Estimating the Climate Niche of Sclerotinia sclerotiorum Using Maximum Entropy Modeling
Abstract
:1. Introduction
2. Materials and Methods
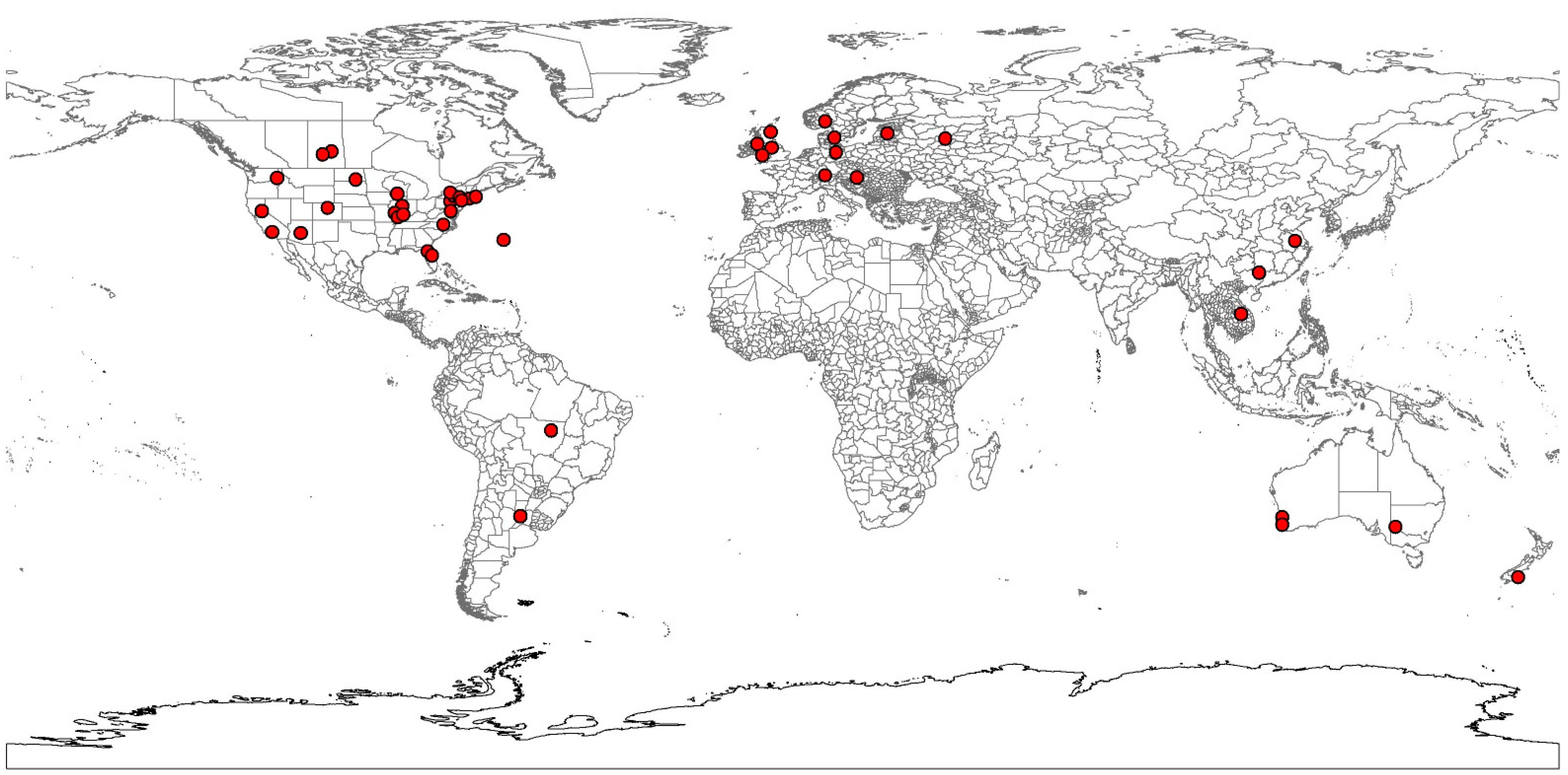
2.1. Species Occurrence Data
2.2. Taxonomy and Life Cycle of Sclerotinia sclerotiorum
2.3. Climate Data and Environmental Variables
2.4. Maxent Model Considerations
2.5. Variable Selection and Climate Niche
2.6. Model Evaluation and Selection
2.7. Fungal Species Habitat Suitability
3. Results
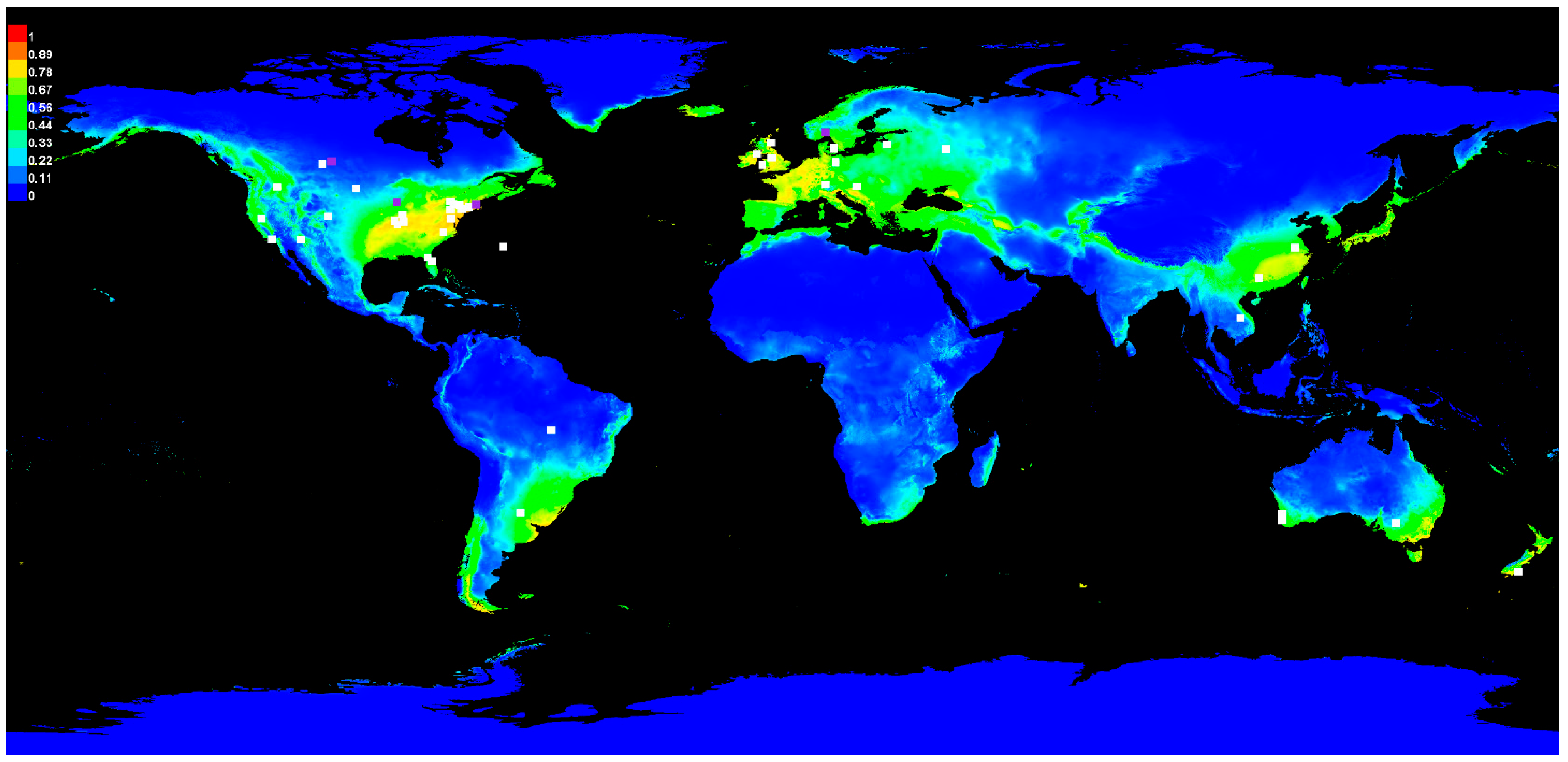
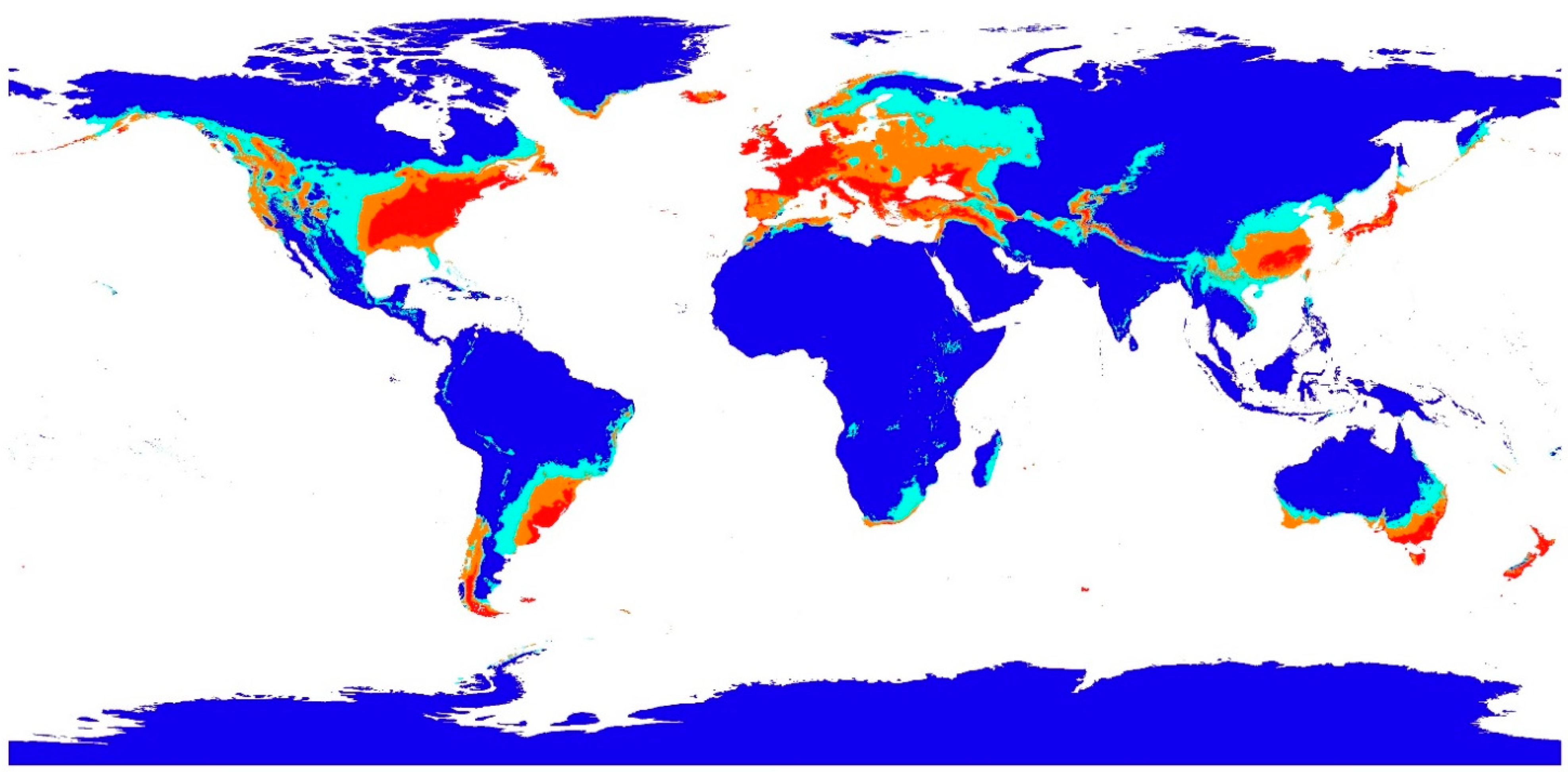
3.1. Sclerotinia Model Prediction
3.2. Climate Responses and Climate Niches
3.3. Geographic Habitat Suitability and Model Validation
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boland, G.J.; Hall, R. Index of plant hosts of Sclerotinia sclerotiorum. Can. J. Plant Pathol. 1994, 16, 93–108. [Google Scholar] [CrossRef]
- Bolton, M.D.; Thomma, B.P.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2006, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- CABI/EPPO. Sclerotinia Sclerotiorum; Distribution Maps of Plant Diseases. No. 971; CAB International: Wallingford, UK, 2005. [Google Scholar]
- Saharan, G.S.; Mehta, N. Sclerotinia Diseases of Crop Plants: Biology, Ecology and Disease Management; Springer Science+Business Media B. V.: Dordrecht, The Netherlands, 2008; p. 485. [Google Scholar] [CrossRef]
- Allen, T.W.; Bradley, C.A.; Sisson, A.J.; Byamukama, E.; Chilvers, M.I.; Coker, C.M.; Collins, A.A.; Damicone, J.P.; Dorrance, A.E.; Dufault, N.S. Soybean yield loss estimates due to diseases in the United States and Ontario, Canada, from 2010 to 2014. Plant Health Prog. 2017, 18, 19–27. [Google Scholar] [CrossRef]
- Peltier, A.J.; Bradley, C.A.; Chilvers, M.I.; Malvick, D.K.; Mueller, D.S.; Wise, K.A.; Esker, P.D. Biology, yield loss and control of Sclerotinia stem rot of soybean. J. Integr. Pest Manag. 2012, 3, B1–B7. [Google Scholar] [CrossRef]
- Willbur, J.F.; Mitchell, P.D.; Fall, M.L.; Byrne, A.M.; Chapman, S.A.; Floyd, C.M.; Bradley, C.A.; Ames, K.; Chilvers, M.I.; Kleczewski, N.M. Meta-analytic and economic approaches for evaluation of pesticide impact on Sclerotinia stem rot control and soybean yield in the North Central United States. Phytopathology 2019, 109, 1157–1170. [Google Scholar] [CrossRef]
- Chaudhary, S.; Lal, M.; Sagar, S.; Tyagi, H.; Kumar, M.; Sharma, S.; Chakrabarti, S. Genetic diversity studies based on morpho-pathological and molecular variability of the Sclerotinia sclerotiorum population infecting potato (Solanum tuberosum L.). World J. Microbiol. Biotechnol. 2020, 36, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.A.; Ferro, C.G.; Lehner, M.d.S.; Paula, T.J., Jr.; Mizubuti, E.S. The population of Sclerotinia sclerotiorum in Brazil is structured by mycelial compatibility groups. Plant Dis. 2021, 105, 3376–3384. [Google Scholar] [CrossRef]
- Faruk, M.I.; Rahman, M. Collection, isolation and characterization of Sclerotinia sclerotiorum, an emerging fungal pathogen causing white mold disease. J. Plant Sci. Phytopathol. 2022, 6, 043–051. [Google Scholar] [CrossRef]
- Adams, P.; Ayers, W. Ecology of Sclerotinia species. Phytopathology 1979, 69, 896–899. [Google Scholar] [CrossRef]
- Mordue, J.; Holliday, P. Sclerotinia Sclerotiorum: CMI Description of Pathogenic Fungi and Bacteria. No. 513; Commonwealth Mycological Institute: Kew, UK, 1976. [Google Scholar]
- Willetts, H.; Wong, J.; Kirst, G.D. The biology of Sclerotinia sclerotiorum, S. trifoliorum, and S. minor with emphasis on specific nomenclature. Bot. Rev. 1980, 46, 101–165. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudik, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudik, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Wollan, A.K.; Bakkestuen, V.; Kauserud, H.; Gulden, G.; Halvorsen, R. Modelling and predicting fungal distribution patterns using herbarium data. J. Biogeogr. 2008, 35, 2298–2310. [Google Scholar] [CrossRef]
- Banasiak, L.; Pietras, M.; Wrzosek, M.; Okrasinska, A.; Gorczak, M.; Kolanowska, M.; Pawlowska, J. Aureoboletus projectellus (Fungi, Boletales)-Occurence data, environmental layers and habitat suitability models for North America and Europe. Data Brief 2019, 23, 103779. [Google Scholar] [CrossRef]
- Copot, O.; Tanase, C. Maxent modelling of the potential distribution of Ganoderma lucidum in north-eastern region of Romania. J. Plant Dev. 2017, 24, 133–143. [Google Scholar]
- Yuan, H.S.; Wei, Y.L.; Wang, X.G. Maxent modeling for predicting the potential distribution of Sanghuang, an important group of medicinal fungi in China. Fungal Ecol. 2015, 17, 140–145. [Google Scholar] [CrossRef]
- Berthon, K.; Esperon-Rodriguez, M.; Beaumont, L.; Carnegie, A.; Leishman, M. Assessment and prioritisation of plant species at risk from myrtle rust (Austropuccinia psidii) under current and future climates in Australia. Biol. Conserv. 2018, 218, 154–162. [Google Scholar] [CrossRef]
- Cohen, S.D. Predicting the worldwide climate suitability for mycoparasites of Sclerotinia sclerotiorum. Phytopathology 2020, 110, S2.140. [Google Scholar] [CrossRef]
- Borah, T.R.; Dutta, S.; Barman, A.R.; Helim, R.; Sen, K. Variability and host range of Sclerotinia sclerotiorum in Eastern and North Eastern India. J. Plant Pathol. 2021, 103, 809–822. [Google Scholar] [CrossRef]
- Kass, J.M.; Vilela, B.; Aiello-Lammens, M.E.; Muscarella, R.; Merow, C.; Anderson, R.P. Wallace: A flexible platform for reproducible modeling of species niches and distributions built for community expansion. Methods Ecol. Evol. 2018, 9, 1151–1156. [Google Scholar] [CrossRef]
- Kohn, L.M. Delimitation of the economically important plant pathogenic Sclerotinia species. Phytopathology 1979, 69, 881–886. [Google Scholar] [CrossRef]
- Michael, P.J.; Lui, K.Y.; Thomson, L.L.; Lamichhane, A.R.; Bennett, S.J. Impact of Preconditioning Temperature and Duration Period on Carpogenic Germination of Diverse Sclerotinia sclerotiorum Populations in Southwestern Australia. Plant Dis. 2021, 105, 1798–1805. [Google Scholar] [CrossRef] [PubMed]
- Purdy, L. Sclerotinia sclerotiorum: History, diseases and symptomatology, host range, geographic distribution, and impact. Phytopathology 1979, 69, 875–880. [Google Scholar] [CrossRef]
- Wang, R.; Yang, H.; Luo, W.; Wang, M.; Lu, X.; Huang, T.; Zhao, J.; Li, Q. Predicting the potential distribution of the Asian citrus psyllid, Diaphorina citri (Kuwayama), in China using the MaxEnt model. PeerJ 2019, 7, e7323. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, G. Study of methods to improve the temporal transfer ability of niche model. J. China Agric. Univ. 2017, 22, 98–105. [Google Scholar]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Phillips, S.J. A Brief Tutorial on Maxent. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 27 January 2022).
- Cao, Y.-T.; Lu, Z.-P.; Gao, X.-Y.; Liu, M.-L.; Sa, W.; Liang, J.; Wang, L.; Yin, W.; Shang, Q.-H.; Li, Z.-H. Maximum Entropy Modeling the Distribution Area of Morchella Dill. ex Pers. Species in China under Changing Climate. Biology 2022, 11, 1027. [Google Scholar] [CrossRef]
- Pearman, P.; Guisan, A.; Broennimann, O.; Randin, C. Niche dynamics in space and time. Trends Ecol. Evol. 2008, 23, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, G.E. Concluding remarks cold spring harbor symposium on quantitative biology. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Hutchinson, G. An Introduction to Population Biology; Yale University Press: New Haven, CT, USA, 1978; p. 260. [Google Scholar]
- Hutchinson, G.E. Population studies: Animal ecology and demography. Bull. Math. Biol. 1991, 53, 193–213. [Google Scholar] [CrossRef]
- Huang, H.; Kozub, G. Temperature requirements for carpogenic germination of sclerotia of Sclerotinia sclerotiorum isolates of different geographic origin. Bot. Bull. Acad. Sin. 1991, 32, 279–286. [Google Scholar]
- Hao, J.; Subbarao, K.; Duniway, J. Germination of Sclerotinia minor and S. sclerotiorum sclerotia under various soil moisture and temperature combinations. Phytopathology 2003, 93, 443–450. [Google Scholar] [CrossRef]
- Sun, P.; Yang, X. Light, temperature, and moisture effects on apothecium production of Sclerotinia sclerotiorum. Plant Dis. 2000, 84, 1287–1293. [Google Scholar] [CrossRef]
- Schwartz, H.; Steadman, J. Factors affecting sclerotium populations of, and apothecium production by, Sclerotinia sclerotiorum. Phytopathology 1978, 68, 383–388. [Google Scholar] [CrossRef]
- Weiss, A.; Kerr, E.; Steadman, J. Temperature and moisture influences on development of white mold disease (Sclerotinia sclerotiorum) on great northern beans. Plant Dis. 1980, 64, 757–759. [Google Scholar] [CrossRef]
- Moore, W.D. Relation of rainfall and temperatures to the incidence of Sclerotinia sclerotiorum in vegetables in south Florida during the years 1944 to 1954. Plant Dis. Rep. 1955, 39, 470–472. [Google Scholar]
- Thuiller, W.; Lavorel, S.; Araújo, M.B. Niche properties and geographical extent as predictors of species sensitivity to climate change. Glob. Ecol. Biogeogr. 2005, 14, 347–357. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Větrovský, T.; Kohout, P.; Kopecký, M.; Machac, A.; Man, M.; Bahnmann, B.D.; Brabcová, V.; Choi, J.; Meszárošová, L.; Human, Z.R. A meta-analysis of global fungal distribution reveals climate-driven patterns. Nat. Commun. 2019, 10, 5142. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifah, D.H.M.; Damra, E.; Khalaf, S.M.; Hozzein, W.N. Biogeography of Black Mold Aspergillus niger: Global Situation and Future Perspective under Several Climate Change Scenarios Using MaxEnt Modeling. Diversity 2022, 14, 845. [Google Scholar] [CrossRef]
- Wakelin, S.A.; Gomez-Gallego, M.; Jones, E.; Smaill, S.; Lear, G.; Lambie, S. Climate change induced drought impacts on plant diseases in New Zealand. Australas. Plant Pathol. 2018, 47, 101–114. [Google Scholar] [CrossRef]
- Aidoo, O.F.; Souza, P.G.C.; da Silva, R.S.; Júnior, P.A.S.; Picanço, M.C.; Osei-Owusu, J.; Sétamou, M.; Ekesi, S.; Borgemeister, C. A machine learning algorithm-based approach (MaxEnt) for predicting invasive potential of Trioza erytreae on a global scale. Ecol. Inform. 2022, 71, 101792. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Li, C.; Liu, Z. Optimized maxent model predictions of climate change impacts on the suitable distribution of Cunninghamia lanceolata in China. Forests 2020, 11, 302. [Google Scholar] [CrossRef]
- Yang, Z.; Bai, Y.; Alatalo, J.M.; Huang, Z.; Yang, F.; Pu, X.; Wang, R.; Yang, W.; Guo, X. Spatio-temporal variation in potential habitats for rare and endangered plants and habitat conservation based on the maximum entropy model. Sci. Total Environ. 2021, 784, 147080. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J.; May, T.W. Enhancing repository fungal data for biogeographic analyses. Fungal Ecol. 2021, 53, 101097. [Google Scholar] [CrossRef]
- West, A.M.; Kumar, S.; Brown, C.S.; Stohlgren, T.J.; Bromberg, J. Field validation of an invasive species Maxent model. Ecol. Inform. 2016, 36, 126–134. [Google Scholar] [CrossRef]

| Bioclimatic Variable * | Unit | Percentage | Permutation |
|---|---|---|---|
| Contribution | Importance | ||
| BIO1 = Annual Mean Temperature | °C | __ | __ |
| BIO2 = Mean Diurnal Range (Mean of monthly (max temp − min temp)) | °C | __ | __ |
| BIO3 = Isothermality (BIO2/BIO7) (* 100) | __ | 8.5 | 11.9 |
| BIO4 = Temperature Seasonality (standard deviation * 100) | C of V | 0.5 | 6.4 |
| BIO5 = Max Temperature of Warmest Month | °C | 2.6 | 2.9 |
| BIO6 = Min Temperature of Coldest Month | °C | 0.4 | 0.0 |
| BIO7 = Temperature Annual Range (BIO5–BIO6) | °C | __ | ___ |
| BIO8 = Mean Temperature of Wettest Quarter | °C | __ | ___ |
| BIO9 = Mean Temperature of Driest Quarter | °C | __ | ___ |
| BIO10 = Mean Temperature of Warmest Quarter | °C | __ | ___ |
| BIO11 = Mean Temperature of Coldest Quarter | °C | 31.1 | 55.6 |
| BIO12 = Annual Precipitation | mm | 14.2 | 14.1 |
| BIO13 = Precipitation of Wettest Month | mm | __ | __ |
| BIO14 = Precipitation of Driest Month | mm | 11.5 | 1.6 |
| BIO15 = Precipitation Seasonality (Coefficient of Variation) | C of V | 7.0 | 0.3 |
| BIO16 = Precipitation of Wettest Quarter | mm | __ | ___ |
| BIO17 = Precipitation of Driest Quarter | mm | __ | ___ |
| BIO18 = Precipitation of Warmest Quarter | mm | __ | ___ |
| BIO19 = Precipitation of Coldest Quarter | mm | 24.2 | 7.1 |
| Species | Training # | Test # | AUC Training | AUC Test | AUC Difference | AUC Average |
|---|---|---|---|---|---|---|
| Fungal pathogen | ||||||
| S. sclerotiorum | ||||||
| Non-Random Model | 34 | 11 | 0.936 | 0.903 | 0.033 | 0.920 |
| Random Model 0 | 40 | 5 | 0.934 | 0.917 | 0.017 | 0.926 |
| Random Model 1 | 40 | 5 | 0.937 | 0.875 | 0.062 | 0.906 |
| Random Model 2 | 40 | 5 | 0.935 | 0.879 | 0.056 | 0.907 |
| Random Model 3 | 40 | 5 | 0.930 | 0.970 | −0.04 | 0.950 |
| Random Model 4 | 40 | 5 | 0.929 | 0.955 | −0.02 | 0.942 |
| Random Model 5 | 41 | 4 | 0.929 | 0.941 | −0.012 | 0.935 |
| Random Model 6 | 41 | 4 | 0.937 | 0.861 | 0.076 | 0.900 |
| Random Model 7 | 41 | 4 | 0.931 | 0.939 | −0.008 | 0.935 |
| Random Model 8 | 41 | 4 | 0.941 | 0.831 | 0.110 | 0.886 |
| Random Model 9 | 41 | 4 | 0.936 | 0.842 | 0.094 | 0.889 |
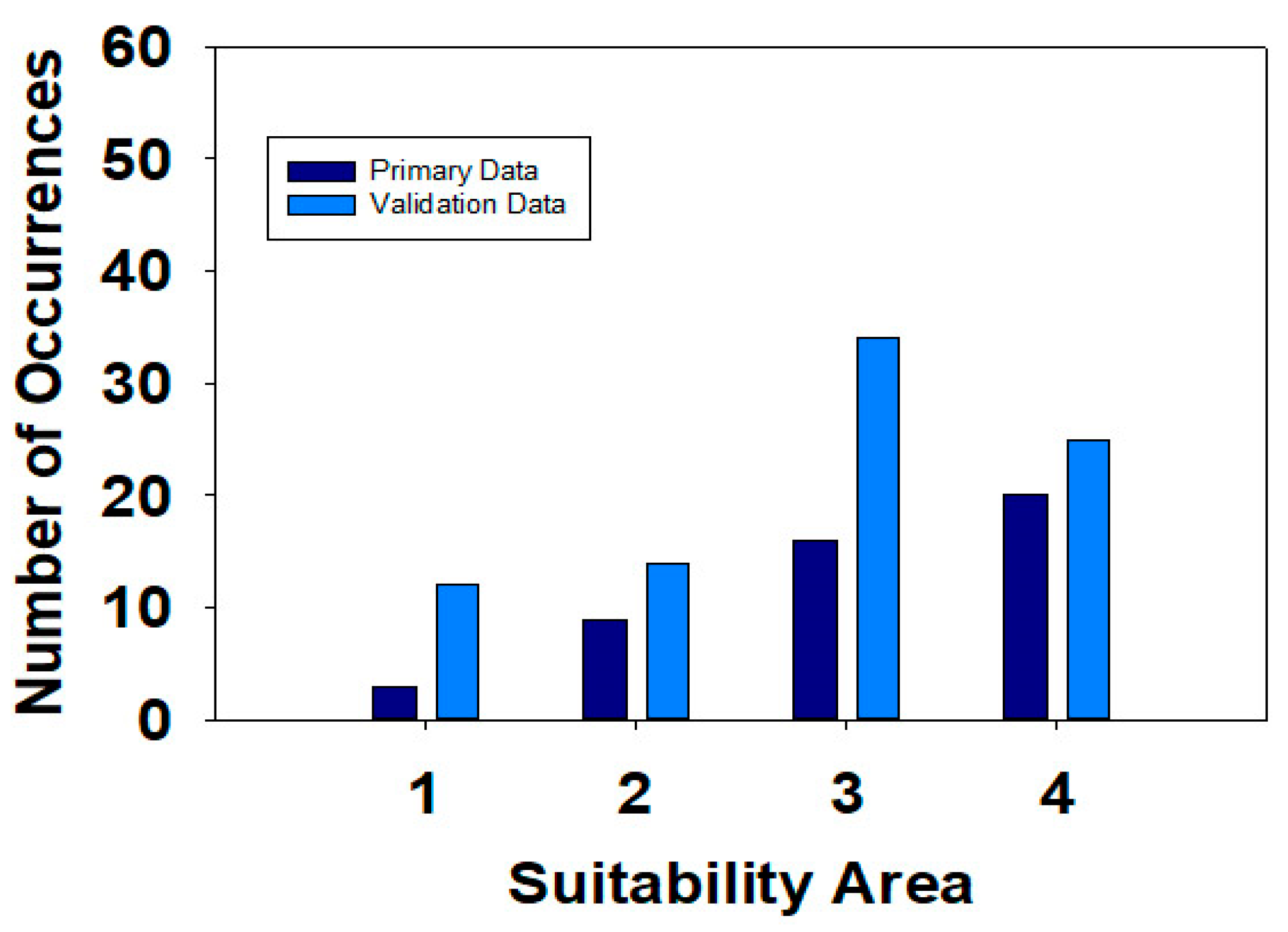
| Suitability Categories * | Number of Occurrences | Z-Score ** | p Value ** | |
|---|---|---|---|---|
| Maxent Model Dataset | Validation Dataset | |||
| Low Suitability | 3 | 12 | −0.624 | 0.533 |
| Moderate Suitability | 9 | 14 | −0.342 | 0.732 |
| High Suitability | 13 | 34 | −0.369 | 0.712 |
| Very High Suitability | 20 | 25 | 0.731 | 0.465 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, S.D. Estimating the Climate Niche of Sclerotinia sclerotiorum Using Maximum Entropy Modeling. J. Fungi 2023, 9, 892. https://doi.org/10.3390/jof9090892
Cohen SD. Estimating the Climate Niche of Sclerotinia sclerotiorum Using Maximum Entropy Modeling. Journal of Fungi. 2023; 9(9):892. https://doi.org/10.3390/jof9090892
Chicago/Turabian StyleCohen, Susan D. 2023. "Estimating the Climate Niche of Sclerotinia sclerotiorum Using Maximum Entropy Modeling" Journal of Fungi 9, no. 9: 892. https://doi.org/10.3390/jof9090892
APA StyleCohen, S. D. (2023). Estimating the Climate Niche of Sclerotinia sclerotiorum Using Maximum Entropy Modeling. Journal of Fungi, 9(9), 892. https://doi.org/10.3390/jof9090892






