Introductory Review of Soft Implantable Bioelectronics Using Conductive and Functional Hydrogels and Hydrogel Nanocomposites
Abstract
1. Introduction
2. Conductive Hydrogels and Hydrogel Nanocomposites
2.1. Intrinsically Conductive Hydrogels
2.1.1. Conjugated Polymer Backbone
2.1.2. Ionic Conductive Hydrogel
2.1.3. Redox-Active Hydrogels
2.2. Hydrogel Nanocomposites
2.2.1. Constituting Materials
2.2.2. Synthesis Methods
3. Functional Hydrogel and Hydrogel Nanocomposites for Advanced Biointerfacing
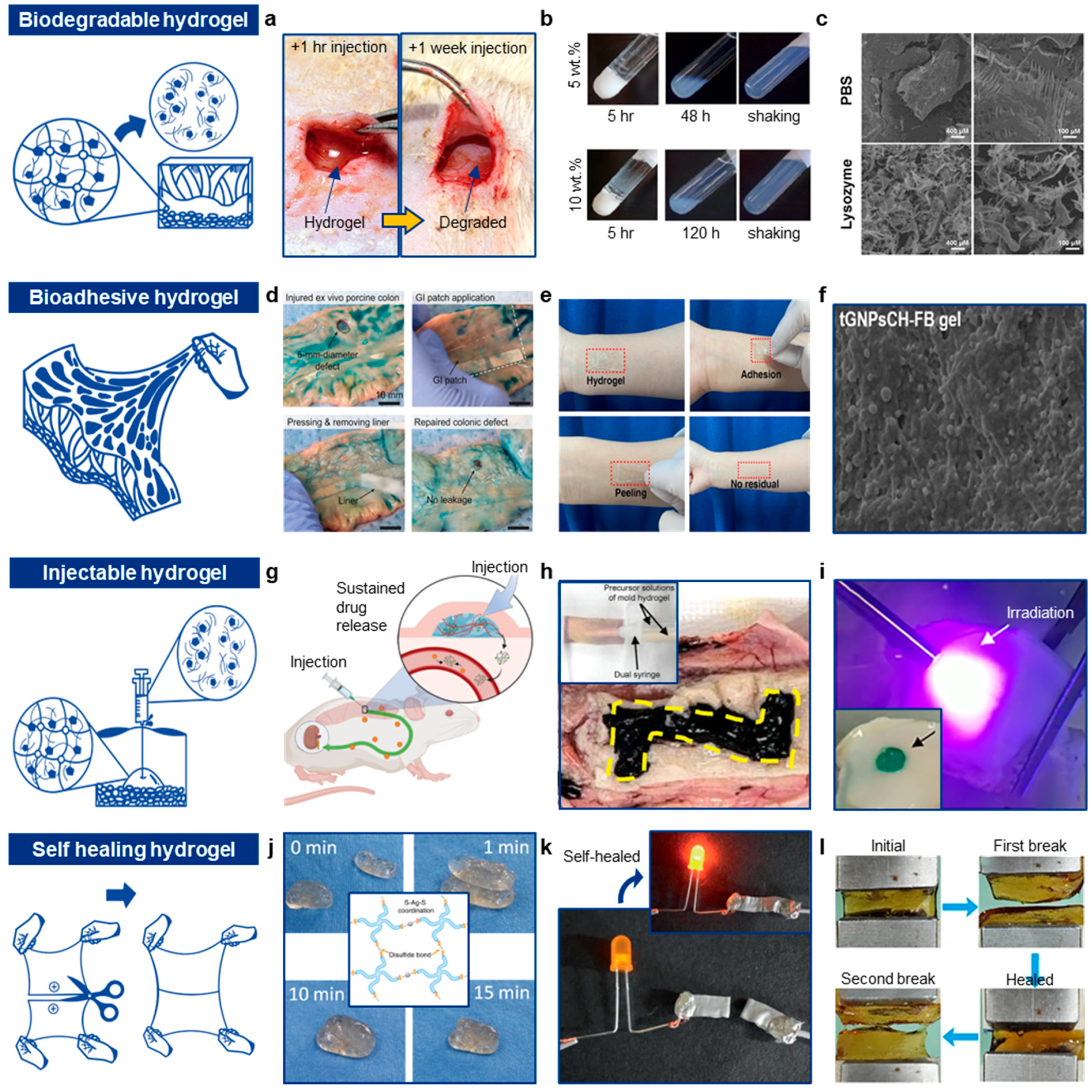
3.1. Biodegradable Hydrogels and Hydrogel Nanocomposites
3.1.1. Degradation Mechanisms
3.1.2. Constituting Hydrogels
3.2. Bioadhesive Hydrogels and Hydrogel Nanocomposites
3.2.1. Physical Interactions
3.2.2. Chemical Interactions
3.2.3. Electrostatic Interactions
3.2.4. Mechanical Interactions
3.2.5. Molecular Recognitions and Mucosal Applications
3.3. Injectable Hydrogels
3.3.1. Thermosensitive Injectable Hydrogels
3.3.2. pH-Sensitive Gelation
3.3.3. Ion-Induced Gelation
3.3.4. Enzymatic Crosslinking
3.3.5. Photocrosslinking Hydrogel
3.4. Self-Healing Hydrogel
Self-Healing Mechanisms
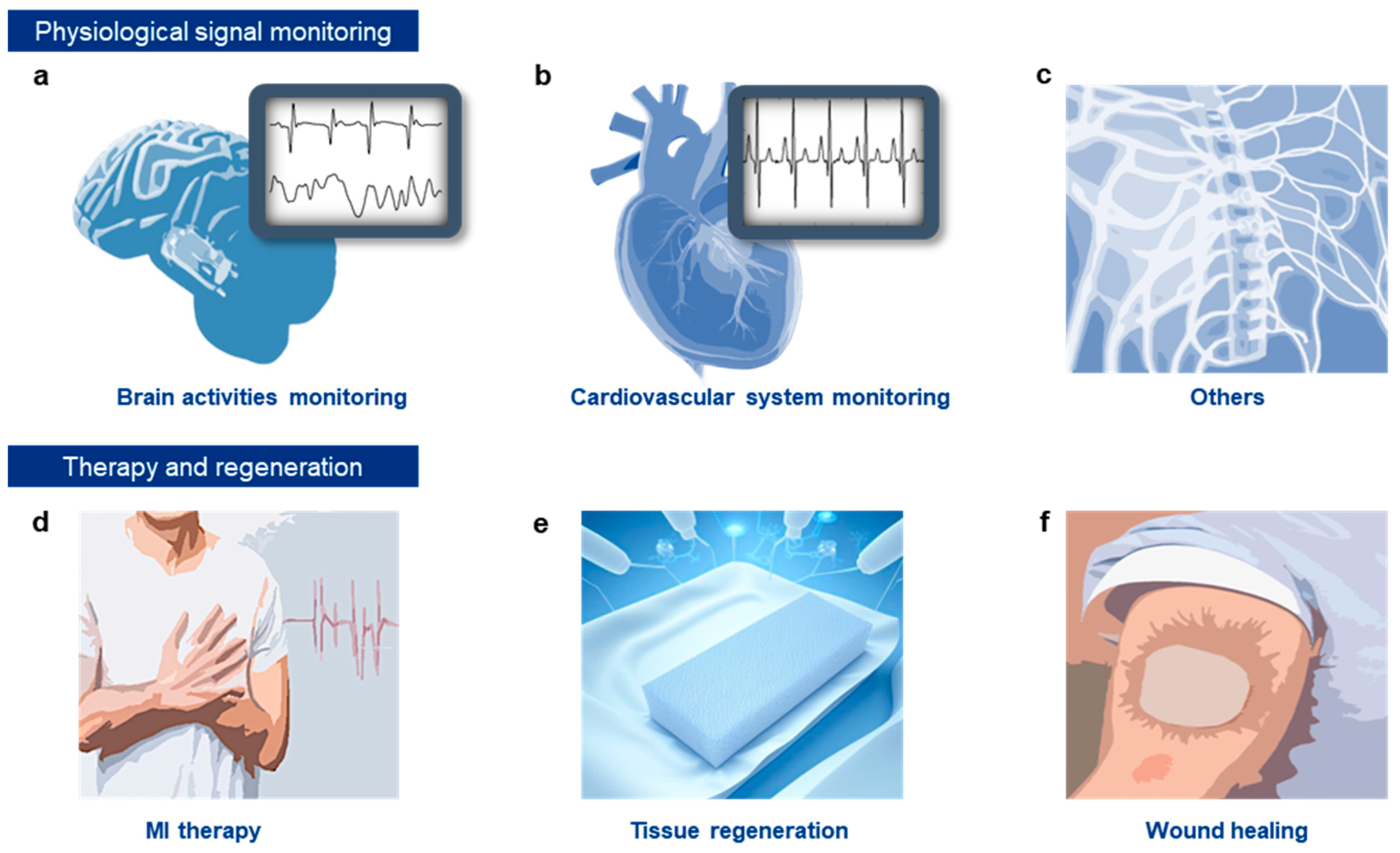
4. Functional Conductive Hydrogels for Monitoring Biological Signals and Therapeutic Applications
4.1. Monitoring of Physiological Electrical Signals
4.1.1. Brain Activity Monitoring
4.1.2. ECG Monitoring
4.1.3. Other Applications
4.2. Therapy
4.2.1. Disease Treatment
4.2.2. Tissue Regeneration
4.2.3. Wound Healing
5. Applications of Hydrogels in the Biological and Therapeutic Domain
5.1. Applications of Conductive Hydrogels and Nanocomposites in Neural Signal Recording
5.2. Applications of Conductive Hydrogels and Nanocomposites for ECG Monitoring and MI Therapy
5.3. Applications of Conductive Hydrogels and Nanocomposites in Neural Signal Recording and Stimulation
6. Current Limitations and Prospects
6.1. Advantages of Soft Conductive Hydrogels for Implantable Bioelectronics
6.2. Current Obstacles and Endeavors
6.3. Prospects for Next-Generation Biomedical Conductive Hydrogels
Funding
Conflicts of Interest
References
- Malekoshoaraie, M.H.; Wu, B.; Krahe, D.D.; Ahmed, Z.; Pupa, S.; Jain, V.; Cui, X.T.; Chamanzar, M. Fully Flexible Implantable Neural Probes for Electrophysiology Recording and Controlled Neurochemical Modulation. Microsyst. Nanoeng. 2024, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Chae, U.; Woo, J.; Cho, Y.; Han, J.K.; Yang, S.H.; Yang, E.; Shin, H.; Kim, H.; Yu, H.Y.; Justin Lee, C.; et al. A Neural Probe for Concurrent Real-Time Measurement of Multiple Neurochemicals with Electrophysiology in Multiple Brain Regions in Vivo. Proc. Natl. Acad. Sci. USA 2023, 120, e2219231120. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.N.; Wang, Y.; Le Friec, A.; Nabavi, S.; Dong, M.; Seliktar, D.; Chen, M. Wireless Electromagnetic Neural Stimulation Patch with Anisotropic Guidance. NPJ Flex. Electron. 2023, 7, 34. [Google Scholar] [CrossRef]
- Woodington, B.J.; Lei, J.; Carnicer-Lombarte, A.; Güemes-González, A.; Naegele, T.E.; Hilton, S.; El-Hadwe, S.; Trivedi, R.A.; Malliaras, G.G.; Barone, D.G. Flexible Circumferential Bioelectronics to Enable 360-Degree Recording and Stimulation of the Spinal Cord. Sci. Adv. 2024, 10, eadl1230. [Google Scholar] [CrossRef]
- Choi, Y.S.; Yin, R.T.; Pfenniger, A.; Koo, J.; Avila, R.; Benjamin Lee, K.; Chen, S.W.; Lee, G.; Li, G.; Qiao, Y.; et al. Fully Implantable and Bioresorbable Cardiac Pacemakers without Leads or Batteries. Nat. Biotechnol. 2021, 39, 1228–1238. [Google Scholar] [CrossRef]
- Giancaterino, S.; Lupercio, F.; Nishimura, M.; Hsu, J.C. Current and Future Use of Insertable Cardiac Monitors. JACC Clin. Electrophysiol. 2018, 4, 1383–1396. [Google Scholar] [CrossRef]
- Zhong, H.; Shan, W.; Liang, L.; Jiang, X.; Heliyon, L.W. High Stretchable and Self-Adhesive Multifunctional Hydrogel for Wearable and Flexible Sensors. Heliyon 2024, 10, 2405–8440. [Google Scholar] [CrossRef]
- Yang, S.; Cheng, J.; Shang, J.; Hang, C.; Qi, J.; Zhong, L.; Rao, Q.; He, L.; Liu, C.; Ding, L.; et al. Stretchable Surface Electromyography Electrode Array Patch for Tendon Location and Muscle Injury Prevention. Nat. Commun. 2023, 14, 6494. [Google Scholar] [CrossRef]
- Adams, D.S.; Levin, M. Endogenous Voltage Gradients as Mediators of Cell-Cell Communication: Strategies for Investigating Bioelectrical Signals during Pattern Formation. Cell Tissue Res. 2013, 352, 95–122. [Google Scholar] [CrossRef]
- Sunwoo, S.-H.; Ihn Han, S.; Joo, H.; Doo Cha, G.; Kim, D.; Hong Choi, S.; Hyeon, T.; Kim, D.-H. Advances in Soft Bioelectronics for Brain Research and Clinical Neuroengineering. Matter 2020, 3, 1923–1947. [Google Scholar] [CrossRef]
- Sunwoo, S.H.; Lee, J.S.; Bae, S.; Shin, J.; Kim, C.S.; Yeon Joo, S.; Choi, H.S.; Suh, M.; Kim, S.W.; Choi, Y.J.; et al. Chronic and Acute Stress Monitoring by Electrophysiological Signals from Adrenal Gland. Proc. Natl. Acad. Sci. USA 2019, 116, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, S.H.; Ha, K.H.; Lee, S.; Lu, N.; Kim, D.H. Wearable and Implantable Soft Bioelectronics: Device Designs and Material Strategies. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 359–391. [Google Scholar] [CrossRef]
- Shim, H.J.; Sunwoo, S.-H.; Kim, Y.; Koo, J.H.; Kim, D.-H. Functionalized Elastomers for Intrinsically Soft and Biointegrated Electronics. Adv. Healthc. Mater. 2021, 10, 2002105. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tsai, Y. Design Strategies of Conductive Hydrogel for Biomedical Applications. Molecules 2020, 25, 5296. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, P.; Qu, X.; Zhang, F.; Ning, S.; Ma, L.; Yang, K.; Su, Y.; Zang, J.; Jiang, W.; et al. Robust Neural Interfaces with Photopatternable, Bioadhesive, and Highly Conductive Hydrogels for Stable Chronic Neuromodulation. ACS Nano 2023, 17, 885–895. [Google Scholar] [CrossRef]
- Rogers, Z.J.; Zeevi, M.P.; Koppes, R.; Bencherif, S.A. Electroconductive Hydrogels for Tissue Engineering: Current Status and Future Perspectives. Bioelectricity 2020, 2, 279–292. [Google Scholar] [CrossRef]
- Fu, L.; Yu, A.; Lai, G. Conductive Hydrogel-Based Electrochemical Sensor: A Soft Platform for Capturing Analyte. Chemosensors 2021, 9, 282. [Google Scholar] [CrossRef]
- Choi, E.J.; Shin, J.; Khaleel, Z.H.; Cha, I.; Yun, S.H.; Cho, S.W.; Song, C. Synthesis of Electroconductive Hydrogel Films by an Electro-Controlled Click Reaction and Their Application to Drug Delivery Systems. Polym. Chem. 2015, 6, 4473–4478. [Google Scholar] [CrossRef]
- Saito, M.; Yamada, H.; Kranthiraja, K.; Jeon, J.; Do Kim, H.; Mikie, T.; Saeki, A.; Ohkita, H.; Osaka, I. Ordered π-Conjugated Polymer Backbone in Amorphous Blend for High Efficiency Nonfullerene Organic Photovoltaics. Commun. Mater. 2023, 4, 72. [Google Scholar] [CrossRef]
- Lu, H.; Li, X.; Lei, Q. Conjugated Conductive Polymer Materials and Its Applications: A Mini-Review. Front. Chem. 2021, 9, 732132. [Google Scholar] [CrossRef]
- Morais, J.; Bernardino, D.V.; da Silva Batista, B.; Pereira, W.O.; Amaral, F.M.B.; Branca, M.C.M.P.; Gasparin, F.P.; dos Santos, A.O.; Sombra, A.S.B.; Macêdo, A.A.M. Conductive Polymer Blend Based on Polyaniline and Galactomannan: Optical and Electrical Properties. Synth. Met. 2023, 295, 117346. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Rashid, S.A.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N.; Yoo, D.J.; Vinothkannan, M. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef] [PubMed]
- Ateh, D.D.; Navsaria, H.A.; Vadgama, P. Polypyrrole-Based Conducting Polymers and Interactions with Biological Tissues. J. R. Soc. Interface 2006, 3, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Yussuf, A.; Al-Saleh, M.; Al-Enezi, S.; Abraham, G. Synthesis and Characterization of Conductive Polypyrrole: The Influence of the Oxidants and Monomer on the Electrical, Thermal, and Morphological Properties. Int. J. Polym. Sci. 2018, 2018, 4191747. [Google Scholar] [CrossRef]
- Hu, F.; Xue, Y.; Xu, J.; Lu, B. PEDOT-Based Conducting Polymer Actuators. Front. Robot. AI 2019, 6, 114. [Google Scholar] [CrossRef]
- Nie, S.; Li, Z.; Yao, Y.; Jin, Y. Progress in Synthesis of Conductive Polymer Poly(3,4-Ethylenedioxythiophene). Front. Chem. 2021, 9, 803509. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, H.S.; Hong, Y.J.; Sunwoo, S.H.; Park, O.K.; Choi, S.H.; Kim, D.H.; Lee, S. Low-Impedance Tissue-Device Interface Using Homogeneously Conductive Hydrogels Chemically Bonded to Stretchable Bioelectronics. Sci. Adv. 2024, 10, eadi7724. [Google Scholar] [CrossRef]
- Lim, C.; Hong, Y.J.; Jung, J.; Shin, Y.; Sunwoo, S.H.; Baik, S.; Park, O.K.; Choi, S.H.; Hyeon, T.; Kim, J.H.; et al. Tissue-like Skin-Device Interface for Wearable Bioelectronics by Using Ultrasoft, Mass-Permeable, and Low-Impedance Hydrogels. Sci. Adv. 2021, 7, eabd3716. [Google Scholar] [CrossRef]
- Yan, M.; Wang, L.; Wu, Y.; Liao, X.; Zhong, C.; Wang, L.; Lu, Y. Conducting Polymer-Hydrogel Interpenetrating Networks for Improving the Electrode–Neural Interface. ACS Appl. Mater. Interfaces 2023, 15, 41310–41323. [Google Scholar] [CrossRef]
- Zhou, T.; Yuk, H.; Hu, F.; Wu, J.; Tian, F.; Roh, H. 3D Printable High-Performance Conducting Polymer Hydrogel for All-Hydrogel Bioelectronic Interfaces. Nat. Mater. 2023, 22, 895–902. [Google Scholar] [CrossRef]
- Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J. Pure Pedot: Pss Hydrogels. Nat. Commun. 2019, 10, 1043. [Google Scholar] [CrossRef] [PubMed]
- Won, D.; Kim, J.; Choi, J.; Kim, H.J.; Han, S.; Ha, I.; Bang, J.; Kim, K.K.; Lee, Y.; Kim, T.S.; et al. Digital Selective Transformation and Patterning of Highly Conductive Hydrogel Bioelectronics by Laser-Induced Phase Separation. Sci. Adv. 2022, 8, eabo3209. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, L.; Wang, S.; Zhang, L.; Chen, L.; Xu, X.; Song, Z.; Liu, H.; Chen, C. Strong, Tough, Ionic Conductive, and Freezing-Tolerant All-Natural Hydrogel Enabled by Cellulose-Bentonite Coordination Interactions. Nat. Commun. 2022, 13, 3408. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, S.; Lee, M.; Kim, H.-S.; Lee, J.Y.; Park, J.; Lee, S.; Lee, M.; Lee, J.Y.; Kim, H.-S. Injectable Conductive Hydrogels with Tunable Degradability as Novel Implantable Bioelectrodes. Small 2023, 19, 2300250. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, H.; Lee, D.; Lee, Y. Highly Conductive and Flexible Silver Nanowire-Based Microelectrodes on Biocompatible Hydrogel. ACS Appl. Mater. Interfaces 2014, 6, 18401–18407. [Google Scholar] [CrossRef]
- Lim, C.; Shin, Y.; Jung, J.; Kim, J.; Lee, S. Stretchable Conductive Nanocomposite Based on Alginate Hydrogel and Silver Nanowires for Wearable Electronics. APL Mater. 2019, 7, 031502. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Q.; Chen, M.; Long, K.; Su, C.; Li, J.; Huang, M.; Lu, A.; Guo, S. Stretchable, adhesive, conductive hydrogel initiated by liquid metal complex for multi-functional sensing. Chem. Eng. J. 2024, 496, 153674. [Google Scholar] [CrossRef]
- Xu, Y.; Rothe, R.; Voigt, D.; Hauser, S.; Cui, M.; Miyagawa, T.; Patino Gaillez, M.; Kurth, T.; Bornhäuser, M.; Pietzsch, J.; et al. Convergent Synthesis of Diversified Reversible Network Leads to Liquid Metal-Containing Conductive Hydrogel Adhesives. Nat. Commun. 2021, 12, 2407. [Google Scholar] [CrossRef]
- Ma, Z.; Shi, W.; Yan, K.; Pan, L. Doping Engineering of Conductive Polymer Hydrogels and Their Application in Advanced Sensor Technologies. Chem. Sci. 2019, 10, 6223–6394. [Google Scholar] [CrossRef]
- Zhang, C.; Hsieh, M.-H.; Wu, S.-Y.; Li, S.-H.; Wu, J.; Liu, S.-M.; Wei, H.-J.; Weisel, R.D.; Sung, H.-W.; Li, R.-K. A Self-Doping Conductive Polymer Hydrogel That Can Restore Electrical Impulse Propagation at Myocardial Infarct to Prevent Cardiac Arrhythmia and Preserve Ventricular. Biomaterials 2019, 231, 119672. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, Y.; Pan, L. Multifunctional Nanostructured Conductive Polymer Gels: Synthesis, Properties, and Applications. Acc. Chem. Res. 2017, 50, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaidi, N.S.; Al-Garawi, Z.S.; Al-Mahdawi, A.S. Polyaniline Doping with Nanoparticles: A Review on the Potential of Electrical Properties. J. Phys. 2021, 1853, 12055. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, B.; Wang, Y.; Fu, T. P-Doped PANI/AgMWs Nano/Micro Coating towards High-Efficiency Flame Retardancy and Electromagnetic Interference Shielding. Compos. Part B Eng. 2022, 238, 109944. [Google Scholar] [CrossRef]
- Ke, Z.; Abtahi, A.; Hwang, J.; Chen, K.; Chaudhary, J.; Song, I.; Perera, K.; You, L.; Baustert, K.N.; Graham, K.R.; et al. Highly Conductive and Solution-Processable n-Doped Transparent Organic Conductor. J. Am. Chem. Soc. 2023, 145, 3706–3715. [Google Scholar] [CrossRef]
- Tang, H.; Liang, Y.; Liu, C.; Hu, Z.; Deng, Y.; Guo, H. Solution-Processed n-Type Conducting Polymer with Ultrahigh Conductivity. Nature 2022, 611, 271–277. [Google Scholar] [CrossRef]
- Lee, C.-J.; Wu, H.; Hu, Y.; Young, M.; Wang, H.; Lynch, D.; Xu, F.; Cong, H.; Cheng, G. Ionic Conductivity of Polyelectrolyte Hydrogels. ACS Appl. Mater. Interfaces 2018, 10, 5845–5852. [Google Scholar] [CrossRef]
- Tian, H.; Wang, C.; Chen, Y.; Zheng, L.; Jing, H.; Xu, L.; Wang, X.; Liu, Y.; Hao, J. Optically Modulated Ionic Conductivity in a Hydrogel for Emulating Synaptic Functions. Sci. Adv. 2023, 9, eadd6950. [Google Scholar] [CrossRef]
- Sun, W.; Xu, Z.; Qiao, C.; Lv, B.; Gai, L.; Ji, X.; Jiang, H.; Liu, L. Antifreezing Proton Zwitterionic Hydrogel Electrolyte via Ionic Hopping and Grotthuss Transport Mechanism toward Solid Supercapacitor Working at −50 °C. Adv. Sci. 2022, 9, 2201679. [Google Scholar] [CrossRef]
- Chandra, S.; Sekhon, S.; Srivastava, R. Proton-Conducting Gel Electrolyte. Solid. State Ion. 2002, 154–155, 609–619. [Google Scholar] [CrossRef]
- Nguyen, T.; Lee, D.; Song, Y. High-Ionic-Conductivity Sodium-Based Ionic Gel Polymer Electrolyte for High-Performance and Ultrastable Microsupercapacitors. ACS Appl. Mater. Interfaces 2023, 15, 3054–3068. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar Verma, D.; Tiwari, R.; Kumar, D.; Parwati, K.; Rai, R.; Adhikary, P.; Krishnamoorthi, S. Sodium-Ion-Conducting Hydrogel Material: Synthesis, Characterization and Conductivity Studies. ChemistrySelect 2023, 8, e202302589. [Google Scholar] [CrossRef]
- Babu, B.; Neumann, C.; Muench, S. Diglyme-Based Gel Polymer Electrolytes for K-Ion Capacitors. Energy Storage Mater. 2023, 56, 342–350. [Google Scholar] [CrossRef]
- Yang, H.; Wang, W.; Huang, Z.; Wang, Z.; Hu, L.; Wang, M.; Yang, S.; Jiao, S.; Yang, H.; Wang, W.; et al. Weak Electrostatic Force on K+ in Gel Polymer Electrolyte Realizes High Ion Transference Number for Quasi Solid-State Potassium Ion Batteries. Adv. Mater. 2024, 36, 2401008. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lin, C.; Tseng, L. Superior Hydrogel Electrolytes in Both Ionic Conductivity and Electrochemical Window from the Immobilized Pair Ions for Carbon-Based Supercapacitors. J. Taiwan Inst. Chem. Eng. 2021, 118, 152–158. [Google Scholar] [CrossRef]
- Przyłuski, J.; Połtarzewski, Z. Proton-Conducting Hydrogel Membranes. Polymer 1998, 39, 4343–4347. [Google Scholar] [CrossRef]
- Sutar, P.; Nath Das, T.; Jena, R.; Dutta, D.; Jiban Bhattacharyya, A.; Kumar Maji, T. Proton Conductivity in a Metal–Organic Cube-Based Framework and Derived Hydrogel with Tubular Morphology. Langmuir 2024, 40, 5913–5922. [Google Scholar] [CrossRef]
- Kim, H.; Prakash, S.; Mustain, W. Sol–Gel Based Sulfonic Acid-Functionalized Silica Proton Conductive Membrane. J. Power Sources 2009, 193, 562–569. [Google Scholar] [CrossRef]
- Furtmair, M.; Timm, J. Sulfonation of Porous Materials and Their Proton Conductivity. Microporous Mesoporous Mater. 2021, 312, 110745. [Google Scholar] [CrossRef]
- Chakraborty, P.; Das, B.; Pal, P.; Datta, S.; Bera, S.; Dastidar, P. A Supramolecular Hydrogel Derived from a Simple Organic Salt Capable of Proton Conduction. Chem. Commun. 2020, 56, 5251–5254. [Google Scholar] [CrossRef]
- Shmukler, L.; Fadeeva, Y. Conductivity of Gel Polymer Electrolytes Doped with Solutions of Phosphonic Acid or Protic Ionic Liquids. Chem. Phys. Lett. 2018, 697, 1–6. [Google Scholar] [CrossRef]
- Umeda, J.; Suzuki, M.; Kato, M. Proton Conductive Inorganic–Organic Hybrid Membranes Functionalized with Phosphonic Acid for Polymer Electrolyte Fuel Cell. J. Power Sources 2010, 195, 5882–5888. [Google Scholar] [CrossRef]
- Parangi, T.F.; Chudasama, U.V. Synthesis, Characterization, and Proton Conduction Behavior of Thorium and Cerium Phosphonates. ACS Omega 2019, 4, 3716–3725. [Google Scholar] [CrossRef]
- Gan, D.; Shuai, T.; Wang, X.; Huang, Z.; Ren, F.; Fang, L.; Wang, K.; Xie, C.; Lu, X. Mussel-Inspired Redox-Active and Hydrophilic Conductive Polymer Nanoparticles for Adhesive Hydrogel Bioelectronics. Nano-Micro Lett. 2020, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, W.; Fan, X.; Shi, X.; Jiang, Y.; Yan, L. A Polyphenol-Derived Redox-Active and Conductive Nanoparticle-Reinforced Hydrogel with Wet Adhesiveness for Myocardial Infarction Repair by Simultaneously. Nano Today 2024, 55, 102157. [Google Scholar] [CrossRef]
- Lin, J.H.; Du, X.S. Self-Healable and Redox Active Hydrogel Obtained via Incorporation of Ferric Ion for Supercapacitor Applications. Chem. Eng. J. 2022, 446, 137244. [Google Scholar] [CrossRef]
- Gan, D.; Huang, Z.; Wang, X.; Xu, D.; Rao, S.; Wang, K.; Ren, F.; Jiang, L.; Xie, C.; Lu, X. Bioadhesive and Electroactive Hydrogels for Flexible Bioelectronics and Supercapacitors Enabled by a Redox-Active Core–Shell PEDOT@ PZIF-71 System. Mater. Horiz. 2023, 10, 2169–2180. [Google Scholar] [CrossRef]
- Karchoubi, F.; Ghotli, R. New Insights into Nanocomposite Hydrogels; a Review on Recent Advances in Characteristics and Applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 54–78. [Google Scholar] [CrossRef]
- Jing, X.; Wang, X.; Mi, H. Stretchable Gelatin/Silver Nanowires Composite Hydrogels for Detecting Human Motion. Mater. Lett. 2019, 237, 53–56. [Google Scholar] [CrossRef]
- Liu, C.; Gou, S.; Bi, Y.; Gao, Q.; Sun, J. Smart DNA-Gold Nanoparticle Hybrid Hydrogel Film Based Portable, Cost-Effective and Storable Biosensing System for the Colorimetric Detection of Lead (II) and Uranyl. Biosens. Bioelectron. 2022, 210, 114290. [Google Scholar] [CrossRef]
- Roshanbinfar, K.; Kolesnik-Gray, M.; Angeloni, M.; Schruefer, S.; Fiedler, M.; Schubert, D.W.; Ferrazzi, F.; Krstic, V.; Engel, F.B. Collagen Hydrogel Containing Polyethylenimine-Gold Nanoparticles for Drug Release and Enhanced Beating Properties of Engineered Cardiac Tissues. Adv. Healthc. Mater. 2023, 12, 2202408. [Google Scholar] [CrossRef]
- Batool, Z.; Muhammad, G.; Mudassir Iqbal, M.; Shahbaz Aslam, M.; Arshad Raza, M.; Sajjad, N.; Abdullah, M.; Akhtar, N.; Syed, A.; Elgorban, A.M.; et al. Hydrogel Assisted Synthesis of Gold Nanoparticles with Enhanced Microbicidal and in Vivo Wound Healing Potential. Sci. Rep. 2022, 12, 6575. [Google Scholar] [CrossRef] [PubMed]
- López-Díaz, A.; Vázquez, A. Hydrogels in Soft Robotics: Past, Present, and Future. ACS Nano 2024, 18, 20817–20826. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X. Light-Driven Soft Microrobots Based on Hydrogels and LCEs: Development and Prospects. RSC Adv. 2024, 14, 14278–14288. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Han, S.; Kim, D.; Hyeon, T. High-Performance Stretchable Conductive Nanocomposites: Materials, Processes, and Device Applications. Chem. Soc. Rev. 2019, 48, 1566–1595. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, M.; Ahn, D.; Yeo, S. Electrical Percolation Threshold of Carbon Black in a Polymer Matrix and Its Application to Antistatic Fibre. Sci. Rep. 2019, 9, 6338. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Chen, H.; Li, M.; Liu, L. Percolation Threshold and Electrical Conductivity of Conductive Polymer Composites Filled with Curved Fibers in Two-Dimensional Space. Soft Matter 2023, 19, 7149. [Google Scholar] [CrossRef]
- Sánchez-Romate, X.; Jiménez-Suárez, A. Novel Approach to Percolation Threshold on Electrical Conductivity of Carbon Nanotube Reinforced Nanocomposites. Rsc Adv. 2016, 6, 43418–43428. [Google Scholar] [CrossRef]
- Mohammadpour-Haratbar, A.; Zare, Y. Simulation of Electrical Conductivity for Polymer Silver Nanowires Systems. Sci. Rep. 2023, 13, 5. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, B.; Zhang, D.; Yang, M.; Huang, X. Conductive Hydrogels Incorporating Carbon Nanoparticles: A Review of Synthesis, Performance and Applications. Particuology 2023, 83, 212–231. [Google Scholar] [CrossRef]
- Chen, G.; Guo, Y.; Hsiao, S.; Hou, K. Tough, Conductive Hydrogels with Double-Network Based on Hydrophilic Polymer Assistant Well-Dispersed Carbon Nanotube for Innovative Force Sensor. Sci. China Technol. Sci. 2022, 65, 1160–1168. [Google Scholar] [CrossRef]
- Park, J.; Jeon, N.; Lee, S.; Choe, G.; Lee, E. Conductive Hydrogel Constructs with Three-Dimensionally Connected Graphene Networks for Biomedical Applications. Chem. Eng. J. 2022, 446, 137344. [Google Scholar] [CrossRef]
- Talebian, S.; Mehrali, M.; Raad, R.; Safaei, F.; Xi, J.; Liu, Z.; Foroughi, J. Electrically Conducting Hydrogel Graphene Nanocomposite Biofibers for Biomedical Applications. Front. Chem. 2020, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Jia, X.; Bai, Y.; Yang, J.; Song, H. Graphene Thermally Conductive Hydrogel with a Solid–Liquid Interpenetrating Heat Conduction Network. ACS Appl. Mater. Interfaces 2023, 16, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Li, C.; Ji, X.; Tao, Y.; Lu, J.; Cheng, Y.; Du, J.; Wang, H. Highly Tough and Conductive Hydrogel Based on Defect-Patched Reduction Graphene Oxide for High-Performance Self-Powered Flexible Sensing Micro-System. Chem. Eng. J. 2023, 466, 143358. [Google Scholar] [CrossRef]
- Sun, P.; Engineering, Q.L.-M.M. Tension-responsive Graphene Oxide Conductive Hydrogel with Robust Mechanical Properties and High Sensitivity for Human Motion Monitoring. Mater. Eng. 2023, 308, 2200529. [Google Scholar] [CrossRef]
- Wei, J.; Wang, R.; Pan, F. Polyvinyl Alcohol/Graphene Oxide Conductive Hydrogels via the Synergy of Freezing and Salting out for Strain Sensors. Sensors 2022, 22, 3015. [Google Scholar] [CrossRef]
- Liu, X.W.; Huang, Y.X.; Sun, X.F.; Sheng, G.P.; Zhao, F.; Wang, S.G.; Yu, H.Q. Conductive Carbon Nanotube Hydrogel as a Bioanode for Enhanced Microbial Electrocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 8158–8164. [Google Scholar] [CrossRef]
- Hsiao, L.; Jing, L.; Li, K.; Yang, H.; Li, Y. Carbon Nanotube-Integrated Conductive Hydrogels as Multifunctional Robotic Skin. Carbon 2020, 161, 784–793. [Google Scholar] [CrossRef]
- Yao, S.; Yang, Y.; Li, C.; Yang, K.; Song, X.; Li, C. Axon-like Aligned Conductive CNT/GelMA Hydrogel Fibers Combined with Electrical Stimulation for Spinal Cord Injury Recovery. Bioact. Mater. 2024, 35, 534–548. [Google Scholar] [CrossRef]
- Cho, K.W.; Sunwoo, S.H.; Hong, Y.J.; Koo, J.H.; Kim, J.H.; Baik, S.; Hyeon, T.; Kim, D.H. Soft Bioelectronics Based on Nanomaterials. Chem. Rev. 2022, 122, 5068–5143. [Google Scholar] [CrossRef]
- Joo, H.; Jung, D.; Sunwoo, S.-H.; Koo, J.H.; Kim, D.-H. Material Design and Fabrication Strategies for Stretchable Metallic Nanocomposites. Small 2020, 16, 1906270. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Park, C.; Sunwoo, S.; Kim, M.; Lee, H. Soft Conductive Nanocomposites for Recording Biosignals on Skin. Soft Sci. 2023, 3. [Google Scholar] [CrossRef]
- Kulkarni, M.; Express, S.G.-N. Microfluidic Devices for Synthesizing Nanomaterials—A Review. Nano Express 2020, 1, 32004. [Google Scholar] [CrossRef]
- Granata, F.; Pirillo, N.; Alabastri, A.; Schirato, A.; Bruno, L.; Costa, R.; Malara, N.; Onesto, V.; Coluccio, M.L.; Iodice, M.; et al. Synthesis of Plasmonic Gold Nanoparticles on Soft Materials for Biomedical Applications. Micro Nano Eng. 2023, 19, 100207. [Google Scholar] [CrossRef]
- Im, J.; Trindade, G.F.; Quach, T.T.; Sohaib, A.; Wang, F.; Austin, J.; Turyanska, L.; Roberts, C.J.; Wildman, R.; Hague, R.; et al. Functionalized Gold Nanoparticles with a Cohesion Enhancer for Robust Flexible Electrodes. ACS Appl. Nano Mater. 2022, 2022, 6716. [Google Scholar] [CrossRef]
- Devaki, S.; Narayanan, R. Electrically Conducting Silver Nanoparticle–Polyacrylic Acid Hydrogel by in Situ Reduction and Polymerization Approach. Mater. Lett. 2014, 116, 135–138. [Google Scholar] [CrossRef]
- Babaei, Z.; Rezaei, B.; Pisheh, M.K.; Afshar-Taromi, F. In Situ Synthesis of Gold/Silver Nanoparticles and Polyaniline as Buffer Layer in Polymer Solar Cells. Mater. Chem. Phys. 2020, 248, 122879. [Google Scholar] [CrossRef]
- Han, J.; Kim, J.; Kim, B.-K.; Park, K. Hydrogel-Based Electrodeposition of Copper Nanoparticles for Selective Detection for Hydrogen Peroxide. Chemosensors 2023, 11, 384. [Google Scholar] [CrossRef]
- Chen, K.; Hu, Y.; Liu, M.; Wang, F.; Liu, P.; Yu, Y.; Feng, Q.; Xiao, X. Highly Stretchable, Tough, and Conductive Ag@ Cu Nanocomposite Hydrogels for Flexible Wearable Sensors and Bionic Electronic Skins. Macromol. Mater. Eng. 2021, 306, 2100341. [Google Scholar] [CrossRef]
- GhavamiNejad, P.; GhavamiNejad, A.; Zheng, H.; Dhingra, K.; Samarikhalaj, M.; Poudineh, M. A conductive hydrogel microneedle-based assay integrating PEDOT: PSS and Ag-Pt nanoparticles for real-time, enzyme-less, and electrochemical sensing of glucose. Adv. Healthc. Mater. 2023, 12, 2202362. [Google Scholar] [CrossRef]
- Sunwoo, S.H.; Han, S.I.; Jung, D.; Kim, M.; Nam, S.; Lee, H.; Choi, S.; Kang, H.; Cho, Y.S.; Yeom, D.H.; et al. Stretchable low-impedance conductor with Ag–Au–Pt core–shell–shell nanowires and in situ formed pt nanoparticles for wearable and implantable device. Acs Nano 2023, 17, 7550–7561. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ma, S.; Wang, Z.; Tan, Z.; Hou, M.; Ma, P.; Cao, R.; Lu, X. Silver nanowire-doped conductive polymer hydrogel anode for increasing electron transfer and COD removal rate simultaneously in microbial fuel cell. Electrochim. Acta 2024, 497, 144566. [Google Scholar] [CrossRef]
- Wunnemann, P.; Noyong, M.; Kreuels, K.; Brüx, R.; Gordiichuk, P.; van Rijn, P.; Plamer, F.A.; Simon, U.; Boker, A. Microstructured hydrogel templates for the formation of conductive gold nanowire arrays. Macromol. Rapid Commun. 2016, 37, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Qu, K.Y.; Zhang, F.; Jiang, H.N.; Zhang, N.; Nihad, C.; Liu, C.M.; Wu, K.H.; Wang, X.W.; Huang, N.P.; et al. High-aspect-ratio water-dispersed gold nanowires incorporated within gelatin methacrylate hydrogels for constructing cardiac tissues in vitro. J. Mater. Chem. B 2020, 8, 7213–7224. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, H.; Wang, R.; Wang, X.; Zhai, H.; Wang, T.; Jin, Q.; Sun, J. Highly stretchable and conductive copper nanowire based fibers with hierarchical structure for wearable heaters. ACS Appl. Mater. Interfaces 2016, 8, 32925–32933. [Google Scholar] [CrossRef]
- Lee, W.; Yun, H.; Song, J. Nanoscale Materials and Deformable Device Designs for Bioinspired and Biointegrated Electronics. Acc. Mater. Res. 2021, 2, 266–281. [Google Scholar] [CrossRef]
- Kiyotake, E.A.; Thomas, E.E.; Homburg, H.B.; Milton, C.K.; Smitherman, A.D.; Donahue, N.D.; Fung, K.M.; Wilhelm, S.; Michael, D.; Detamore, M.S. Conductive and injectable hyaluronic acid/gelatin/gold nanorod hydrogels for enhanced surgical translation and bioprinting. J. Biomed. Mater. Res. Part A 2022, 110, 365–382. [Google Scholar] [CrossRef]
- Navaei, A.; Saini, H.; Christenson, W.; Sullivan, R.T.; Ros, R.; Nikkhah, M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater. 2016, 41, 133–146. [Google Scholar] [CrossRef]
- Wang, M.; Feng, X.; Hu, S.; Zhang, C.; Qi, H. Facile gelation of a fully polymeric conductive hydrogel activated by liquid metal nanoparticles. J. Mater. Chem. A 2021, 9, 24539–24547. [Google Scholar] [CrossRef]
- Qin, H.; Sun, M.; Li, P.; Li, J.; Zhang, Z.; Dai, S.; Huang, M.; Lu, B.; Pan, X.; Wu, L. Soft, Stretchable, and Conductive Hydrogel Based on Liquid Metal for Accurately Facial Expression Monitoring. Adv. Mater. Technol. 2023, 8, 2300406. [Google Scholar] [CrossRef]
- Nam, S.; Doo Cha, G.; Sunwoo, S.-H.; Hwan Jeong, J.; Kang, H.; Kyu Park, O.; Lee, K.-Y.; Oh, S.; Hyeon, T.; Hong Choi, S.; et al. Needle-like Multifunctional Biphasic Microfiber for Minimally Invasive Implantable Bioelectronics. Adv. Mater. 2024, 36, 2404101. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, M.; Du, Y.; Ding, X.; Xiao, C.; Wang, Y.; Yang, Y.; Zhuo, Y.; Zheng, K.; Liu, X.; et al. Self-Healing Liquid Metal Hydrogel for Human–Computer Interaction and Infrared Camouflage. Mater. Horiz. 2023, 10, 2945. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, B.; Chen, D.; Liu, X.; Wang, H.; Song, Z.; Yu, D.; Li, G.; Ge, S.; Liu, W. Highly Sensitive and Self-Healing Conductive Hydrogels Fabricated from Cationic Cellulose Nanofiber-Dispersed Liquid Metal for Strain Sensors. Sci. China Mater. 2023, 2023, 1923–1933. [Google Scholar] [CrossRef]
- Wang, L.; Daoud, W.A. Hybrid conductive hydrogels for washable human motion energy harvester and self-powered temperature-stress dual sensor. Nano Energy 2019, 66, 104080. [Google Scholar] [CrossRef]
- Nicodemus, G.D.; Bryant, S.J. Cell Encapsulation in Biodegradable Hydrogels for Tissue Engineering Applications. Tissue Eng. Part. B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef]
- Cui, X.; Lee, J.J.; Chen, W.N. Eco-friendly and biodegradable cellulose hydrogels produced from low cost okara: Towards non-toxic flexible electronics. Sci. Rep. 2019, 9, 18166. [Google Scholar] [CrossRef]
- Turioni, C.; Guerrini, G.; Squartini, A.; Morari, F.; Maggini, M.; Gross, S. Biodegradable Hydrogels: Evaluation of Degradation as a Function of Synthesis Parameters and Environmental Conditions. GrossSoil Syst. 2021, 5, 47. [Google Scholar] [CrossRef]
- Pan, Z.; Brassart, L. Constitutive modelling of hydrolytic degradation in hydrogels. J. Mech. Phys. Solids 2022, 167, 105016. [Google Scholar] [CrossRef]
- Vetrík, M.; Přádný, M.; Hrubý, M.; Michálek, J. Hydrazone-based hydrogel hydrolytically degradable in acidic environment. Polym. Degrad. Stab. 2011, 96, 756–759. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Michlovská, L.; Vojtová, L.; Humpa, O.; Kučerík, J.; Žídek, J.; Jančář, J. Hydrolytic stability of end-linked hydrogels from PLGA–PEG–PLGA macromonomers terminated by α, ω-itaconyl groups. RSC Adv. 2016, 6, 16808–16816. [Google Scholar] [CrossRef]
- Gohil, S.V.; Padmanabhan, A.; Kan, H.M.; Khanal, M.; Nair, L.S. Degradation-Dependent Protein Release from Enzyme Sensitive Injectable Glycol Chitosan Hydrogel. Tissue Eng. Part A 2021, 27, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Knipe, J.; Chen, F. Enzymatic Biodegradation of Hydrogels for Protein Delivery Targeted to the Small Intestine. Biomacromolecules 2015, 16, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yu, L. PEG-Based Thermosensitive and Biodegradable Hydrogels. Acta Biomater. 2021, 128, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, C.C.; Du, F.S.; Li, Z.C. Supersensitive Oxidation-Responsive Biodegradable PEG Hydrogels for Glucose-Triggered Insulin Delivery. ACS Appl. Mater. Interfaces 2017, 9, 25905–25914. [Google Scholar] [CrossRef]
- Jensen, B.; Dávila, I. Poly (Vinyl Alcohol) Physical Hydrogels: Matrix-Mediated Drug Delivery Using Spontaneously Eroding Substrate. J. Phys. Chem. B 2016, 120, 5916–5926. [Google Scholar] [CrossRef]
- Woodard, L.N.; Grunlan, M.A. Hydrolytic Degradation and Erosion of Polyester Biomaterials. ACS Macro Lett. 2018, 7, 976–982. [Google Scholar] [CrossRef]
- Reay, S.L.; Jackson, E.L.; Ferreira, A.M.; Hilkens, C.M.U.; Novakovic, K. In Vitro Evaluation of the Biodegradability of Chitosan–Genipin Hydrogels. Mater. Adv. 2022, 3, 7946. [Google Scholar] [CrossRef]
- Yanev, P.; Van Tilborg, G.A.F.; Boere, K.W.M.; Stowe, A.M.; Van Der Toorn, A.; Viergever, M.A.; Hennink, W.E.; Vermonden, T.; Dijkhuizen, R.M. Thermosensitive Biodegradable Hydrogels for Local and Controlled Cerebral Delivery of Proteins: MRI-Based Monitoring of In Vitro and In Vivo Protein Release. ACS Biomater. Sci. Eng. 2023, 9, 760–772. [Google Scholar] [CrossRef]
- Doo Cha, G.; Hee Lee, W.; Sunwoo, S.-H.; Kang, D.; Kang, T.; Won Cho, K.; Kim, M.; Kyu Park, O.; Jung, D.; Lee, J.; et al. Multifunctional Injectable Hydrogel for In Vivo Diagnostic and Therapeutic Applications. ACS Nano 2022, 16, 567. [Google Scholar]
- Pertici, V.; Pin-Barre, C.; Rivera, C.; Pellegrino, C.; Laurin, J.; Gigmes, D.; Trimaille, T. Degradable and Injectable Hydrogel for Drug Delivery in Soft Tissues. Biomacromolecules 2018, 20, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Xiao, L.; Chen, T.; Roa, P.; Cocco, E.; Peng, Z.; Yu, L.; Wu, M.; Liu, J.; Zhao, X.; et al. Injectable Nanocomposite Hydrogels Improve Intraperitoneal Co-Delivery of Chemotherapeutics and Immune Checkpoint Inhibitors for Enhanced Peritoneal. ACS Nano 2024, 18, 18963–18979. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yuk, H.; Sarrafian, T.L.; Guo, C.F.; Griffiths, L.G.; Nabzdyk, C.S.; Zhao, X. An Off-the-Shelf Bioadhesive Patch for Sutureless Repair of Gastrointestinal Defects. Sci. Transl. Med. 2022, 14, eabh2857. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, Y.; Zu, B.; Lei, D.; Guo, Y.; Wang, M.; Dou, X. Reversible Adhesive Hydrogel with Enhanced Sampling Efficiency Boosted by Hydrogen Bond and van Der Waals Force for Visualized Detection. Chem. Eng. J. 2023, 455, 140493. [Google Scholar] [CrossRef]
- Sundaram, M.N.; Kaliannagounder, V.K.; Selvaprithiviraj, V.; Suresh, M.K.; Biswas, R.; Vasudevan, A.K.; Varma, P.K.; Jayakumar, R. Bioadhesive, Hemostatic, and Antibacterial in Situ Chitin–Fibrin Nanocomposite Gel for Controlling Bleeding and Preventing Infections at Mediastinum. ACS Sustain. Chem. Eng. 2018, 6, 7826–7840. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, R.S.; Stiles, W.R.; Jo, M.; Zeng, L.; Rho, S.; Baek, Y.; Kim, J.; Kim, M.S.; Kang, H. Injectable Thermosensitive Hydrogels for a Sustained Release of Iron Nanochelators. Adv. Sci. 2022, 9, 2200872. [Google Scholar] [CrossRef]
- Cha, G.D.; Kim, M.; Park, O.K.; Sunwoo, S.H.; Kang, T.; Lee, W.H.; Nam, S.; Hyeon, T.; Choi, S.H.; Kim, D.H. Minimally-Invasive and In-Vivo Hydrogel Patterning Method for In Situ Fabrication of Implantable Hydrogel Devices. Small Methods 2023, 7, 2300032. [Google Scholar] [CrossRef]
- Hua, Y.; Xia, H.; Jia, L.; Zhao, J.; Zhao, D.; Yan, X.; Zhang, Y.; Tang, S.; Zhou, G.; Zhu, L. Ultrafast, Tough, and Adhesive Hydrogel Based on Hybrid Photocrosslinking for Articular Cartilage Repair in Water-Filled Arthroscopy. Sci. Adv. 2021, 7, eabg0628. [Google Scholar] [CrossRef]
- Han, L.; Yan, L.; Wang, K.; Fang, L.; Zhang, H.; Tang, Y.; Ding, Y.; Weng, L.-T.; Xu, J.; Weng, J. Tough, Self-Healable and Tissue-Adhesive Hydrogel with Tunable Multifunctionality. NPG Asia Mater. 2017, 9, e372. [Google Scholar] [CrossRef]
- Zhao, Y.; Ohm, Y.; Liao, J.; Luo, Y.; Cheng, H. A Self-Healing Electrically Conductive Organogel Composite. Nat. Electron. 2023, 6, 206–215. [Google Scholar] [CrossRef]
- Bakravi, A.; Ahamadian, Y.; Hashemi, H.; Namazi, H. Synthesis of gelatin-based biodegradable hydrogel nanocomposite and their application as drug delivery agent. Adv. Polym. Technol. 2018, 37, 2625–2635. [Google Scholar] [CrossRef]
- Marciano, J.S.; Ferreira, R.R.; de Souza, A.G.; Barbosa, R.F.; de Moura Junior, A.J.; Rosa, D.S. Biodegradable gelatin composite hydrogels filled with cellulose for chromium (VI) adsorption from contaminated water. Int. J. Biol. Macromol. 2021, 181, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Materials, K.M. Injectable, Biodegradable Hydrogels for Tissue Engineering Applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Chitosan, N.; Guillén-Carvajal, K.; Valdez-Salas, B.; Beltrán-Partida, E.; Salomón-Carlos, J.; Cheng, N. Chitosan, Gelatin, and Collagen Hydrogels for Bone Regeneration. Polymers 2023, 15, 2762. [Google Scholar] [CrossRef]
- Suvarnapathaki, S.; Nguyen, M.A.; Wu, X.; Nukavarapu, S.P.; Camci-Unal, G. Synthesis and Characterization of Photocrosslinkable Hydrogels from Bovine Skin Gelatin. RSC Adv. 2019, 9, 13016–13025. [Google Scholar] [CrossRef]
- Leach, J.B.; Bivens, K. Photocrosslinked Hyaluronic Acid Hydrogels: Natural, Biodegradable Tissue Engineering Scaffolds. Biotechnol. Bioeng. 2003, 82, 578–589. [Google Scholar] [CrossRef]
- Fan, M.; Ma, Y.; Zhang, Z.; Mao, J.; Tan, H.; Hu, X. Biodegradable hyaluronic acid hydrogels to control release of dexamethasone through aqueous Diels–Alder chemistry for adipose tissue engineering. Mater. Sci. Eng. C 2015, 56, 311–317. [Google Scholar] [CrossRef]
- Shi, M.; Dong, R.; Hu, J.; Guo, B. Conductive self-healing biodegradable hydrogel based on hyaluronic acid-grafted-polyaniline as cell recruitment niches and cell delivery carrier for myogenic differentiation and skeletal muscle regeneration. Chem. Eng. J. 2023, 457, 141110. [Google Scholar] [CrossRef]
- Patterson, J.; Siew, R.; Herring, S.W.; Lin, A.S.; Guldberg, R.; Stayton, P.S. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials 2010, 31, 6772–6781. [Google Scholar] [CrossRef]
- Sakai, S.; Ohi, H.; Taya, M. Gelatin/hyaluronic acid content in hydrogels obtained through blue light-induced gelation affects hydrogel properties and adipose stem cell behaviors. Biomolecules 2019, 9, 342. [Google Scholar] [CrossRef]
- Camci-Unal, G.; Cuttica, D.; Annabi, N.; Demarchi, D.; Khademhosseini, A. Synthesis and Characterization of Hybrid Hyaluronic Acid-Gelatin Hydrogels. Biomacromolecules 2013, 14, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, N.; Galbis, E.; Valencia, C.; Díaz-Blanco, M.J.; Lacroix, B.; de-Paz, M.V. Biodegradable double cross-linked chitosan hydrogels for drug delivery: Impact of chemistry on rheological and pharmacological performance. Int. J. Biol. Macromol. 2020, 165, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar] [PubMed]
- Li, B.; Wang, L.; Xu, F.; Gang, X.; Demirci, U.; Wei, D.; Li, Y.; Feng, Y.; Jia, D.; Zhou, Y. Hydrosoluble, UV-crosslinkable and injectable chitosan for patterned cell-laden microgel and rapid transdermal curing hydrogel in vivo. Acta Biomater. 2015, 22, 59–69. [Google Scholar] [CrossRef]
- Giri, T.K.; Thakur, A.; Alexander, A.; Badwaik, H.; Tripathi, D.K. Modified chitosan hydrogels as drug delivery and tissue engineering systems: Present status and applications. Acta Pharm. Sin. B 2012, 2, 439–449. [Google Scholar] [CrossRef]
- Arafa, E.G.; Sabaa, M.W.; Mohamed, R.R.; Kamel, E.M.; Elzanaty, A.M.; Mahmoud, A.M.; Abdel-Gawad, O.F. Eco-friendly and biodegradable sodium alginate/quaternized chitosan hydrogel for controlled release of urea and its antimicrobial activity. Carbohydr. Polym. 2022, 291, 119555. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, M.B.; Torbati, M.; Akbarzadeh, A. Alginate-Based Hydrogels as Drug Delivery Vehicles in Cancer Treatment and Their Applications in Wound Dressing and 3D Bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar]
- Ji, D.; Park, J.M.; Oh, M.S.; Nguyen, T.L.; Shin, H.; Kim, J.S.; Kim, D.; Park, H.S.; Kim, J. Superstrong, Superstiff, and Conductive Alginate Hydrogels. Nat. Commun. 2022, 13, 3019. [Google Scholar] [CrossRef]
- Van Tomme, S.R.; Hennink, W.E. Biodegradable Dextran Hydrogels for Protein Delivery Applications. Expert. Rev. Med. Devices 2007, 4, 147–164. [Google Scholar] [CrossRef]
- Pitarresi, G.; Palumbo, F.S.; Giammona, G.; Casadei, M.A.; Moracci, F.M. Biodegradable hydrogels obtained by photocrosslinking of dextran and polyaspartamide derivatives. Biomaterials 2003, 24, 4301–4313. [Google Scholar] [CrossRef]
- Salber, J.; Zoso, A.; Carmagnola, I.; Sobczak, M. Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems—State of Knowledge and Future Prospects. Int. J. Mol. Sci. 2022, 23, 4421. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Benoit, D.S. Degradable poly (ethylene glycol)(PEG)-based hydrogels for spatiotemporal control of siRNA/nanoparticle delivery. J. Control. Release 2018, 287, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Tanan, W.; Panichpakdee, J.; Suwanakood, P.; Saengsuwan, S. Biodegradable hydrogels of cassava starch-g-polyacrylic acid/natural rubber/polyvinyl alcohol as environmentally friendly and highly efficient coating material for slow-release urea fertilizers. J. Ind. Eng. Chem. 2021, 101, 237–252. [Google Scholar] [CrossRef]
- Hiemstra, C.; Zhong, Z.; Li, L.; Dijkstra, P.J.; Feijen, J. In-Situ Formation of Biodegradable Hydrogels by Stereocomplexation of PEG−(PLLA)8 and PEG−(PDLA)8 Star Block Copolymers. Biomacromolecules 2006, 7, 2790–2795. [Google Scholar] [CrossRef]
- Henise, J.; Hearn, B.R.; Ashley, G.W.; Santi, D.V. Biodegradable Tetra-PEG Hydrogels as Carriers for a Releasable Drug Delivery System. Bioconjugate Chem. 2015, 26, 270–278. [Google Scholar] [CrossRef]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J. Poly (vinyl alcohol) hydrogels: The old and new functional materials. Int. J. Polym. Sci. 2021, 2021, 2225426. [Google Scholar] [CrossRef]
- Grosjean, M.; Girard, E.; Bethry, A.; Chagnon, G.; Garric, X.; Nottelet, B. Degradable Bioadhesives Based on Star PEG–PLA Hydrogels for Soft Tissue Applications. Biomacromolecules 2023, 24, 4430–4443. [Google Scholar] [CrossRef]
- Hardman, D.; George Thuruthel, T.; Iida, F. Self-Healing Ionic Gelatin/Glycerol Hydrogels for Strain Sensing Applications. NPG Asia Mater. 2022, 14, 11. [Google Scholar] [CrossRef]
- Choi, H.; Kim, Y.; Kim, S.; Jung, H.; Lee, S.; Kim, K.; Han, H.S.; Kim, J.Y.; Shin, M.; Son, D. Adhesive Bioelectronics for Sutureless Epicardial Interfacing. Nat. Electron. 2023, 6, 779–789. [Google Scholar] [CrossRef]
- Song, F.; Zhang, J.; Lu, J.; Cheng, Y.; Tao, Y.; Shao, C.; Wang, H. A Mussel-Inspired Flexible Chitosan-Based Bio-Hydrogel as a Tailored Medical Adhesive. Int. J. Biol. Macromol. 2021, 189, 183–193. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Bai, H.; Zhang, S.; Ma, P.; Dong Li, W.Z.; Wang, D.; Bai, H.; Zhang, S.; Ma, P.; et al. Photo-crosslinking Strategy Constructs Adhesive, Superabsorbent, and Tough PVA-based Hydrogel through Controlling the Balance of Cohesion and Adhesion. Macromol. Mater. Eng. 2020, 305, 1900623. [Google Scholar] [CrossRef]
- Gong, C.; Lu, C.; Li, B.; Shan, M.; Wu, G. Injectable Dopamine-modified Poly (α, Β-aspartic Acid) Nanocomposite Hydrogel as Bioadhesive Drug Delivery System. J. Biomed. Mater. Res. Part A 2017, 105, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, X.; Zhang, W.; Wang, M.; Yan, L.; Wang, K.; Han, L.; Lu, X. Infant Skin Friendly Adhesive Hydrogel Patch Activated at Body Temperature for Bioelectronics Securing and Diabetic Wound Healing. ACS Nano 2022, 16, 8662–8676. [Google Scholar] [CrossRef] [PubMed]
- Strehin, I.; Nahas, Z.; Arora, K.; Nguyen, T.; Biomaterials, J.E. A Versatile PH Sensitive Chondroitin Sulfate–PEG Tissue Adhesive and Hydrogel. Biomaterials 2010, 31, 2788–2797. [Google Scholar] [CrossRef]
- Tavafoghi, M.; Sheikhi, A.; Tutar, R.; Jahangiry, J.; Baidya, A.; Haghniaz, R.; Khademhosseini, A. Engineering Tough, Injectable, Naturally Derived, Bioadhesive Composite Hydrogels. Adv. Healthc. Mater. 2020, 9, 1901722. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Z.; Huang, Y.; Yu, R.; Guo, B. Dual-Dynamic-Bond Cross-Linked Antibacterial Adhesive Hydrogel Sealants with on-Demand Removability for Post-Wound-Closure and Infected Wound Healing. ACS Nano 2021, 15, 7078–7093. [Google Scholar] [CrossRef]
- Zhao, P.; Guo, Z.; Wang, H.; Zhou, B.; Huang, F. A Multi-Crosslinking Strategy of Organic and Inorganic Compound Bio-Adhesive Polysaccharide-Based Hydrogel for Wound Hemostasis. Biomater. Adv. 2023, 152, 213481. [Google Scholar] [CrossRef]
- Tian, G.; Liu, Y.; Yu, M.; Liang, C.; Yang, D.; Huang, J.; Zhao, Q.; Zhang, W.; Chen, J.; Wang, Y.; et al. Electrostatic Interaction-Based High Tissue Adhesive, Stretchable Microelectrode Arrays for the Electrophysiological Interface. ACS Appl. Mater. 2022, 14, 4861. [Google Scholar] [CrossRef]
- Huang, G.; Tang, Z.; Peng, S.; Zhang, P.; Sun, T.; Wei, W.; Zeng, L.; Guo, H.; Guo, H.; Meng, G. Modification of Hydrophobic Hydrogels into a Strongly Adhesive and Tough Hydrogel by Electrostatic Interaction. Macromolecules 2021, 55, 156–165. [Google Scholar] [CrossRef]
- Pan, P.; Svirskis, D.; Waterhouse, G. Hydroxypropyl Methylcellulose Bioadhesive Hydrogels for Topical Application and Sustained Drug Release: The Effect of Polyvinylpyrrolidone on the Physicomechanical. Pharmaceutics 2023, 15, 2360. [Google Scholar] [CrossRef]
- Michel, R.; Poirier, L.; Van Poelvoorde, Q.; Legagneux, J.; Manassero, M.; Corté, L. Interfacial Fluid Transport Is a Key to Hydrogel Bioadhesion. Proc. Natl. Acad. Sci. USA 2019, 116, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; O’Cearbhaill, E.D.; Sisk, G.C.; Park, K.M.; Cho, W.K.; Villiger, M.; Bouma, B.E.; Pomahac, B.; Karp, J.M. A Bio-Inspired Swellable Microneedle Adhesive for Mechanical Interlocking with Tissue. Nat. Commun. 2013, 4, 1702. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Fang, Y.; Zhou, W.; Yan, L.; Xu, Y.; Zhu, H.; Horizons, H.L.-M. Mussel Foot Protein Inspired Tough Tissue-Selective Underwater Adhesive Hydrogel. Mater. Horiz. 2021, 8, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liang, Y.; Huang, Y.; He, J.; Han, Y.; Guo, B. Physical Double-Network Hydrogel Adhesives with Rapid Shape Adaptability, Fast Self-Healing, Antioxidant and NIR/PH Stimulus-Responsiveness for Multidrug-Resistant Bacterial Infection and Removable Wound Dressing. Adv. Funct. Mater. 2020, 30, 1910748. [Google Scholar] [CrossRef]
- Xu, J.; Strandman, S.; Zhu, J.; Barralet, J.; Biomaterials, M.C. Genipin-Crosslinked Catechol-Chitosan Mucoadhesive Hydrogels for Buccal Drug Delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef]
- Vakili, M.; Mohammed-Saeid, W. Development of Mucoadhesive Hydrogels Based on Polyacrylic Acid Grafted Cellulose Nanocrystals for Local Cisplatin Delivery. Carbohydr. Polym. 2021, 255, 117332. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In Situ Forming Injectable Hydrogels for Drug Delivery and Wound Repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Shin, G.R.; Kim, H.E.; Kim, J.H.; Choi, S.; Kim, M.S. Advances in Injectable in Situ-Forming Hydrogels for Intratumoral Treatment. Pharmaceutics 2021, 13, 1953. [Google Scholar] [CrossRef]
- Maria Alonso, J.; Andrade del Olmo, J.; Perez Gonzalez, R.; Saez-Martinez Citation, V.; del Olmo, A.; Gonzalez, P.; Velasco, H. Injectable Hydrogels: From Laboratory to Industrialization. Polymers 2021, 13, 650. [Google Scholar] [CrossRef]
- Shukla, A.; Singh, A.P.; Maiti, P. Injectable Hydrogels of Newly Designed Brush Biopolymers as Sustained Drug-Delivery Vehicle for Melanoma Treatment. Signal Transduct. Target. Ther. 2021, 6, 63. [Google Scholar] [CrossRef]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Qi, X.; Chen, Y. Thermo-Sensitive Hydrogels for Delivering Biotherapeutic Molecules: A Review. Saudi Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Pena-Francesch, A.; Montero, L.; Langmuir, S.B. Tailoring the LCST of Thermosensitive Hydrogel Thin Films Deposited by ICVD. Langmuir 2014, 30, 7162–7167. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ramirez, C.; Miljkovic, N.; Li, H. Thermosensitive Injectable Hyaluronic Acid Hydrogel for Adipose Tissue Engineering. Biomaterials 2009, 30, 6844–6853. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Q.; Javed Ansari, M.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A. Poly(N-Isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art. Gels 2022, 8, 454. [Google Scholar] [CrossRef]
- Majstorovic, N.; Materials, S. Thermosensitive Fluorescence of an UCST-Type Hybrid Functional Hydrogel. ACS Appl. Polym. Mater. 2021, 3, 4992–4999. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Lai, P.-L.; Lin, Y.-K.; Peng, S.; Lee, L.-Y.; Chen, C.-N.; Chu, I.-M. A Poloxamer-Polypeptide Thermosensitive Hydrogel as a Cell Scaffold and Sustained Release Depot. Polym. Chem. 2016, 7, 2976. [Google Scholar] [CrossRef]
- Shriky, B.; Kelly, A.; Isreb, M. Pluronic F127 Thermosensitive Injectable Smart Hydrogels for Controlled Drug Delivery System Development. J. Colloid Interface Sci. 2020, 565, 119–130. [Google Scholar] [CrossRef]
- García-Couce, J.; Tomás, M.; Fuentes, G.; Que, I.; Almirall, A. Chitosan/Pluronic F127 Thermosensitive Hydrogel as an Injectable Dexamethasone Delivery Carrier. Gels 2022, 8, 44. [Google Scholar] [CrossRef]
- Raghuwanshi, V.; Mendoza, D. Effect of Temperature on the Conformation and Functionality of Poly (N-Isopropylacrylamide)(PNIPAM)-Grafted Nanocellulose Hydrogels. J. Colloid Interface Sci. 2023, 652, 1609–1619. [Google Scholar] [CrossRef]
- Taylor, M.J.; Tomlins, P.; Sahota, T.S. Thermoresponsive Gels. Gels 2017, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Gong, C.; Qi, T.; Wei, X.; Qu, Y.; Wu, Q.; Luo, F.; Qian, Z. Thermosensitive Polymeric Hydrogels as Drug Delivery Systems. Curr. Med. Chem. 2013, 20, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, Y.; He, Y.; Hu, L.; Liang, L. Thermosensitive Hydrogel Microneedles for Controlled Transdermal Drug Delivery. Acta Biomater. 2022, 153, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Jung, J.; Science, D.L.-B. Recent Strategies to Develop PH-Sensitive Injectable Hydrogels. Biomater. Sci. 2023, 11, 1948–1961. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Ma, P.; Guo, B.; Du, Y. PH-Responsive Injectable Hydrogels with Mucosal Adhesiveness Based on Chitosan-Grafted-Dihydrocaffeic Acid and Oxidized Pullulan for Localized Drug Delivery. J. Colloid Interface Sci. 2019, 536, 224–234. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. PH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, X.; Li, H.; Zhang, R. Injectable and Body Temperature Sensitive Hydrogels Based on Chitosan and Hyaluronic Acid for PH Sensitive Drug Release. Carbohydr. Polym. 2018, 186, 82–90. [Google Scholar] [CrossRef]
- Andrade, F.; Mercé Roca-Melendres, M.; Durán-Lara, E.F.; Rafael, D.; Schwartz, S.; Durán-Lara, M.M.; Rafael, E.F. Stimuli-Responsive Hydrogels for Cancer Treatment: The Role of PH, Light, Ionic Strength and Magnetic Field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef]
- Liu, X.; Tong, Z.; Hu, O. Swelling Equilibria of Hydrogels with Sulfonate Groups in Water and in Aqueous Salt Solutions Macromolecules. Macromolecules 1995, 28, 3813–3817. [Google Scholar] [CrossRef]
- Lima, D.; Tenorio-Neto, E. PH-Responsive Alginate-Based Hydrogels for Protein Delivery. J. Mol. Liq. 2018, 262, 29–36. [Google Scholar] [CrossRef]
- Chen, Y.; Polymers, P.S. PH-Sensitive Polyampholyte Microgels of Poly(Acrylic Acid-Co-Vinylamine) as Injectable Hydrogel for Controlled Drug Release. Polymers 2019, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Hendi, A.; Hassan, M.U.; Elsherif, M.; Alqattan, B.; Park, S.; Yetisen, A.K.; Butt, H. Healthcare Applications of PH-Sensitive Hydrogel-Based Devices: A Review. Int. J. Nanomed. 2020, 3887–3901. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ma, D.; Wu, D.; Qiu, X.; Yang, S. A PH-Responsive, Injectable and Self-Healing Chitosan-Coumarin Hydrogel Based on Schiff Base and Hydrogen Bonds. Int. J. Biol. Macromol. 2024, 255, 128122. [Google Scholar] [CrossRef] [PubMed]
- Eswaramma, S.; Reddy, N.S.; Rao, K.K. Carbohydrate Polymer Based PH-Sensitive IPN Microgels: Synthesis, Characterization and Drug Release Characteristics. Mater. Chem. Phys. 2017, 195, 176–186. [Google Scholar] [CrossRef]
- Kocak, F.; Talari, A.; Yar, M. In-Situ Forming PH and Thermosensitive Injectable Hydrogels to Stimulate Angiogenesis: Potential Candidates for Fast Bone Regeneration Applications. Int. J. Mol. Sci. 2020, 21, 1633. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable Antibacterial Conductive Hydrogels with Dual Response to an Electric Field and PH for Localized “Smart” Drug Release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef]
- Xiao, Y.; Kang, S.; Liu, Y.; Guo, X.; Li, M. Effect and Mechanism of Calcium Ions on the Gelation Properties of Cellulose Nanocrystals-Whey Protein Isolate Composite Gels. Food Hydrocoll. 2021, 111, 10640. [Google Scholar] [CrossRef]
- Topuz, F.; Henke, A.; Richtering, W.; Groll, J. Magnesium Ions and Alginate Do Form Hydrogels: A Rheological Study. Soft Matter 2012, 8, 4877–4881. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-Induced Gelation of Alginate: Mechanisms and Applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Hoang, N.H.; Le Thanh, T.; Sangpueak, R.; Treekoon, J.; Saengchan, C.; Thepbandit, W.; Papathoti, N.K.; Kamkaew, A.; Buensanteai, N. Chitosan Nanoparticles-Based Ionic Gelation Method: A Promising Candidate for Plant Disease Management. Polymers 2022, 14, 662. [Google Scholar] [CrossRef]
- Lee, J.; Chang, K.; Kim, S.; Gite, V.; Chung, H.; Sohn, D. Phase Controllable Hyaluronic Acid Hydrogel with Iron (III) Ion–Catechol Induced Dual Cross-Linking by Utilizing the Gap of Gelation Kinetics. Macromolecules 2016, 49, 7450–7459. [Google Scholar] [CrossRef]
- Kovrlija, I.; Locs, J.; Loca, D.; Piedade, A.P. Incorporation of Barium Ions into Biomaterials: Dangerous Liaison or Potential Revolution? Materials 2021, 14, 5772. [Google Scholar] [CrossRef] [PubMed]
- Badali, E.; Hosseini, M.; Mohajer, M.; Hassanzadeh, S.; Saghati, S.; Hilborn, J.; Khanmohammadi, M. Enzymatic Crosslinked Hydrogels for Biomedical Application. Polym. Sci. Ser. A 2021, 63, S1–S22. [Google Scholar] [CrossRef]
- Le Thi, P.; Lee, Y.; Nguyen, D.H.; Park, K.D. In Situ Forming Gelatin Hydrogels by Dual-Enzymatic Cross-Linking for Enhanced Tissue Adhesiveness. J. Mater. Chem. B 2017, 5, 757–764. [Google Scholar] [CrossRef]
- Hense, D.; Gels, O.S. Fibrillogenesis and Hydrogel Formation from Fibrinogen Induced by Calcium Salts. Gels 2023, 9, 175. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Ren, X.; Long, H.; Qian, H.; Ma, K.; Guo, Y. Enzymatically Crosslinked Gelatin Hydrogel Promotes the Proliferation of Adipose Tissue-Derived Stromal Cells. PeerJ 2016, 4, e2497. [Google Scholar] [CrossRef]
- Hasturk, O.; Jordan, K.; Choi, J. Enzymatically Crosslinked Silk and Silk-Gelatin Hydrogels with Tunable Gelation Kinetics, Mechanical Properties and Bioactivity for Cell Culture and Encapsulation. Biomaterials 2020, 232, 119720. [Google Scholar] [CrossRef]
- Wachendörfer, M.; Miriam Buhl, E.; Ben Messaoud, G.; Richtering, W.; Fischer, H.; Wachendörfer, M.; Fischer, H. PH and Thrombin Concentration Are Decisive in Synthesizing Stiff, Stable, and Open-Porous Fibrin-Collagen Hydrogel Blends without Chemical Cross-Linker. Adv. Healthc. Mater. 2023, 12, 2203302. [Google Scholar] [CrossRef]
- Naranjo-Alcazar, R.; Bendix, S.; Groth, T.; Gallego Ferrer, G. Research Progress in Enzymatically Cross-Linked Hydrogels as Injectable Systems for Bioprinting and Tissue Engineering. Gels 2023, 9, 230. [Google Scholar] [CrossRef]
- Wang, R.; Xu, D.-L.; Liang, L.; Xu, T.-T.; Liu, W.; Ouyang, P.-K.; Chi, B.; Xu, H. Enzymatically Crosslinked Epsilon-Poly-L-Lysine Hydrogels with Inherent Antibacterial Properties for Wound Infection Prevention. RSC Adv. 2016, 6, 8620–8627. [Google Scholar] [CrossRef]
- Teixeira, L.S.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-Catalyzed Crosslinkable Hydrogels: Emerging Strategies for Tissue Engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz-Turan, S.; Lopez-Sanchez, P. Revealing the Mechanisms of Hydrogel Formation by Laccase Crosslinking and Regeneration of Feruloylated Arabinoxylan from Wheat Bran. Food Hydrocoll. 2022, 128, 107575. [Google Scholar] [CrossRef]
- Lee, F.; Bae, K.H.; Kurisawa, M. Injectable Hydrogel Systems Crosslinked by Horseradish Peroxidase. Biomed. Mater. 2015, 11, 014101. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.; Lee, S. Influence of Thrombin Concentration on the Mechanical and Morphological Properties of Cell-Seeded Fibrin Hydrogels. Acta Biomater. 2007, 3, 59–67. [Google Scholar] [CrossRef]
- Png, R.; Chia, P.; Tang, J. High-Performance Polymer Semiconducting Heterostructure Devices by Nitrene-Mediated Photocrosslinking of Alkyl Side Chains. Nat. Mater. 2010, 9, 152–158. [Google Scholar] [CrossRef]
- Kushibiki, T.; Mayumi, Y.; Nakayama, E.; Azuma, R.; Ojima, K.; Horiguchi, A.; Ishihara, M. Photocrosslinked Gelatin Hydrogel Improves Wound Healing and Skin Flap Survival by the Sustained Release of Basic Fibroblast Growth Factor. Sci. Rep. 2021, 11, 23094. [Google Scholar] [CrossRef]
- Jeon, O.; Bouhadir, K.; Mansour, J. Photocrosslinked Alginate Hydrogels with Tunable Biodegradation Rates and Mechanical Properties. Biomaterials 2009, 30, 2724–2734. [Google Scholar] [CrossRef]
- Xiang, L.; Cui, W. Biomedical Application of Photo-Crosslinked Gelatin Hydrogels. J. Leather Sci. Eng. 2021, 3, 3. [Google Scholar] [CrossRef]
- Xia, C.; Chen, P.; Mei, S.; Ning, L.; Lei, C.; Wang, J. Photo-Crosslinked HAMA Hydrogel with Cordycepin Encapsulated Chitosan Microspheres for Osteoarthritis Treatment. Oncotarget 2017, 8, 2835. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, M.; Wang, J.; Zhang, W.; Lu, W.; Gao, Y.; Zhang, B.; Guo, Y. Development of a Photo-Crosslinking, Biodegradable GelMA/PEGDA Hydrogel for Guided Bone Regeneration Materials. Materials 2018, 11, 1345. [Google Scholar] [CrossRef]
- Burke, G.; Barron, V.; Geever, T. Evaluation of the Materials Properties, Stability and Cell Response of a Range of PEGDMA Hydrogels for Tissue Engineering Applications. J. Mech. Behav. Biomed. Mater. 2019, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T. Photochemically Crosslinked Cell-laden Methacrylated Collagen Hydrogels with High Cell Viability and Functionality. J. Biomed. Mater. Res. Part A 2019, 107, 1541–1550. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Pérez-Álvarez, L. Photocrosslinkable and Self-Healable Hydrogels of Chitosan and Hyaluronic Acid. Int. J. Biol. Macromol. 2022, 216, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Hwang, H.J.; Jeon, H.R.; Park, S.J.; Bae, I.S.; Yang, Y.J. Photocrosslinkable Natural Polymers in Tissue Engineering. Front. Bioeng. Biotechnol. 2023, 11, 1127757. [Google Scholar] [CrossRef] [PubMed]
- Devi, A.; Shyam, R.; Palaniappan, A.; Kumar Jaiswal, A.; Oh, T.-H.; Nathanael, A.J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef] [PubMed]
- Rumon, M.; Akib, A.; Sultana, F. Self-Healing Hydrogels: Development, Biomedical Applications, and Challenges. Polymers 2022, 14, 4539. [Google Scholar] [CrossRef]
- Yin, H.; Liu, F.; Abdiryim, T. Self-Healing Hydrogels: From Synthesis to Multiple Applications. ACS Mater. Lett. 2023, 5, 1787–1830. [Google Scholar] [CrossRef]
- Xu, J.; Hsu, S.H. Self-Healing Hydrogel as an Injectable Implant: Translation in Brain Diseases. J. Biomed. Sci. 2023, 30, 43. [Google Scholar] [CrossRef]
- Tu, Y.; Chen, N.; Li, C.; Liu, H.; Zhu, R.; Chen, S.; Xiao, Q. Advances in Injectable Self-Healing Biomedical Hydrogels. Acta Biomater. 2019, 90, 1–20. [Google Scholar] [CrossRef]
- Perera, M.M.; Ayres, N. Dynamic Covalent Bonds in Self-Healing, Shape Memory, and Controllable Stiffness Hydrogels. Polym. Chem. 2020, 11, 1410–1423. [Google Scholar] [CrossRef]
- Liu, X.; Ren, Z.; Liu, F.; Zhao, L. Multifunctional Self-Healing Dual Network Hydrogels Constructed via Host–Guest Interaction and Dynamic Covalent Bond as Wearable Strain Sensors for Monitoring. ACS Appl. Mater. Interfaces 2021, 13, 14612–14622. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Luo, J.; Zhang, W.; Sun, J.; Wang, J.; Qin, C.; Zhuo, Q.; Dai, L. A Novel Polyvinyl Alcohol-based Hydrogel with Ultra-fast Self-healing Ability and Excellent Stretchability Based on Multi Dynamic Covalent Bond Cross-linking. Macromol. Mater. Eng. 2023, 308, 2200525. [Google Scholar] [CrossRef]
- Quan, L.; Xin, Y.; Wu, X. Mechanism of Self-Healing Hydrogels and Application in Tissue Engineering. Polymers 2022, 14, 2184. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, R.; Rana, N. Consequence of Imine Bond Origination: Fabrication of Rapid Self-Healing Chitosan Hydrogel as a Drug Delivery Candidate for Water-Soluble Drug. Eur. Polym. J. 2022, 180, 111605. [Google Scholar] [CrossRef]
- Basu, S.; Pacelli, S. Self-Healing DNA-Based Injectable Hydrogels with Reversible Covalent Linkages for Controlled Drug Delivery. Acta Biomater. 2020, 105, 159–169. [Google Scholar] [CrossRef]
- Mredha, M.; Na, J.; Seon, J.; Cui, J. Multifunctional Poly (Disulfide) Hydrogels with Extremely Fast Self-Healing Ability and Degradability. Chem. Eng. J. 2020, 394, 124941. [Google Scholar]
- An, H.; Bo, Y.; Chen, D.; Wang, Y.; Wang, H.; He, Y. Cellulose-Based Self-Healing Hydrogel through Boronic Ester Bonds with Excellent Biocompatibility and Conductivity. RSC Adv. 2020, 10, 11300–11310. [Google Scholar] [CrossRef]
- Figueiredo, T.; Jing, J.; Jeacomine, I.; Olsson, J.; Gerfaud, T.; Boiteau, J.-G.; Rome, C.; Harris, C.; Auzeíy-Velty, R. Injectable Self-Healing Hydrogels Based on Boronate Ester Formation between Hyaluronic Acid Partners Modified with Benzoxaborin Derivatives and Saccharides. Biomacromolecules 2019, 21, 230–239. [Google Scholar] [CrossRef]
- Yang, H.; Cho, S.; Eom, Y.; Park, S. Preparation of Self-Healable and Spinnable Hydrogel by Dynamic Boronate Ester Bond from Hyperbranched Polyglycerol and Boronic Acid-Containing Polymer. Macromol. Res. 2021, 29, 140–148. [Google Scholar] [CrossRef]
- Fan, L.; Ge, X.; Qian, Y.; Wei, M.; Zhang, Z.; Yuan, W.E.; Ouyang, Y. Advances in Synthesis and Applications of Self-Healing Hydrogels. Front. Bioeng. Biotechnol. 2020, 8, 654. [Google Scholar] [CrossRef]
- Sharma, P.K.; Taneja, S.; Singh, Y. Hydrazone-Linkage-Based Self-Healing and Injectable Xanthan–Poly (Ethylene Glycol) Hydrogels for Controlled Drug Release and 3D Cell Culture. ACS Appl. Mater. Interfaces 2018, 10, 30936–30945. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xiao, Q.; Qi, G.; Chen, F.; Tu, B.; Zhang, S.; Li, Y.; Chen, Y.; Yu, H.; Duan, P. A Hydrogen Bonds-Crosslinked Hydrogels With Self-Healing and Adhesive Properties for Hemostatic. Front. Bioeng. Biotechnol. 2022, 10, 855013. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, Y.; Woo, J.; Park, H.; Hur, K. Fast Healing of Ionic Bonds in Tough Hydrogels under an Acoustic Excitation. Extrem. Mech. Lett. 2019, 33, 100572. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, Q.; Sun, J.; Jiang, G. Double Network Self-Healing Hydrogel Based on Hydrophobic Association and Ionic Bond for Formation Plugging. Pet. Sci. 2022, 19, 2150–2164. [Google Scholar] [CrossRef]
- Hong, S.; Park, T.; Lee, J.; Ji, Y.; Walsh, J.; Yu, T.; Park, J.Y.; Lim, J.; Alston, C.B.; Solorio, L. Rapid Self-Healing Hydrogel with Ultralow Electrical Hysteresis for Wearable Sensing. ACS Sens. 2024, 9, 662–673. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Y.; Wang, J.; You, L.; Zhang, R. Chitosan-Based Mussel-Inspired Hydrogel for Rapid Self-Healing and High Adhesion of Tissue Adhesion and Wound Dressings. Carbohydr. Polym. 2023, 316, 121083. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Takashima, Y.; Yamaguchi, H.; Niu, P.; Liu, B.; Li, H. Room-Temperature Self-Healing Elastomer Based on Van Der Waals Forces in Air and under Water. J. Phys. Conf. Ser. 2021, 2083, 022066. [Google Scholar]
- Park, J.; Kim, J.Y.; Heo, H.; Kim, Y.; Kim, S.A.; Park, K.; Lee, Y.; Jin, Y.; Shin, S.R.; Kim, D.W. Intrinsically Nonswellable Multifunctional Hydrogel with Dynamic Nanoconfinement Networks for Robust Tissue-Adaptable Bioelectronics. Adv. Sci. 2023, 10, 2207237. [Google Scholar] [CrossRef]
- Peng, X.; Xia, X.; Xu, X.; Yang, X.; Yang, B.; Zhao, P.; Yuan, W.; Chiu, P.W.Y.; Bian, L. Ultrafast Self-Gelling Powder Mediates Robust Wet Adhesion to Promote Healing of Gastrointestinal Perforations. Sci. Adv. 2021, 7, eabe8739. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, T.; Yu, Z.; Wang, F.; Shi, D.; Ni, Z.; Chen, M. Effects of Surfactant and Ionic Concentration on Properties of Dual Physical Crosslinking Self-Healing Hydrogels by Hydrophobic Association and Ionic Interactions. New J. Chem. 2020, 44, 4061–4070. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Liu, R.; Liang, Z.; Yang, J.; Zhang, R.; Zhou, Z.; Nie, Y. Design of Self-Healing Rubber by Introducing Ionic Interaction to Construct a Network Composed of Ionic and Covalent Cross-Linking. Ind. Eng. Chem. Res. 2019, 58, 14848–14858. [Google Scholar] [CrossRef]
- Qin, T.; Liao, W.; Yu, L.; Zhu, J.; Wu, M.; Peng, Q.; Han, L.; Zeng, H. Recent Progress in Conductive Self-healing Hydrogels for Flexible Sensors. J. Polym. Sci. 2022, 60, 2607–2634. [Google Scholar] [CrossRef]
- Bertran, O.; Saldías, C.; Díaz, D. Molecular Dynamics Simulations on Self-Healing Behavior of Ionene Polymer-Based Nanostructured Hydrogels. Polymer 2020, 211, 123072. [Google Scholar] [CrossRef]
- Yang, B.; Song, J.; Jiang, Y.; Li, M.; Wei, J.; Qin, J.; Peng, W.; Lasaosa, F.L.; He, Y.; Mao, H. Injectable Adhesive Self-Healing Multicross-Linked Double-Network Hydrogel Facilitates Full-Thickness Skin Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 57782–57797. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.G.; Zhu, X.X. Self-Healing Supramolecular Hydrogel Made of Polymers Bearing Cholic Acid and β-Cyclodextrin Pendants. Chem. Mater. 2015, 27, 387–393. [Google Scholar] [CrossRef]
- Xiong, H.; Li, Y.; Ye, H.; Huang, G. Self-Healing Supramolecular Hydrogels through Host–Guest Interaction between Cyclodextrin and Carborane. J. Mater. Chem. B 2020, 8, 10309–10313. [Google Scholar] [CrossRef]
- Nichifor, M. Role of hydrophobic associations in self-healing hydrogels based on amphiphilic polysaccharides. Polymers 2023, 15, 1065. [Google Scholar] [CrossRef]
- Zhao, D.; Tang, Q.; Zhou, Q.; Peng, K.; Yang, H.; Zhang, X. A photo-degradable injectable self-healing hydrogel based on star poly (ethylene glycol)-b-polypeptide as a potential pharmaceuticals delivery carrier. Soft Matter 2018, 14, 7420–7428. [Google Scholar] [CrossRef]
- Kumar, A.; Biswas, A.; Jewrajka, S.K.; Anuradha. Ijectable amphiphilic hydrogel systems from the self-assembly of partially alkylated poly (2-dimethyl aminoethyl) methacrylate with inherent antimicrobial property and sustained release behaviour. Eur. Polym. J. 2022, 179, 111559. [Google Scholar] [CrossRef]
- Tang, L.; Chen, X.; Wang, L.; Qu, J. Metallo-Supramolecular Hydrogels Based on Amphiphilic Polymers Bearing a Hydrophobic Schiff Base Ligand with Rapid Self-Healing and Multi-Stimuli Responsive. Polym. Chem. 2017, 8, 4680. [Google Scholar] [CrossRef]
- Yang, X.; Guo, M.; Wu, Y.; Xue, S.; Xia, Y.; Zhang, R.; Wang, H.; Guo, Q. A facile approach for polymer hydrogels with enhanced strength, self−healing and multi−responsive shape memory properties. Mater. Res. Express 2019, 6, 125340. [Google Scholar] [CrossRef]
- Tong, X.; Du, L.; Xu, Q. Tough, adhesive and self-healing conductive 3D network hydrogel of physically linked functionalized-boron nitride/clay/poly (N-isopropylacrylamide). J. Mater. Chem. A 2018, 6, 3091–3099. [Google Scholar] [CrossRef]
- Kamiyama, Y.; Tamate, R.; Hiroi, T.; Samitsu, S.; Fujii, K.; Ueki, T. Highly Stretchable and Self-Healable Polymer Gels from Physical Entanglements of Ultrahigh–Molecular Weight Polymers. Sci. Adv. 2022, 8, eadd0226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, J.; Lang, C.; Qiao, S.; An, G.; Fan, X.; Zhao, L.; Hou, C.; Liu, J. Enzyme-Regulated Fast Self-Healing of a Pillararene-Based Hydrogel. Biomacromolecules 2017, 18, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhong, Y.; Wang, X.; Hao, J. Enzyme-Regulated Healable Polymeric Hydrogels. ACS Cent. Sci. 2020, 6, 1507–1522. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lin, Z.; Yang, Y.; Jiang, T.; Shang, J.; Luo, Z. Biocompatible conductive hydrogels: Applications in the field of biomedicine. Int. J. Mol. Sci. 2022, 23, 4578. [Google Scholar] [CrossRef]
- Mo, F.; Zhou, P.; Lin, S.; Zhong, J.; Wang, Y. A Review of Conductive Hydrogel-Based Wearable Temperature Sensors. Adv. Healthc. Mater. 2024, 2401503. [Google Scholar] [CrossRef]
- Progress, C.; Cao, J.; Wu, B.; Yuan, P.; Liu, Y.; Hu, C. Progress of Research on Conductive Hydrogels in Flexible Wearable Sensors. Gels 2024, 10, 144. [Google Scholar] [CrossRef]
- Dawit, H.; Zhao, Y.; Wang, J.; Pei, R. Advances in conductive hydrogels for neural recording and stimulation. Biomater. Sci. 2024, 12, 2786–2800. [Google Scholar] [CrossRef]
- Hsieh, J.-C.; He, W.; Venkatraghavan, D.; Schnyer, D.M.; Baird, B.; Wang, H.; Koptelova, V.B.; Ahmad, Z.J.; Pyatnitskiy, I.; Wang, W.; et al. Design of an Injectable, Self-Adhesive, and Highly Stable Hydrogel Electrode for Sleep Recording. Device 2024, 2, 100182. [Google Scholar] [CrossRef]
- Liang, Q.; Shen, Z.; Sun, X.; Yu, D.; Liu, K.; Mugo, S.M.; Chen, W.; Wang, D.; Zhang, Q.; Liang, Q.; et al. Electron Conductive and Transparent Hydrogels for Recording Brain Neural Signals and Neuromodulation. Adv. Mater. 2023, 35, 2211159. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Xu, M.; Lu, F.; Chang, Q. Adipogenesis or osteogenesis: Destiny decision made by mechanical properties of biomaterials. RSC Adv. 2022, 12, 24501–24510. [Google Scholar] [CrossRef] [PubMed]
- Şahin, B.; İlgün, G. Risk Factors of Deaths Related to Cardiovascular Diseases in World Health Organization (WHO) Member Countries. Health Soc. Care Community 2020, 30, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, S.-H.; Han, S.I.; Kang, H.; Cho, S.; Jung, D.; Lim, C.; Lim, C.; Cha, M.-J.; Lee, S.-P.; Hyeon, T.; et al. Stretchable Low-impedance Nanocomposite Comprised of Ag–Au Core–Shell Nanowires and Pt Black for Epicardial Recording and Stimulation. Adv. Mater. Technol. 2019, 5, 1900768. [Google Scholar] [CrossRef]
- He, Y.; Li, Q.; Chen, P.; Duan, Q.; Zhan, J.; Cai, X.; Wang, L.; Hou, H.; Qiu, X. A Smart Adhesive Janus Hydrogel for Non-Invasive Cardiac Repair and Tissue Adhesion Prevention. Nat. Commun. 2022, 13, 7666. [Google Scholar] [CrossRef]
- Oh, B.; Lim, Y.S.; Ko, K.W.; Seo, H.; Kim, D.J.; Kong, D.; You, J.M.; Kim, H.; Kim, T.S.; Park, S.; et al. Ultra-soft and highly stretchable tissue-adhesive hydrogel based multifunctional implantable sensor for monitoring of overactive bladder. Biosens. Bioelectron. 2023, 225, 115060. [Google Scholar] [CrossRef]
- Shi, Y.; Ding, Y.; Wang, W.; Yu, D. High performance zwitterionic hydrogels for ECG/EMG signals monitoring. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 132081. [Google Scholar] [CrossRef]
- Sunwoo, S.H.; Cha, M.J.; Han, S.I.; Kang, H.; Cho, Y.S.; Yeom, D.H.; Park, C.S.; Park, N.K.; Choi, S.W.; Kim, S.J.; et al. Ventricular Tachyarrhythmia Treatment and Prevention by Subthreshold Stimulation with Stretchable Epicardial Multichannel Electrode Array. Sci. Adv. 2023, 9, eadf6856. [Google Scholar] [CrossRef]
- Sunwoo, S.H.; Han, S.I.; Park, C.S.; Kim, J.H.; Georgiou, J.S.; Lee, S.P.; Hyeon, T. Soft bioelectronics for the management of cardiovascular diseases. Nat. Rev. Bioeng. 2024, 2, 8–24. [Google Scholar] [CrossRef]
- Yu, C.; Shi, M.; He, S.; Yao, M.; Sun, H.; Yue, Z.; Qiu, Y.; Liu, B.; Liang, L.; Zhao, Z.; et al. Chronological Adhesive Cardiac Patch for Synchronous Mechanophysiological Monitoring and Electrocoupling Therapy. Nat. Commun. 2023, 14, 6226. [Google Scholar] [CrossRef]
- Zhang, L.; Li, T.; Yu, Y.; Shi, K.; Bei, Z.; Qian, Y.; Qian, Z. An injectable conductive hydrogel restores electrical transmission at myocardial infarct site to preserve cardiac function and enhance repair. Bioact. Mater. 2023, 20, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhao, C.; Wang, Y.; Qin, Q.; Liu, Z.; Hu, Y.; Xu, Z.; Wang, K.; Jiang, X.; Lu, X. Adhesive and conductive hydrogel-based therapy simultaneously targeting neuroinflammation and neurofunctional damage after brain injury. Nano Today 2023, 51, 101934. [Google Scholar] [CrossRef]
- Fu, F.; Wang, J.; Zeng, H.; Yu, J. Functional Conductive Hydrogels for Bioelectronics. ACS Mater. Lett. 2020, 2, 1287–1301. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Cao, X.; Zhao, Y. Developing conductive hydrogels for biomedical applications. Smart Med. 2024, 3, e20230023. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, H.; Zhou, Q.; Zhou, F.; Zhang, Q.; Su, J. Smart Hydrogels for Bone Reconstruction via Modulating the Microenvironment. Research 2023, 6, 0089. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, G.; Sun, L.; Ye, F.; Shen, X.; Zhao, Y. Claw-inspired microneedle patches with liquid metal encapsulation for accelerating incisional wound healing. Chem. Eng. J. 2021, 406, 126741. [Google Scholar] [CrossRef]
- Kim, S.D.; Park, K.; Lee, S.; Kum, J.; Kim, Y.; An, S.; Kim, H.; Shin, M.; Son, D. Injectable and tissue-conformable conductive hydrogel for MRI-compatible brain-interfacing electrodes. Soft Sci. 2023, 3, 18. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D printing of conducting polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef]
- Tringides, C.M.; Vachicouras, N.; de Lázaro, I.; Wang, H.; Trouillet, A.; Seo, B.R.; Elosegui-Artola, A.; Fallegger, F.; Shin, Y.; Casiraghi, C.; et al. Viscoelastic surface electrode arrays to interface with viscoelastic tissues. Nat. Nanotechnol. 2021, 16, 1019–1029. [Google Scholar] [CrossRef]
- Huang, S.; Liu, X.; Lin, S.; Glynn, C.; Felix, K.; Sahasrabudhe, A.; Maley, C.; Xu, J.; Chen, W.; Hong, E.; et al. Control of Polymers’ Amorphous-Crystalline Transition Enables Miniaturization and Multifunctional Integration for Hydrogel Bioelectronics. Nat. Commun. 2024, 15, 3525. [Google Scholar] [CrossRef]
- Chong, J.; Sung, C.; Nam, K.S.; Kang, T.; Kim, H.; Lee, H.; Park, H.; Park, S.; Kang, J. Highly conductive tissue-like hydrogel interface through template-directed assembly. Nat. Commun. 2023, 14, 2206. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xue, Y.; Chen, X.; Zhang, P.; Shan, L.; Duan, Q.; Xing, J.; Lan, Y.; Lu, B.; Liu, J.; et al. 3D Printed Implantable Hydrogel Bioelectronics for Electrophysiological Monitoring and Electrical Modulation. Adv. Funct. Mater. 2024, 34, 2314471. [Google Scholar] [CrossRef]
- Yang, M.; Wang, L.; Liu, W.; Li, W.; Huang, Y.; Jin, Q.; Zhang, L.; Jiang, Y.; Luo, Z. Highly-Stable, Injectable, Conductive Hydrogel for Chronic Neuromodulation. Nat. Commun. 2024, 15, 7993. [Google Scholar] [CrossRef] [PubMed]





| Hydrogel Matrix | Conductive Filler | Mechanical Modulus | Conductivity | Functionality | Application | Disease Model | Chronic Application | Ref. |
|---|---|---|---|---|---|---|---|---|
| PEDOT:PSS | Au NP | 57 MPa | 670 S/cm | Monitoring, stimulation | Brain, nerve | 4 weeks | [32] | |
| HA, Tyramine | PEDOT:PSS | 0.2 MPa | 0.0179 S/cm | Biodegradable | Monitoring | Brain | 4 Weeks | [307] |
| PEDOS:PSS | 1.1 MPa | 155 S/cm | Monitoring | Brain | 2 weeks | [308] | ||
| Aiginate | Graphene, CNT | 1 MPa | 35 S/m | Self-healing | Monitoring | Brain, muscle, heart | [309] | |
| PVA | CNT | 2.8 MPa | Injectable | Monitoring, optogenetics | Brain | 10 weeks | [310] | |
| PVA | PANI | 1.35 S/m | Adhesive, self-healing, injectable | Monitoring, regeneration | Heart | 4 weeks | [300] | |
| PAA | PEDOT:PSS | 25 kPa | 247 S/cm | Adhesive | Monitoring, stimulation | Heart, muscle | 2 weeks | [311] |
| PEDOT:PSS | 650 kPa | 9 S/m | Adhesive | Monitoring, stimulation | Heart | 2 weeks | [312] | |
| HPU | PEDOT:PSS | 1 MPa | 11 S/cm | Adhesive | Monitoring, stimulation | Heart, nerve | 8 weeks | [30] |
| Gelatin | PPy | 0.00052 S/cm | Biodegradable | Regeneration | Heart | 4 weeks | [301] | |
| PVA | CNT | 2.8 Mpa | 670 S/cm | Optogenetics | 3 month | [302] | ||
| HA, PEDOT | PEDOT | 5 kOhm | Monitoring | Brain | 4 weeks | [303] | ||
| PEDOT | PEDOT | 1 MPa | 11 S/cm | Adhesive | Monitoring | Heart | 2 months | [304] |
| Phenylborate | Au NP | 0.01 S/cm | injectable | Regeneration, Monitoring | Muscle, Nerve | 4 weeks | [305] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Shin, Y.; Han, J.; Kim, H.J.; Sunwoo, S.-H. Introductory Review of Soft Implantable Bioelectronics Using Conductive and Functional Hydrogels and Hydrogel Nanocomposites. Gels 2024, 10, 614. https://doi.org/10.3390/gels10100614
Kim S, Shin Y, Han J, Kim HJ, Sunwoo S-H. Introductory Review of Soft Implantable Bioelectronics Using Conductive and Functional Hydrogels and Hydrogel Nanocomposites. Gels. 2024; 10(10):614. https://doi.org/10.3390/gels10100614
Chicago/Turabian StyleKim, San, Yumin Shin, Jaewon Han, Hye Jin Kim, and Sung-Hyuk Sunwoo. 2024. "Introductory Review of Soft Implantable Bioelectronics Using Conductive and Functional Hydrogels and Hydrogel Nanocomposites" Gels 10, no. 10: 614. https://doi.org/10.3390/gels10100614
APA StyleKim, S., Shin, Y., Han, J., Kim, H. J., & Sunwoo, S.-H. (2024). Introductory Review of Soft Implantable Bioelectronics Using Conductive and Functional Hydrogels and Hydrogel Nanocomposites. Gels, 10(10), 614. https://doi.org/10.3390/gels10100614






