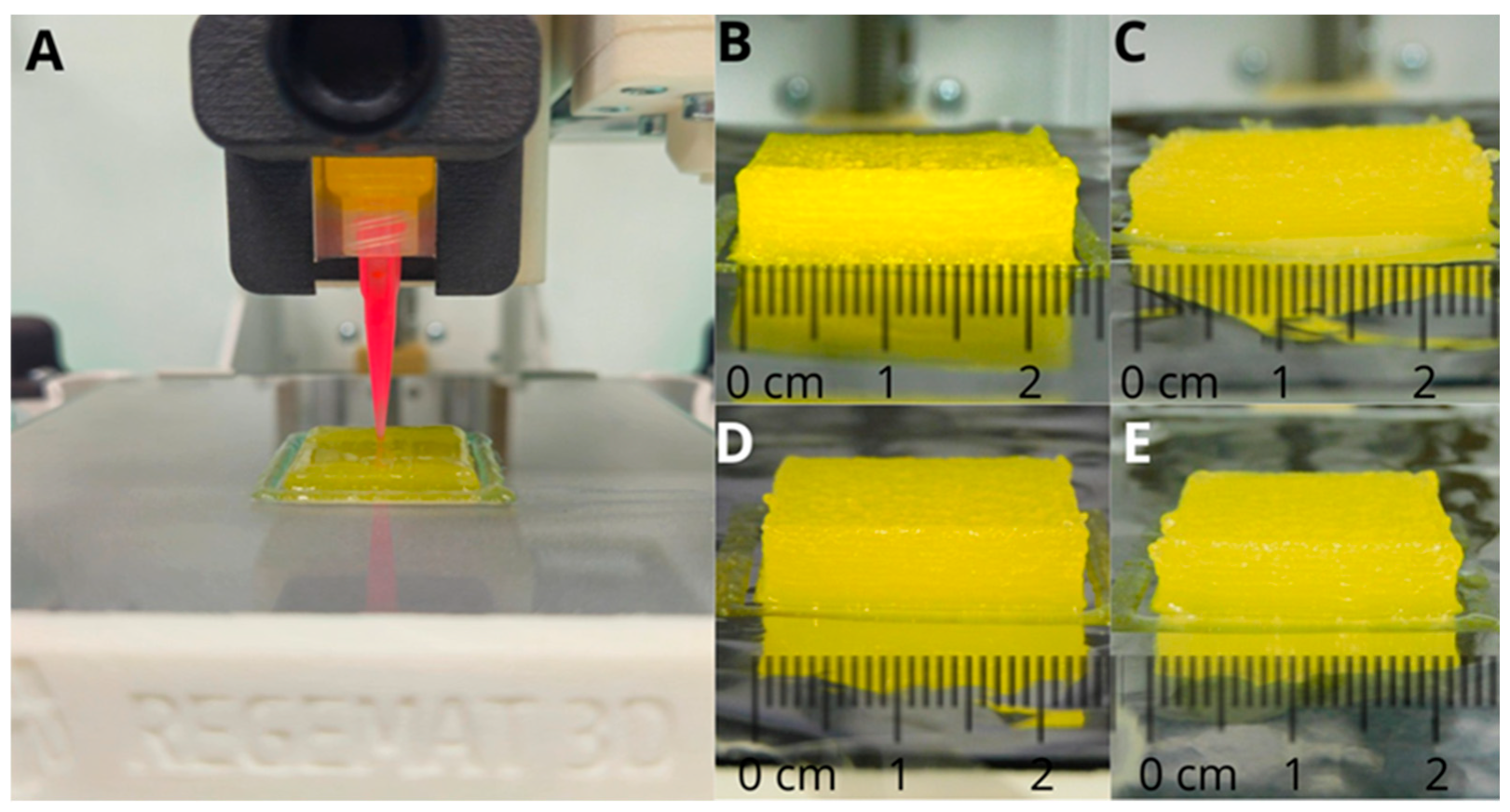
2.2.2. 3D Printing SSE
2.3. Content Uniformity and Mass Uniformity
2.4. Physicochemical Characterization and Organoleptic Properties
2.4.1. Powder X-ray Diffraction
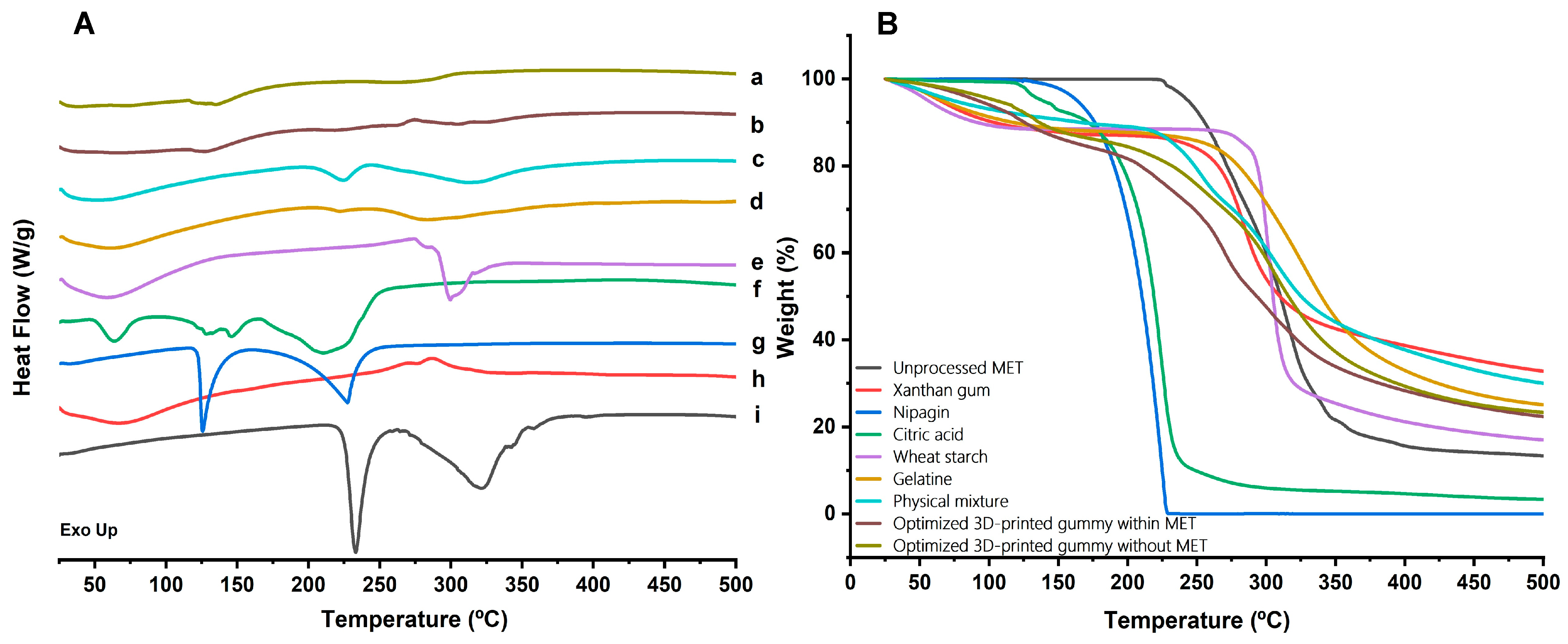
2.4.2. DSC-TGA Analysis
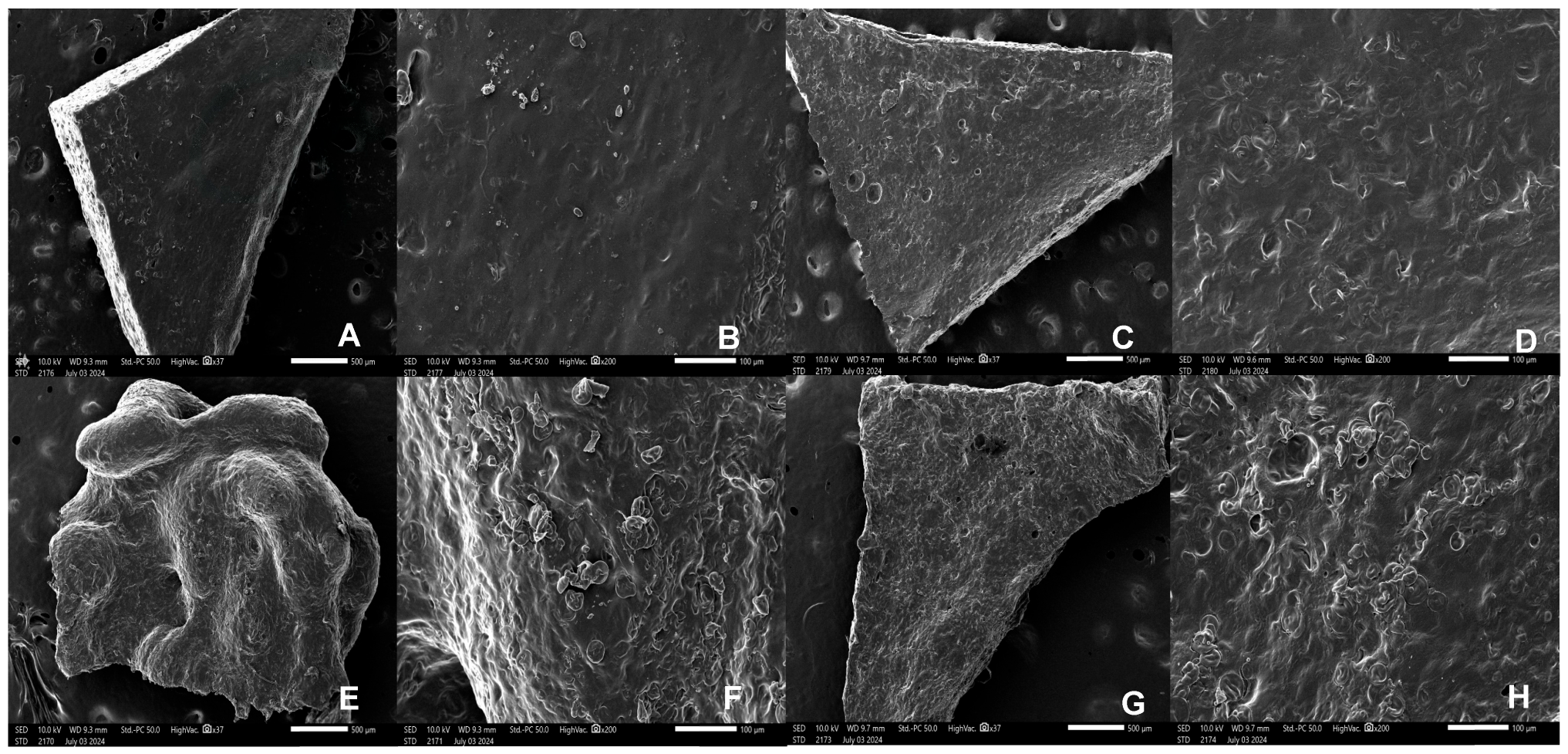
2.4.3. Scanning Electron Microscopy (SEM)
2.4.4. Organoleptic Properties
2.5. Mechanical Strength
2.6. Rheological Evaluation
2.7. Dissolution Profile
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Quality by Design Approach
4.3. Gummy Preparation
4.3.1. Casting Method
4.3.2. 3D Printing Semisolid Extrusion
4.3.3. Search for Optimum Formulation and Validation Studies
4.4. Content Uniformity and Mass Uniformity
4.5. Physicochemical Characterization
4.5.1. Powder X-ray Diffraction
4.5.2. Differential Scanning Calorimetry
4.5.3. Scanning Electron Microscopy
4.5.4. Organoleptic Evaluation
- Soft and squishy: Extremely soft to the touch, yielding easily with little pressure, minimal resistance when biting. Tends to dissolve quickly in the mouth.
- Soft and slightly chewy: Soft but with a little resistance when pressed. Offers a more satisfying bite but is still easy to chew and dissolves relatively quickly.
- Moderately firm and chewy: Balanced between firmness and softness. Requires noticeable effort to bite and chew, offering a consistent chew throughout.
- Firm and very chewy: Solid texture with significant resistance when biting. Takes longer to chew and breaks down more slowly.
- Hard and sticky: Very firm to the touch and chewy with a sticky texture. Difficult to bite into and sticks to teeth when chewing.
4.6. Mechanical Strength
4.7. Rheology
4.8. Dissolution Profile
Quantification of MET by High-Performance Liquid Chromatography (HPLC)
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, H.; Verre, M.C. Type 2 Diabetes Mellitus in Children. Am. Fam. Physician. 2018, 98, 590–594. [Google Scholar] [PubMed]
- Di Cicco, M.; Ghezzi, M.; Kantar, A.; Song, W.J.; Bush, A.; Peroni, D.; D’Auria, E. Pediatric obesity and severe asthma: Targeting pathways driving inflammation. Pharmacol. Res. 2023, 188, 106658. [Google Scholar] [CrossRef] [PubMed]
- Argelich, E.; Alemany, M.E.; Amengual-Miralles, B.; Argüelles, R.; Bandiera, D.; Barceló, M.A.; Beinbrech, B.; Bouzas, C.; Capel, P.; Lònia Cerdà, A.; et al. Paediatric teams in front of childhood obesity: A qualitative study within the STOP project. An. Pediatr. 2021, 95, 174–185. [Google Scholar] [CrossRef]
- Lasarte-Velillas, J.J.; Lamiquiz-Moneo, I.; Lasarte-Sanz, I.; Sala-Fernández, L.; Marín-Andrés, M.; Rubio-Sánchez, P.; Moneo-Hernández, M.I.; Hernández-Aguilar, M.T. Prevalence of overweight and obesity in Aragón and variations according to health determinants. An. Pediatr. 2023, 98, 157–164. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Tamborlane Editor, W.V. Contemporary Endocrinology Series Editor: Leonid Poretsky Diabetes in Children and Adolescents A Guide to Diagnosis and Management [Internet]. Available online: http://www.springer.com/ (accessed on 30 August 2024).
- Chou, Y.H.; Su, Y.T.; Lo, F.S.; Chiu, C.F.; Huang, Y.C. Influencing factors for treatment escalation from metformin monotherapy in youth-onset type 2 diabetes in Northern Taiwan. Pediatr Neonatol. 2024, 65, 435–440. [Google Scholar] [CrossRef]
- Siller, A.F.; Tosur, M.; Relan, S.; Astudillo, M.; McKay, S.; Dabelea, D.; Redondo, M.J. Challenges in the diagnosis of diabetes type in pediatrics. In Pediatric Diabetes; Blackwell Publishing Ltd.: Oxford, UK, 2020; Volume 21, pp. 1064–1073. [Google Scholar]
- Tamborlane, W.; Shehadeh, N. Unmet Needs in the Treatment of Childhood Type 2 Diabetes: A Narrative Review. Adv. Ther. 2023, 40, 4711–4720. [Google Scholar] [CrossRef]
- Or, T.; Lm, T.; Mr, P. Type 2 diabetes mellitus in children and adolescents: A relatively new clinical problem within pediatric practice. J. Med. Life 2016, 9, 235. [Google Scholar]
- Newton, K.P.; Wilson, L.A.; Crimmins, N.A.; Fishbein, M.H.; Molleston, J.P.; Xanthakos, S.A.; Behling, C.; Schwimmer, J.B.; Garner, D.; Hertel, P.; et al. Incidence of Type 2 Diabetes in Children With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 1261–1270. [Google Scholar] [CrossRef]
- Barrett, T.; Jalaludin, M.Y.; Turan, S.; Hafez, M.; Shehadeh, N. Rapid progression of type 2 diabetes and related complications in children and young people—A literature review. Pediatr. Diabetes 2020, 21, 158–172. [Google Scholar] [CrossRef]
- Popoviciu, M.S.; Kaka, N.; Sethi, Y.; Patel, N.; Chopra, H.; Cavalu, S. Type 1 Diabetes Mellitus and Autoimmune Diseases: A Critical Review of the Association and the Application of Personalized Medicine. J. Pers. Med. 2023, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Uribe, N.; Hormazábal-Aguayo, I.A.; Izquierdo, M.; García-Hermoso, A. Youth with type 1 diabetes mellitus are more inactive and sedentary than apparently healthy peers: A systematic review and meta-analysis. In Diabetes Research and Clinical Practice; Elsevier Ireland Ltd.: Dublin, Ireland, 2023; Volume 200. [Google Scholar]
- Alfaraidi, H.; Samaan, M.C. Metformin therapy in pediatric type 2 diabetes mellitus and its comorbidities: A review. Front. Endocrinol. 2023, 13, 1072879. [Google Scholar] [CrossRef] [PubMed]
- Axon, E.; Atkinson, G.; Richter, B.; Metzendorf, M.I.; Baur, L.; Finer, N.; Corpeleijn, E.; O’Malley, C.; Ells, L.J.; Cochrane Metabolic Endocrine Disorders Group. Drug interventions for the treatment of obesity in children and adolescents. In Cochrane Database of Systematic Reviews; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2016; Volume 2016. [Google Scholar]
- Sikorskaya, K.; Zarzecka, I.; Ejikeme, U.; Russell, J. The use of metformin as an add-on therapy to insulin in the treatment of poorly controlled type 1 diabetes mellitus in adolescents. Metabol. Open. 2021, 9, 100080. [Google Scholar] [CrossRef] [PubMed]
- Roep, B.O.; Thomaidou, S.; van Tienhoven, R.; Zaldumbide, A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nature Reviews Endocrinology. Nat. Res. 2021, 17, 150–161. [Google Scholar]
- Satterwhite, L.E. Metformin Extended-Release Oral Solution. Clin. Diabetes 2021, 39, 226–227. [Google Scholar] [CrossRef]
- Racaniello, G.F.; Silvestri, T.; Pistone, M.; D’Amico, V.; Arduino, I.; Denora, N.; Lopedota, A.A. Innovative Pharmaceutical Techniques for Paediatric Dosage Forms: A Systematic Review on 3D Printing, Prilling/Vibration and Microfluidic Platform. J. Pharm. 2024, 113, 1726–1748. [Google Scholar] [CrossRef]
- Lajoinie, A.; Janiaud, P.; Henin, E.; Gleize, J.C.; Berlion, C.; Nguyen, K.A.; Nony, P.; Gueyffier, F.; Maucort-Boulch, D.; Koupaï, B.K. Assessing the effects of solid versus liquid dosage forms of oral medications on adherence and acceptability in children. Cochrane Database Syst. Rev. 2017, 2017, 9. [Google Scholar]
- Bryson, S.P. Patient-centred, administration friendly medicines for children—An evaluation of children’s preferences and how they impact medication adherence. Int. J. Pharm. 2014, 469, 257–259. [Google Scholar] [CrossRef]
- Venables, R.; Batchelor, H.; Hodson, J.; Stirling, H.; Marriott, J. Determination of formulation factors that affect oral medicines acceptability in a domiciliary paediatric population. Int. J. Pharm. 2015, 480, 55–62. [Google Scholar] [CrossRef]
- Mistry, P.; Batchelor, H. Evidence of acceptability of oral paediatric medicines: A review. J. Pharm. Pharmacol. 2017, 69, 361–376. [Google Scholar] [CrossRef]
- Meyers, R.S. The Past, Present, and Future of Oral Dosage Forms for Children. J. Pediatr. Pharmacol. Ther. 2024, 29, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Cram, A.; Breitkreutz, J.; Desset-Brèthes, S.; Nunn, T.; Tuleu, C. Challenges of developing palatable oral paediatric formulations. Int. J. Pharm. 2009, 365, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Pan, H.; Su, Y.; Fang, D.; Qiao, S.; Ding, P.; Pan, W. Opportunities and challenges of three-dimensional printing technology in pharmaceutical formulation development. Acta Pharm. Sin. B 2021, 11, 2488–2504. [Google Scholar] [CrossRef] [PubMed]
- Malebari, A.M.; Kara, A.; Khayyat, A.N.; Mohammad, K.A.; Serrano, D.R. Development of Advanced 3D-Printed Solid Dosage Pediatric Formulations for HIV Treatment. Pharmaceuticals 2022, 15, 435. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient acceptability of 3D printed medicines. Int. J. Pharm. 2017, 530, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Anaya, B.J.; Cerda, J.R.; D’Atri, R.M.; Yuste, I.; Luciano, F.C.; Kara, A.; Ruiz, H.K.; Ballesteros, M.P.; Serrano, D.R. Engineering of 3D printed personalized polypills for the treatment of the metabolic syndrome. Int. J. Pharm. 2023, 642, 123194. [Google Scholar] [CrossRef]
- Rouaz-El Hajoui, K.; Herrada-Manchón, H.; Rodríguez-González, D.; Fernández, M.A.; Aguilar, E.; Suné-Pou, M.; Nardi-Ricart, A.; Pérez-Lozano, P.; García-Montoya, E. Pellets and gummies: Seeking a 3D printed gastro-resistant omeprazole dosage for paediatric administration. Int. J. Pharm. 2023, 643, 123289. [Google Scholar] [CrossRef]
- Lee, J.; Song, C.; Noh, I.; Rhee, Y.S. Applications of the design of additive manufacturing (DfAM) in the development of pharmaceutical dosage forms. J. Pharm. Investig. 2024, 54, 175–193. [Google Scholar] [CrossRef]
- Ganatra, P.; Jyothish, L.; Mahankal, V.; Sawant, T.; Dandekar, P.; Jain, R. Drug-loaded vegan gummies for personalized dosing of simethicone: A feasibility study of semi-solid extrusion-based 3D printing of pectin-based low-calorie drug gummies. Int. J. Pharm. 2024, 651, 123777. [Google Scholar] [CrossRef]
- Herrada-Manchon, H.; Rodriguez-Gonzalez, D.; Alejandro Fernandez, M.; Sune-Pou, M.; Perez-Lozano, P.; Garcia-Montoya, E.; Aguilar, E. 3D printed gummies: Personalized drug dosage in a safe and appealing way. Int. J. Pharm. 2020, 587, 119687. [Google Scholar] [CrossRef]
- Tagami, T.; Ito, E.; Kida, R.; Hirose, K.; Noda, T.; Ozeki, T. 3D printing of gummy drug formulations composed of gelatin and an HPMC-based hydrogel for pediatric use. Int. J. Pharm. 2021, 594, 120118. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O.; et al. 3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals. Pharmaceutics 2023, 15, 313. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Elshaer, A.; Sareh, P.; Elsayed, M.; Hassanin, H. Additive Manufacturing Technologies for Drug Delivery Applications. Int. J. Pharm. 2020, 580, 119245. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Durga Prasad Reddy, R.; Sharma, V. Additive manufacturing in drug delivery applications: A review. Int. J. Pharm. 2020, 15, 589. [Google Scholar] [CrossRef]
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaisford, S.; Goyanes, A.; Basit, A.W. Vat photopolymerization 3D printing for advanced drug delivery and medical device applications. J. Control. Release 2021, 329, 743–757. [Google Scholar] [CrossRef]
- Rodríguez-Pombo, L.; de Castro-López, M.J.; Sánchez-Pintos, P.; Giraldez-Montero, J.M.; Januskaite, P.; Duran-Piñeiro, G.; Bóveda, M.D.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A.; et al. Paediatric clinical study of 3D printed personalised medicines for rare metabolic disorders. Int. J. Pharm. 2024, 25, 657. [Google Scholar] [CrossRef]
- Zhu, C.; Tian, Y.; Zhang, E.; Gao, X.; Zhang, H.; Liu, N.; Han, X.; Sun, Y.; Wang, Z.; Zheng, A. Semisolid Extrusion 3D Printing of Propranolol Hydrochloride Gummy Chewable Tablets: An Innovative Approach to Prepare Personalized Medicine for Pediatrics. AAPS PharmSciTech 2022, 23, 166. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Januskaite, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A. Semi-solid extrusion 3D printing in drug delivery and biomedicine: Personalised solutions for healthcare challenges. J. Control. Release 2021, 332, 367–389. [Google Scholar] [CrossRef]
- Díaz-Torres, E.; Rodríguez-Pombo, L.; Ong, J.J.; Basit, A.W.; Santoveña-Estévez, A.; Fariña, J.B.; Alvarez-Lorenzo, C.; Goyanes, A. Integrating pressure sensor control into semi-solid extrusion 3D printing to optimize medicine manufacturing. Int. J. Pharm. X 2022, 4, 100133. [Google Scholar] [CrossRef]
- Junnila, A.; Mortier, L.; Arbiol, A.; Harju, E.; Tomberg, T.; Hirvonen, J.; Viitala, T.; Karttunen, A.P.; Peltonen, L. Rheological insights into 3D printing of drug products: Drug nanocrystal-poloxamer gels for semisolid extrusion. Int. J. Pharm. 2024, 655, 124070. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Zhang, J.; Ma, J.; Zhang, J. Perspectives on 3D printed personalized medicines for pediatrics. Int. J. Pharm. 2024, 653, 123867. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced Bioinks for 3D Printing: A Materials Science Perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Lille, M.; Nurmela, A.; Nordlund, E.; Metsä-Kortelainen, S.; Sozer, N. Applicability of protein and fiber-rich food materials in extrusion-based 3D printing. J. Food Eng. 2018, 220, 20–27. [Google Scholar] [CrossRef]
- Niu, D.; Zhang, M.; Tang, T.; Mujumdar, A.S.; Li, J. Investigation of 3D printing of children starch gummies with precise and special shape based on change of model parameters. J. Food Eng. 2023, 356, 111568. [Google Scholar] [CrossRef]
- Wang, R.; Hartel, R.W. Confectionery gels: Gelling behavior and gel properties of gelatin in 1 concentrated sugar solutions. Food Hydrocoll. 2021, 124, 107132. [Google Scholar] [CrossRef]
- Agencia Española de Medicamentos y Productos Sanitarios. FICHA TECNICA METFORMINA STADA 850 mg COMPRIMIDOS RECUBIERTOS CON PELICULA EFG [Internet]. 2023. Available online: https://cima.aemps.es/cima/dochtml/ft/69709/FichaTecnica_69709.html (accessed on 28 August 2024).
- Rong, L.; Chen, X.; Shen, M.; Yang, J.; Qi, X.; Li, Y.; Xie, J. The application of 3D printing technology on starch-based product: A review. Trends Food Sci. Technol. 2023, 134, 149–161. [Google Scholar] [CrossRef]
- Serrano, D.R.; Walsh, D.; O’Connell, P.; Mugheirbi, N.A.; Worku, Z.A.; Bolas-Fernandez, F.; Galiana, C.; Dea-Ayuela, M.A.; Healy, A.M. Optimising the in vitro and in vivo performance of oral cocrystal formulations via spray coating. Eur. J. Pharm. Biopharm. 2018, 124, 13–27. [Google Scholar] [CrossRef]
- U.S.P. Reagents: Test Solutions. Available online: https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/pf-legacy-pdf/pf-2015_vol-41.pdf (accessed on 17 August 2024).
- Galana Gerlin, M.C.; Mazon Cardoso, T.F.; Souza, J.B.G.D.; Baroni, A.C.D.M.; Amaral, M.S.D.; Kassab, N.M. Desenvolvimento e validação de método analítico por CLAE para determinação simultânea de atorvastatina, losartana e metformina em formulações farmacêuticas magistrais. Rev. Colomb. Cienc. Quím. Farm. 2022, 51, 955–970. [Google Scholar]
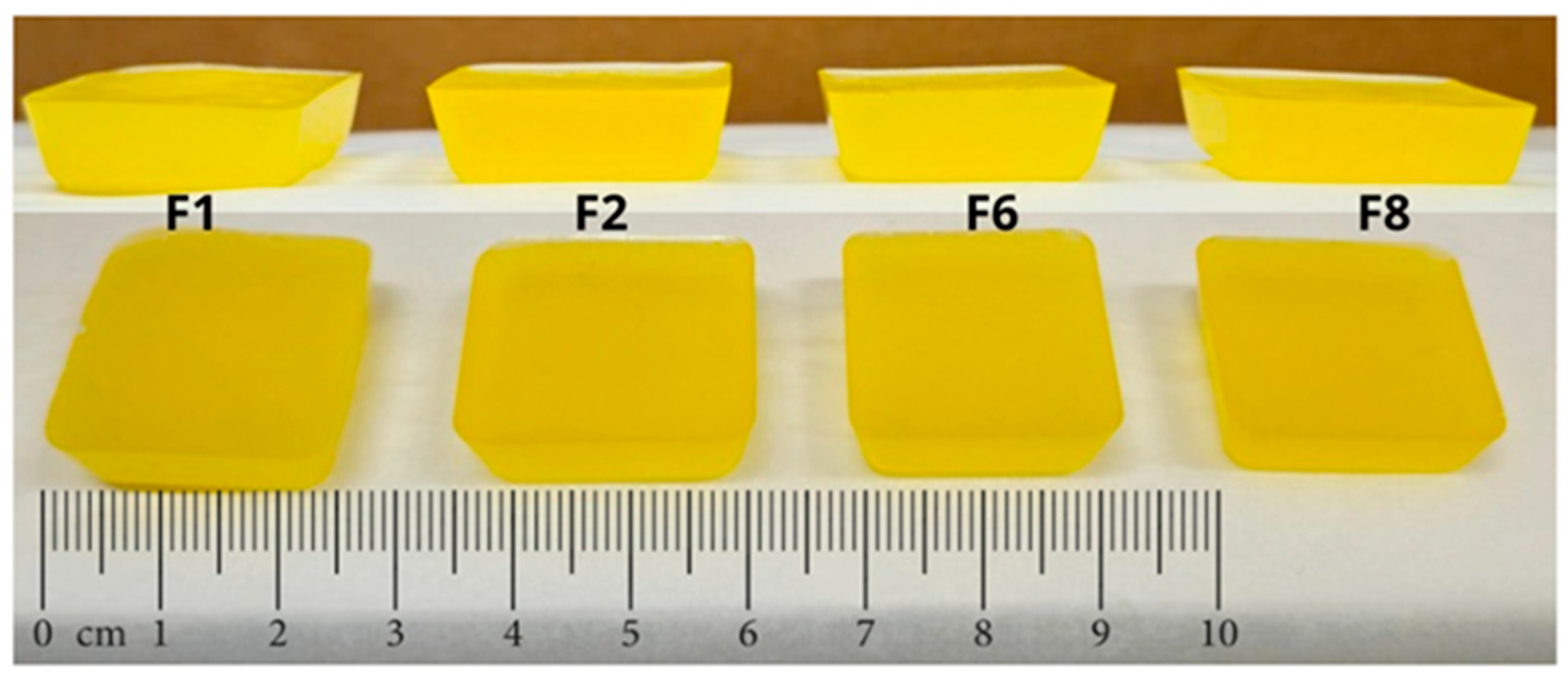




| Formulations | Height (mm) | Width (mm) | Length (mm) | Weight (g) |
|---|---|---|---|---|
| F1 | 7.4 | 24.5 | 24.7 | 4.3193 |
| F2 | 7.3 | 24.7 | 24.5 | 4.1445 |
| F6 | 7.1 | 24.9 | 24.5 | 4.2605 |
| F8 | 6.9 | 24.5 | 24.7 | 4.2318 |
| Formulations | Height (mm) | Width (mm) | Length (mm) | Weight (g) |
|---|---|---|---|---|
| F3 | 6.1 | 23.8 | 24.0 | 4.1217 |
| F4 | 4.3 | 24.0 | 24.3 | 2.6848 |
| F5 | 5.7 | 24.1 | 23.8 | 4.0355 |
| F7 | 5.9 | 24.5 | 24.1 | 3.9868 |
| Gelatin (%) | Starch (%) | Process | |
|---|---|---|---|
| F1 | 15 | 5.0 | Casting |
| F2 | 20 | 7.5 | Casting |
| F3 | 15 | 5.0 | 3DP |
| F4 | 15 | 7.5 | 3DP |
| F5 | 20 | 5.0 | 3DP |
| F6 | 20 | 5.0 | Casting |
| F7 | 20 | 7.5 | 3DP |
| F8 | 15 | 7.5 | Casting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santamaría, K.J.; Anaya, B.J.; Lalatsa, A.; González-Barranco, P.; Cantú-Cárdenas, L.; Serrano, D.R. Engineering 3D Printed Gummies Loaded with Metformin for Paediatric Use. Gels 2024, 10, 620. https://doi.org/10.3390/gels10100620
Santamaría KJ, Anaya BJ, Lalatsa A, González-Barranco P, Cantú-Cárdenas L, Serrano DR. Engineering 3D Printed Gummies Loaded with Metformin for Paediatric Use. Gels. 2024; 10(10):620. https://doi.org/10.3390/gels10100620
Chicago/Turabian StyleSantamaría, Karla J., Brayan J. Anaya, Aikaterini Lalatsa, Patricia González-Barranco, Lucía Cantú-Cárdenas, and Dolores R. Serrano. 2024. "Engineering 3D Printed Gummies Loaded with Metformin for Paediatric Use" Gels 10, no. 10: 620. https://doi.org/10.3390/gels10100620
APA StyleSantamaría, K. J., Anaya, B. J., Lalatsa, A., González-Barranco, P., Cantú-Cárdenas, L., & Serrano, D. R. (2024). Engineering 3D Printed Gummies Loaded with Metformin for Paediatric Use. Gels, 10(10), 620. https://doi.org/10.3390/gels10100620









