Effect of Fiber Characteristics on the Structure and Properties of Quartz Fiber Felt Reinforced Silica-Polybenzoxazine Aerogel Composites
Abstract
1. Introduction
2. Results and Discussion
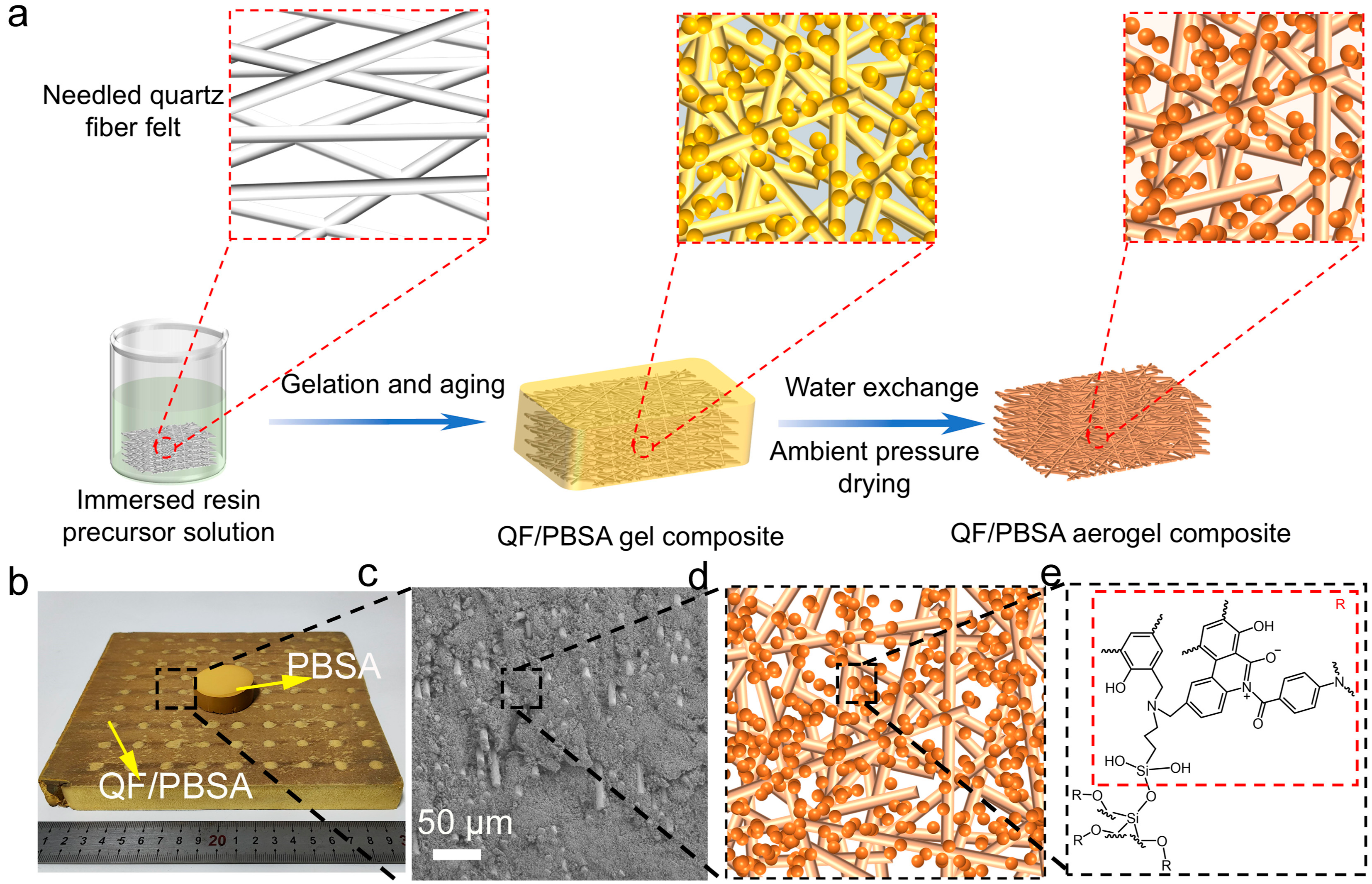
2.1. Influence of Fiber Diameter on Morphology and Textural Properties of QF/PBSAs
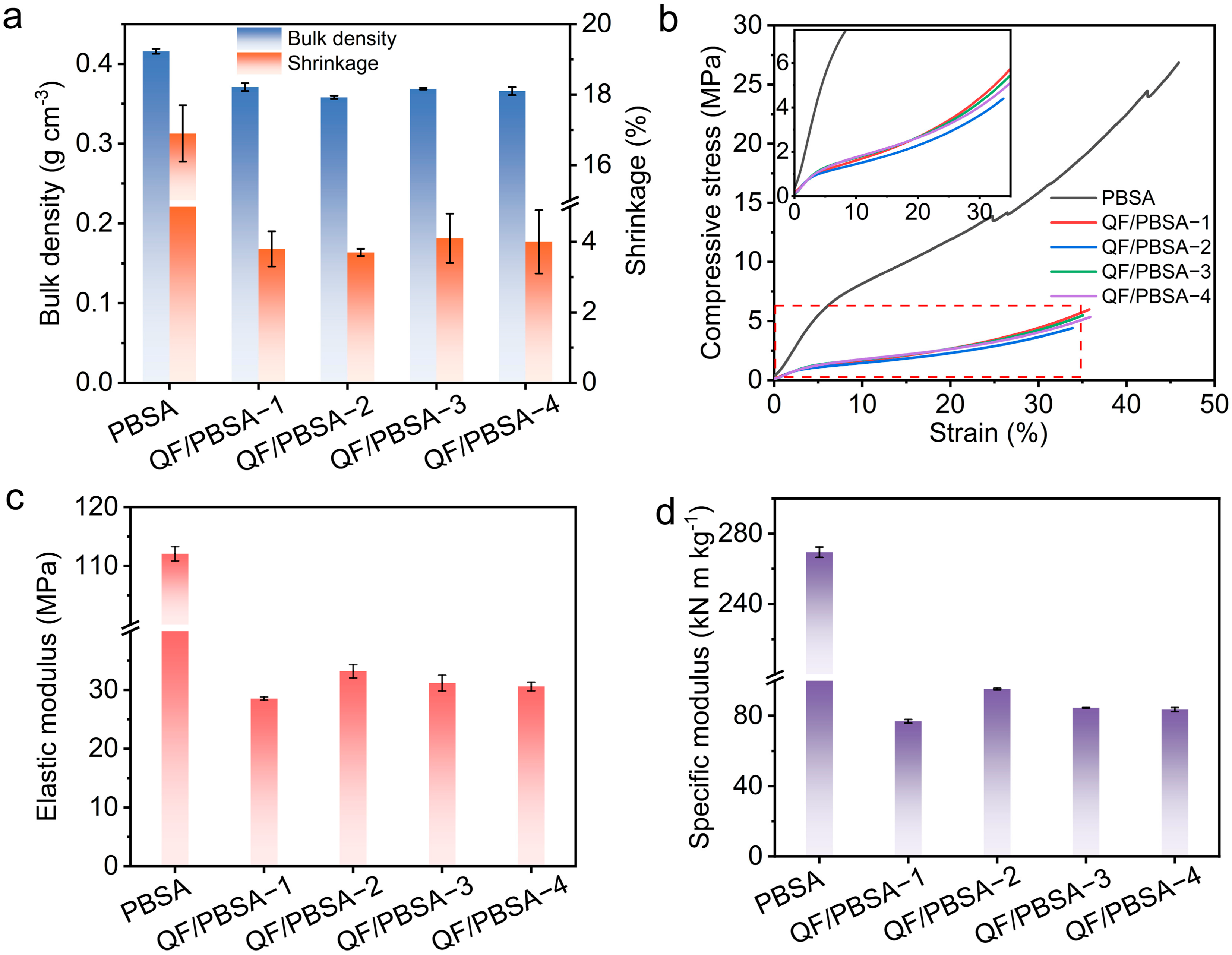
2.2. Influence of Fiber Diameter on the Mechanical Properties of QF/PBSAs
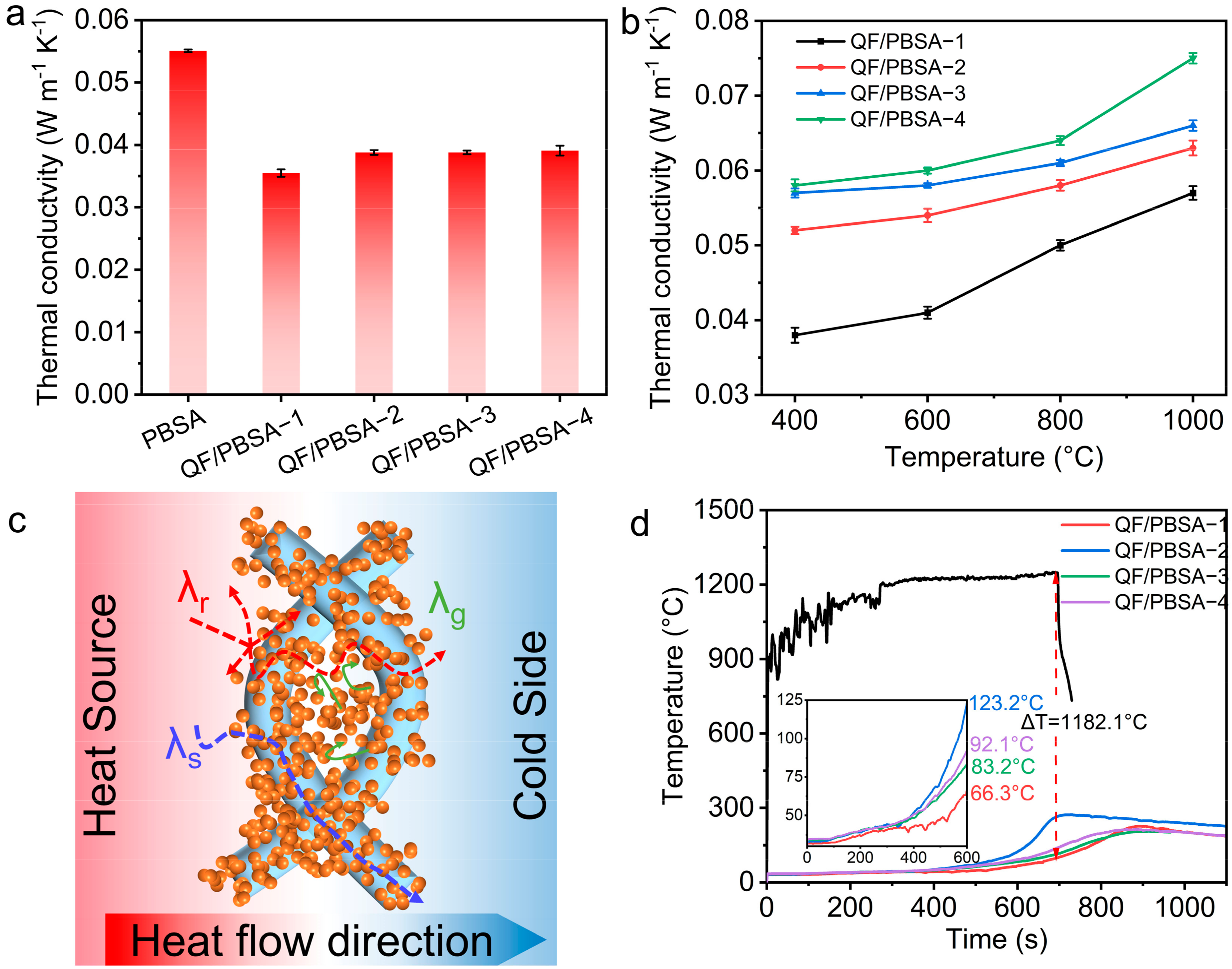
2.3. Influence of Fiber Diameter on the Thermal Insulation Properties of QF/PBSAs
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of QF/PBSAs
4.3. Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, M.; Hu, C.; Li, J.; Zhao, R.; Pang, S.; Liang, B.; Tang, S.; Liu, G.; Cheng, H.M. An Unusual Carbon–Ceramic Composite with Gradients in Composition and Porosity Delivering Outstanding Thermal Protection Performance up to 1900 °C. Adv. Funct. Mater. 2022, 32, 2204133. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, P.; Hong, C.; Zhang, B.; Hui, D. Improved ablation resistance of carbon-phenolic composites by introducing zirconium diboride particles. Compos. Part B Eng. 2013, 47, 320–325. [Google Scholar] [CrossRef]
- Zhao, S.; Pei, L.; He, J.; Zhang, X.; Hu, W.; Yan, H.; Zhao, G.; Zhang, C.; Wang, Z. Curing mechanism, thermal and ablative properties of hexa-(4-amino-phenoxy) cyclotriphosphazene/benzoxazine blends. Compos. Part B Eng. 2021, 216, 108838. [Google Scholar] [CrossRef]
- Jin, X.; Xu, J.; Pan, Y.; Wang, H.; Ma, B.; Liu, F.; Yan, X.; Wu, C.; Huang, H.; Cheng, H.; et al. Lightweight and multiscale needle quartz fiber felt reinforced siliconoxycarbide modified phenolic aerogel nanocomposite with enhanced mechanical, insulative and flame-resistant properties. Compos. Sci. Technol. 2022, 217, 109100. [Google Scholar] [CrossRef]
- Xu, J.; Hong, C.; Geng, J.; Jin, X.; Pan, Y.; Wang, H.; Luo, X.; Zhang, X. Facile synthesis, mechanical toughening, low thermal conductivity and fire-retardant of lightweight quartz fiber reinforced polymer nanocomposites. Compos. Sci. Technol. 2021, 211, 108836. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.-B.; Wang, M.; Qian, X.; Dasari, A.; Yu, Z.-Z. Phenolic resin-enhanced three-dimensional graphene aerogels and their epoxy nanocomposites with high mechanical and electromagnetic interference shielding performances. Compos. Sci. Technol. 2017, 152, 254–262. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, Y.; Tebyetekerwa, M.; Meng, S.; Zhu, M.; Lu, Y. “Stiff–Soft” Binary Synergistic Aerogels with Superflexibility and High Thermal Insulation Performance. Adv. Funct. Mater. 2019, 29, 1806407. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, R.; Liang, B.; Pang, S.; Hu, C.; Li, J.; Cheng, H.-M.; Tang, S. Construction of C/SiC–Cu3Si–Cu interpenetrating composites for long-duration thermal protection at 2500 °C by cooperative active-passive cooling. Compos. Part B Eng. 2023, 266, 111015. [Google Scholar] [CrossRef]
- Maddu, S.; Srivastava, T.; Katari, N.K.; Merugu, K.; Gundla, R.; Krishna Mohan, S. Studies on Ablative Performance of Silicone Low-Density Ablative Material. Silicon 2023, 15, 3599–3608. [Google Scholar] [CrossRef]
- Jia, X.; Song, W.; Chen, W.; Ma, C.; Wang, J.; Qiao, W.; Ling, L. Facile fabrication of lightweight mullite fiber/phenolic ablator with low thermal conductivity via ambient pressure impregnation. Ceram. Int. 2021, 47, 28032–28036. [Google Scholar] [CrossRef]
- Milos, F.S.; Chen, Y.-K. Ablation and thermal response property model validation for phenolic impregnated carbon ablator. J. Spacecr. Rocket. 2010, 47, 786–805. [Google Scholar] [CrossRef]
- Hong, C.; Han, J.; Zhang, X.; David, H.; Li, W.; Chen, Y.; Du, S. Novel phenolic impregnated 3-D Fine-woven pierced carbon fabric composites: Microstructure and ablation behavior. Compos. Part B Eng. 2012, 43, 2389–2394. [Google Scholar] [CrossRef]
- Cheng, H.; Hong, C.; Zhang, X.; Xue, H.; Meng, S.; Han, J. Super flame-retardant lightweight rime-like carbon-phenolic nanofoam. Sci. Rep. 2016, 6, 33480. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Yang, T.; Huang, Z.; Qin, Y.; Wang, Y. Thermal stability and ablation resistance, and ablation mechanism of carbon–phenolic composites with different zirconium silicide particle loadings. Compos. Part B Eng. 2018, 154, 313–320. [Google Scholar] [CrossRef]
- Wu, C.; Huang, H.; Jin, X.; Yan, X.; Wang, H.; Pan, Y.; Zhang, X.; Hong, C. Water-assisted synthesis of phenolic aerogel with superior compression and thermal insulation performance enabled by thick-united nano-structure. Chem. Eng. J. 2023, 464, 142805. [Google Scholar] [CrossRef]
- Liu, L.; Li, L.; Hu, Y.; Feng, J.; Jiang, Y.; Feng, J. High Mass Residual Silica/Polybenzoxazine Nanoporous Aerogels for High-Temperature Thermal Protection. ACS Appl. Nano Mater. 2024, 7, 19527–19537. [Google Scholar] [CrossRef]
- Mahadik-Khanolkar, S.; Donthula, S.; Bang, A.; Wisner, C.; Sotiriou-Leventis, C.; Leventis, N. Polybenzoxazine Aerogels. 2. Interpenetrating Networks with Iron Oxide and the Carbothermal Synthesis of Highly Porous Monolithic Pure Iron(0) Aerogels as Energetic Materials. Chem. Mater. 2014, 26, 1318–1331. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Ishida, H.; Qutubuddin, S. Carbon Aerogels with Excellent CO2 Adsorption Capacity Synthesized from Clay-Reinforced Biobased Chitosan-Polybenzoxazine Nanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 1286–1295. [Google Scholar] [CrossRef]
- Lorjai, P.; Chaisuwan, T.; Wongkasemjit, S. Porous structure of polybenzoxazine-based organic aerogel prepared by sol–gel process and their carbon aerogels. J. Sol.-Gel. Sci. Technol. 2009, 52, 56–64. [Google Scholar] [CrossRef]
- Periyasamy, T.; Asrafali, S.P.; Lee, J. High-Performance Supercapacitor Electrodes from Fully Biomass-Based Polybenzoxazine Aerogels with Porous Carbon Structure. Gels 2024, 10, 462. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, L.; Cai, H.; Liu, F.; Zhang, S.; Feng, J.; Jiang, Y.; Feng, J. In situ co-polymerization of high-performance polybenzoxazine/silica aerogels for flame-retardancy and thermal insulation. J. Appl. Polym. Sci. 2020, 138, e50333. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, L.; Liu, F.; Zhang, S.; Feng, J.; Jiang, Y.; Feng, J. Compressible, Flame-Resistant and Thermally Insulating Fiber-Reinforced Polybenzoxazine Aerogel Composites. Materials 2020, 13, 2809. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Han, Y.; Chen, F.; Luo, Z.; Li, H.; Zhao, T. The effect of structure on thermal stability and anti-oxidation mechanism of silicone modified phenolic resin. Polym. Degrad. Stabil. 2016, 124, 68–76. [Google Scholar] [CrossRef]
- Dueramae, I.; Jubsilp, C.; Takeichi, T.; Rimdusit, S. High thermal and mechanical properties enhancement obtained in highly filled polybenzoxazine nanocomposites with fumed silica. Compos. Part B Eng. 2014, 56, 197–206. [Google Scholar] [CrossRef]
- Wu, C.; Chen, Z.; Wang, F.; Hu, Y.; Rao, Z.; Wang, E.; Zhang, X. Preparation and characterization of ultralight glass fiber wool/phenolic resin aerogels with a spring-like structure. Compos. Sci. Technol. 2019, 179, 125–133. [Google Scholar] [CrossRef]
- Wang, H.; Quan, X.; Yin, L.; Jin, X.; Pan, Y.; Wu, C.; Huang, H.; Hong, C.; Zhang, X. Lightweight quartz fiber fabric reinforced phenolic aerogel with surface densified and graded structure for high temperature thermal protection. Compos. Part A Appl. Sci. Manuf. 2022, 159, 107022. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, Z.; Hong, C.; Zhang, X. Lightweight multiscale hybrid carbon-quartz fiber fabric reinforced phenolic-silica aerogel nanocomposite for high temperature thermal protection. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106313. [Google Scholar] [CrossRef]
- Liu, F.; He, C.; Jiang, Y.; Feng, J.; Li, L.; Tang, G.; Feng, J. Ultralight Ceramic Fiber Aerogel for High-Temperature Thermal Superinsulation. Nanomaterials 2023, 13, 380. [Google Scholar] [CrossRef]
- Liang, H.W.; Guan, Q.F.; Chen, L.F.; Zhu, Z.; Zhang, W.J.; Yu, S.H. Macroscopic-scale template synthesis of robust carbonaceous nanofiber hydrogels and aerogels and their applications. Angew. Chem. Int. Ed. Engl. 2012, 51, 5101–5105. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, H.; Zhan, G.; Liu, X.; Yang, Y.; Zhuang, Q.; Qian, J. Preparing Multifunctional High-Performance Cross-Linked Polybenzoxazole Aerogels from Polybenzoxazine. ACS Appl. Mater. Interfaces 2021, 3, 2352–2362. [Google Scholar] [CrossRef]
- Liu, L.; Li, L.; Hu, Y.; Liu, F.; Jiang, Y.; Feng, J.; Feng, J. Facile preparation of low shrinkage polybenzoxazine aerogels for high efficiency thermal insulation. Sci. China Mater. 2024. [Google Scholar] [CrossRef]
- Mahadik-Khanolkar, S.; Donthula, S.; Sotiriou-Leventis, C.; Leventis, N. Polybenzoxazine Aerogels. 1. High-Yield Room-Temperature Acid-Catalyzed Synthesis of Robust Monoliths, Oxidative Aromatization, and Conversion to Microporous Carbons. Chem. Mater. 2014, 26, 1303–1317. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Lin, X.C.; Li, S.L.; Li, W.X.; Wang, Z.H.; Zhang, J.Y.; Liu, B.W.; Fu, T.; Zhao, H.B.; Wang, Y.Z. Thermo-Responsive Self-Ceramifiable Robust Aerogel with Exceptional Strengthening and Thermal Insulating Performance at Ultrahigh Temperatures. Adv. Funct. Mater. 2023, 33, 2214913. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Cao, L.; Shi, Y.; Yang, H.; Yang, R.; Xie, F.; Zhang, X. Biomimetic porous silicon oxycarbide ceramics with improved specific strength and efficient thermal insulation. J. Mater. Sci. Technol. 2024, 168, 185–193. [Google Scholar] [CrossRef]
- Wang, W.; You, Q.; Wu, Z.; Cui, S.; Shen, W. Fabrication of the SiC/HfC Composite Aerogel with Ultra-Low Thermal Conductivity and Excellent Compressive Strength. Gels 2024, 10, 292. [Google Scholar] [CrossRef]
- Ge, R.; Zhang, J.; Yang, N.; Fan, Z.; Yin, R.; Cheng, H.; Hong, C.; Zhang, X. Constructing lightweight ternary interpenetrating network of carbon fabric/siloxane/phenolic aerogels for long-time high-temperature thermal protection. Compos. Commun. 2024, 48, 101914. [Google Scholar] [CrossRef]
- Yang, D.; Dong, S.; Xin, J.; Liu, C.; Hu, P.; Xia, L.; Hong, C.; Zhang, X. Robust and thermostable C/SiOC composite aerogel for efficient microwave absorption, thermal insulation and flame retardancy. Chem. Eng. J. 2023, 469, 143851. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Zhang, Z.; Shi, Y.; Zhao, Y.; Shen, S.; Yao, X.; Shen, J. A Facile Method for Fabricating a Monolithic Mullite Fiber-Reinforced Alumina Aerogel with Excellent Mechanical and Thermal Properties. Gels 2022, 8, 380. [Google Scholar] [CrossRef]
- Hu, F.; Wu, S.; Sun, Y. Hollow-Structured Materials for Thermal Insulation. Adv. Mater. 2019, 31, e1801001. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Zhu, C.-Y.; Zhao, X.-P. A theoretical and numerical study on the gas-contributed thermal conductivity in aerogel. Int. J. Heat. Mass. Transf. 2017, 108, 1982–1990. [Google Scholar] [CrossRef]
- Huang, B.; Li, J.; Gong, L.; Dai, P.; Zhu, C. The Influence of Reinforced Fibers and Opacifiers on the Effective Thermal Conductivity of Silica Aerogels. Gels 2024, 10, 300. [Google Scholar] [CrossRef] [PubMed]
- Merillas, B.; Gómez Álvarez-Arenas, T.E.; Villafañe, F.; Rodríguez-Pérez, M.Á. Reaching a near zero radiative heat transfer by the inclusion of modified multiwalled-carbon nanotubes (MWCNTs) in polyurethane-polyisocyanurate aerogels. Mater. Today Chem. 2023, 34, 101789. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, A.; Ren, J.; Liu, Y.; Han, R. Mechanically robust hydrogel from thermosetting polybenzoxazine with dual crosslinking network. Polymer 2023, 289, 126490. [Google Scholar] [CrossRef]
- Yin, R.; Cheng, H.; Hong, C.; Zhang, X. Synthesis and characterization of novel phenolic resin/silicone hybrid aerogel composites with enhanced thermal, mechanical and ablative properties. Compos. Part A Appl. Sci. Manuf. 2017, 101, 500–510. [Google Scholar] [CrossRef]

| Samples | SBET (m2 g−1) | Smeso (m2 g−1) | Smic (m2 g−1) | Vtotal (cm3 g−1) | V1.7~300nm (cm3 g−1) | Vmic (cm3 g−1) | Porosity (%) | Average Pore Diameter (nm) | |
|---|---|---|---|---|---|---|---|---|---|
| 4Vtotal/SBET | from Hg Intrusion | ||||||||
| PBSA | 87.74 | 78.39 | 9.35 | 1.82 | 0.5343 | 0.0039 | 75.8 | 83.1 | 42.2 |
| QF/PBSA−1 | 104.45 | 88.28 | 16.17 | 2.17 | 0.5396 | 0.0078 | 80.4 | 80.9 | 107.3 |
| QF/PBSA−2 | 71.52 | 60.58 | 10.94 | 2.28 | 0.3314 | 0.0057 | 81.1 | 127.3 | 180.7 |
| QF/PBSA−3 | 120.47 | 120.47 | 0 | 2.19 | 0.3332 | 0 | 80.5 | 72.6 | 103.2 |
| QF/PBSA−4 | 147.19 | 147.19 | 0 | 2.21 | 0.3770 | 0 | 80.7 | 60.1 | 108.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Li, L.; Hu, Y.; Feng, J.; Jiang, Y.; Feng, J. Effect of Fiber Characteristics on the Structure and Properties of Quartz Fiber Felt Reinforced Silica-Polybenzoxazine Aerogel Composites. Gels 2024, 10, 613. https://doi.org/10.3390/gels10100613
Liu L, Li L, Hu Y, Feng J, Jiang Y, Feng J. Effect of Fiber Characteristics on the Structure and Properties of Quartz Fiber Felt Reinforced Silica-Polybenzoxazine Aerogel Composites. Gels. 2024; 10(10):613. https://doi.org/10.3390/gels10100613
Chicago/Turabian StyleLiu, Lanfang, Liangjun Li, Yijie Hu, Junzong Feng, Yonggang Jiang, and Jian Feng. 2024. "Effect of Fiber Characteristics on the Structure and Properties of Quartz Fiber Felt Reinforced Silica-Polybenzoxazine Aerogel Composites" Gels 10, no. 10: 613. https://doi.org/10.3390/gels10100613
APA StyleLiu, L., Li, L., Hu, Y., Feng, J., Jiang, Y., & Feng, J. (2024). Effect of Fiber Characteristics on the Structure and Properties of Quartz Fiber Felt Reinforced Silica-Polybenzoxazine Aerogel Composites. Gels, 10(10), 613. https://doi.org/10.3390/gels10100613






