Pitaya Juice Consumption Protects against Oxidative Damage Induced by Aflatoxin B1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Red Pitaya Juice
2.2. Drugs and Reagents
2.3. Animals
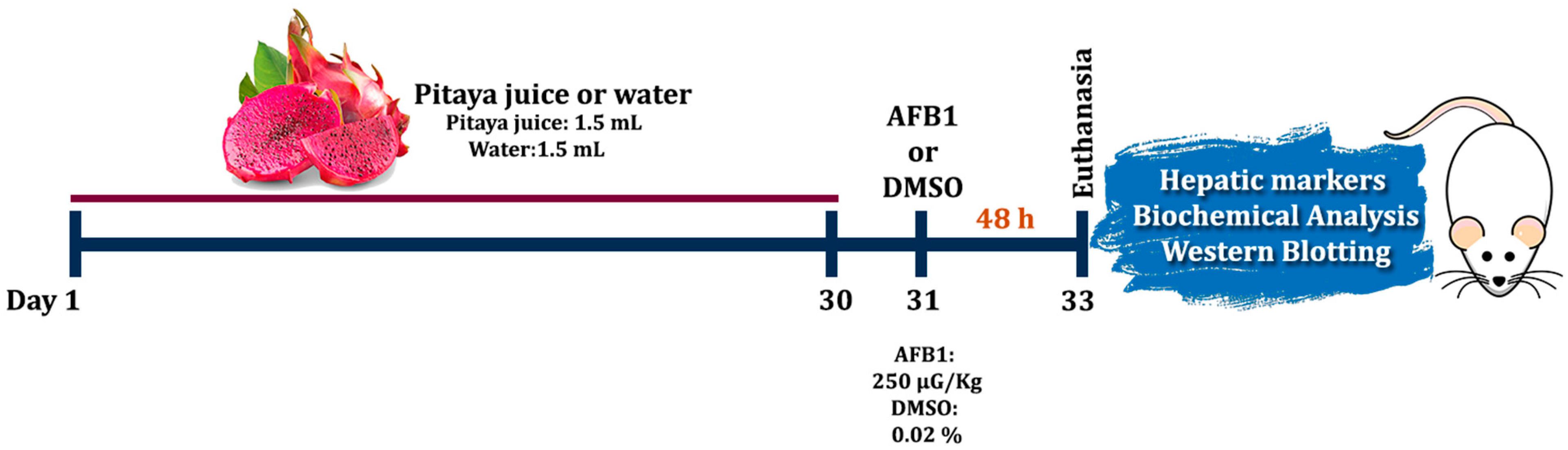
2.4. Experimental Design and Pre-Treatment
2.5. Liver Damage Markers
2.6. Parameters of Oxidative Stress
2.6.1. Thiobarbituric acid Reactive Species (TBARS)
2.6.2. Reactive Species Levels (RS)
2.6.3. Enzyme Activity of Catalase (CAT)
2.6.4. Enzyme Activity of Superoxide Dismutase (SOD)
2.6.5. Enzymatic Activity of Glutathione-S-Transferase (GST)
2.6.6. Fluorimetric Assay of Reduced Glutathione (GSH)
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. Pitaya Juice Partially Attenuates AFB1-Induced Liver Toxicity
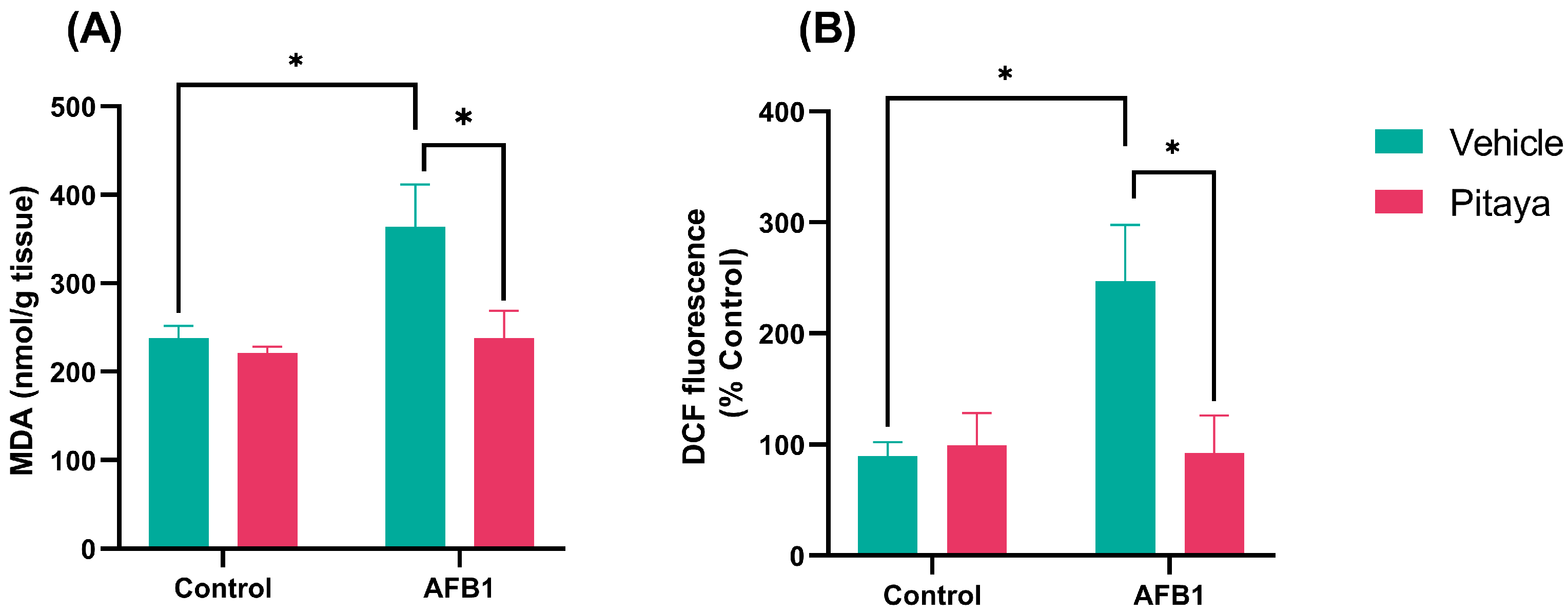
3.2. Pitaya Pre-Treatment Protected Rats from Lipid Peroxidation and AFB1-Induced RS
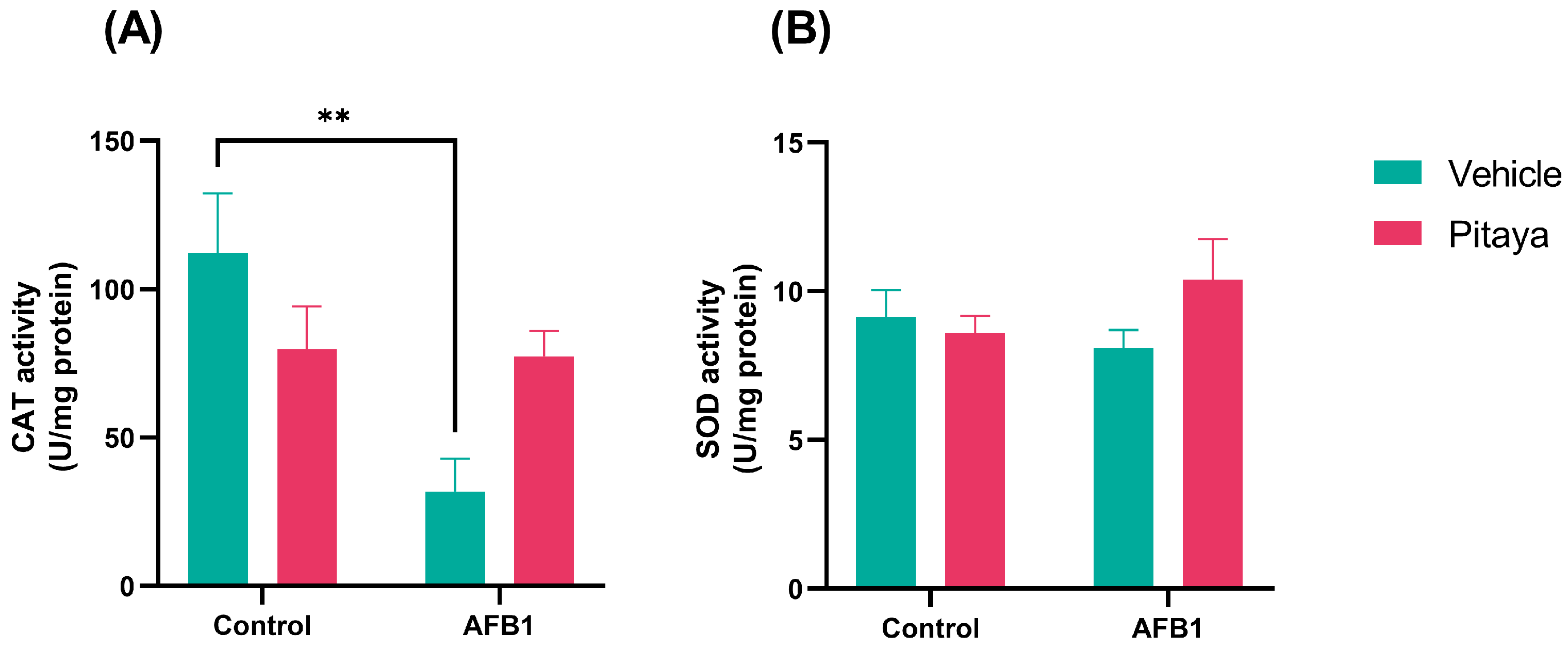
3.3. Pitaya Attenuated the CAT Enzymatic Activity Altered by AFB1 but Not Significantly
3.4. AFB1 Alters GST and GSH, with Protective Effect of Pitaya on GST
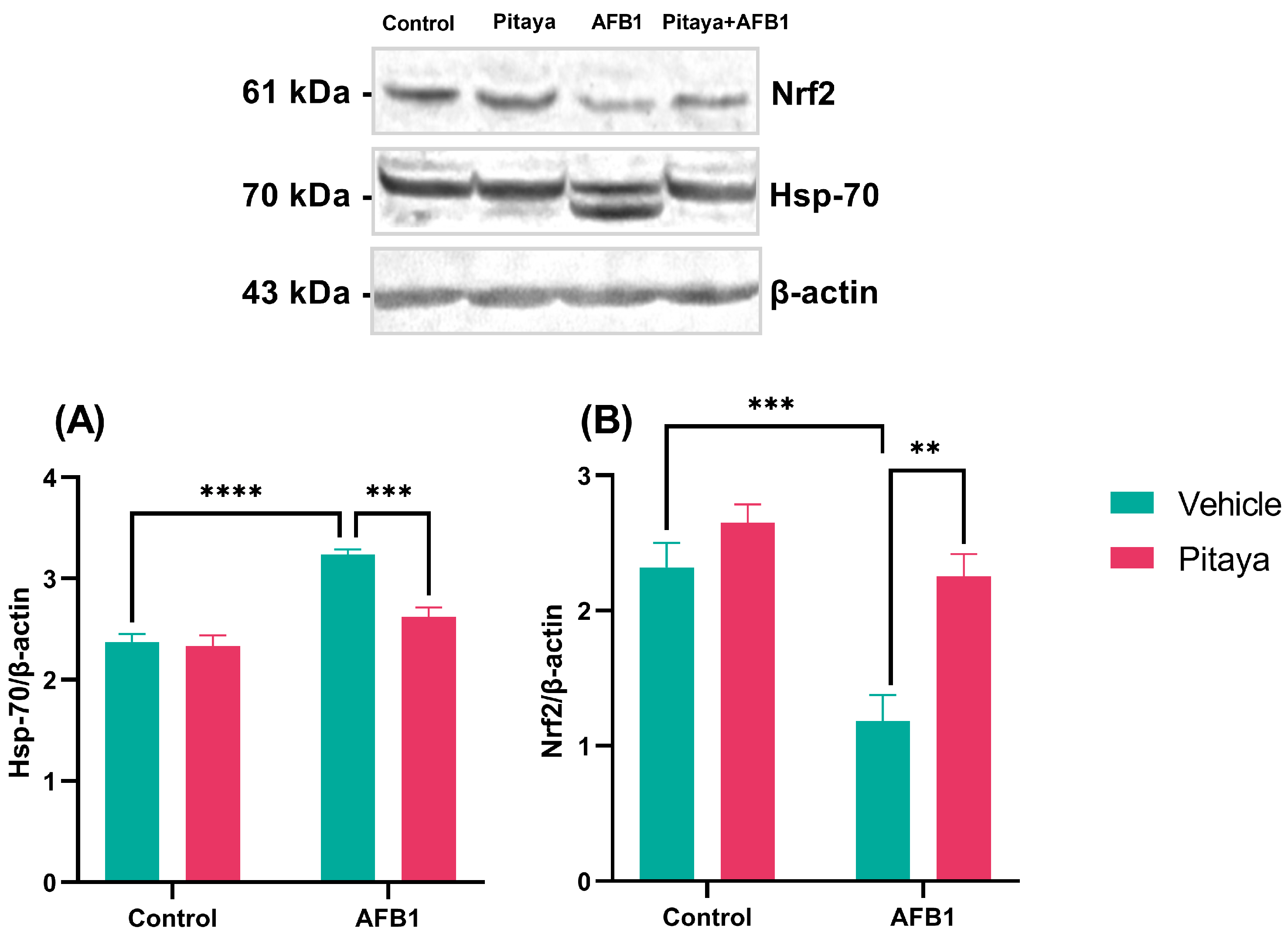
3.5. Pitaya Modulated the Expression of Hsp-70 and Nrf2 Markers in Mice Exposed to AFB1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gimeno, A.; Martins, M.L. Micotoxinas y Micotoxicosis en Animales y Humanos; Special Nutrients Inc.: Miami, FL, USA, 2011. [Google Scholar]
- Patriarca, A.; Pinto, V.F. Prevalence of mycotoxins in foods and decontamination. Curr. Opin. Food Sci. 2017, 14, 50–60. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2019, 60, 2773–2789. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Mazo, J.D.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.C.; Nebbia, C.S.; et al. Risk assessment of aflatoxins in food. EFSA J. 2020, 18, 6040. [Google Scholar] [CrossRef]
- Liu, J.; Song, W.J.; Zhang, N.Y.; Tan, J.; Krumm, C.S.; Sun, L.H.; Qi, D.S. Biodetoxification of aflatoxin B1 in cottonseed meal by fermentation of Cellulosimicrobium funkei in duckling diet. Poult. Sci. 2017, 96, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef]
- Moon, H.; Han, K.H.; Yu, J.H. Upstream Regulation of Development and Secondary Metabolism in Aspergillus Species. Cells 2022, 12, 2. [Google Scholar] [CrossRef]
- Jallow, A.; Xie, H.; Tang, X.; Qi, Z.; Li, P. Worldwide aflatoxin contamination of agricultural products and foods: From occurrence to control. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2332–2381. [Google Scholar] [CrossRef]
- Kew, M.C. Aflatoxins as a cause of hepatocellular carcinoma. J. Gastrointest. Liver Dis. 2013, 22, 305–310. [Google Scholar]
- International Agency for Research on Cancer (IARC). Monographs on the Evaluation of Carcinogenic Risks to Humans. Agents Classified by the IARC Monographs. Available online: https://monographs.iarc.who.int/agents-classified-by-the-iarc/ (accessed on 22 June 2023).
- Afsah-Hejri, L.; Jinap, S.; Hajeb, P.; Radu, S.; Shakibazadeh, S. A review on mycotoxins in food and feed: Malaysia case study. Compr. Rev. Food Sci. Food Saf. 2013, 12, 629–651. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Q.; Wang, L.; Gao, X.; Zhu, W.; Um, P.; Deng, Y. Aflatoxin B1 Induces Neurotoxicity through Reactive Oxygen Species Generation, DNA Damage, Apoptosis, and S-Phase Cell Cycle Arrest. Int. J. Mol. Sci. 2020, 21, 6517. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Towner, R.A.; Qian, S.Y.; Kadiiska, M.B.; Mason, R.P. In vivo identification of aflatoxin-induced free redicals in rat bile. Free Radic. Biol. Med. 2003, 35, 1330–1340. [Google Scholar] [CrossRef]
- Dai, C.; Tin, E.; Hao, Z.; Tang, S.; Wang, Z.; Sharma, G.; Jiang, H.; Shen, J. Aflatoxin B1 Toxicity and Protective Effects of Curcumin: Molecular Mechanisms and Clinical Implications. Antioxidants 2021, 11, 2031. [Google Scholar] [CrossRef] [PubMed]
- Safari, N.; Ardakani, M.M.; Hemmati, R.; Parroni, A.; Beccaccioli, M.; Reverberi, M. The Potential of Plant-Based Bioactive Compounds on Inhibition of Aflatoxin B1 Biosynthesis and Down-regulation of aflR, aflM and aflP Genes. Antibiotics 2020, 9, 728. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, O.Y.; Salazar, C.J. Pitahaya (Hylocereus spp.): A short review. Comun. Sci. 2012, 3, 220–237. [Google Scholar] [CrossRef]
- Esquivel, P.; Stintzing, F.; Carle, R. Phenolic compound profiles and their corresponding antioxidant capacity of purple pitaya (Hylocereus sp.) genotypes. Z. Naturforschung C 2007, 62, 636–644. [Google Scholar] [CrossRef]
- Joshi, M.; Prabhakar, B. Phytoconstituents and pharmaco-therapeutic benefits of pitaya: A wonder fruit. J. Food Biochem. 2020, 44, e13260. [Google Scholar] [CrossRef]
- Song, H.; Zheng, Z.; Wu, J.; Lai, J.; Chu, Q.; Zheng, X. White Pitaya (Hylocereus undatus) Juice Attenuates Insulin Resistance and Hepatic Steatosis in Diet-Induced Obese Mice. PLoS ONE 2016, 11, 0149670. [Google Scholar] [CrossRef]
- García-Cruz, L.; Dueñas, M.; Santos-Buelgas, C.; Valle-Guadarrama, S.; Salinas-Moreno, Y. Betalains and phenolic compounds profiling and antioxidant capacity of pitaya (Stenocereus spp.) fruit from two species (S. Pruinosus and S. stellatus). Food Chem. 2017, 234, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Souto, N.S.; Braga, A.C.M.; de Freitas, M.L.; Fighera, M.R.; Royes, L.F.F.; Oliveira, M.S.; Furian, A.F. Aflatoxin B1 reduces non-enzymatic antioxidant defenses and increases protein kinase C activation in the cerebral cortex of young rats. Nutr. Neurosci. 2017, 21, 268–275. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Severiano, F.; Santamaría, A.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Ríos, C.; Segovia, J. Increased formation of reactive oxygen species, but no changes in glutathione peroxidase activity, in striata of mice transgenic for the Huntington’s disease mutation. Neurochem. Res. 2004, 29, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Sies, H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Guerra, G.P.; Mello, C.F.; Bochi, G.V.; Pazini, A.M.; Rosa, M.M.; Ferreira, J.; and Rubin, M.A. Spermidine-induced improvement of memory involves a cross-talk between protein kinases C and A. J. Neurochem. 2012, 122, 363–373. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Romero-Calvo, I.; Ocón, B.; Martínez-Moya, P.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; de Medina, F.S. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal. Biochem. 2010, 401, 318–320. [Google Scholar] [CrossRef]
- Ellis, W.O.; Smith, J.P.; Simpson, B.K.; Oldham, J.H. Aflatoxins in food: Occurrence, biosynthesis, effects on organisms, detection, and methods of control. Crit. Rev. Food Sci. Nutr. 1991, 30, 403–439. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; John, S. Cholestatic Jaundice; StatPearls: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482279/ (accessed on 1 July 2023).
- Chapman, S.E.; Hostutler, R.A. A laboratory diagnostic approach to hepatobiliary disease in small animals. Vet. Clin. N. Am. Small Anim. Pr. 2013, 43, 1209–1225. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Liu, M.; Zhou, X.; Wang, M.; Cao, K.; Jin, S.; Shan, A.; Feng, X. Curcumin mitigates aflatoxin B1-induced liver injury via regulating the NLRP3 inflammasome and Nrf2 signaling pathway. Food Chem. Toxicol. 2022, 161, 112823. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.D.; Mamat, S.S.; Kanisan, F.H.; Yahya, F.; Kamarolzaman, M.F.F.; Nasir, N.; Mahtarrudin, N.; Tohid, S.F.; Zakaria, Z.A. Amelioration of Paracetamol-Induced Hepatotoxicity in Rat by the Administration of Methanol Extract of Muntingia calabura L. Leaves. BioMed Res. Int. 2014, 2014, 695678. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, A.R.; Rosa, É.V.F.; Sari, M.H.M.; Sampaio, T.B.; Dos Santos, J.T.; Jardim, N.S.; Furian, A.F. Therapeutic potential of beta-caryophyllene against aflatoxin B1-Induced liver toxicity: Biochemical and molecular insights in rats. Chem. Biol. Interact. 2021, 348, 109635. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W. Aflatoxin B1 impairs mitochondrial functions, activates ROS generation, induces apoptosis and involves Nrf2 signal pathway in primary broiler hepatocytes. Anim. Sci. J. 2016, 87, 1490–1500. [Google Scholar] [CrossRef]
- Mughal, M.J.; Peng, X.; Zhou, Y.; Fang, J. Aflatoxin B1 invokes apoptosis via death receptor pathway in hepatocytes. Oncotarget 2017, 8, 8239–8249. [Google Scholar] [CrossRef]
- Forman, H.J. Redox signaling: An evolution from free radicals to aging. Free Radic. Biol. Med. 2016, 97, 398–407. [Google Scholar] [CrossRef]
- Chen, J.J.; Yu, B.Y. Alterations in mitochondrial membrane fluidity by lipid peroxidation products. Free Radic. Biol. Med. 1994, 17, 411–418. [Google Scholar] [CrossRef]
- Ferrante, M.C.; Meli, R.; Raso, G.M.; Esposito, E.; Severino, L.; Di Carlo, G.; Lucisano, A. Effect of fumonisin B1 on structure and function of macrophage plasma membrane. Toxicol. Lett. 2002, 129, 181–187. [Google Scholar] [CrossRef]
- Zhang, N.Y.; Qi, M.; Zhao, L.; Zhu, M.K.; Guo, J.; Liu, J.; Gu, C.Q.; Rajput, S.A.; Krumm, C.S.; Qi, D.S.; et al. Curcumin Prevents Aflatoxin B1 Hepatoxicity by Inhibition of Cytochrome P450 Isozymes in Chick Liver. Toxins 2016, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Cueto, R.; Effi, C.; Zhang, Y.; Tan, H.; Qin, X.; Ji, Y.; Yang, X.; Wang, H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019, 26, 101284. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, R.; Xia, S.; Wei, G.; Ishfaq, M.; Zhang, Y.; Zhang, X. Protective role of curcumin on aflatoxin B1-induced TLR4/RIPK pathway mediated-necroptosis and inflammation in chicken liver. Ecotoxicol. Environ. Saf. 2022, 233, 113319. [Google Scholar] [CrossRef] [PubMed]
- Andarwulan, N.; Puspita, N.C.; Saraswati; Średnicka-Tober, D. Antioxidants Such as Flavonoids and Carotenoids in the Diet of Bogor, Indonesia Residents. Antioxidants 2021, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, P.M.; Rubio, M.A.G.; Escudero, P.H.; Carmona, F.G.; Herrero, F.G. Health-promoting potential of betalains in vivo and their relevance as functional ingredients: A review. Trends Food Sci. Technol. 2022, 122, 66–82. [Google Scholar] [CrossRef]
- Lanneau, D.; Brunet, M.; Frisan, E.; Solary, E.; Fontenay, M.; Garrido, C. Heat shock proteins: Essential proteins for apoptosis regulation. J. Cell. Mol. Med. 2008, 12, 743–761. [Google Scholar] [CrossRef]
- Harahap, N.S.; Lelo, A.; Purba, A.; Sibuea, A.; Amelia, R.; Zulaini, Z. The effect of red-fleshed pitaya (Hylocereus polyrhizus) on heat shock protein 70 and cortisol expression in strenuous exercise induced rats. F1000Research 2021, 8, 130. [Google Scholar] [CrossRef]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011, 16, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Tang, L.; Rao, G.; Zhong, G.; Jiang, X.; Wu, S.; Huang, R.; Tang, Z.; Ruan, z.; Chen, Z. Curcumin activates the Nrf2 Pathway to alleviate AFB1-induced immunosuppression in the spleen of ducklings. Toxicon 2022, 209, 18–27. [Google Scholar] [CrossRef]
- Zhou, Y.; Jin, Y.; Yu, H.; Shan, A.; Shen, J.; Zhou, C.; Zhao, Y.; Fang, H.; Wang, X.; Wang, H.; et al. Resveratrol inhibits aflatoxin B1-induced oxidative stress and apoptosis in bovine mammary epithelial cells and is involved the Nrf2 signaling pathway. Toxicon 2019, 164, 10–15. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Ou, Z.; Meng, Y.; Chen, Y.; Lin, R.; Hashim, J.H.; Hashim, Z.; Wieslander, G.; Chen, Q.; et al. Indoor microbiome, microbial and plant metabolites, chemical compounds, and asthma symptoms in junior high school students: A multicentre association study in Malaysia. Eur. Respir. J. 2022, 60, 2200260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, S.; Luo, H.; Xu, F.; Liang, J.; Ma, C.; Ren, L.; Wang, H.; Hou, Y. Aflatoxin B1 induces microglia cells apoptosis mediated by oxidative stress through NF-κB signaling pathway in mice spinal cords. Environ. Toxicol. Pharmacol. 2022, 90, 103794. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madalosso, L.M.; Balok, F.R.M.; Bortolotto, V.C.; Dahleh, M.M.M.; Backes, L.G.; Escalante, E.S.S.; Benites, F.V.; da Silva e Silva, F.A.; Segat, H.J.; Boeira, S.P. Pitaya Juice Consumption Protects against Oxidative Damage Induced by Aflatoxin B1. J. Fungi 2023, 9, 874. https://doi.org/10.3390/jof9090874
Madalosso LM, Balok FRM, Bortolotto VC, Dahleh MMM, Backes LG, Escalante ESS, Benites FV, da Silva e Silva FA, Segat HJ, Boeira SP. Pitaya Juice Consumption Protects against Oxidative Damage Induced by Aflatoxin B1. Journal of Fungi. 2023; 9(9):874. https://doi.org/10.3390/jof9090874
Chicago/Turabian StyleMadalosso, Luiggi Müller, Franciéle Romero Machado Balok, Vandreza Cardoso Bortolotto, Mustafa Munir Mustafa Dahleh, Lucas Gabriel Backes, Elizabeth Sabryna Sarquis Escalante, Fernanda Vilhalba Benites, Francisco Andrey da Silva e Silva, Hecson Jesser Segat, and Silvana Peterini Boeira. 2023. "Pitaya Juice Consumption Protects against Oxidative Damage Induced by Aflatoxin B1" Journal of Fungi 9, no. 9: 874. https://doi.org/10.3390/jof9090874
APA StyleMadalosso, L. M., Balok, F. R. M., Bortolotto, V. C., Dahleh, M. M. M., Backes, L. G., Escalante, E. S. S., Benites, F. V., da Silva e Silva, F. A., Segat, H. J., & Boeira, S. P. (2023). Pitaya Juice Consumption Protects against Oxidative Damage Induced by Aflatoxin B1. Journal of Fungi, 9(9), 874. https://doi.org/10.3390/jof9090874






