Biosynthesis and Characterization of Cross-Linked Fmoc Peptide-Based Hydrogels for Drug Delivery Applications
Abstract
:1. Introduction
2. Results and Discussion
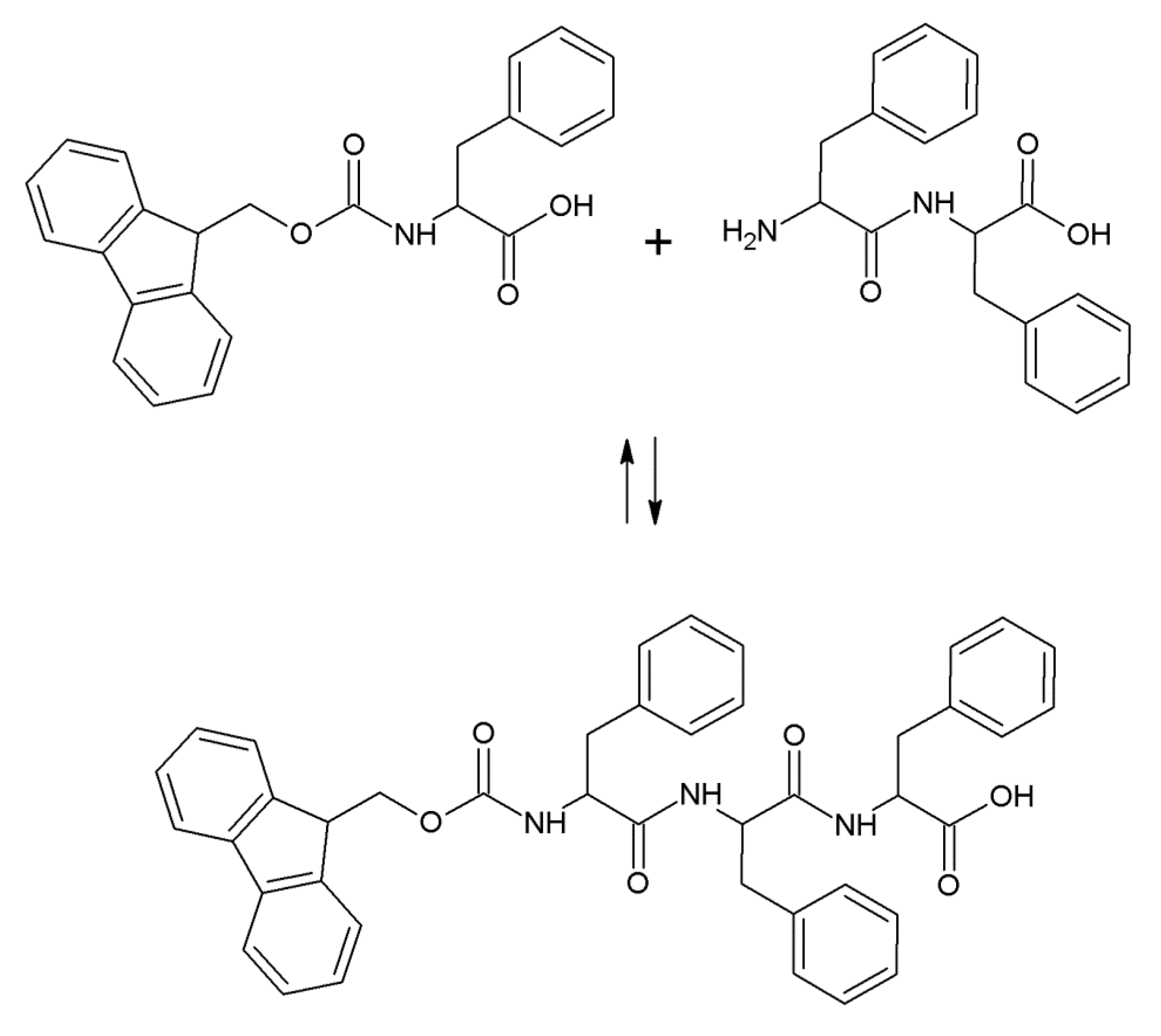
2.1. Hydrogel Biosynthesis
| Material | Reaction Yield (%) |
|---|---|
| FmocF*F*F* | 50 ± 2 |
| Genipin-crosslinked FmocF*F*F* | 53 ± 3 |
| FmocFFF | 27 ± 2 |
| Genipin-crosslinked FmocFFF | 32 ± 2 |
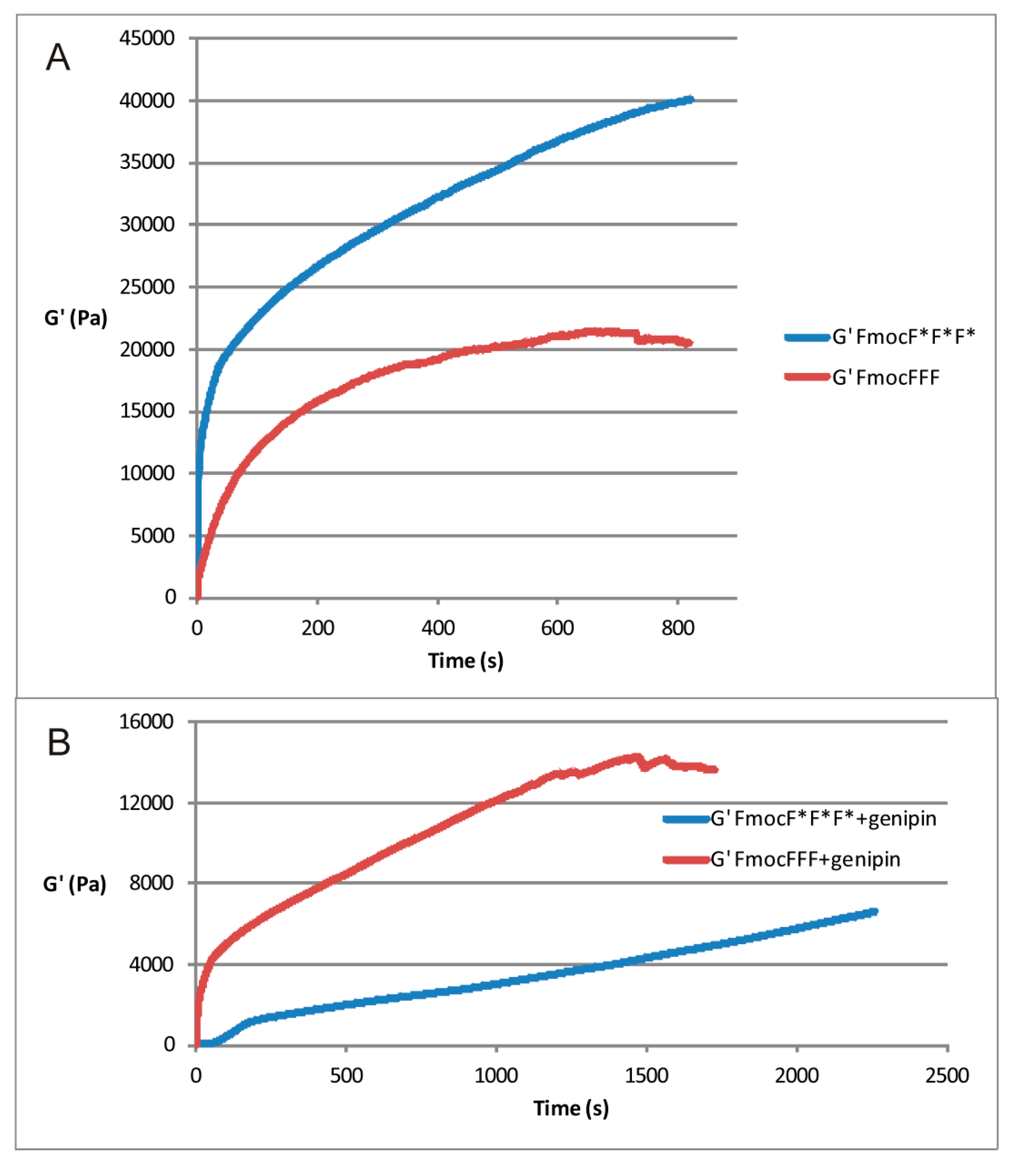
2.2. Rheological Measurements

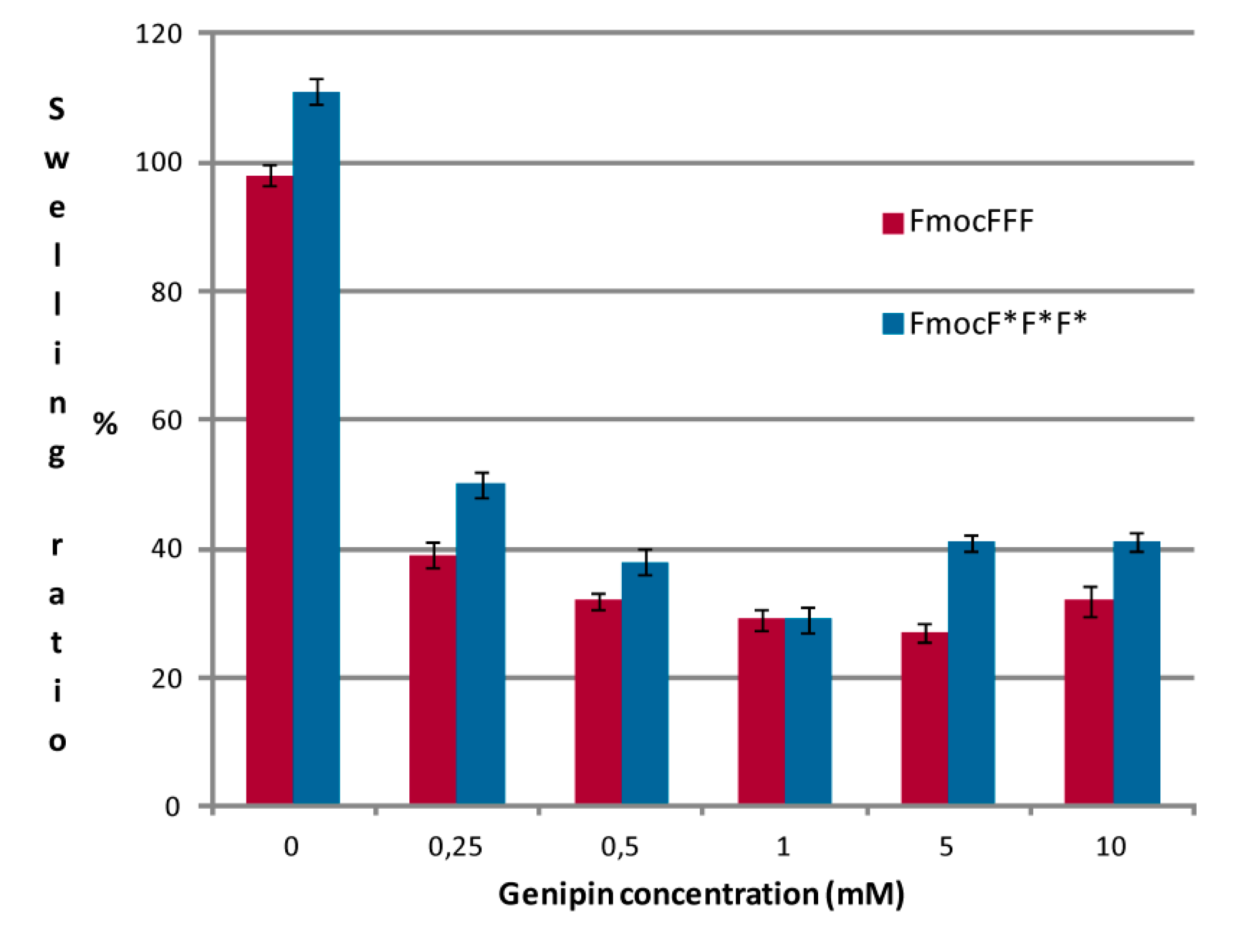
2.3. Swelling Ratio and Weight Loss Ratio Measurements

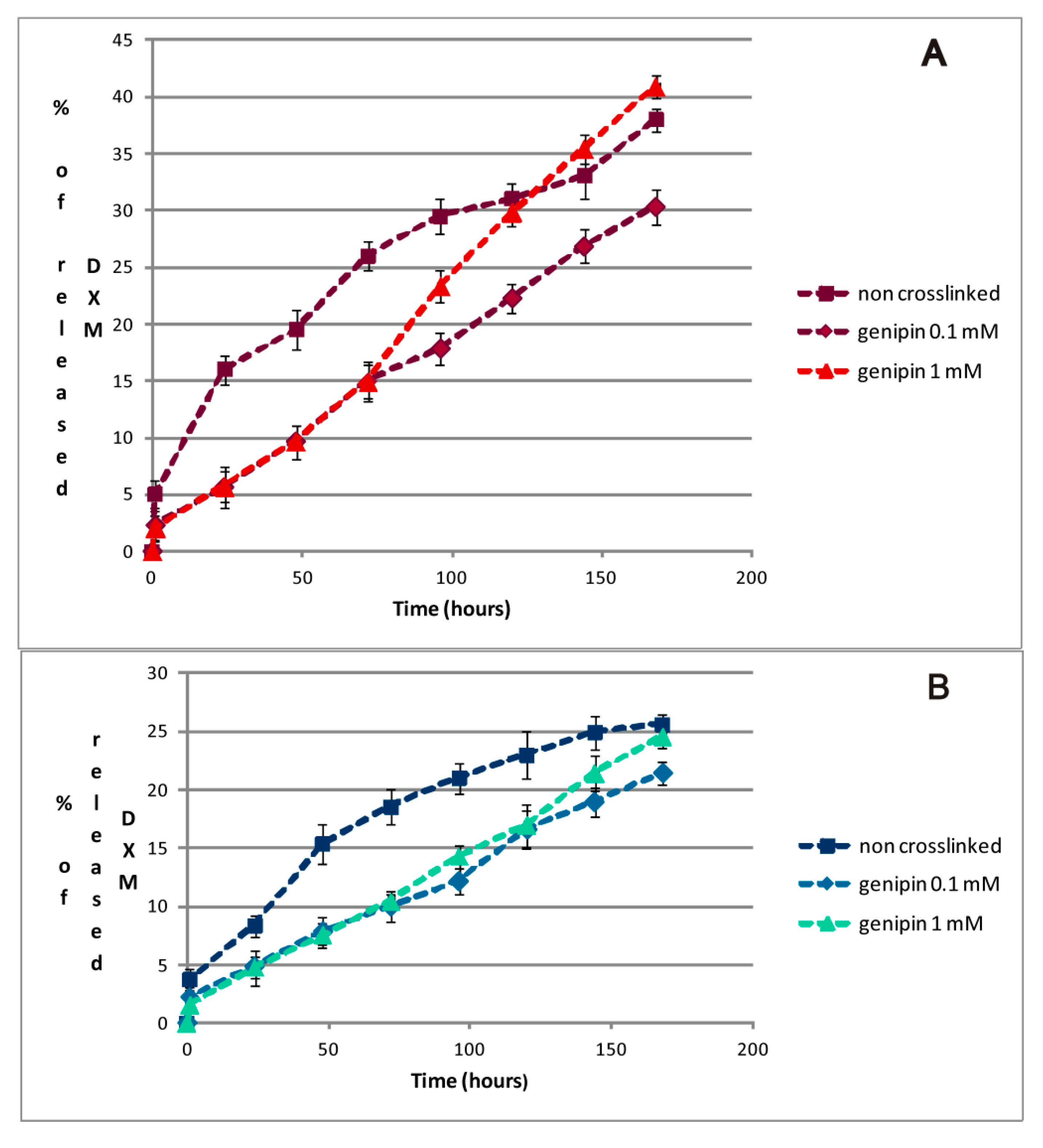
2.4. DXM in Vitro Release Studies
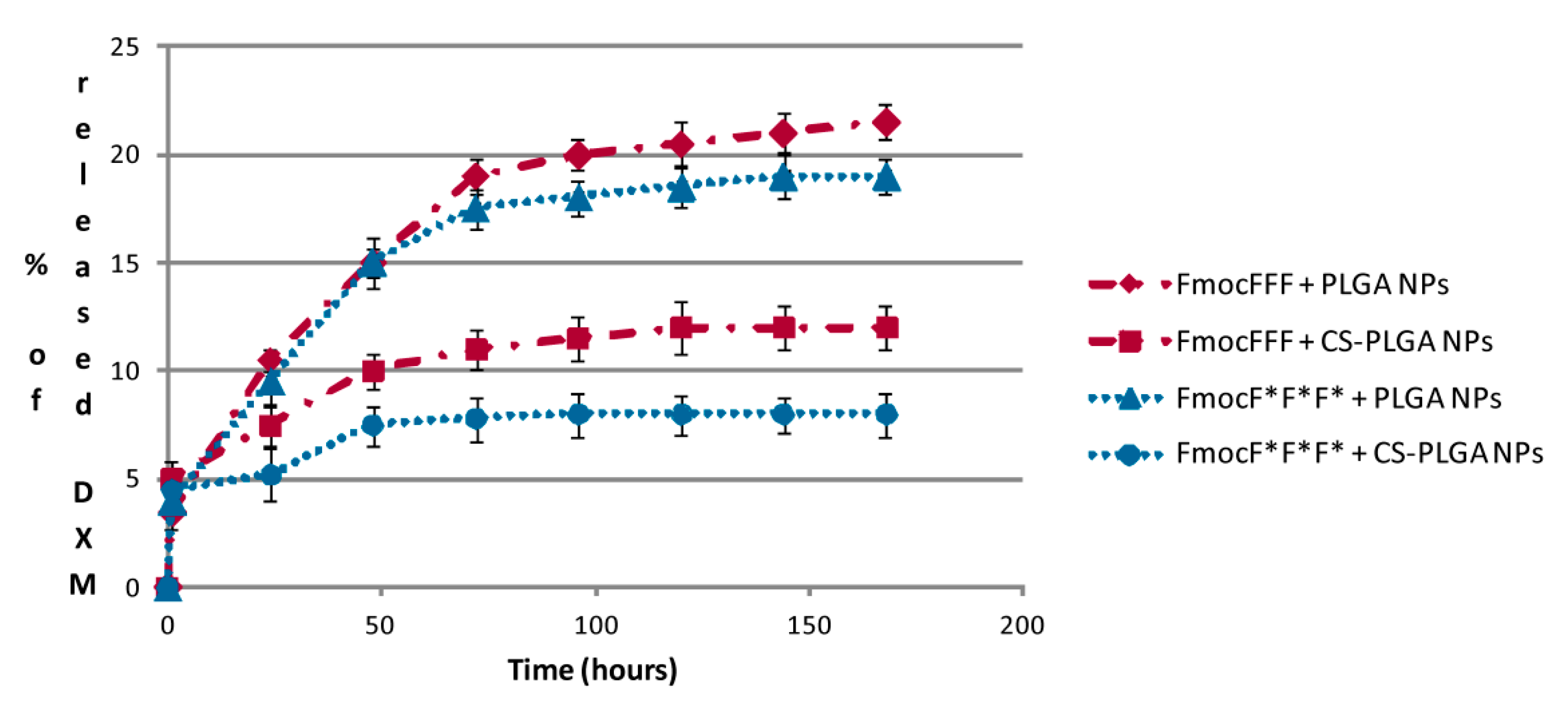
2.5. SEM and AFM Measurements
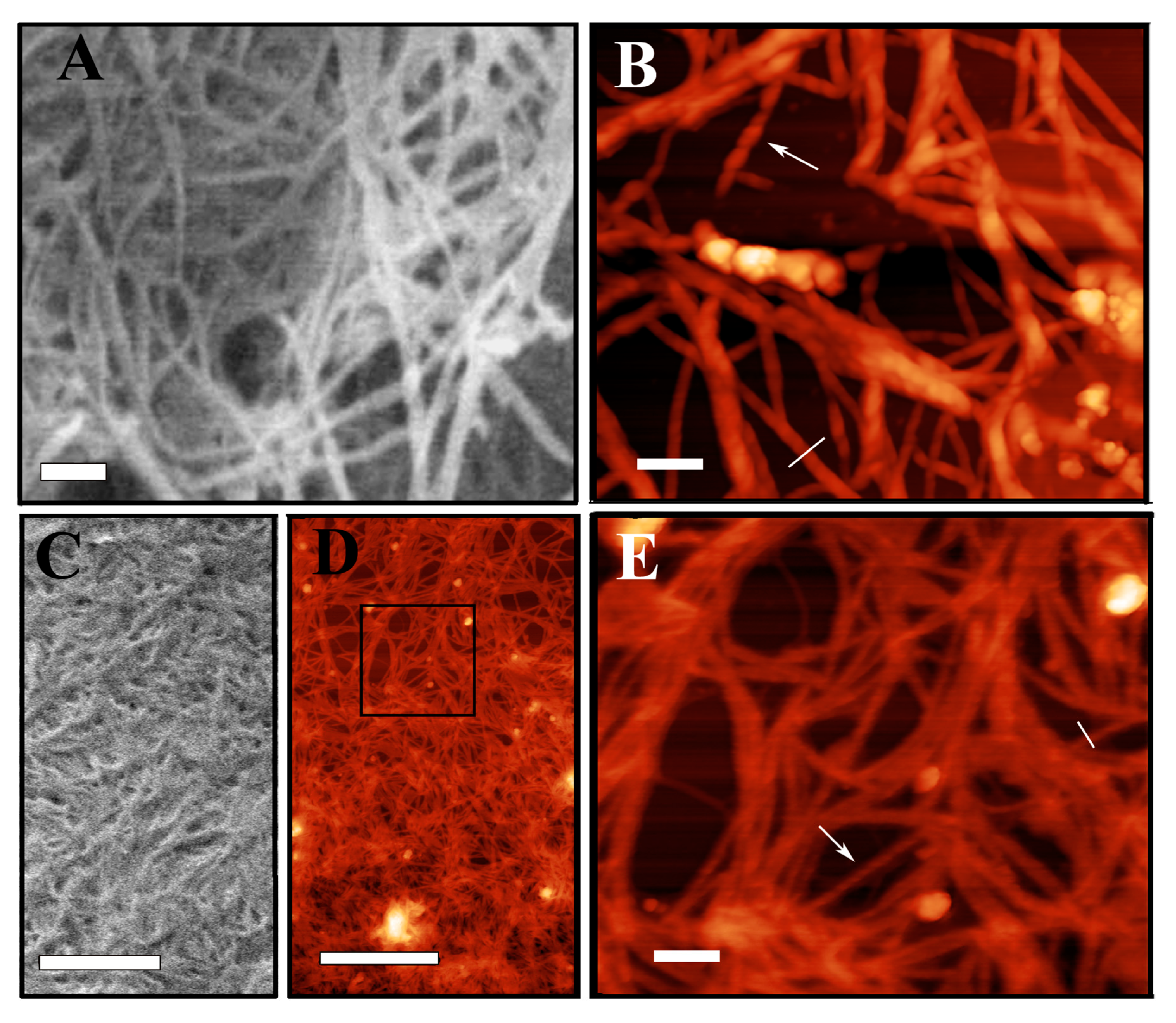
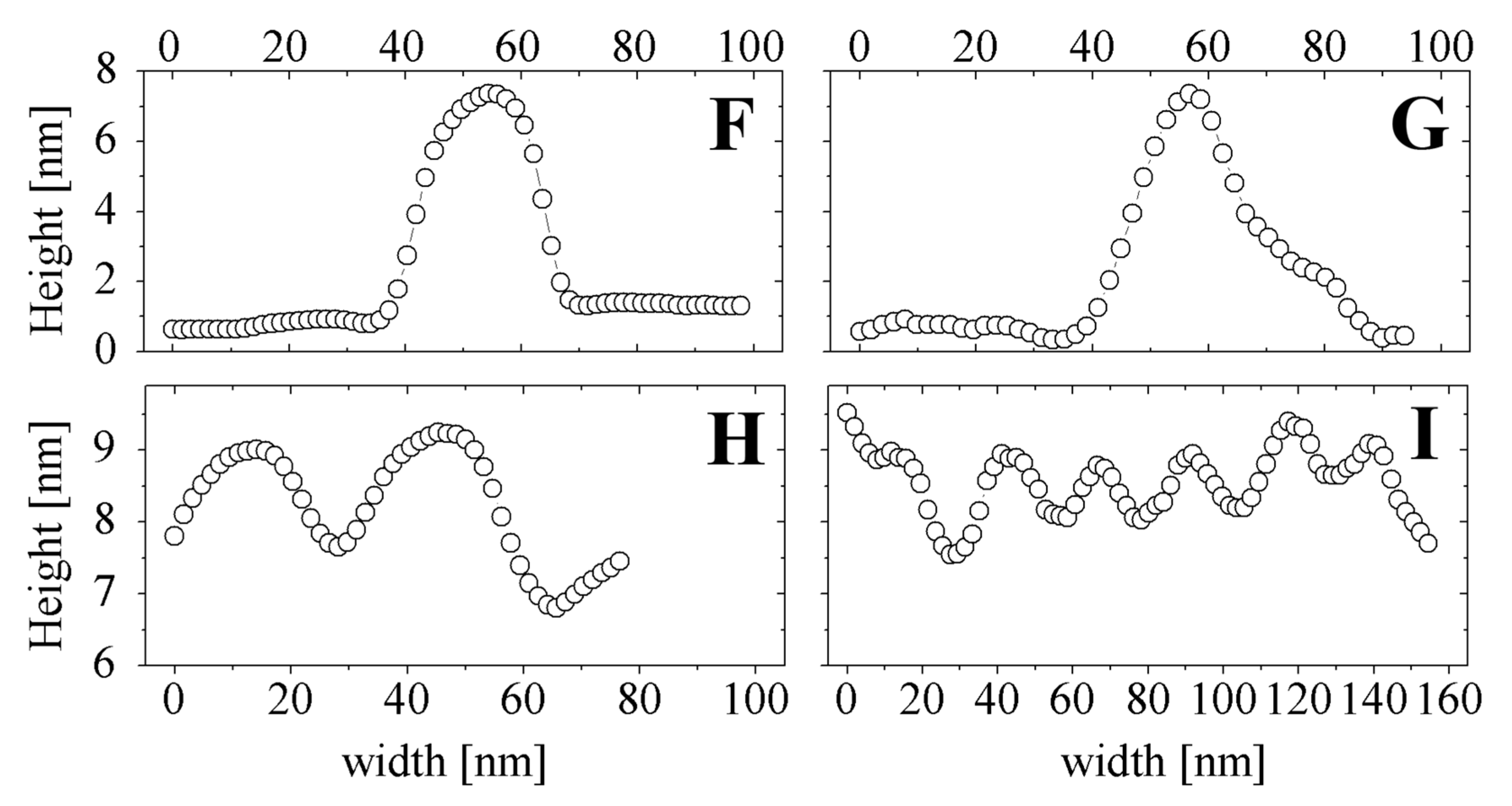
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Biosynthesis of Peptide Hydrogels
4.3. Rheological Measurements
4.4. Swelling and Stability Studies
4.5. DXM in Vitro Release Experiments
4.6. SEM and AFM Measurements
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sinagoga, K.L.; Wells, J.M. Generating human intestinal tissues from pluripotent stem cells to study development and disease. EMBO J. 2015, 34, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Solanki, A.; Hamilos, A.; Levy, O.; Wen, K.; Yin, X.; Karp, J.M. Application of biomaterials to advance induced pluripotent stem cell research and therapy. EMBO J. 2015, 34, 987–1008. [Google Scholar] [CrossRef] [PubMed]
- Kirchmajer, D.M.; Gorkin, R., III; in het Panhuis, M. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. J. Mater. Chem. B Mater. Biol. Med. 2015, 3, 4105–4117. [Google Scholar] [CrossRef]
- Kehr, N.S.; Prasetyanto, E.A.; Benson, K.; Ergün, B.; Galstyan, A.; Galla, H.J. Periodic mesoporous organosilica-based nanocomposite hydrogels as three-dimensional scaffolds. Angew. Chem. Int. Ed. Engl. 2013, 52, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Asghar, W.; Kim, Y.T.; Ilyas, A.; Sankaran, J.; Wan, Y.; Iqbal, S.M. Synthesis of nano-textured biocompatible scaffolds from chicken eggshells. Nanotechnology 2012, 23, 475601–475609. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.M.; Silva, T.H.; Reis, R.L.; Mano, J.F. Hierarchical fibrillar scaffolds obtained by non-conventional layer-by-layer electrostatic self-assembly. Adv. Healthc. Mater. 2013, 2, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.C.; Baran, E.T.; Lisboa, P.; Reis, R.L.; Azevedo, H.S. Microfluidic fabrication of self-assembled peptide-polysaccharide microcapsules as 3D environments for cell culture. Biomacromolecules 2012, 13, 4039–4048. [Google Scholar] [CrossRef] [PubMed]
- Mehrban, N.; Zhu, B.; Tamagnini, F.; Young, F.I.; Wasmuth, A.; Hudson, K.L.; Thomson, A.R.; Birchall, M.A.; Randall, A.D.; Song, B.; et al. Functionalized α-helical peptide hydrogels for neural tissue engineering. ACS Biomater. Sci. Eng. 2015, 1, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.H.; Li, S.; Howard, O.M.Z.; Dunlap, M.; Trivett, A.; Schneider, J.P.; Oppenheim, J.J. Enhanced immunostimulatory effects of DNA-encapsulated peptide hydrogels. Biomaterials 2015, 53, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Lorenzoni, S.; Masci, G.; Dentini, M.; Togna, A.R.; Togna, G.; Bordi, F.; Palocci, C. Lipase-supported synthesis of peptidic hydrogels. Soft Matter 2010, 6, 2525–2532. [Google Scholar] [CrossRef]
- Yamamoto, M.; Miura, N.; Ohtake, N.; Amagaya, S.; Ishige, A.; Sasaki, H.; Komatsu, Y.; Fukuda, K.; Ito, T.; Terasawa, K. Genipin, a metabolite derived from the herbal medicine Inchin-ko-to, and suppression of Fas-induced lethal liver apoptosis in mice. Gastroenterology 2000, 118, 380–389. [Google Scholar] [CrossRef]
- Bae, Y.A.; Chang, I.M.; Lee, N.K. Dyeing method, blue-dye production method, and a kit therefor. PCT Int. Appl. WO 2012060522 A1, 10 May 2012. [Google Scholar]
- Touyama, R.; Inoue, K.; Takeda, Y.; Yatsuzuka, M.; Ikumoto, T.; Moritome, N.; Shingu, T.; Yokoi, T.; Inouye, H. Studies on the blue pigments produced from genipin and methylamine. II. On the formation mechanisms of brownish-red intermediates leading to the blue pigment formation. Chem. Pharm. Bull. 1994, 42, 1571–1578. [Google Scholar] [CrossRef]
- Mehta, G.K.; Kondaveeti, S.; Siddhanta, A.K. Facile synthesis of agarose-l-phenylalanine ester hydrogels. Polym. Chem. 2011, 2, 2334–2340. [Google Scholar] [CrossRef]
- Bedran-Russo, A.K.; Pereira, P.N.; Duarte, W.R.; Drummond, J.L.; Yamauchi, M. Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Schek, R.M.; Michalek, A.J.; Iatridis, J.C. Genipin-crosslinked fibrin hydrogels as a potential adhesive to augment intervertebral disc annulus repair. Eur. Cell Mater. 2011, 21, 373–383. [Google Scholar] [PubMed]
- Baiguera, S.; Del Gaudio, C.; Lucatelli, E.; Kuevda, E.; Boieri, M.; Mazzanti, B.; Bianco, A.; Macchiarini, P. Electrospun gelatin scaffolds incorporating rat decellularized brain extracellular matrix for neural tissue engineering. Biomaterials 2014, 35, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.S.; Chen, P.S.; Hsieh, P.L.; Chen, B.Y. Silicification of genipin-cross-linked polypeptide hydrogels toward biohybrid materials and mesoporous oxides. ACS Appl. Mater. Interfaces 2012, 4, 6865–6874. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Togna, A.R.; Guarguaglini, G.; Masci, G.; Giammaruco, F.; Togna, G.I.; Palocci, C. Self-assembling peptide hydrogels promote microglial cells proliferation and NGF production. Soft Matter 2012, 8, 5784–5790. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Sennato, S.; Bordi, F.; Giannella, D.; Di Nitto, A.; Barbetta, A.; Dentini, M.; Togna, A.R.; Togna, G.I.; Moschini, S.; et al. Designing unconventional Fmoc-peptide-based biomaterials: Structure and related properties. Soft Matter 2014, 10, 1944–1952. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.; Prasad, K.; Siddhanta, A.K. Development of a stable hydrogel network based on agar– kappa-carrageenan blend cross-linked with genipin. Food Hydrocoll. 2009, 23, 497–509. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Massimi, M.; Giardi, M.F.; Cametti, C.; Conti Devirgiliis, L.; Dentini, M.; Palocci, C. Chitosan-coated PLGA nanoparticles: a sustained drug release strategy for cell cultures. Colloids Surf. B Biointerfaces 2013, 103, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Fratoddi, I.; Palocci, C.; Venditti, I.; Russo, M.V. Osmosis based method drives the self-assembly of polymeric chains into micro- and nanostructures. Langmuir 2009, 25, 11940–11946. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Cutonilli, A.; Cametti, C.; Dentini, M.; Palocci, C. PLGA-based nanoparticles: Effect of chitosan in the aggregate stabilization. A dielectric relaxation spectroscopy study. Colloids Surf. B Biointerfaces 2012, 97, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, R. Science of Microscopy; Hawkes, P.W., Spence, J.C.H., Eds.; Springer: New York, NY, USA, 2007; Volume I, pp. 133–272. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chronopoulou, L.; Margheritelli, S.; Toumia, Y.; Paradossi, G.; Bordi, F.; Sennato, S.; Palocci, C. Biosynthesis and Characterization of Cross-Linked Fmoc Peptide-Based Hydrogels for Drug Delivery Applications. Gels 2015, 1, 179-193. https://doi.org/10.3390/gels1020179
Chronopoulou L, Margheritelli S, Toumia Y, Paradossi G, Bordi F, Sennato S, Palocci C. Biosynthesis and Characterization of Cross-Linked Fmoc Peptide-Based Hydrogels for Drug Delivery Applications. Gels. 2015; 1(2):179-193. https://doi.org/10.3390/gels1020179
Chicago/Turabian StyleChronopoulou, Laura, Silvia Margheritelli, Yosra Toumia, Gaio Paradossi, Federico Bordi, Simona Sennato, and Cleofe Palocci. 2015. "Biosynthesis and Characterization of Cross-Linked Fmoc Peptide-Based Hydrogels for Drug Delivery Applications" Gels 1, no. 2: 179-193. https://doi.org/10.3390/gels1020179
APA StyleChronopoulou, L., Margheritelli, S., Toumia, Y., Paradossi, G., Bordi, F., Sennato, S., & Palocci, C. (2015). Biosynthesis and Characterization of Cross-Linked Fmoc Peptide-Based Hydrogels for Drug Delivery Applications. Gels, 1(2), 179-193. https://doi.org/10.3390/gels1020179









