Co-Treatment with Ritonavir or Sertraline Enhances Itraconazole Efficacy Against Azole-Resistant Trichophyton indotineae Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Type II and I Specific PCR, qPCR, and DNA Sequencing
2.3. Medical Stock Preparations and Concentration Ranges
2.4. Microplate Laser Nephelometry (MLN) Assays
2.5. Graphical Image Preparation, Data Performance, and Statistical Analysis
3. Results
3.1. Erg11B Copy Number Determination and Type II and I Specific PCR to Verify Strain Genotypes
3.2. Treatment of Itraconazole in Combination with Quinine Hydrochloride Showed a Weak Effect on Inhibitory Concentrations of 50% (IC50) of T. indotineae Strains
3.3. Co-Treatment with Ritonavir Showed a 50% Reduction in Itraconazole IC50 Values
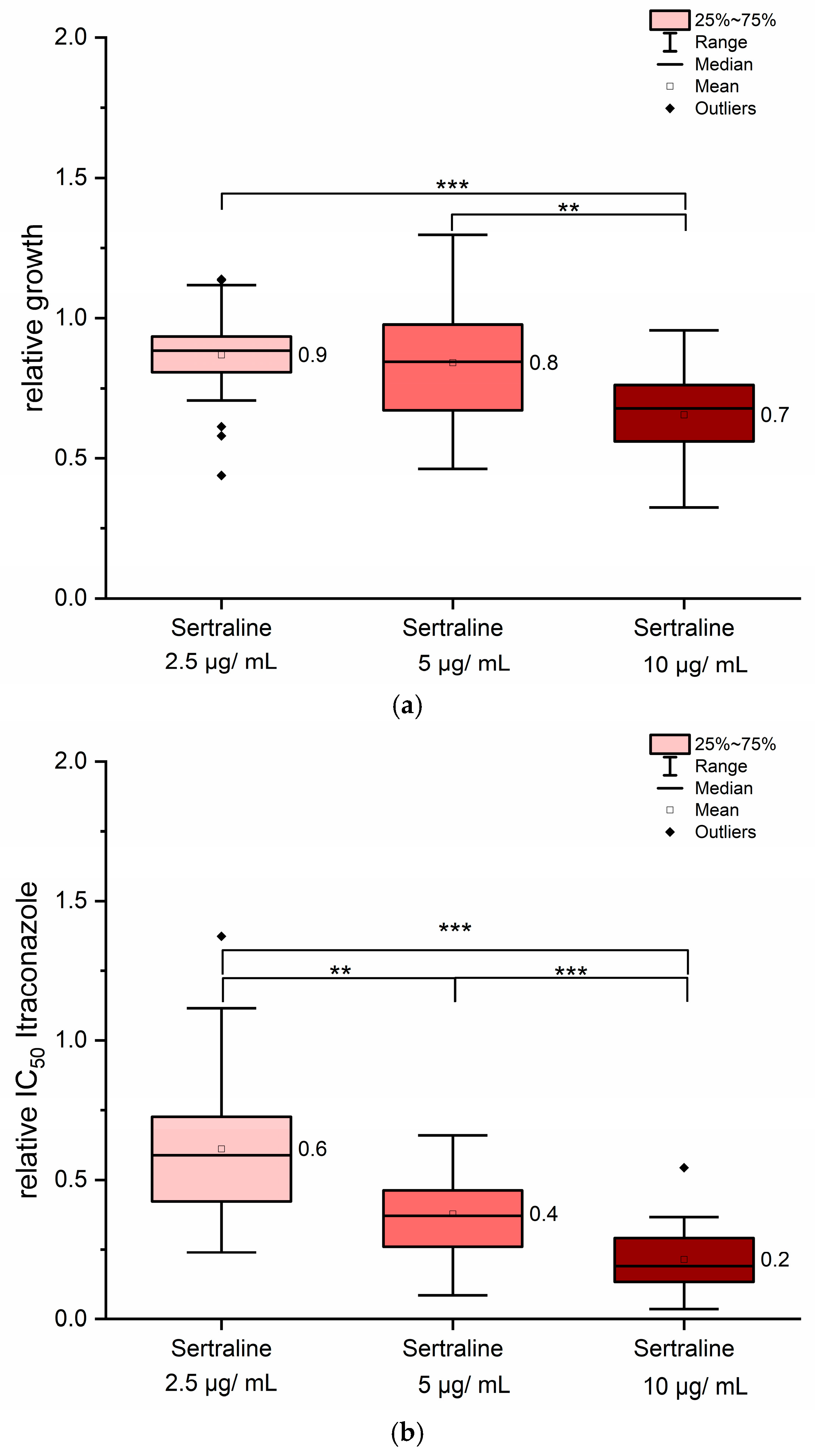
3.4. Sertraline Showed Antifungal Properties and Synergistic Effects in Combination with Itraconazole
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU | Colony-forming units |
| DMSO | Dimethyl sulfoxide |
| FICI | Fractional inhibitory concentration index |
| IC | Inhibitory concentration |
| ITZ | Itraconazole |
| MDR | Multiple drug resistance |
| MFS | Major facilitator superfamily |
| MIC | Minimal inhibitory concentration |
| MLN | Microplate laser nephelometry |
| MOPS | 3-(N-morpholino)propane sulfonic acid |
| RPMI | Roswell Park Memorial Institute |
| SG | Sabouraud-glucose medium |
References
- Gupta, A.K.; Talukder, M.; Carviel, J.L.; Cooper, E.A.; Piguet, V. Combatting antifungal resistance: Paradigm shift in the diagnosis and management of onychomycosis and dermatomycosis. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1706–1717. [Google Scholar] [CrossRef]
- Jabet, A.; Normand, A.-C.; Brun, S.; Dannaoui, E.; Bachmeyer, C.; Piarroux, R.; Hennequin, C.; Moreno-Sabater, A. Trichophyton indotineae from epidemiology to therapeutic. J. Med. Mycol. 2023, 33, 101383. [Google Scholar] [CrossRef]
- Gupta, A.K.; Wang, T.; Mann, A.; Ravi, S.P.; Talukder, M.; Lincoln, S.A.; Foreman, H.-C.; Kaplan, B.; Galili, E.; Piguet, V.; et al. Antifungal resistance in dermatophytes—Review of the epidemiology, diagnostic challenges, and treatment strategies for managing Trichophyton indotineae infections. Expert Rev. Anti-Infect. Ther. 2024, 22, 739–751. [Google Scholar] [CrossRef]
- Sonego, B.; Corio, A.; Mazzoletti, V.; Zerbato, V.; Benini, A.; di Meo, N.; Zalaudek, I.; Stinco, G.; Errichetti, E.; Zelin, E. Trichophyton indotineae, an emerging drug-resistant dermatophyte: A review of the treatment options. J. Clin. Med. 2024, 13, 3558. [Google Scholar] [CrossRef]
- Singh, A.; Masih, A.; Khurana, A.; Singh, P.K.; Gupta, M.; Hagen, F.; Meis, J.F.; Chowdhary, A. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses 2018, 61, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, S.M.; Shankarnarayan, S.A.; Dogra, S.; Shaw, D.; Mushtaq, K.; Paul, R.A.; Narang, T.; Chakrabarti, A. Mutation in the squalene epoxidase gene of Trichophyton interdigitale and Trichophyton rubrum associated with allylamine resistance. Antimicrob. Agents Chemother. 2018, 62, e02522-17. [Google Scholar] [CrossRef]
- Khurana, A.; Masih, A.; Chowdhary, A.; Sardana, K.; Borker, S.; Gupta, A.; Gautam, R.K.; Sharma, P.K.; Jain, D. Correlation of in vitro susceptibility based on MICs and squalene epoxidase mutations with clinical response to terbinafine in patients with tinea corporis/cruris. Antimicrob. Agents Chemother. 2018, 62, e01038-18. [Google Scholar] [CrossRef]
- Nenoff, P.; Verma, S.B.; Vasani, R.; Burmester, A.; Hipler, U.-C.; Wittig, F.; Krüger, M.; Nenoff, K.; Wiegand, C.; Saraswat, A.; et al. The current epidemic of superficial dermatophytosis due to Trichophyton mentagrophytes–a molecular study. Mycoses 2019, 62, 336–356. [Google Scholar] [CrossRef]
- Ebert, A.; Monod, M.; Salamin, K.; Burmester, A.; Uhrlaß, S.; Wiegand, C.; Hipler, U.-C.; Krüger, C.; Koch, D.; Wittig, F.; et al. Alarming India-wide phenomenon of antifungal resistance in dermatophytes: A multicenter study. Mycoses 2020, 63, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Pchelin, I.M.; Azarov, D.V.; Churina, M.A.; Scherbak, S.G.; Apalko, S.V.; Vasilyeva, N.V.; Taraskina, A.E. Species boundaries in the Trichophyton mentagrophytes/T. interdigitale species complex. Med. Mycol. 2019, 57, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Kano, R.; Kimura, U.; Kakurai, M.; Hiruma, J.; Kamata, H.; Suga, Y.; Harada, K. Trichophyton indotineae sp. nov.: A new highly terbinafine-resistant anthropophilic dermatophyte species. Mycopathologia 2020, 185, 947–958. [Google Scholar] [CrossRef]
- Tang, C.; Kong, X.; Ahmed, S.A.; Thakur, R.; Chowdhary, A.; Nenoff, P.; Uhrlaß, S.; Verma, S.B.; Meis, J.F.; Kandemir, H.; et al. Taxonomy of the Trichophyton mentagrophytes/T. interdigitale species complex, harboring the highly virulent, multiresistent genotype T. indotineae. Mycopathologia 2021, 186, 315–326. [Google Scholar] [CrossRef]
- Singh, S.; Chandra, U.; Anchan, V.N.; Verma, P.; Tilak, R. Limited effectiveness of four oral antifungal drugs (fluconazole, griseofulvin, itraconazole and terbinafine) in the current epidemic of altered dermatophytosis in India: Results of a randomized pragmatic trial. Br. J. Dermatol. 2020, 183, 840–846. [Google Scholar] [CrossRef]
- Burmester, A.; Hipler, U.-C.; Elsner, P.; Wiegand, C. Point mutations in the squalene epoxidase erg1 and sterol 14-α demethylase erg11 gene of T. indotineae isolates indicate that the resistant mutant strains evolved independently. Mycoses 2022, 65, 97–102. [Google Scholar] [CrossRef]
- Yamada, T.; Yaguchi, T.; Maeda, M.; Alshahni, M.M.; Salamin, K.; Guenova, E.; Feuermann, M.; Monod, M. Gene amplification of CYP51B: A new mechanism of resistance to azole compounds in Trichophyton indotineae. Antimicrob. Agents Chemother. 2022, 66, e0005922. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.S.I.; Verma, S.B.; Illigner, G.-M.; Uhrlaß, S.; Klonowski, E.; Burmester, A.; Noor, T.; Nenoff, P. Trichophyton mentagrophytes ITS genotype VIII/Trichophyton indotineae infection and antifungal resistance in Bangladesh. J. Fungi 2024, 10, 768. [Google Scholar] [CrossRef]
- Yamada, T.; Maeda, M.; Nagai, H.; Salamin, K.; Chang, Y.-T.; Guenova, E.; Feuermann, M.; Monod, M. Two different types of tandem sequences mediate the overexpression of TinCYP51B in azole-resistant Trichophyton indotineae. Antimicrob. Agents Chemother. 2023, 67, e00933-23. [Google Scholar] [CrossRef] [PubMed]
- Berstecher, N.; Burmester, A.; Gregersen, D.M.; Tittelbach, J.; Wiegand, C. Trichophyton indotineae Erg1Ala448Thr strain expressed constitutively high levels of sterol 14-α demethylase Erg11B mRNA, while transporter MDR3 and Erg11A expression was induced after addition of short chain azoles. J. Fungi 2024, 10, 731. [Google Scholar] [CrossRef]
- Krauße, L.; Burmester, A.; Uhrlaß, S.; Fabri, M.; Nenoff, P.; Tittelbach, J.; Wiegand, C. Significant impact of growth medium on itraconazole susceptibility in azole-resistant versus wild-type Trichophyton indotineae, rubrum, and quinckeanum isolates. Int. J. Mol. Sci. 2025, 26, 7090. [Google Scholar] [CrossRef]
- Monod, M.; Feuermann, M.; Salamin, K.; Fratti, M.; Makino, M.; Alshahni, M.M.; Makimura, K.; Yamada, T. Trichophyton rubrum azole resistance mediated by a new ABC transporter, TruMDR3. Antimicrob. Agents Chemother. 2019, 63, e00863-19. [Google Scholar] [CrossRef] [PubMed]
- Gnat, S.; Lagowski, D.; Nowakiewicz, A.; Dylag, M.; Osinska, M. Complementary effect of mechanism of multidrug resistance in Trichophyton mentagrophytes isolated from human dermatophytosis of animal origin. Mycoses 2021, 64, 537–549. [Google Scholar] [CrossRef]
- Gregersen, D.M.; Berstecher, N.; Burmester, A.; Tittelbach, J.; Wiegand, C. Unexpected perseverance in tinea corporis—Special mutations found in Trichophyton indotineae dermatomycosis. JEADV Clin. Pract. 2025, 4, 867–871. [Google Scholar] [CrossRef]
- Kano, R.; Kimura, U.; Noguchi, H.; Hiruma, M. Clinical isolate of a multi-antifungal-resistant Trichophyton rubrum. Antimicrob. Agents Chemother. 2022, 66, e02393-21. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Kahlmeter, G.; Guinea, J.; Meletiadis, J. How to: Perform antifungal susceptibility testing of microconidia-forming dermatophytes following the new reference EUCAST method E.Def 11.0, exemplified by Trichophyton. Clin. Microbiol. Inf. 2021, 27, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Guinea, J.; Meletiadis, J. Twenty years in EUCAST antifungal susceptibility testing: Progress & remaining challenges. Mycopathologia 2024, 189, 64. [Google Scholar] [CrossRef]
- CLSI Document M38: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017.
- Blanchard, G.; Amarov, B.; Fratti, M.; Salamin, K.; Bontems, O.; Chang, Y.-T.; Sabou, A.M.; Künzle, N.; Monod, M.; Guenova, E. Reliable and rapid identification of terbinafine resistance in dermatophytic nail and skin infections. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 2080–2089. [Google Scholar] [CrossRef]
- Fink, S.; Burmester, A.; Hipler, U.-C.; Neumeister, C.; Götz, M.R.; Wiegand, C. Efficacy of antifungal agents against fungal spores: An in vitro study using microplate laser nephelometry and an artificially infected 3D skin model. MicrobiologyOpen 2022, 11, e1257. [Google Scholar] [CrossRef]
- Winter, P.; Burmester, A.; Tittelbach, J.; Wiegand, C. A new genotype of Trichophyton quinckeanum with point mutations in Erg11A encoding sterol 14-α demethylase exhibits increased itraconazole resistance. J. Fungi 2023, 9, 1006. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yu, A.-M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Phar. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef]
- Basrani, S.; Patil, S.; Chougule, S.; Kotalagi, T.; Yankanchi, S.; Karuppayil, S.M.; Jadhav, A.S. Repurposing of quinine as an antifungal antibiotic: Identification of molecular targets in Candida albicans. Folia Microbiol. 2025. [Google Scholar] [CrossRef]
- Brihlante, R.S.N.; Caetano, E.P.; Riello, G.B.; Guedes, G.M.d.M.; Castelo-Branco, D.; Fechine, M.A.B.; de Oliveira, J.S.; de Camargo, Z.P.; de Mesquita, J.R.L.; Monteiro, A.J.; et al. Antiretroviral drugs saquinavir and ritonavir reduce inhibitory concentration values of itraconazole against Histoplasma capsulatum strains in vitro. Braz. J. Infect. Dis. 2016, 20, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Salama, E.A.; Eldesouky, H.E.; Elgammal, Y.; Abutaleb, N.S.; Seleem, M.N. Lopinavir and ritonavir act synergistically with azoles against Candida auris in vitro and in a mouse model of disseminated candidiasis. Int. J. Antimicrob. Agents 2023, 62, 106906. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C.; Dierich, M.P.; Fuchs, D.; Semenitz, E.; Ledochowski, M. Antifungal activity against Candida species of the selective serotonin-reuptake inhibitor, sertraline. Clin. Inf. Dis. 2001, 33, e135–e136. [Google Scholar] [CrossRef]
- Barbarossa, A.; Rosato, A.; Carrieri, A.; Fumarola, L.; Tardugno, R.; Corbo, F.; Fracchiolla, G.; Carocci, A. Exploring the antibiofilm effect of sertraline in synergy with Cinnamomum verum Essential Oil to counteract Candida species. Pharmaceuticals 2024, 17, 1109. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Martinez-de-Oliveira, J.; Donders, G.G.G.; Palmeira-de-Oliveira, R. Anti-Candida activity of antidepressants sertraline and fluoxetine: Effect upon pre-formed biofilms. Med. Microbiol. Immun. 2018, 207, 195–200. [Google Scholar] [CrossRef]
- Zhai, B.; Wu, C.; Wang, L.; Sachs, M.S.; Lin, X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob. Agents Chemother. 2012, 56, 3758–3766. [Google Scholar] [CrossRef]
- Ke, W.; Xie, Y.; Chen, Y.; Ding, H.; Ye, L.; Qiu, H.; Li, H.; Zhang, L.; Chen, L.; Tian, X.; et al. Fungicide-tolerant persister formation during cryptococcal pulmonary infection. Cell Host Microbe 2024, 32, 276–289. [Google Scholar] [CrossRef]
- Li, W.; Yun, Z.; Ji, C.; Tu, J.; Yang, W.; Li, J.; Liu, N.; Sheng, C. Discovery of novel sertraline derivatives as potent anti-Cryptococcus agents. J. Med. Chem. 2022, 65, 6541–6554. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Liao, Y.; Yang, S.; Yang, R. In vitro antifungal activity of sertraline and synergistic effects in combination with antifungal drugs against planktonic forms and biofilms of clinical Trichosporon asahii isolates. PLoS ONE 2016, 11, e0167903. [Google Scholar] [CrossRef]
- Paul, S.; Mortimer, R.B.; Mitchell, M. Sertraline demonstrates fungicidal activity in vitro for Coccidioides immitis. Mycology 2016, 7, 99–101. [Google Scholar] [CrossRef]
- Rocha, C.H.L.; Rocha, F.M.G.; Bitencourt, T.A.; Martins, M.P.; Sanches, P.R.; Rossi, A.; Martinez-Rossi, N.M. Synergism between the antidepressant sertraline and caspofungin as an approach to minimize the virulence and resistance in the dermatophyte Trichophyton rubrum. J. Fungi 2022, 8, 815. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Parikh, S.K.; Patel, A.D.; Dave, J.B.; Sen, D.J. Development and validation of UV spectrophotometric method for estimation of itraconazole bulk drug and pharmaceutical formulation. Int. Drug Dev. Res. 2011, 3, 324–328. [Google Scholar]
- Hsieh, M.H.; Yu, C.M.; Yu, V.L.; Chow, J.W. Synergy accessed by checkerboard, a critical analysis. Diagn. Microbiol. Infect. Dis. 1993, 16, 343–349. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the checkerboard puts between them. J. Antimicrob. Chem. 2003, 52, 1. [Google Scholar] [CrossRef]
- Scorzoni, L.; Sangalli-Leite, F.; Singulani, J.L.; Silva, A.C.A.P.; Costa-Orlandi, C.B.; Fusco-Almeida, A.M.; Mendes-Gianinni, M.J.S. Searching new antifungals: The use of in vitro and in vivo methods for evaluation of natural compounds. J. Microbiol. Meth. 2016, 123, 68–78. [Google Scholar] [CrossRef]
- Pfeifer, N.D.; Goss, S.L.; Swift, B.; Ghibellini, G.; Ivanovic, M.; Heizer, W.D.; Gangarosa, L.M.; Brouwer, K.L.M. Effect of ritonavir on 99mTechnetium-Mebrofenin disposition in humans: A semi-PBPK modeling and in vitro approach to predict transporter-mediated DDIs. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, e20. [Google Scholar] [CrossRef] [PubMed]
- Sammadar, S.; Redhwan, M.A.M.; Eraiah, M.M.; Koneri, R. Clinical isolates of the anthropophilic dermatophyte Trichophyton tonsurans exhibit transcriptional regulation of multidrug efflux transporters that induce antifungal resistance. Mol. Biol. Rep. 2025, 52, 612. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.M.G.; Rocha, C.H.L.; Martins, M.P.; Sanches, P.R.; Bitencourt, T.A.; Sachs, M.S.; Martinez-Rossi, N.M.; Rossi, A. The antidepressant sertraline affects cell signaling and metabolism in Trichophyton rubrum. J. Fungi 2023, 9, 275. [Google Scholar] [CrossRef]
- Liu, J.; Vanderwyk, K.A.; Donnelley, M.A.; Thompson, G.R. SUBA-itraconazole in the treatment of systemic fungal infections. Future Microbiol. 2024, 14, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, Z.; Tan, Y.; Li, L.; Wang, Z.; Wen, Y.; Huang, S.; Shang, D. Population pharmacokinetic approach to guide personalized sertraline treatment in Chinese patients. Heliyon 2024, 10, e25231. [Google Scholar] [CrossRef] [PubMed]
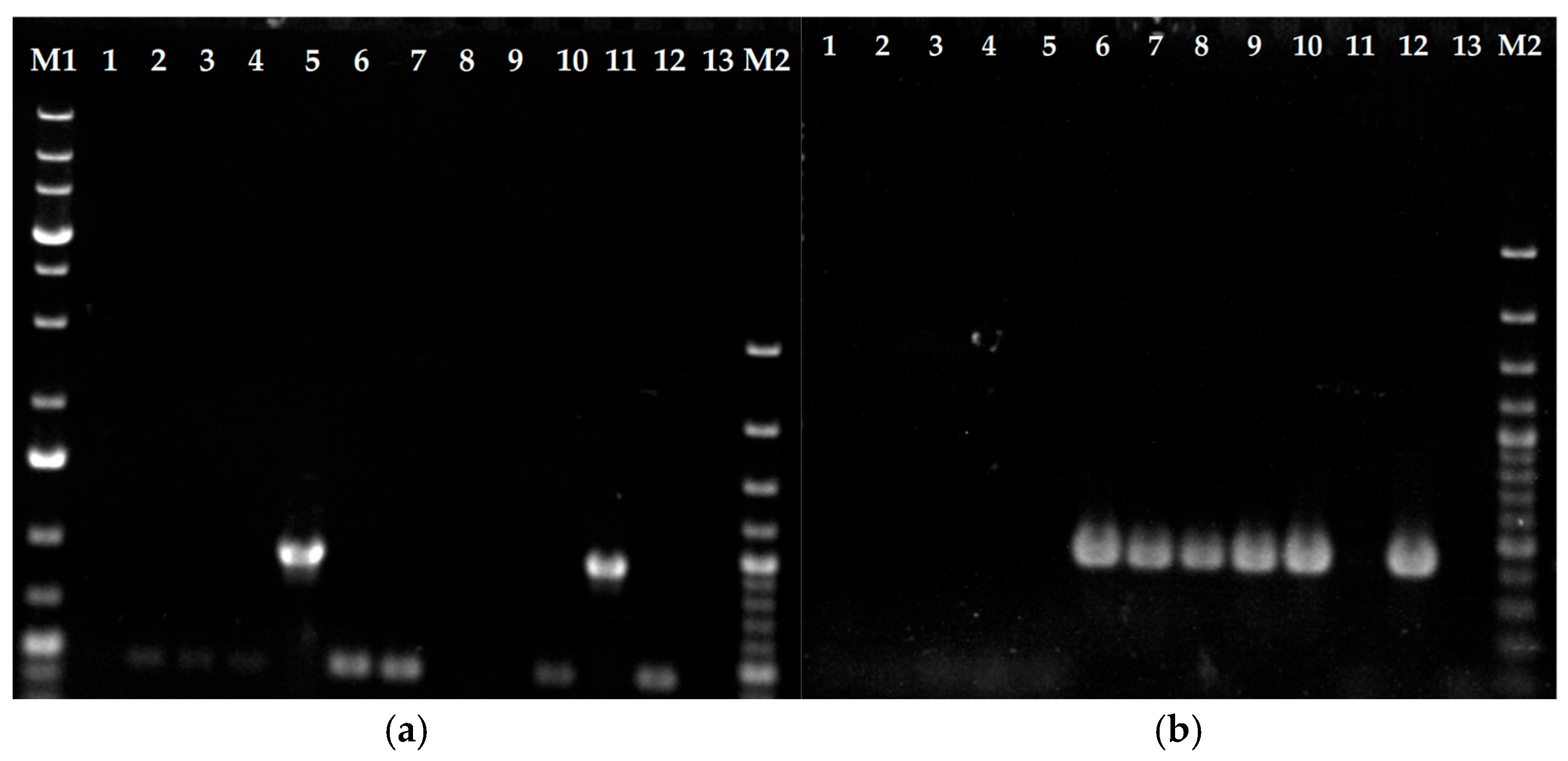
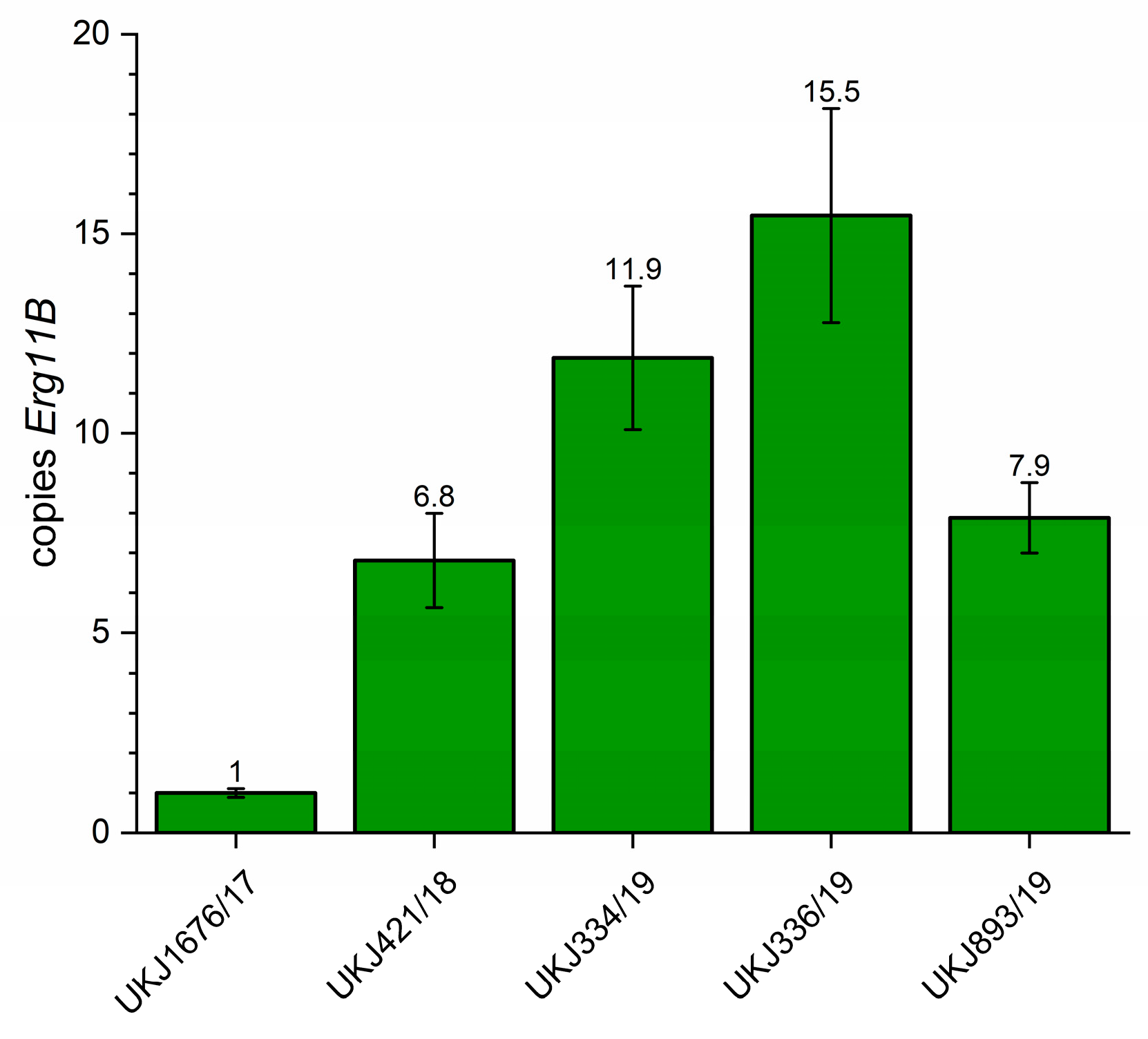
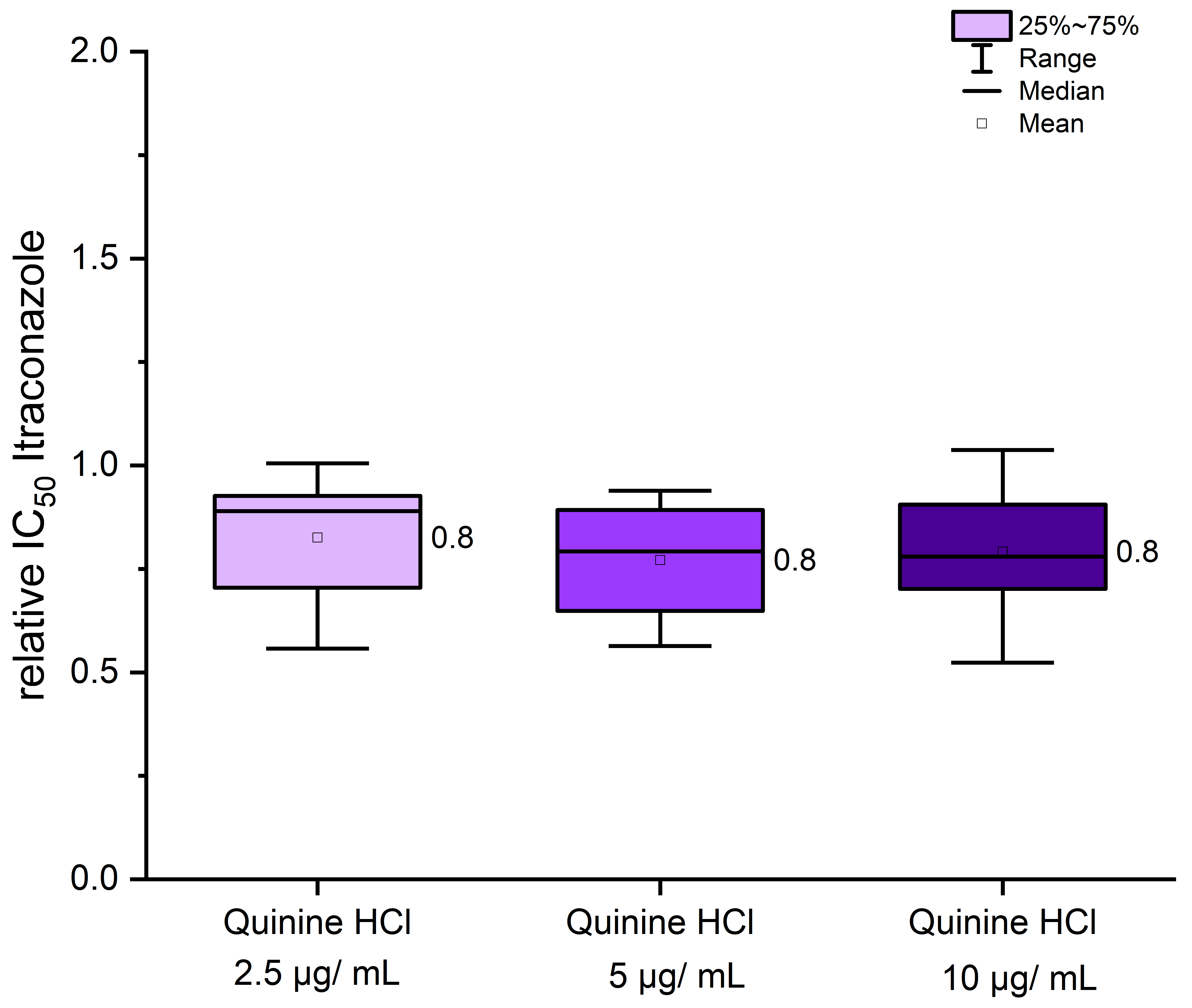
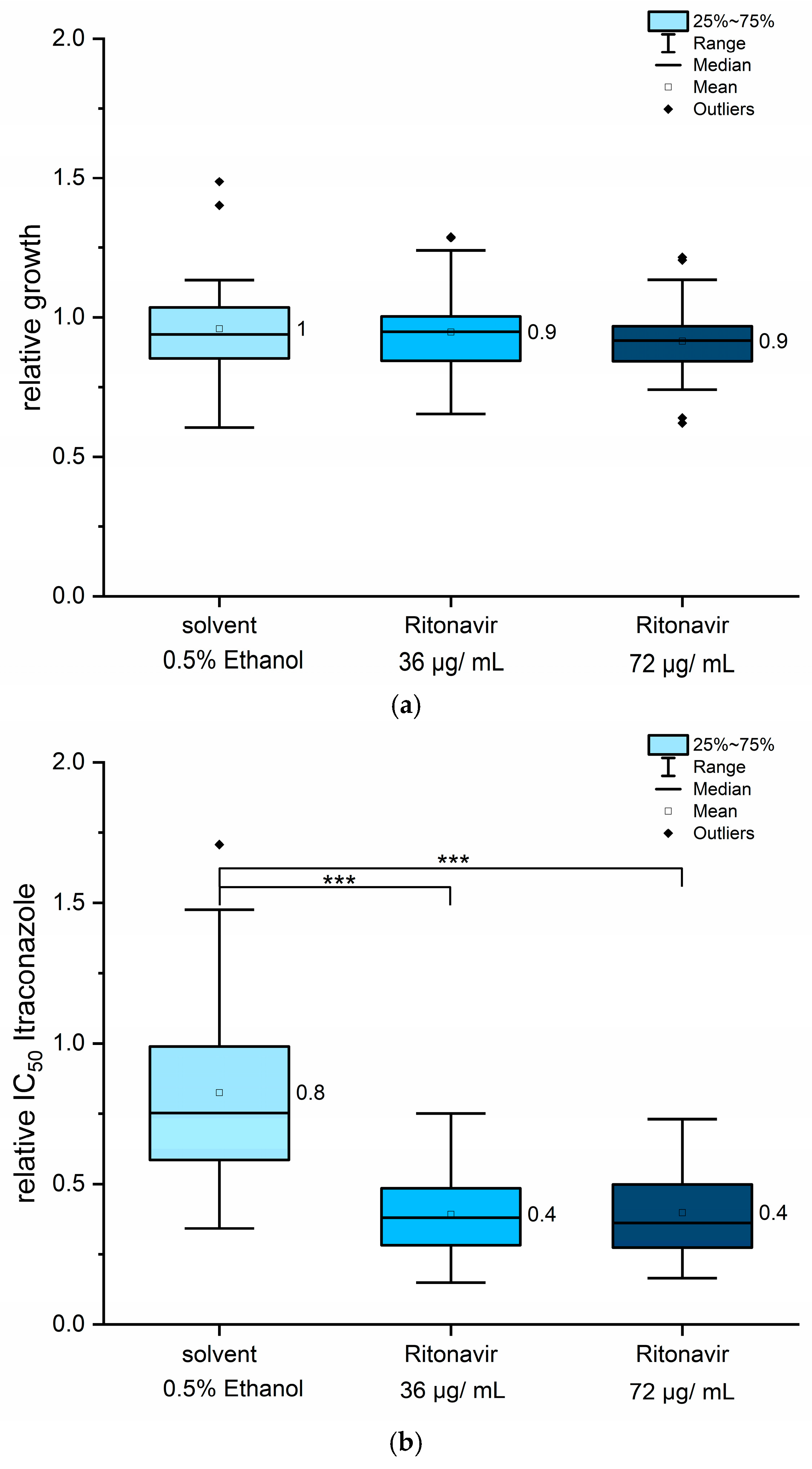
| UKJ Number | Collection Synonyms | Erg1 Amino Acid Exchanges | Erg11B Amino Acid Exchanges | Erg11B Amplification Type | Source, Cited |
|---|---|---|---|---|---|
| UKJ1676/17 | TIMM20114 | Ala448Thr | Wild type | No amplificates | [9,15,17] |
| UKJ1687/17 | TIMM20118 | Phe397Leu | Gly443Glu | Type I | [9,15,17] |
| UKJ1708/17 | Ala448Thr | Wild type | Type II | [9,15,17] | |
| UKJ392/18 | TIMM20117, 200087/18 | Ala448Thr | Wild type | Type II | [9,15,17] |
| UKJ421/18 | TIMM20119, 200123/18 | Phe397Leu | Gly443Glu | Type I | [9,15,17] |
| UKJ334/19 | TIMM201120, 250082/18 | Ala448Thr | Wild type | Type II | [9,17] |
| UKJ336/19 | TIMM201121, 250084/18 | Phe397Leu, Ala448Thr | Wild type | Type II | [9,17] |
| UKJ893/19 | TIMM201123, 600097/19 | Ala448Thr | Wild type | Type II | [9,17] |
| UKJ262/21 | Ala448Thr | Tyr444His | No amplificates | [14,18] | |
| UKJ476/21 | Ala448Thr | Wild type | Type II | [14,18] | |
| UKJ1067/21 | Wild type | Ala230Thr, Tyr444His | No amplificates | [14,18,22] | |
| UKJ1985/21 | Wild type | Ala230Thr, Tyr444His | No amplificates | [18,22] |
| Strain | MIC90 ITZ Alone µg/mL | MIC90 ITZ with Ritonavir µg/mL | FIC ITZ | % Reduction MICcombined | % Growth Reduction of Ritonavir Alone |
|---|---|---|---|---|---|
| UKJ1708/17 | 0.069 | 0.057 | 0.83 | 17 | 3 |
| UKJ336/19 | 0.028 | 0.0058 | 0.21 | 79 | 14 |
| UKJ421/18 | 0.024 | 0.0091 | 0.38 | 62 | 4 |
| UKJ893/19 | 0.024 | 0.0072 | 0.30 | 70 | 6 |
| UKJ476/21 | 0.023 | 0.015 | 0.67 | 33 | 12 |
| UKJ392/18 | 0.022 | 0.0087 | 0.40 | 60 | 0 |
| UKJ1067/21 | 0.017 | 0.0090 | 0.53 | 47 | 3 |
| UKJ1687/18 | 0.013 | 0.0072 | 0.53 | 47 | 3 |
| UKJ334/19 | 0.012 | 0.0053 | 0.43 | 57 | 1 |
| UKJ262/21 | 0.0095 | 0.0040 | 0.43 | 57 | 14 |
| UKJ1985/21 | 0.0032 | 0.0012 | 0.36 | 64 | 14 |
| UKJ1676/17 | 0.0025 | 0.0013 | 0.51 | 49 | 16 |
| Strain | MIC90 ITZ Alone µg/mL | MIC90 ITZ with Sertraline µg/mL | FIC ITZ | % Reduction MICcombined | % Growth Reduction of Sertraline Alone |
|---|---|---|---|---|---|
| UKJ1708/17 | 0.061 | 0.026 | 0.42 | 58 | 11 |
| UKJ421/18 | 0.047 | 0.0056 | 0.12 | 88 | 33 |
| UKJ893/19 | 0.033 | 0.0039 | 0.12 | 88 | 25 |
| UKJ476/21 | 0.028 | 0.011 | 0.40 | 60 | 28 |
| UKJ1687/18 | 0.024 | 0.0025 | 0.11 | 90 | 30 |
| UKJ334/19 | 0.018 | 0.0076 | 0.42 | 58 | 53 |
| UKJ336/19 | 0.017 | 0.0053 | 0.32 | 68 | 40 |
| UKJ1067/21 | 0.015 | 0.0025 | 0.17 | 83 | 49 |
| UKJ392/18 | 0.012 | 0.0023 | 0.19 | 81 | 26 |
| UKJ262/21 | 0.0105 | 0.0028 | 0.26 | 74 | 24 |
| UKJ1676/17 | 0.0032 | 0.0011 | 0.36 | 64 | 33 |
| UKJ1985/21 | 0.0028 | 0.00039 | 0.14 | 86 | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Günther, A.; Burmester, A.; Fabri, M.; Tittelbach, J.; Wiegand, C. Co-Treatment with Ritonavir or Sertraline Enhances Itraconazole Efficacy Against Azole-Resistant Trichophyton indotineae Isolates. J. Fungi 2025, 11, 698. https://doi.org/10.3390/jof11100698
Günther A, Burmester A, Fabri M, Tittelbach J, Wiegand C. Co-Treatment with Ritonavir or Sertraline Enhances Itraconazole Efficacy Against Azole-Resistant Trichophyton indotineae Isolates. Journal of Fungi. 2025; 11(10):698. https://doi.org/10.3390/jof11100698
Chicago/Turabian StyleGünther, Anna, Anke Burmester, Mario Fabri, Jörg Tittelbach, and Cornelia Wiegand. 2025. "Co-Treatment with Ritonavir or Sertraline Enhances Itraconazole Efficacy Against Azole-Resistant Trichophyton indotineae Isolates" Journal of Fungi 11, no. 10: 698. https://doi.org/10.3390/jof11100698
APA StyleGünther, A., Burmester, A., Fabri, M., Tittelbach, J., & Wiegand, C. (2025). Co-Treatment with Ritonavir or Sertraline Enhances Itraconazole Efficacy Against Azole-Resistant Trichophyton indotineae Isolates. Journal of Fungi, 11(10), 698. https://doi.org/10.3390/jof11100698






