Influence of Suillus grevillea on the Root Morphology, Growth and Rhizosphere Soil Properties of Quercus variabilis Blume Seedlings with Root Pruning
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Indicator Determination Method
2.3.1. Biomass
2.3.2. Mycorrhizal Colonization
2.3.3. Root Morphology
2.3.4. Root Endogenous Hormone Content
2.3.5. Nutrient Contents
2.3.6. Soil Physiochemical Properties
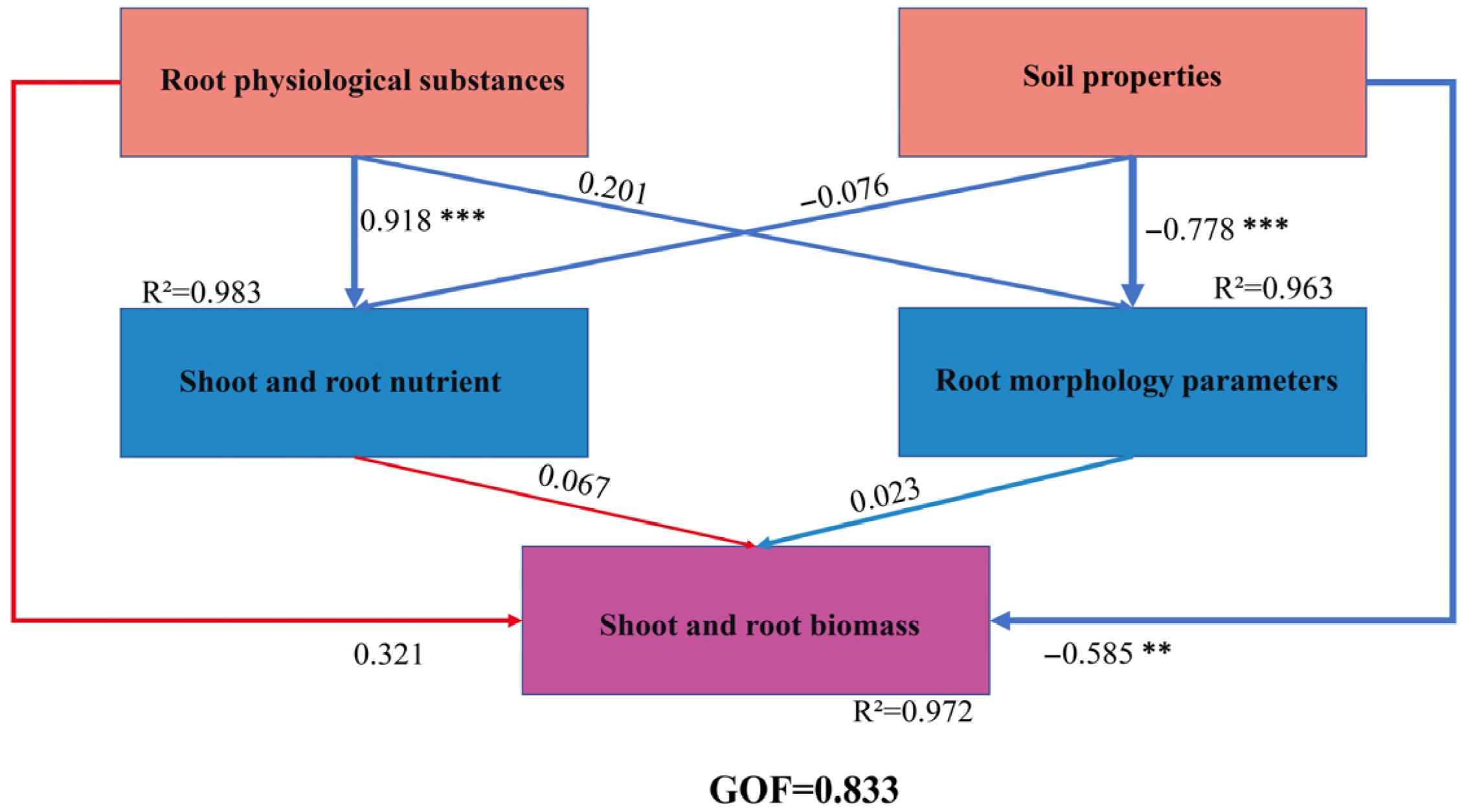
2.4. PLS-SEM Modeling
2.5. Statistical Methods
3. Results
3.1. Mycorrhizal Colonization and Mycelial Density
3.2. Biomass of Seedlings
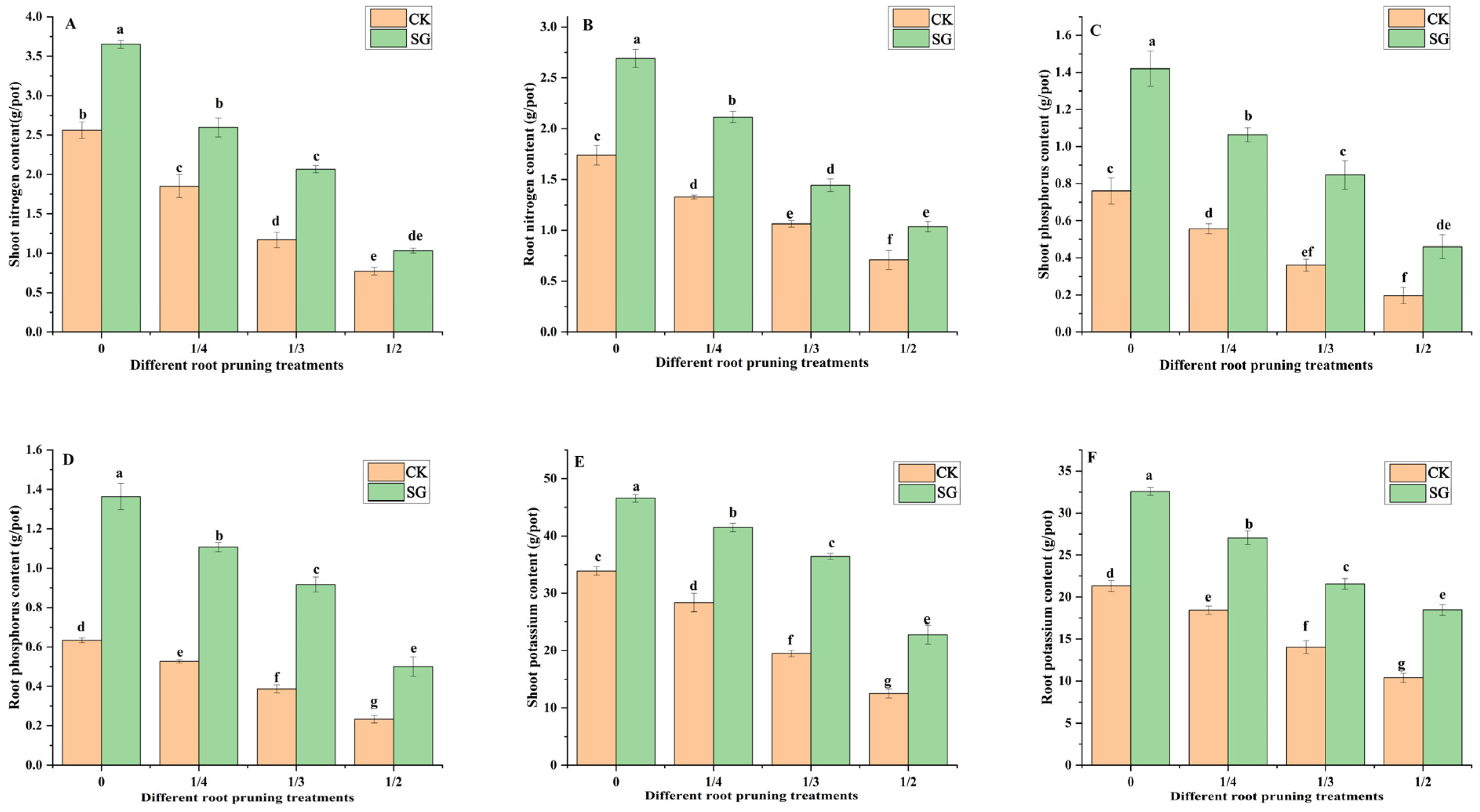
3.3. Mineral Nutrition of the Seedlings
3.4. The Root Morphology of Seedlings
3.5. Endogenous Hormones in the Roots of the Seedlings
3.6. Correlations Between Root Morphology and Other Growth Indicators
3.7. Soil Properties
| Inoculation Treatment | Different Root Pruning Treatments | pH | Conductivity (µS/cm) | Available Phosphorus Content (mg/kg) | Available Nitrogen Content (mg/kg) | Available Potassium Content (mg/kg) | Total Nitrogen Content (%) | Total Carbon Content (%) |
|---|---|---|---|---|---|---|---|---|
| R | 0 | 7.90 ± 0.03 a | 484.33 ± 4.63 cd | 18.91 ± 0.33 g | 535.33 ± 4.06 g | 13.76 ± 0.9 f | 0.37 ± 0.01 g | 2.46 ± 0.06 f |
| M | 0 | 6.87 ± 0.12 f | 794.67 ± 30.9 a | 32.43 ± 0.67 e | 916.67 ± 23.97 a | 31.52 ± 0.44 c | 0.84 ± 0.01 a | 4.68 ± 0.10 a |
| H | 0 | 7.35 ± 0.06 c | 629.67 ± 51.85 b | 26.11 ± 1.06 f | 840 ± 8.14 b | 28.05 ± 0.89 d | 0.64 ± 0.05 d | 3.68 ± 0.05 c |
| R | 1/4 | 7.58 ± 0.03 b | 354 ± 9.85 e | 27.69 ± 1.23 f | 623.67 ± 6.94 f | 22.74 ± 0.88 e | 0.32 ± 0.01 gh | 1.81 ± 0.08 g |
| M | 1/4 | 6.59 ± 0.07 g | 641.33 ± 40.4 b | 47.76 ± 1.35 a | 836.67 ± 11.14 b | 34.4 ± 0.95 b | 0.78 ± 0.01 b | 4.03 ± 0.05 b |
| H | 1/4 | 7.17 ± 0.04 de | 551 ± 19.52 c | 36.41 ± 1.38 d | 750.33 ± 0.67 c | 31.73 ± 0.39 c | 0.64 ± 0.01 d | 3.36 ± 0.10 d |
| R | 1/3 | 7.24 ± 0.05 cd | 257.67 ± 2.03 f | 34.96 ± 1.17 de | 712 ± 7.37 d | 26.45 ± 0.62 d | 0.28 ± 0.01 hi | 1.47 ± 0.04 h |
| M | 1/3 | 6.44 ± 0.06 g | 459 ± 17.78 d | 47.35 ± 1.06 a | 824.67 ± 6.64 b | 37.52 ± 0.56 a | 0.72 ± 0.02 c | 3.9 ± 0.03 b |
| H | 1/3 | 7.02 ± 0.02 ef | 387.67 ± 14.45 e | 38.26 ± 1.21 cd | 766.67 ± 12.72 c | 33.41 ± 0.53 bc | 0.63 ± 0.01 d | 3.14 ± 0.04 e |
| R | 1/2 | 7.02 ± 0.02 ef | 163.33 ± 19.53 g | 40.29 ± 0.58 c | 668.33 ± 8.67 e | 32.33 ± 0.58 c | 0.24 ± 0.01 i | 1.29 ± 0.12 h |
| M | 1/2 | 6.23 ± 0.04 h | 340 ± 10.02 e | 44.32 ± 0.64 b | 761 ± 6.24 c | 37.44 ± 0.66 a | 0.56 ± 0.01 e | 2.99 ± 0.02 e |
| H | 1/2 | 6.90 ± 0.04 f | 233.33 ± 12.03 f | 35.89 ± 0.63 d | 701 ± 5.69 d | 34.54 ± 0.68 b | 0.46 ± 0.02 f | 2.52 ± 0.03 f |
4. Discussion
4.1. Effects of Suillus grevillea on the Physiological Growth of Seedlings
4.2. Effects of Suillus grevillea on the Rhizosphere Soil Properties of Seedlings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, S.; Song, Y.; Jia, L. Influence of the slope aspect on the ectomycorrhizal fungal community of Quercus variabilis Blume in the middle part of the Taihang Mountains, North China. J. For. Res. 2020, 32, 385–400. [Google Scholar] [CrossRef]
- Visick, D.O.; Adams, I.; Marzano, F.S.; Ratnieks, F.L.W. Effect of bark harvest intensity on the formation of cavities and other microhabitats in cork oaks (Quercus suber). Eur. J. For. Res. 2025, 144, 467–479. [Google Scholar] [CrossRef]
- Dou, H.; Sun, J.; Feng, X.; Lyu, H.; Qin, Z.; Ni, R.; Wang, Y.; Sun, H.; Zhou, X.; Tang, W.; et al. Research on the molecular mechanisms and key gene discovery in Quercus variabilis root pruning based on transcriptomics and hormone profiling. Int. J. Mol. Sci. 2024, 25, 11541. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Sun, H.; Feng, X.; Wang, T.; Wang, Y.; Quan, J.; Yang, X. Full-Length transcriptome assembly of Platycladus orientalis root integrated with RNA-Seq to identify genes in response to root pruning. Forests 2024, 15, 1232. [Google Scholar] [CrossRef]
- Sun, J.; Rong, Z.; Yang, L.; Zhu, Q.; Yuan, Y.; Feng, Z.; Li, L.; Li, N.; Zhang, L.; Guo, S. Effects of AMF inoculation on the growth, photosynthesis, and root physiological morphology of root-pruned Robinia pseudoacacia seedlings. Tree Physiol. 2023, 44, tpad130. [Google Scholar] [CrossRef]
- Koji, Y.; Miki, M.; Yoshihiro, H.; Morio, I. Root pruning is effective in alleviating the inhibition of soybean growth caused by anaerobic stress for a short period. J. Integr. Agric. 2023, 22, 1035–1044. [Google Scholar]
- Chang, X.; Zhang, J.; Wan, F.; Xian, L.; Liu, Y. Effects of root pruning and size on growth traits of hybrid Poplar seedlings. Forests 2024, 15, 1770. [Google Scholar] [CrossRef]
- Ureña, C.F.; Maldini, Á.C.; Acevedo, M.; Olate, S.E.M.; Dumroese, K.R.; Olea, S.A.; Ovalle, F.J.; Segura, E.E. Phosphorus fertilization and chemical root pruning: Effects on root traits during the nursery stage in two Mediterranean species from central Chile. Plants 2025, 14, 195. [Google Scholar] [CrossRef]
- Sung-Joon, N.; In-Sik, K.; Jae-Hee, K.; Do-Hyung, L. Growth characteristics of Pinus densiflora seedlings by root pruning intensity. J. Agric. Life Sci. 2014, 48, 15–21. [Google Scholar] [CrossRef]
- Cinantya, A.; Manea, A.; Leishman, M.R. The effect of root shaving and biostimulant application on the transplant success of six common Australian urban tree species. Urban Ecosyst. 2024, 27, 1313–1322. [Google Scholar] [CrossRef]
- Savatin, D.V.; Gramegna, G.; Modesti, V.; Cervone, F. Wounding in the plant tissue: The defense of a dangerous passage. Front. Plant Sci. 2014, 5, 470. [Google Scholar] [CrossRef]
- Jian, Y.; Gong, D.; Wang, Z.; Liu, L.; He, J.; Han, X.; Tsuda, K. How plants manage pathogen infection. EMBO Rep. 2024, 25, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Xu, L. De novo root regeneration from leaf explants: Wounding, auxin, and cell fate transition. Curr. Opin. Plant Biol. 2018, 41, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Se, P.J. The early hormone signaling network underlying wound-induced de novo root regeneration. J. Exp. Bot. 2025, 76, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, S.; Ruiz-Cano, H.; Fernández, M.A.; Sánchez-García, A.B.; Villanova, J.; Micol, J.L.; Pérez-Pérez, J.M. A network-guided genetic approach to identify novel regulators of adventitious root formation in Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 461. [Google Scholar] [CrossRef]
- Jung, J.K.H.; McCouch, S. Getting to the roots of it: Genetic and hormonal control of root architecture. Front. Plant Sci. 2013, 4, 186. [Google Scholar] [CrossRef]
- Rivas-San, V.M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Fang, X.; Tran, S.; Zhai, N.; Yang, Z.; Guo, F.; Chen, L.; Yu, J.; Ison, M.S.; et al. Transcriptional landscapes of de novo root regeneration from detached Arabidopsis leaves revealed by time-lapse and single-cell RNA sequencing analyses. Plant Commun. 2022, 3, 100306. [Google Scholar] [CrossRef]
- Ediriweera, A.N.; Lu, W.; Moreno, J.P.; Kalamulla, R.; Mayadunna, N.; Pelewatta, A.; Dissanayake, G.; Maduwanthi, I.; Wijesooriya, M.; Dai, D.-Q.; et al. Ectomycorrhizal fungal symbiosis on plant nutrient acquisition in tropical ecosystems. N. Z. J. Bot. 2025, 63, 1871–1894. [Google Scholar] [CrossRef]
- Zhang, R.; Peng, F.-R.; Yan, P.; Cao, F.; Liu, Z.-Z.; Le, D.-L.; Tan, P.-P. Effects of root pruning on germinated pecan seedlings. Hortic. Sci. 2015, 50, 1549–1552. [Google Scholar] [CrossRef]
- Koizumi, T.; Nara, K. Strong climatic effects on ectomycorrhizal fungal communities at seedling establishment stage in ice-age relict forests. Fungal Ecol. 2025, 75, 101410. [Google Scholar] [CrossRef]
- Yan, X.; Zhou, L.; Chen, Y.; Liu, R.; Guo, L.; Li, N.; Kang, A.; Zhai, K.; Zhou, G.; Zhou, X. Ectomycorrhizal fungi explain more variation in rhizosphere nutrient availability than root traits in temperate forests. Appl. Soil Ecol. 2025, 207, 105923. [Google Scholar] [CrossRef]
- Qi, J.; Yin, D. Effects of Suillus luteus on the growth, photosynthesis, stomata, and root system of Pinus tabulaeformis under drought stress. J. Plant Growth Regul. 2022, 42, 3486–3497. [Google Scholar] [CrossRef]
- De Quesada, G.; Xu, J.; Salmon, Y.; Lintunen, A.; Poque, S.; Himanen, K.; Heinonsalo, J. The effect of ectomycorrhizal fungal exposure on nursery-raised Pinus sylvestris seedlings: Plant transpiration under short-term drought, root morphology and plant biomass. Tree Physiol. 2024, 44, tpae029. [Google Scholar] [CrossRef]
- Johnson, D.; Liu, X.; Burslem, D.F.R.P. Symbiotic control of canopy dominance in subtropical and tropical forests. Trends Plant Sci. 2023, 28, 995–1003. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, Y.; Li, P.; Xu, L.; Fu, Q. Ectomycorrhizal fungi and dark septate endophyte inoculation improve growth and tolerance of Pinus tabulaeformis under cadmium stress. Pedosphere 2024, 34, 473–483. [Google Scholar] [CrossRef]
- Marko, K.; Saša, K.; Srđan, S.; Gavranović, A.M.; Milica, Z.; Lazar, K.; Victo, F. A fine-tuning of the plant hormones, polyamines and osmolytes by ectomycorrhizal fungi enhances drought tolerance in pedunculate oak. Int. J. Mol. Sci. 2023, 24, 7510. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, J.; Xue, Z.; Liang, J.; Chen, Y.; Liu, Y. Synergistic effect of phytohormone-producing ectomycorrhizal fungus Suillus luteus and fertilizer GGR6 on Pinus massoniana growth. J. Plant Interact. 2022, 17, 643–655. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, Y.; Tu, G.; Luo, X.; Zhang, Z. Light Drought stress positively influenced root morphological and endogenous hormones in Pinus massoniana seedlings inoculated with Suillus luteus. Forests 2024, 15, 1997. [Google Scholar] [CrossRef]
- Sebastiana, M.; Pereira, T.V.; Alcântara, A.; Pais, S.M.; Silva, B.A. Ectomycorrhizal inoculation with Pisolithus tinctorius increases the performance of Quercus suber L. (cork oak) nursery and field seedlings. New For. 2013, 44, 937–949. [Google Scholar] [CrossRef]
- Jones, G.H.G. A simple pot culture technique for studying the relative “early availability” of phosphatic fertilizers when added to different soil types. East Afr. Agric. J. 2015, 14, 201–209. [Google Scholar] [CrossRef]
- Adithya, S.; Nunna, S.A.D.; Chinnadurai, C.; Balachandar, D. Effects of rice cropping method and growth stage on rhizosphere bacterial diversity and soil biological attributes. Pedosphere 2025, 35, 983–994. [Google Scholar] [CrossRef]
- Li, C.; Jiao, N.; Liu, S.; Qu, L.; Zhang, Y.; Zhang, N. Patterns of rhizosphere microbial community assembly affect blueberry growth: Environmental and host effects. Rhizosphere 2025, 36, 101228. [Google Scholar] [CrossRef]
- Tateishi, T.; Yokoyama, K.; Kohno, N.; Okabe, H.; Marumoto, T. Estimation of mycorrhizal colonization of the roots of oak seedlings inoculated with an ectomycorrhizal fungus, Laccaria amethystea. Soil Sci. Plant Nutr. 2003, 49, 641–645. [Google Scholar] [CrossRef]
- Feng, Z.P.; Kong, D.L.; Kong, Y.H.; Zhang, B.H.; Yang, X.T. Coordina-tion of root growth with root morphology, physiology and defensefunction in response to root pruning in Piatycladus orientlis. J. Adv. Res. 2022, 36, 187–199. [Google Scholar] [CrossRef]
- Wen, S.; Miao, D.; Cui, H.; Li, S.; Gu, Y.; Jia, R.; Leng, Y. Physiology and transcriptomic analysis of endogenous hormonesregulating in vitro adventitious root formation in tree peony. Sci. Hortic. 2023, 318, 112122. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agricultural Chemistry Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Ma, D.; Teng, W.; Mo, Y.-T.; Yi, B.; Chen, W.-L.; Pang, Y.-P.; Wang, L. Effects of nitrogen, phosphorus, and potassium fertilization on plant growth, element levels in plants and soil, and the relationships among nutrient concentrations, plant yield, and nutrient status in Erythropalum scandens (Blume). J. Plant Nutr. 2024, 47, 82–96. [Google Scholar] [CrossRef]
- Cebrián-Piqueras, M.A.; Trinogga, J.; Trenkamp, A.; Minden, V.; Maier, M.; Mantilla-Contreras, J. Digging into the roots: Understanding direct and indirect drivers of ecosystem service trade-offs in coastal grasslands via plant functional traits. Environ. Monit. Assess. 2021, 193, 271. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, Y.; Chen, L.; Pei, H.; Wang, P.; Wang, B.; Yang, T. Impact of ecological governance policies on county ecosystem change in national key ecological functional zones: A case study of Tianzhu County, Gansu Province. Ecol. Indic. 2023, 154, 110748. [Google Scholar] [CrossRef]
- Tripolskaja, L.; Toleikiene, M.; Skersiene, A.; Versulieneet, A. Biomass of shoots and roots of multicomponent grasslands and their impact on soil carbon accumulation in Arenosol Rich in stones. Land 2024, 13, 1098. [Google Scholar] [CrossRef]
- Xie, L.; Palmroth, S.; Yin, C.; Oren, R. Extramatrical mycelial biomass is mediated by fine root mass and ectomycorrhizal fungal community composition across tree species. Sci. Total Environ. 2024, 950, 175175. [Google Scholar] [CrossRef] [PubMed]
- Truong, C.; Gabbarini, A.L.; Moretto, A.; Escobar, M.J.; Smith, E.M. Ectomycorrhizal fungi and the nitrogen economy of Nothofagus in southern Patagonia. Ecol. Evol. 2024, 14, e70299. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.D.; Zhang, D.J.; Hu, X.C.; Wu, Q.S.; Jiang, C.J.; Xia, T.J.; Gao, X.B.; Kuča, K. Mycorrhiza-induced changes in root growth and nutrient absorption of tea plants. Plant Soil Environ. 2018, 64, 283–289. [Google Scholar] [CrossRef]
- Yang, S.; Xing, S.; Liu, C.; Du, Z.; Wang, H.; Xu, Y. Effects of root pruning on the vegetative growth and fruit quality of Zhanhuadongzao trees. Hortic. Sci. 2010, 37, 14–21. [Google Scholar] [CrossRef]
- Parasquive, V.; Brisson, J.; Laliberté, E.; Chagnon, P.L. Arbuscular and ectomycorrhizal tree seedling growth is inhibited by competition from neighboring roots and associated fungal hyphae. Plant Soil 2024, 507, 571–584. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, Y. Adaptive growth: Shaping auxin-mediated root system architecture. Trends Plant Sci. 2020, 25, 121–123. [Google Scholar] [CrossRef]
- He, Y.; Fan, X.; Zhang, G.; Li, B.; Li, T.; Zu, Y.; Zhan, F. Effects of arbuscular mycorrhizal fungi and dark septate endophytes on maize performance and root traits under a high cadmium stress. S. Afr. J. Bot. 2020, 134, 415–423. [Google Scholar] [CrossRef]
- Xiong, H.; Chen, P.; Chen, W.; Yang, Y.; Jin, Y.; Tian, S.; Masabni, J.; Yuan, D.; Zou, F. Effect of an ectomycorrhizal fungus on the growth of Castanea henryi seedlings and the seasonal variation of root tips’ structure and physiology. Forests 2021, 12, 1643. [Google Scholar] [CrossRef]
- Zhai, N.; Sun, B.; Wu, S.; Zhou, F.; Jiao, Y.; Xu, L. Cytokinin facilitates the patterning of the adventitious root apical meristem from leaf cuttings. Mol. Hortic. 2024, 4, 11. [Google Scholar] [CrossRef]
- Zawaski, C.; Busov, V.B. Roles of gibberellin catabolism and signaling in growth and physiological response to drought and short-day photoperiods in Populus trees. PLoS ONE 2014, 9, e86217. [Google Scholar] [CrossRef]
- Wang, W.X.; Zhang, F.; Chen, Z.L.; Liu, J.; Gu, C.; He, J.D.; Zou, Y.N.; Wu, Q.S. Responses of phytohormones and gas exchange to mycorrhizal colonization in trifoliate orange subjected to drought stress. Arch. Agron. Soil Sci. 2017, 63, 14–23. [Google Scholar] [CrossRef]
- Tervonen, K.; Oldén, A.; Halme, P. Ectomycorrhizal fungi in wood-pastures: Communities are determined by trees and soil properties, not by grazing. Agric. Ecosyst. Environ. 2019, 269, 13–21. [Google Scholar] [CrossRef]
- Mohammadnia, S.; Haghighi, M.; Mozafarian, M.; Geösel, A. Impact of mycorrhiza inoculations and iron amino chelate on growth and physiological changes of cucumber seedlings across different pH levels. Plants 2025, 14, 341. [Google Scholar] [CrossRef] [PubMed]
- Serafim, C.; Ramos, M.A.; Yilmaz, T.; Sousa, N.R.; Yu, K.; Van Geel, M.; Ceulemans, T.; Saudreau, M.; Somers, B.; Améglio, T.; et al. Substrate pH mediates growth promotion and resilience to water stress of Tilia tomentosa seedlings after ectomycorrhizal inoculation. BMC Plant Biol. 2024, 24, 1001. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Zhang, M.; Cao, G.; Zhu, J.; Zhang, A.; Bai, H.; Dai, C.; Jia, Y. Endofungal bacteria and ectomycorrhizal fungi synergistically promote the absorption of organic phosphorus in Pinus massoniana. Plant Cell Environ. 2023, 47, 600–610. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Hui, D.; Wang, Y.; Li, J.; Chen, J.; Chen, G.; Zhu, Y.; Zhang, L.; Zhang, D.; et al. Mycorrhizal fungi alleviate acidification-induced phosphorus limitation: Evidence from a decade-long field experiment of simulated acid deposition in a tropical forest in south China. Glob. Change Biol. 2022, 28, 3605–3619. [Google Scholar] [CrossRef]
- Zhang, T.; Meng, F.; Yin, D. Effects of ectomycorrhizal fungi on the activation and uptake of phosphorus in the mycorrhizal and hypodermal layers of Populus davidiana× P. bolleana. J. Plant Growth Regul. 2024, 44, 2504–2513. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Hartley, I.P.; Dungait, J.A.J.; Wen, X.; Li, D.; Guo, Z.; Quine, T.A. Contrasting rhizosphere soil nutrient economy of plants associated with arbuscular mycorrhizal and ectomycorrhizal fungi in karst forests. Plant Soil 2021, 470, 81–93. [Google Scholar] [CrossRef]
- Zhou, J.; Gube, M.; Holz, M.; Song, B.; Shan, I.; Shi, L.; Kuzyakov, Y.; Dippold, M.A.; Pausch, J. Ectomycorrhizal and non-mycorrhizal rhizosphere fungi increase root-derived C input to soil and modify enzyme activities: A 14 C pulse labeling of Picea abies seedlings. Plant Cell Environ. 2022, 45, 3122–3133. [Google Scholar] [CrossRef]
- Adamczyk, S.; Shrestha, R.; Adamczyk, B.; Liang, C.; Biasi, C.; Heinonsalo, J.; Karhu, K. Interaction between ectomycorrhizal and ericoid mycorrhizal plants decelerates stable soil organic matter decomposition. Appl. Soil Ecol. 2024, 198, 105395. [Google Scholar] [CrossRef]
- Yang, N.; Wan, C.; Ren, D.; Zhang, Y.; Chelli, S.; Zhu, Z.; Traversari, S.; Emiliani, G.; Mao, L. Three ectomycorrhizal fungal inoculum modifies the growth performance and tolerance of Quercus nuttallii seedlings under salt stress. For. Ecol. Manag. 2025, 601, 123353. [Google Scholar] [CrossRef]
- Tan, Q.; You, L.; Hao, C.; Wang, J.; Liu, Y. Effects of four bolete species on ectomycorrhizae formation and development in Pinus thunbergii and Quercus acutissima. BMC Ecol. Evol. 2024, 24, 54. [Google Scholar] [CrossRef]
- Cheng, K.; Liu, Y.; Tang, M.; Zhang, H. Suillus grevillei and Suillus luteus promote lead tolerance of Pinus tabulaeformis and biomineralize lead to pyromorphite. Front. Microbiol. 2024, 15, 1296512. [Google Scholar] [CrossRef]
- Zong, K.; Huang, J.; Nara, K.; Chen, Y.; Shen, Z.; Lian, C. Inoculation of ectomycorrhizal fungi contributes to the survival of tree seedlings in a copper mine tailing. J. For. Res. 2015, 20, 493–500. [Google Scholar] [CrossRef]





| Inoculation Treatment | Different Root Pruning Treatments | Mycorrhizal Colonization (%) | Mycelial Density (g/cm) |
|---|---|---|---|
| CK | 0 | 0.00 ± 0.00 e | 0.00 ± 0.00 e |
| SG | 0 | 86.6 ± 9.07 a | 6.83 ± 0.61 a |
| CK | 1/4 | 0.00 ± 0.00 e | 0.00 ± 0.00 e |
| SG | 1/4 | 63.33 ± 3.33 b | 3.58 ± 0.32 b |
| CK | 1/3 | 0.00 ± 0.00 e | 0.00 ± 0.04 e |
| SG | 1/3 | 43.4 ± 1.95 c | 2.95 ± 0.05 c |
| CK | 1/2 | 0.00 ± 0.00 e | 0.00 ± 0.00 e |
| SG | 1/2 | 29.9 ± 4.51 d | 1.72 ± 0.18 d |
| Inoculation Treatment | Different Root Pruning Treatments | RL (cm) | RPA (cm2) | RSA (cm2) | ARD (mm) | RD (cm/m3) | RV (cm3) | RT |
|---|---|---|---|---|---|---|---|---|
| CK | 0 | 315.79 ± 3.41 bc | 50.64 ± 3.22 c | 231.95 ± 4.86 c | 1.74 ± 0.26 bcd | 45.81 ± 1.89 c | 5.76 ± 0.34 b | 1545 ± 151.31 b |
| SG | 0 | 397.38 ± 31.64 a | 92.05 ± 1.12 a | 362.65 ± 19.30 a | 2.58 ± 0.09 a | 82.69 ± 5.55 a | 8.19 ± 0.40 a | 2144.67 ± 298.14 a |
| CK | 1/4 | 277.10 ± 1.86 cd | 31.46 ± 0.53 d | 145.49 ± 9.56 d | 1.57 ± 0.13 cd | 35.78 ± 1.44 cd | 4.63 ± 0.14 c | 824.67 ± 36.04 c |
| SG | 1/4 | 342.06 ± 15.81 b | 60.97 ± 3.47 b | 277.69 ± 7.95 b | 2.29 ± 0.35 ab | 66.58 ± 8.08 b | 6.23 ± 0.53 b | 1684.33 ± 107.91 b |
| CK | 1/3 | 247.44 ± 13.81 d | 20.51 ± 0.55 e | 108.99 ± 8.80 ef | 1.34 ± 0.27 cd | 28.43 ± 4.42 de | 3.55 ± 0.23 d | 451.67 ± 15.98 cd |
| SG | 1/3 | 272.46 ± 15.41 cd | 36.65 ± 4.57 d | 132.29 ± 18.84 de | 1.92 ± 0.04 bc | 43.08 ± 0.73 c | 4.36 ± 0.15 cd | 781.67 ± 26.61 c |
| CK | 1/2 | 172.17 ± 10.27 e | 19.34 ± 0.26 e | 86.58 ± 1.46 f | 1.17 ± 0.04 d | 17.09 ± 2.23 e | 1.50 ± 0.15 e | 301.33 ± 34.65 d |
| SG | 1/2 | 176.28 ± 6.97 e | 23.40 ± 1.34 e | 91.59 ± 2.24 f | 1.23 ± 0.03 d | 20.79 ± 0.31 e | 1.95 ± 0.13 e | 375.33 ± 11.79 d |
| Latent Variable | Observed Variable | Loading | CA | CR | AVE |
|---|---|---|---|---|---|
| Root physiological substances | IAA | 0.987 | 0.983 | 0.968 | 0.989 |
| CTK | 0.971 | ||||
| GA | 0.967 | ||||
| ABA | 0.936 | ||||
| ZR | 0.971 | ||||
| SA | 0.975 | ||||
| Soil properties | Conductivity | 0.949 | 0.780 | 0.693 | 0.580 |
| TC | 0.750 | ||||
| TN | 0.695 | ||||
| Shoot and root nutrient | Shoot Nitrogen | 0.965 | 0.989 | 0.974 | 0.949 |
| Root Nitrogen | 0.971 | ||||
| Shoot Phosphorus | 0.980 | ||||
| Root Phosphorus | 0.970 | ||||
| Shoot Potassium | 0.977 | ||||
| Root Potassium | 0.982 | ||||
| Root morphological parameters | RL | 0.939 | 0.986 | 0.968 | 0.894 |
| RPA | 0.969 | ||||
| RSA | 0.976 | ||||
| ARD | 0.851 | ||||
| RD | 0.951 | ||||
| RV | 0.964 | ||||
| RT | 0.962 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Sun, J.; Zhao, S.; Yang, L.; Liang, Y.; Yang, X.; Shen, L.; Guo, E.; Li, Q.; Jia, Y.; Zhang, L.; et al. Influence of Suillus grevillea on the Root Morphology, Growth and Rhizosphere Soil Properties of Quercus variabilis Blume Seedlings with Root Pruning. J. Fungi 2026, 12, 6. https://doi.org/10.3390/jof12010006
Sun J, Zhao S, Yang L, Liang Y, Yang X, Shen L, Guo E, Li Q, Jia Y, Zhang L, et al. Influence of Suillus grevillea on the Root Morphology, Growth and Rhizosphere Soil Properties of Quercus variabilis Blume Seedlings with Root Pruning. Journal of Fungi. 2026; 12(1):6. https://doi.org/10.3390/jof12010006
Chicago/Turabian StyleSun, Jinhua, Shu Zhao, Liu Yang, Yazhen Liang, Xitian Yang, Lianfeng Shen, Erhui Guo, Qingxin Li, Yishuo Jia, Lin Zhang, and et al. 2026. "Influence of Suillus grevillea on the Root Morphology, Growth and Rhizosphere Soil Properties of Quercus variabilis Blume Seedlings with Root Pruning" Journal of Fungi 12, no. 1: 6. https://doi.org/10.3390/jof12010006
APA StyleSun, J., Zhao, S., Yang, L., Liang, Y., Yang, X., Shen, L., Guo, E., Li, Q., Jia, Y., Zhang, L., Liu, H., & Sun, R. (2026). Influence of Suillus grevillea on the Root Morphology, Growth and Rhizosphere Soil Properties of Quercus variabilis Blume Seedlings with Root Pruning. Journal of Fungi, 12(1), 6. https://doi.org/10.3390/jof12010006





