Mycotoxins from Tomato Pathogenic Alternaria alternata and Their Combined Cytotoxic Effects on Human Cell Lines and Male Albino Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Isolation
2.2. Molecular Characterization
2.2.1. DNA Extraction and PCR Amplification
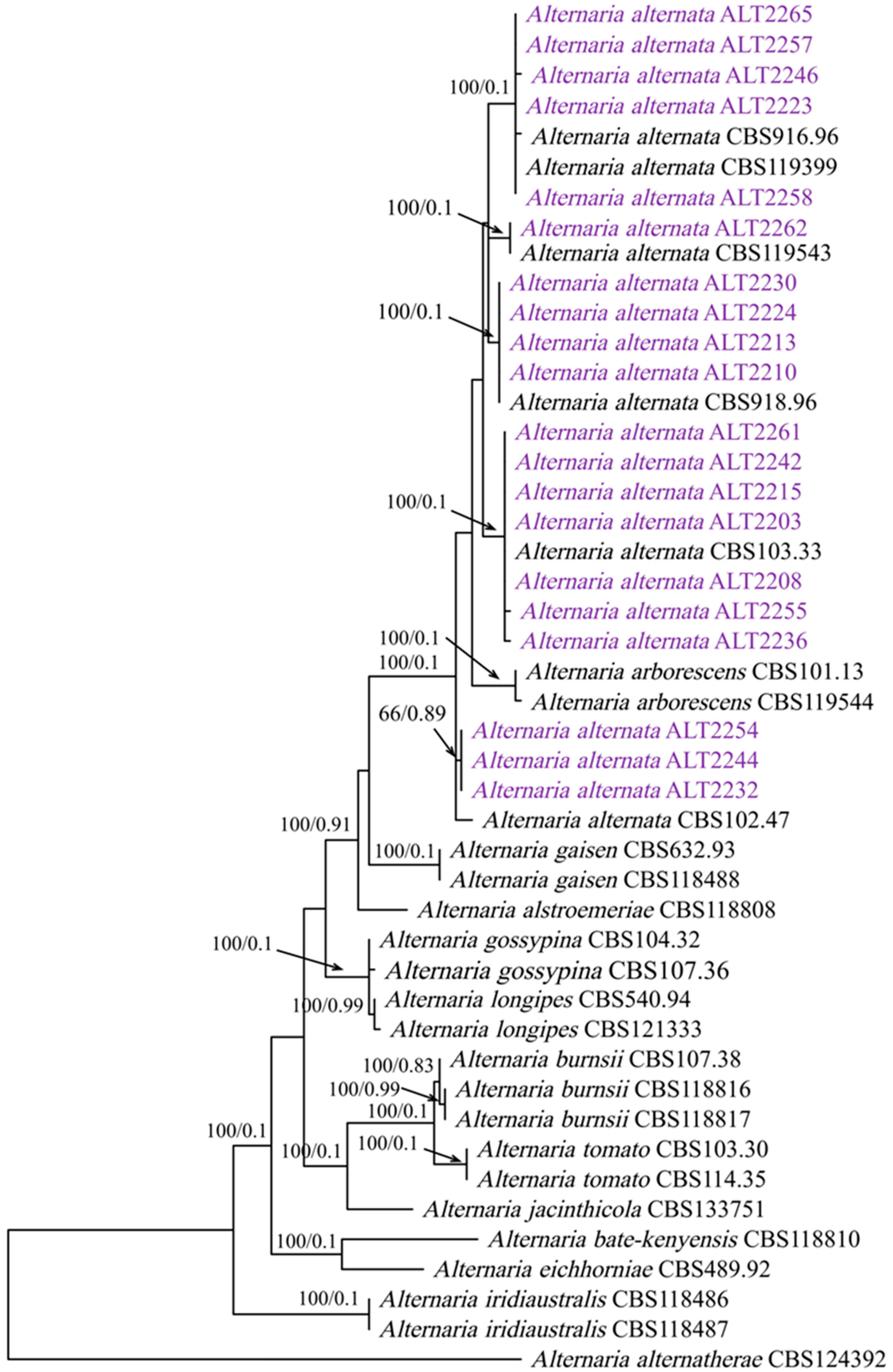
2.2.2. Phylogenetic Analysis
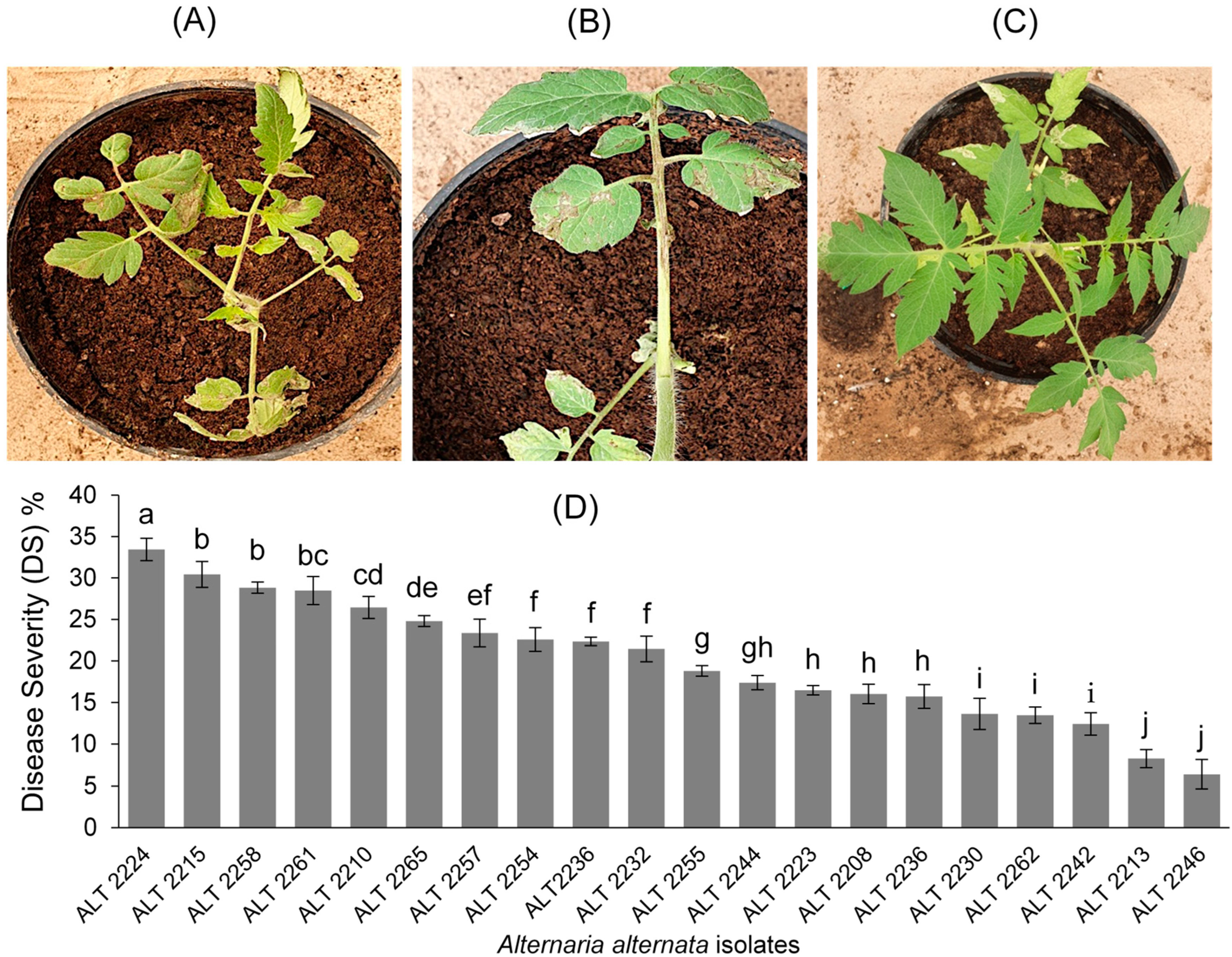
2.3. Pathogenicity Tests
2.4. Mycotoxin Production and Extraction
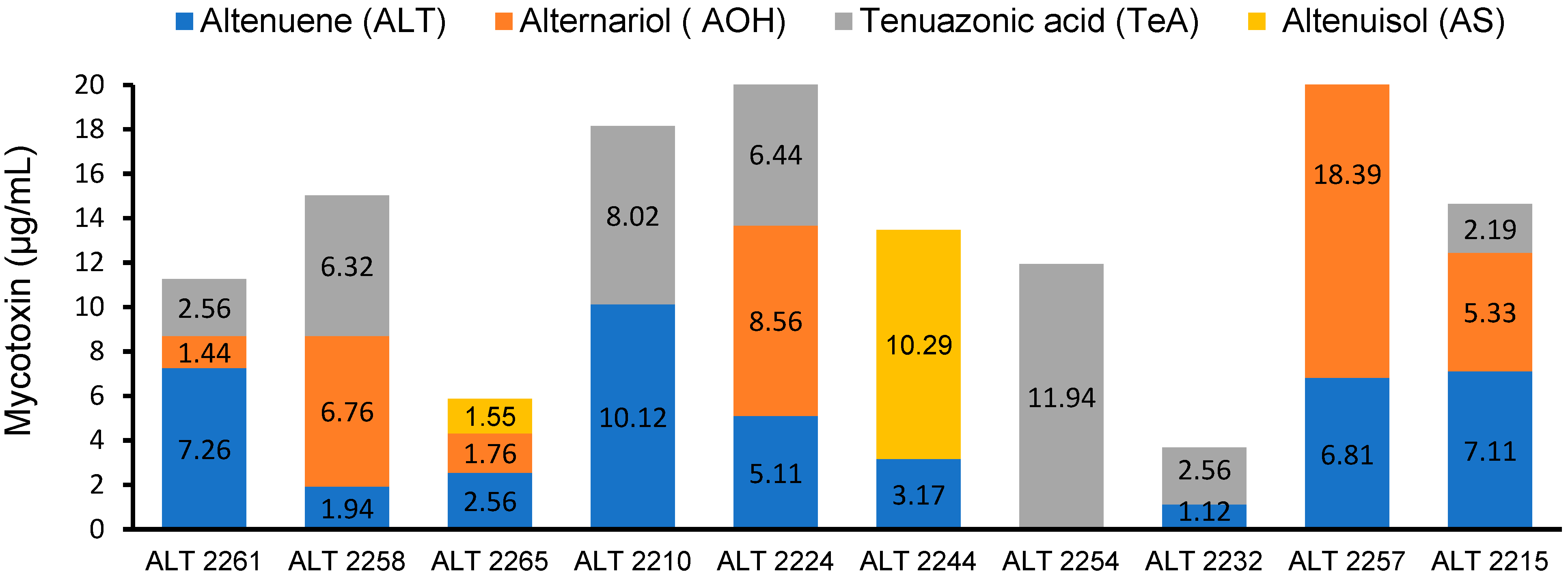
2.5. HPLC Analysis
2.6. Cytotoxicity Assays
2.6.1. Cell Lines
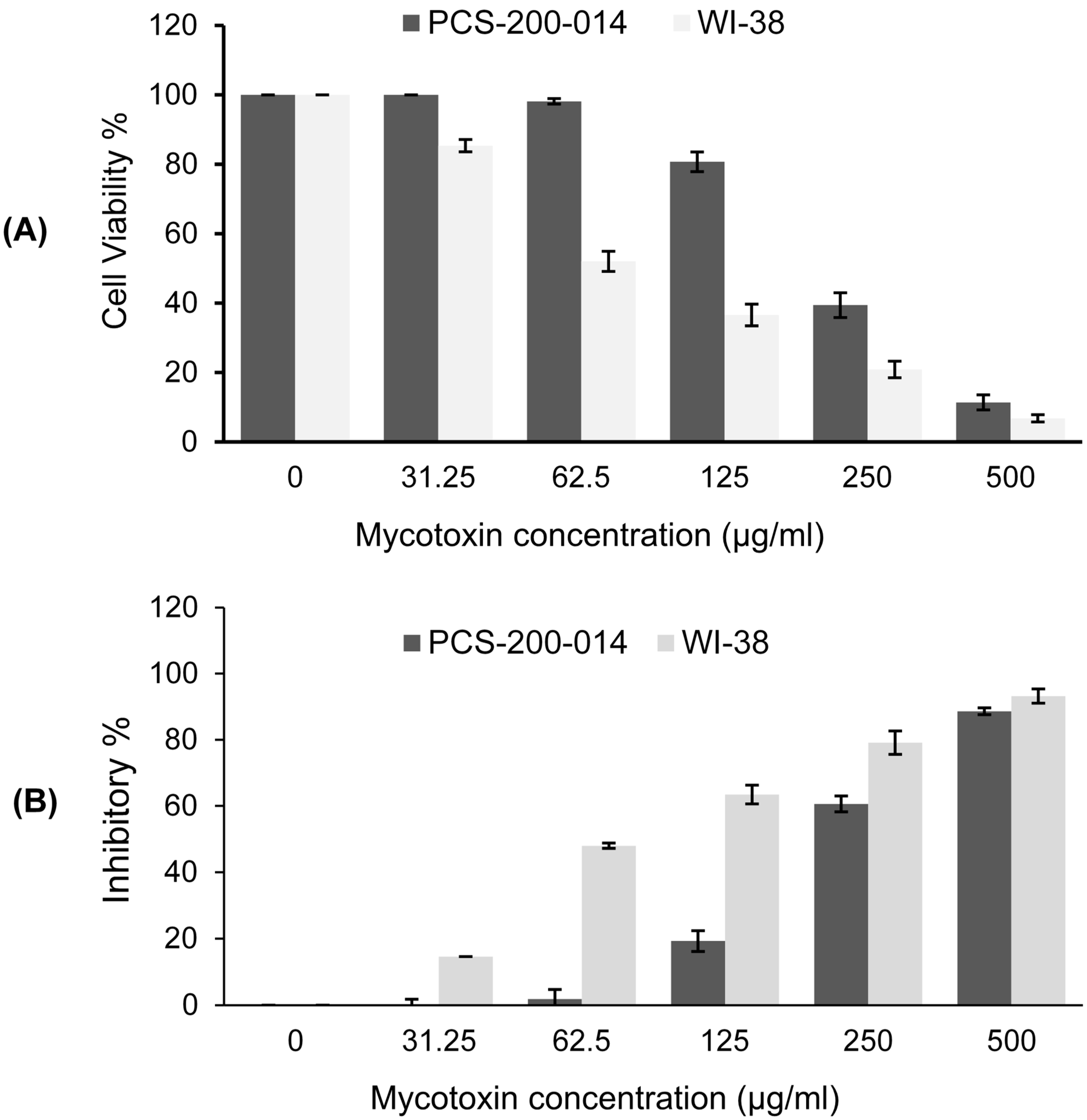
2.6.2. Cell Viability Assay
2.6.3. Microscopic Examination
2.7. Potential Toxicity of the Combined Mycotoxins ALT, AOH, TeA, and AS on Albino Rats
2.8. Gene Expression Levels of Apoptosis-Associated Gene (TNF-α) by RT-PCR
2.9. Hematological Analysis
2.10. Serum Biochemical Analysis
2.11. Histopathological Analysis
2.12. Statistical Analysis
3. Results
3.1. Sampling and Isolation
3.2. Phylogenetic Analysis
3.3. Pathogenicity Tests
3.4. HPLC Analysis
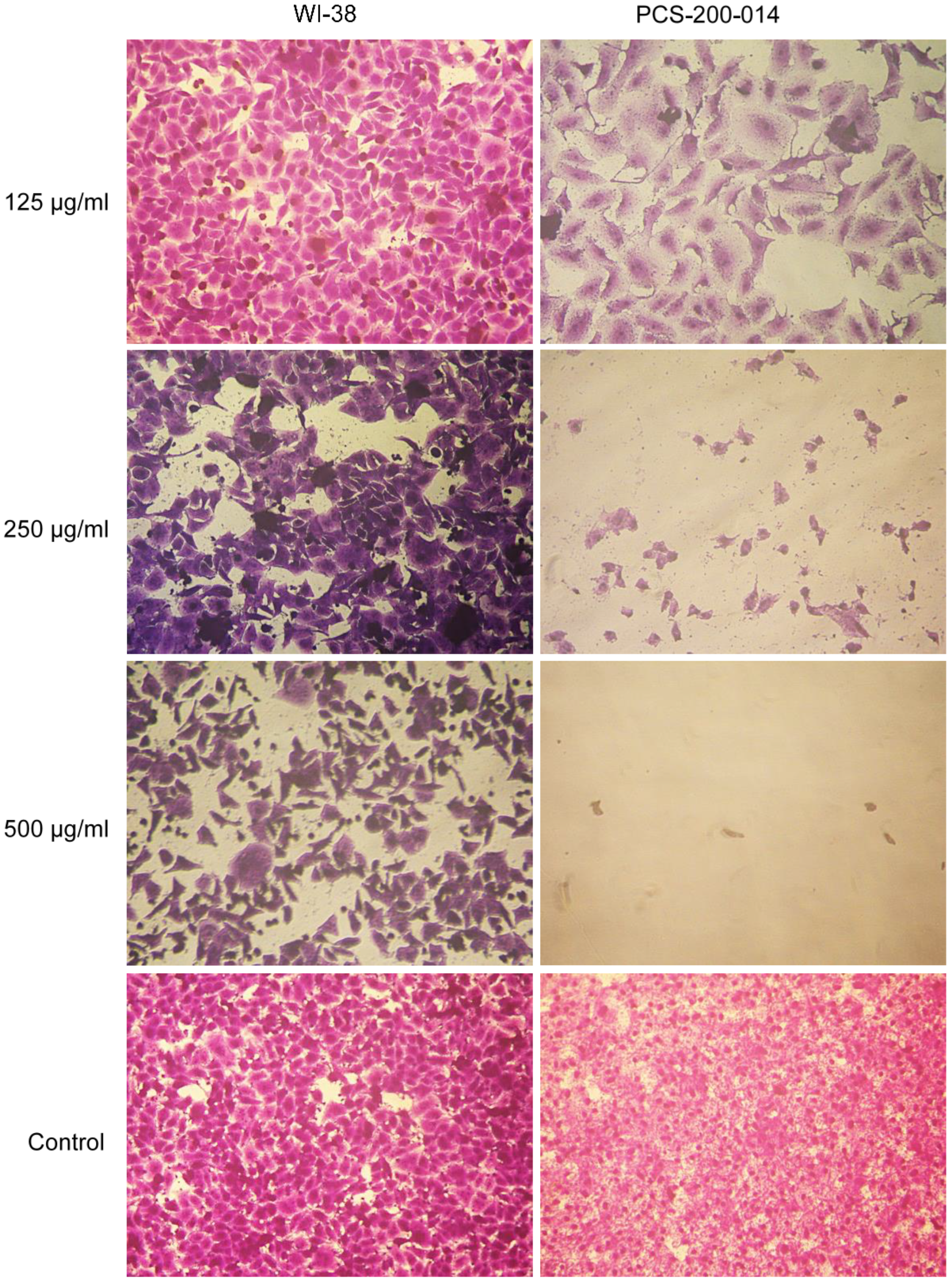
3.5. Cytotoxicity Assay
3.6. Microscopic Examination
3.7. Potential Toxicity of the Combined Mycotoxins ALT, AOH, TeA, and AS on Male Albino Rats
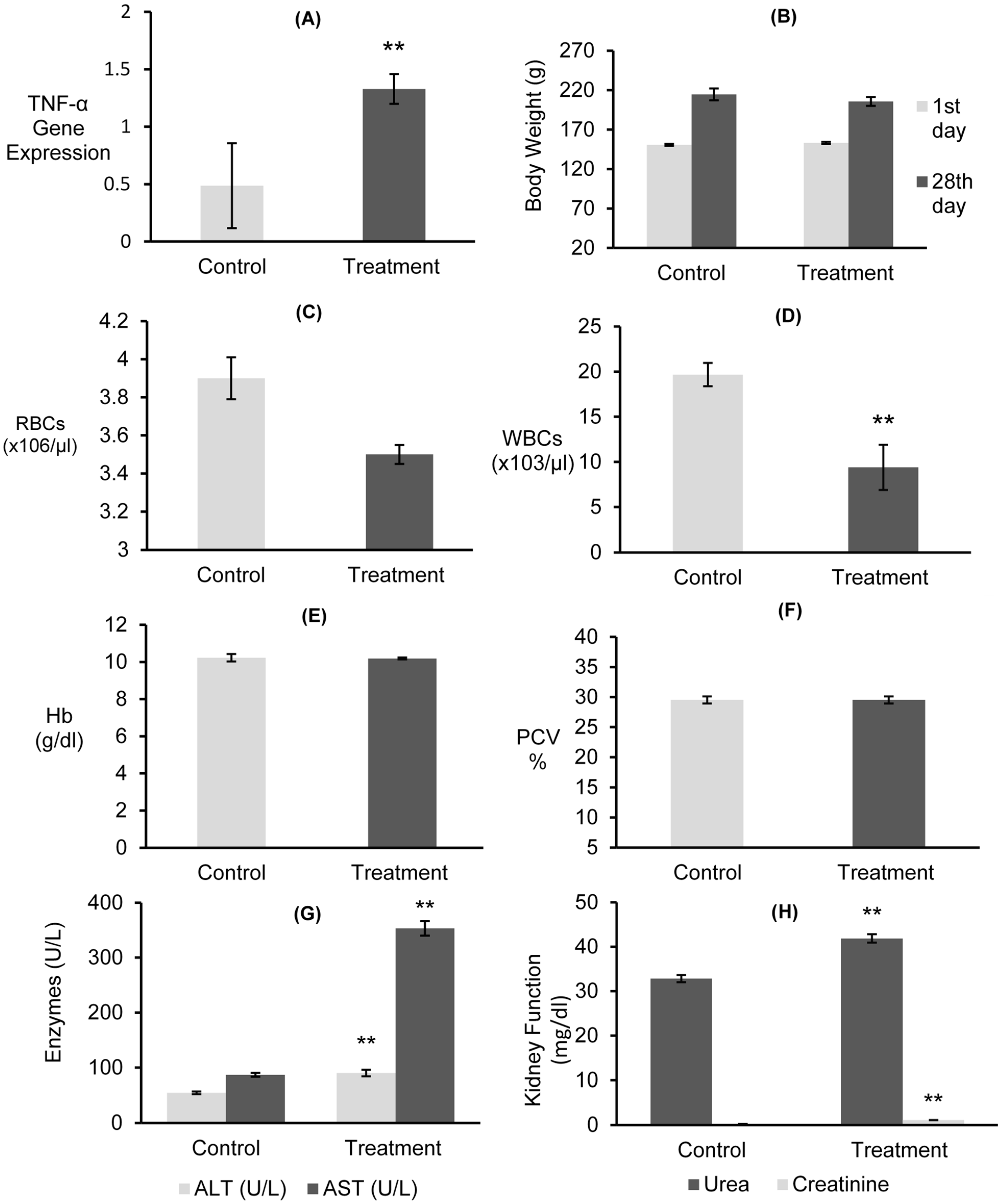
3.7.1. Gene Expressions of mRNAs for TNF-α and Body Weight
3.7.2. Hematological Examinations
3.7.3. Liver and Kidney Functions
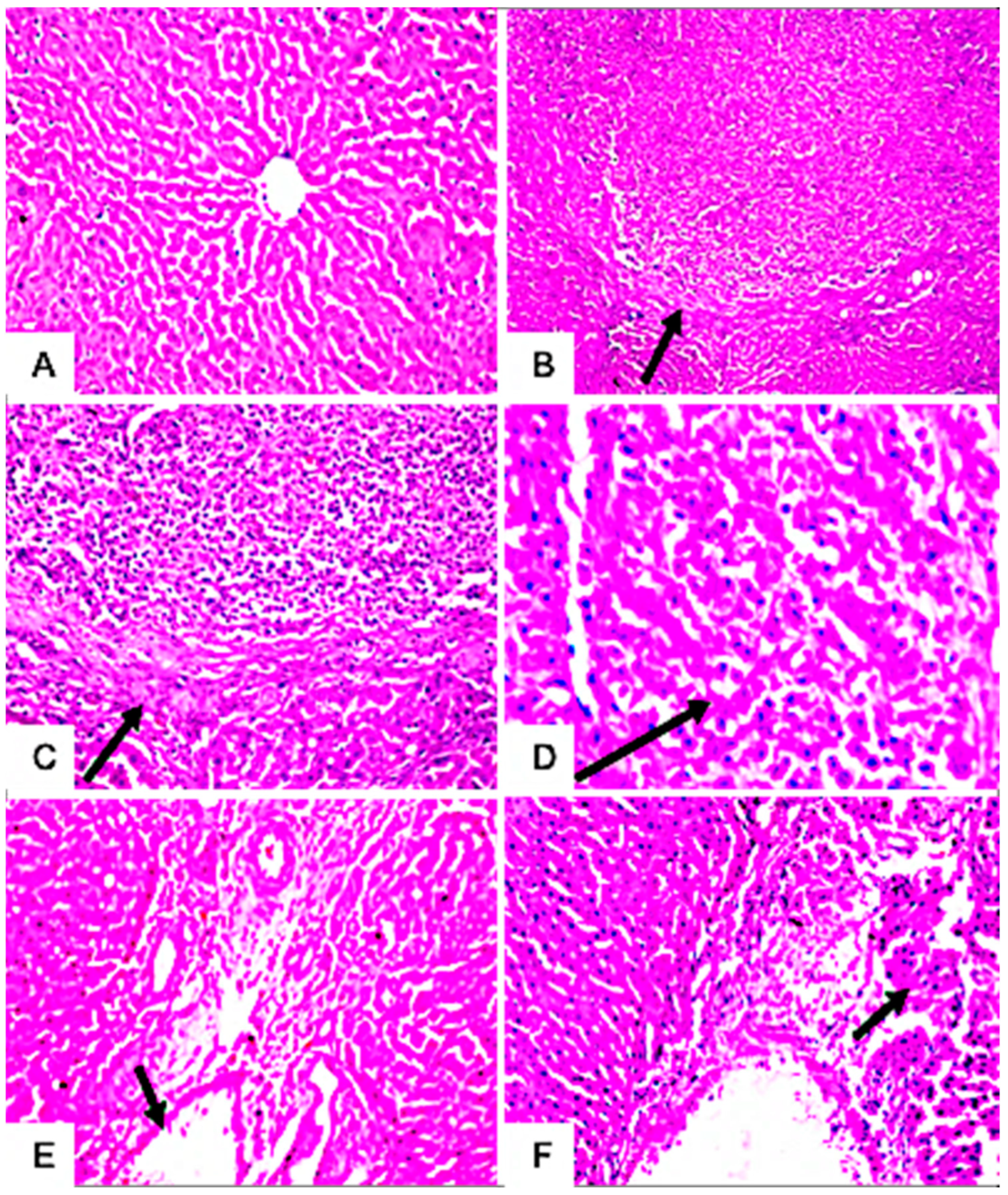
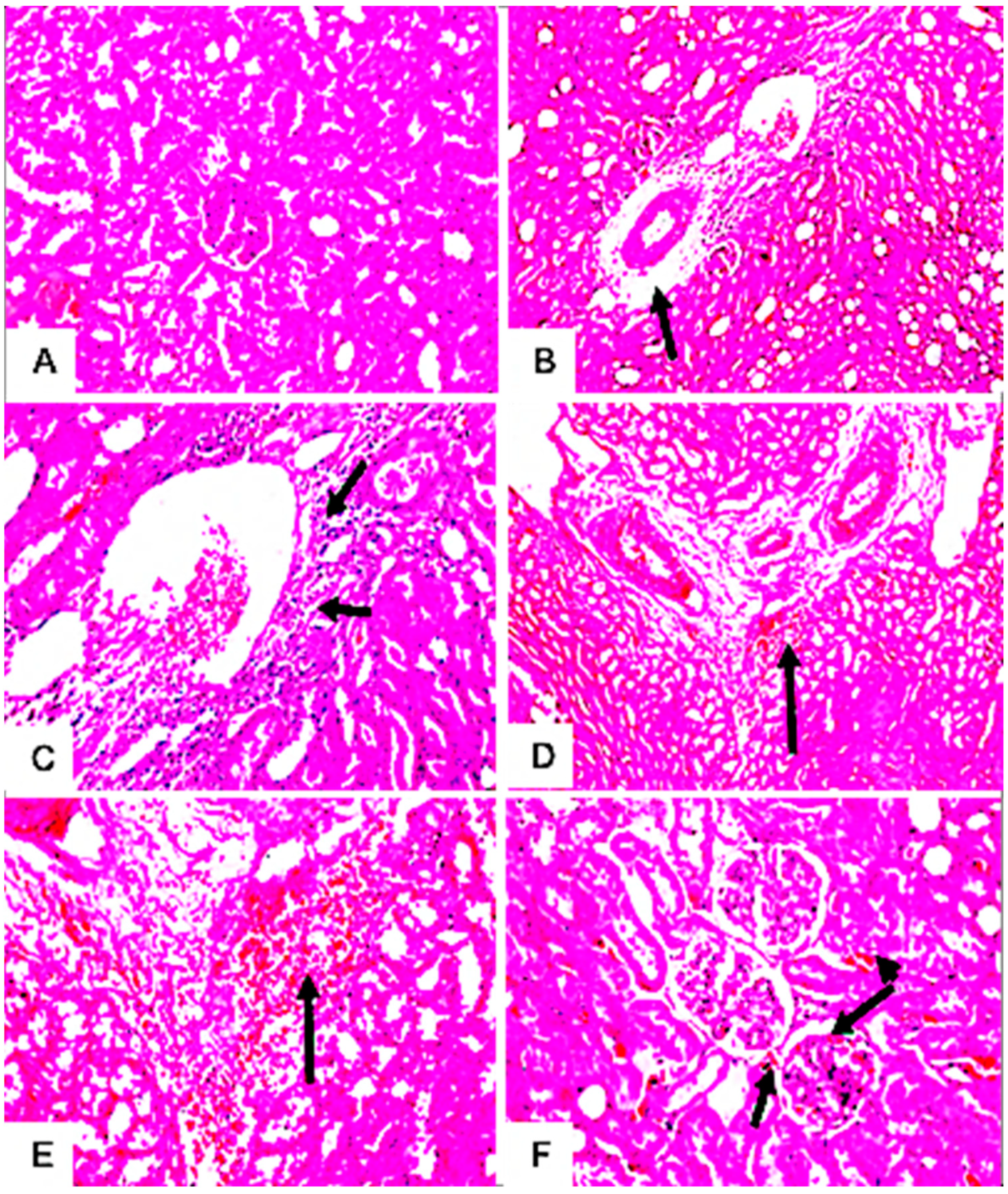
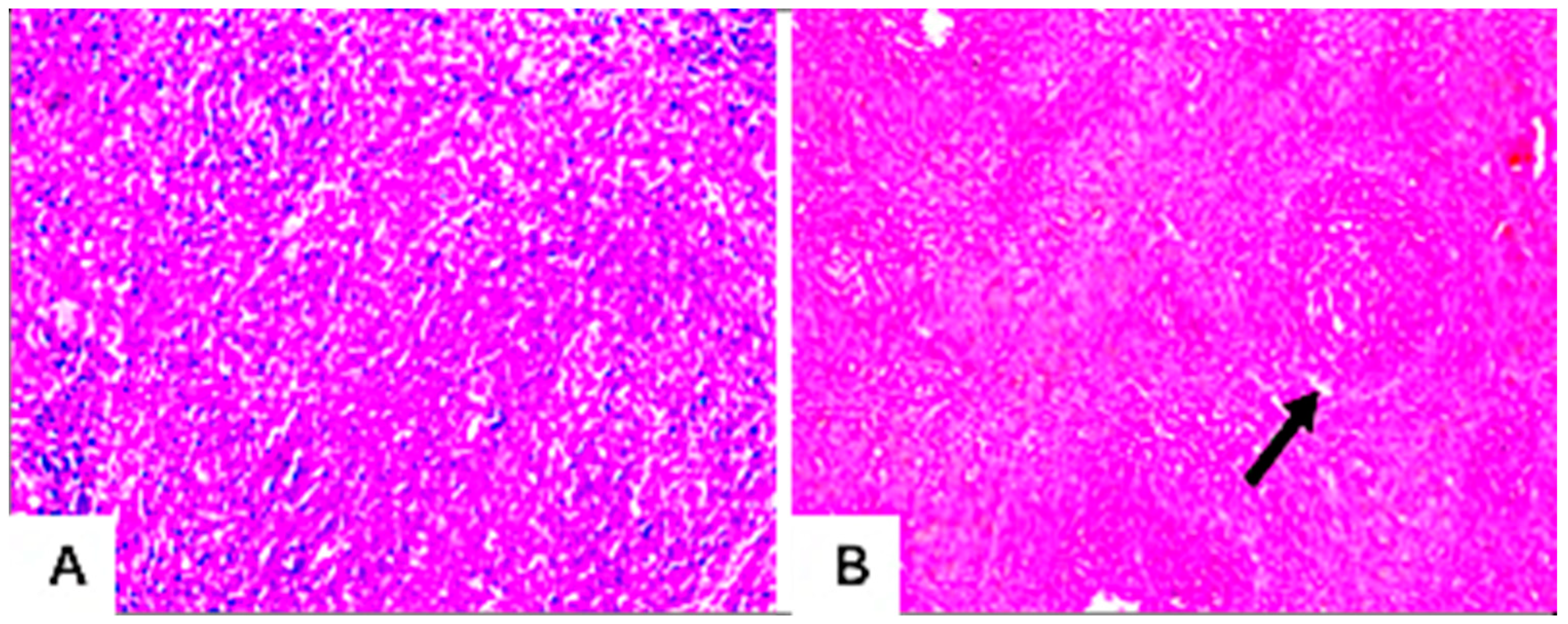
3.7.4. Histopathological Examination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meena, M.; Gupta, S.K.; Swapnil, P.; Zehra, A.; Dubey, M.K.; Upadhyay, R.S. Alternaria toxins: Potential virulence factors and genes related to pathogenesis. Front. Microbiol. 2017, 8, 1451. [Google Scholar] [CrossRef]
- Nash, A.; Gardner, R. Tomato early blight resistance in a breeding line derived from Lycopersicon hirsutum PI 126445. Plant Dis. 1988, 72, 206–209. [Google Scholar] [CrossRef]
- Saharan, G.; Mehta, N.; Meena, P. Alternaria Blight of Crucifers: Biology, Ecology and Disease Management; Springer: Singapore, 2016. [Google Scholar]
- Al-Lami, H.; You, M.; Barbetti, M. Role of foliage component and host age on severity of Alternaria leaf spot (caused by Alternaria japonica and A. brassicae) in canola (Brassica napus) and mustard (B. juncea) and yield loss in canola. Crop Pasture Sci. 2019, 70, 969–980. [Google Scholar] [CrossRef]
- Suganthi, D.; Sharma, O.; Mohan, G.; Pruthi, S.; Kaur, M. Importance of early blight of potato induced by (Alternaria solani) and its management. Biotica Res. Today 2020, 2, 870–873. [Google Scholar]
- Adhikari, P.; Oh, Y.; Panthee, D.R. Current status of early blight resistance in tomato: An update. Int. J. Mol. Sci. 2017, 18, 2019. [Google Scholar] [CrossRef]
- Bessadat, N.; Berruyer, R.; Hamon, B.; Bataille-Simoneau, N.; Benichou, S.; Kihal, M.; Henni, D.E.; Simoneau, P. Alternaria species associated with early blight epidemics on tomato and other Solanaceae crops in northwestern Algeria. Eur. J. Plant Pathol. 2017, 148, 181–197. [Google Scholar] [CrossRef]
- Ren, Z.; Cao, A.; Li, J.; Guo, M.; Wang, Q.; Yan, D.; Ouyang, C.; Li, Y. First Report of Alternaria alternata Causing Black Spot on Tomato (Solanum lycopersicum) in Tongzhou, China. Plant Dis. 2017, 101, 1680. [Google Scholar] [CrossRef]
- El Gobashy, S.F.; Mikhail, W.Z.A.; Ismail, A.M.; Zekry, A.; Moretti, A.; Susca, A.; Soliman, A.S. Phylogenetic, toxigenic and virulence profiles of Alternaria species causing leaf blight of tomato in Egypt. Mycol. Prog. 2018, 17, 1269–1282. [Google Scholar] [CrossRef]
- Puvača, N.; Avantaggiato, G.; Merkuri, J.; Vuković, G.; Bursić, V.; Cara, M. Occurrence and Determination of Alternaria Mycotoxins Alternariol, Alternariol Monomethyl Ether, and Tentoxin in Wheat Grains by QuEChERS Method. Toxins 2022, 14, 791. [Google Scholar] [CrossRef]
- Janik, E.; Niemcewicz, M.; Ceremuga, M.; Stela, M.; Saluk-Bijak, J.; Siadkowski, A.; Bijak, M. Molecular aspects of mycotoxins—A serious problem for human health. Int. J. Mol. Sci. 2020, 21, 8187. [Google Scholar] [CrossRef]
- Qin, Q.; Fan, Y.; Jia, Q.; Duan, S.; Liu, F.; Jia, B.; Wang, G.; Guo, W.; Wang, C. The Potential of Alternaria Toxins Production by A. alternata in Processing Tomatoes. Toxins 2022, 14, 827. [Google Scholar] [CrossRef]
- Saleem, A.; El-Shahir, A.A. Morphological and Molecular Characterization of Some Alternaria Species Isolated from Tomato Fruits Concerning Mycotoxin Production and Polyketide Synthase Genes. Plants 2022, 11, 1168. [Google Scholar] [CrossRef]
- Escrivá, L.; Oueslati, S.; Font, G.; Manyes, L. Alternaria mycotoxins in food and feed: An overview. J. Food Qual. 2017, 2017, 1569748. [Google Scholar] [CrossRef]
- Authority, E.F.S.; Arcella, D.; Eskola, M.; Gómez Ruiz, J.A. Dietary exposure assessment to Alternaria toxins in the European population. EFSA J. 2016, 14, e04654. [Google Scholar]
- Den Hollander, D.; Holvoet, C.; Demeyere, K.; De Zutter, N.; Audenaert, K.; Meyer, E.; Croubels, S. Cytotoxic effects of alternariol, alternariol monomethyl-ether, and tenuazonic acid and their relevant combined mixtures on human enterocytes and hepatocytes. Front. Microbiol. 2022, 13, 849243. [Google Scholar] [CrossRef]
- Mujahid, C.; Savoy, M.-C.; Baslé, Q.; Woo, P.M.; Ee, E.C.Y.; Mottier, P.; Bessaire, T. Levels of Alternaria toxins in selected food commodities including green coffee. Toxins 2020, 12, 595. [Google Scholar] [CrossRef]
- Ostry, V. Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- Motta, S.D.; Soares, L.M.V. A method for the determination of two Alternaria toxins, alternariol and alternariol monomethyl ether, in tomato products. Braz. J. Microbiol. 2000, 31, 315–320. [Google Scholar] [CrossRef]
- Wojciechowska, E.; Weinert, C.H.; Egert, B.; Trierweiler, B.; Schmidt-Heydt, M.; Horneburg, B.; Graeff-Hönninger, S.; Kulling, S.E.; Geisen, R. Chlorogenic acid, a metabolite identified by untargeted metabolome analysis in resistant tomatoes, inhibits the colonization by Alternaria alternata by inhibiting alternariol biosynthesis. Eur. J. Plant Pathol. 2014, 139, 735–747. [Google Scholar] [CrossRef]
- Patriarca, A.; Azcarate, M.; Terminiello, L.; Pinto, V.F. Mycotoxin production by Alternaria strains isolated from Argentinean wheat. Int. J. Food Microbiol. 2007, 119, 219–222. [Google Scholar] [CrossRef]
- Pose, G.; Patriarca, A.; Kyanko, V.; Pardo, A.; Pinto, V.F. Water activity and temperature effects on mycotoxin production by Alternaria alternata on a synthetic tomato medium. Int. J. Food Microbiol 2010, 142, 348–353. [Google Scholar] [CrossRef]
- Woudenberg, J.; Groenewald, J.; Binder, M.; Crous, P. Alternaria redefined. Stud. Mycol. 2013, 75, 171–212. [Google Scholar] [CrossRef]
- Dettman, J.R.; Eggertson, Q. Phylogenomic analyses of Alternaria section Alternaria: A high-resolution, genome-wide study of lineage sorting and gene tree discordance. Mycologia 2021, 113, 1218–1232. [Google Scholar] [CrossRef]
- Pryor, B.M.; Gilbertson, R.L. Molecular phylogenetic relationships amongst Alternaria species and related fungi based upon analysis of nuclear ITS and mt SSU rDNA sequences. Mycol. Res. 2000, 104, 1312–1321. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Gannibal, P.B.; Dugan, F.M.; Pryor, B.M. Characterization of Alternaria isolates from the infectoria species-group and a new taxon from Arrhenatherum, Pseudoalternaria arrhenatheria sp. nov. Mycol. Prog. 2014, 13, 257–276. [Google Scholar] [CrossRef]
- Hsin-Hung, C.; Wen-Shi, W. Phylogenetic analysis of internal transcribed spacer regions of the genus Alternaria, and the significance of filament-beaked conidia. Mycol. Res. 2002, 106, 164–169. [Google Scholar]
- Hong, S.G.; Cramer, R.A.; Lawrence, C.B.; Pryor, B.M. Alt a 1 allergen homologs from Alternaria and related taxa: Analysis of phylogenetic content and secondary structure. Fungal Genet. Biol. 2005, 42, 119–129. [Google Scholar] [CrossRef]
- Woudenberg, J.; Truter, M.; Groenewald, J.Z.; Crous, P.W. Large-spored Alternaria pathogens in section Porri disentangled. Stud. Mycol. 2014, 79, 1–47. [Google Scholar] [CrossRef]
- Woudenberg, J.; Seidl, M.; Groenewald, J.; De Vries, M.; Stielow, J.; Thomma, B.; Crous, P. Alternaria section Alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef]
- Habib, W.; Masiello, M.; El Ghorayeb, R.; Gerges, E.; Susca, A.; Meca, G.; Quiles, J.M.; Logrieco, A.F.; Moretti, A. Mycotoxin profile and phylogeny of pathogenic Alternaria species isolated from symptomatic tomato plants in Lebanon. Toxins 2021, 13, 513. [Google Scholar] [CrossRef]
- Berbee, M.; Pirseyedi, M.; Hubbard, S. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 1999, 91, 964–977. [Google Scholar] [CrossRef]
- Sung, G.-H.; Sung, J.-M.; Hywel-Jones, N.L.; Spatafora, J.W. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol. Phylogenet. Evol. 2007, 44, 1204–1223. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Swofford, D.L. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Available online: https://paup.phylosolutions.com/ (accessed on 25 September 2022).
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Miller, M.; Pfeiffer, W.; Schwartz, T. CIPRES Science Gateway survey. Available online: http://www.phylo.org (accessed on 20 September 2022).
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Latha, P.; Anand, T.; Ragupathi, N.; Prakasam, V.; Samiyappan, R. Antimicrobial activity of plant extracts and induction of systemic resistance in tomato plants by mixtures of PGPR strains and Zimmu leaf extract against Alternaria solani. Biol. Control 2009, 50, 85–93. [Google Scholar] [CrossRef]
- Andersen, B.; Hansen, M.E.; Smedsgaard, J. Automated and unbiased image analyses as tools in phenotypic classification of small-spored Alternaria spp. Phytopathology 2005, 95, 1021–1029. [Google Scholar] [CrossRef]
- Fontana, A.R.; Prendes, L.P.; Morata, V.I.; Bottini, R. High-throughput modified QuEChERS method for the determination of the mycotoxin tenuazonic acid in wine grapes. RSC Adv. 2016, 6, 95670–95679. [Google Scholar] [CrossRef]
- Song, J.; Ma, Q.; Hu, M.; Qian, D.; Wang, B.; He, N. The inhibition of miR-144-3p on cell proliferation and metastasis by targeting TOP2A in HCMV-positive glioblastoma cells. Molecules 2018, 23, 3259. [Google Scholar] [CrossRef]
- Stoev, S.; Denev, S.; Dutton, M. Cytotoxic effect of some mycotoxins and their combinations on human peripheral blood mononuclear cells as measured by the MTT assay. Open Toxinology J. 2009, 2, 1–8. [Google Scholar] [CrossRef]
- Prosperini, A.; Font, G.; Ruiz, M. Interaction effects of Fusarium enniatins (A, A1, B and B1) combinations on in vitro cytotoxicity of Caco-2 cells. Toxicol. In Vitro 2014, 28, 88–94. [Google Scholar] [CrossRef]
- Handayani, D.; Rivai, H.; Mulyana, R.; Suharti, N.; Rasyid, R.; Hertiani, T. Antimicrobial and cytotoxic activities of endophytic fungi isolated from mangrove plant Sonneratia alba Sm. J. Appl. Pharm. Sci. 2018, 8, 49–53. [Google Scholar]
- Abo-Ashour, M.F.; Eldehna, W.M.; Nocentini, A.; Bonardi, A.; Bua, S.; Ibrahim, H.S.; Elaasser, M.M.; Kryštof, V.; Jorda, R.; Gratteri, P. 3-Hydrazinoisatin-based benzenesulfonamides as novel carbonic anhydrase inhibitors endowed with anticancer activity: Synthesis, in vitro biological evaluation and in silico insights. Eur. J. Med. Chem. 2019, 184, 111768. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Wang, D.; Chen, Y.; Zhu, X.; Tang, X.; Zhang, J.; Zhang, L.; Chen, J. General toxicity and genotoxicity of alternariol: A novel 28-day multi-endpoint assessment in male Sprague–Dawley rats. Mycotoxin Res. 2022, 38, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Groestlinger, J.; Spindler, V.; Pahlke, G.; Rychlik, M.; Del Favero, G.; Marko, D. Alternaria alternata mycotoxins activate the aryl hydrocarbon receptor and Nrf2-ARE pathway to alter the structure and immune response of colon epithelial cells. Chem. Res. Toxicol. 2022, 35, 731–749. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Abdelhalim, M.; Alhomida, A.; Al Ayed, M. Transient increase in IL-1β, IL-6 and TNF-α gene expression in rat liver exposed to gold nanoparticles. Genet. Mol. Res. 2013, 12, 5851–5857. [Google Scholar] [CrossRef]
- Ronis, M.J.; Butura, A.; Sampey, B.P.; Shankar, K.; Prior, R.L.; Korourian, S.; Albano, E.; Ingelman-Sundberg, M.; Petersen, D.R.; Badger, T.M. Effects of N-acetylcysteine on ethanol-induced hepatotoxicity in rats fed via total enteral nutrition. Free Radic. Biol. Med. 2005, 39, 619–630. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Patton, C.J.; Crouch, S. Spectrophotometric and kinetics investigation of the Berthelot reaction for the determination of ammonia. Anal. Chem. 1977, 49, 464–469. [Google Scholar] [CrossRef]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing kidney function—Measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, S.; Layton, C.; Bancroft, J.D. Theory and Practice of Histological Techniques; Churchill Livingstone: London, UK, 2013; pp. 173–187. [Google Scholar]
- Snedecor, G.; Cochran, W. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980; p. 507. [Google Scholar]
- El-Gazzar, N.; Ismail, A.M. The potential use of Titanium, Silver and Selenium nanoparticles in controlling leaf blight of tomato caused by Alternaria alternata. Biocatal. Agric. Biotechnol. 2020, 27, 101708. [Google Scholar] [CrossRef]
- Youssef, N.H.; Qari, S.H.; Behiry, S.I.; Dessoky, E.S.; El-Hallous, E.I.; Elshaer, M.M.; Kordy, A.; Maresca, V.; Abdelkhalek, A.; Heflish, A.A. Antimycotoxigenic activity of beetroot extracts against Alternaria alternata mycotoxins on potato crop. Appl. Sci. 2021, 11, 4239. [Google Scholar] [CrossRef]
- El-Ganainy, S.M.; El-Abeid, S.E.; Ahmed, Y.; Iqbal, Z. Morphological and molecular characterization of large-spored Alternaria species associated with potato and tomato early blight in Egypt. Int. J. Agric. Biol. 2021, 25, 1101–1110. [Google Scholar] [CrossRef]
- Hussein, M.A.; Voigt, K. Phylogenetic and enzymatic variability of Alternaria species isolated from various substrates in Qena governorate of Upper Egypt. Arch. Phytopathol. Plant Prot. 2019, 52, 530–541. [Google Scholar] [CrossRef]
- Nagrale, D.T.; Gaikwad, A.P.; Sharma, L. Morphological and cultural characterization of Alternaria alternata (Fr.) Keissler blight of gerbera (Gerbera jamesonii H. Bolus ex JD Hook). J. Appl. Nat. Sci. 2013, 5, 171–178. [Google Scholar] [CrossRef]
- Goyal, P.; Chahar, M.; Mathur, A.; Kumar, A.; Chattopadhyay, C. Morphological and cultural variation in different oilseed Brassica isolates of Alternaria brassicae from different geographical regions of India. Indian J. Agric. Sci. 2011, 81, 1052. [Google Scholar]
- Li, J.; Phookamsak, R.; Jiang, H.; Bhat, D.J.; Camporesi, E.; Lumyong, S.; Kumla, J.; Hongsanan, S.; Mortimer, P.E.; Xu, J. Additions to the inventory of the genus Alternaria section Alternaria (Pleosporaceae, Pleosporales) in Italy. J. Fungi 2022, 8, 898. [Google Scholar] [CrossRef]
- Nishikawa, J.; Nakashima, C. Japanese species of Alternaria and their species boundaries based on host range. Fungal Syst. Evol. 2020, 5, 197. [Google Scholar] [CrossRef]
- Meena, M.; Samal, S. Alternaria host-specific (HSTs) toxins: An overview of chemical characterization, target sites, regulation and their toxic effects. Toxicol. Rep. 2019, 6, 745–758. [Google Scholar] [CrossRef]
- Siciliano, I.; Ortu, G.; Gilardi, G.; Gullino, M.L.; Garibaldi, A. Mycotoxin production in liquid culture and on plants infected with Alternaria spp. isolated from rocket and cabbage. Toxins 2015, 7, 743–754. [Google Scholar] [CrossRef]
- Ramires, F.A.; Masiello, M.; Somma, S.; Villani, A.; Susca, A.; Logrieco, A.F.; Luz, C.; Meca, G.; Moretti, A. Phylogeny and mycotoxin characterization of Alternaria species isolated from wheat grown in Tuscany, Italy. Toxins 2018, 10, 472. [Google Scholar] [CrossRef]
- Garganese, F.; Schena, L.; Siciliano, I.; Prigigallo, M.I.; Spadaro, D.; De Grassi, A.; Ippolito, A.; Sanzani, S.M. Characterization of citrus-associated Alternaria species in Mediterranean areas. PloS ONE 2016, 11, e0163255. [Google Scholar] [CrossRef]
- Yekeler, H.; Bitmiş, K.; Ozçelik, N.; Doymaz, M.Z.; Calta, M. Analysis of toxic effects of Alternaria toxins on esophagus of mice by light and electron microscopy. Toxicol. Pathol. 2001, 29, 492–497. [Google Scholar] [CrossRef]
- Liu, G.; Qian, Y.; Zhang, P.; Dong, Z.; Shi, Z.; Zhen, Y.; Miao, J.; Xu, Y. Relationships between Alternaria alternata and oesophageal cancer. IARC Sci. Publ. 1991, 29, 258–262. [Google Scholar]
- Crudo, F.; Varga, E.; Aichinger, G.; Galaverna, G.; Marko, D.; Dall’Asta, C.; Dellafiora, L. Co-occurrence and combinatory effects of Alternaria mycotoxins and other xenobiotics of food origin: Current scenario and future perspectives. Toxins 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Skrzydlewski, P.; Twarużek, M.; Grajewski, J. Cytotoxicity of Mycotoxins and Their Combinations on Different Cell Lines: A Review. Toxins 2022, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Blanco, C.; Elmo, L.; Waldner, T.; Ruiz, M.-J. Cytotoxic effects induced by patulin, deoxynivalenol and toxin T2 individually and in combination in hepatic cells (HepG2). Food Chem. Toxicol. 2018, 120, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Bensassi, F.; Gallerne, C.; Sharaf el dein, O.; Rabeh Hajlaoui, M.; Bacha, H.; Lemaire, C. Combined effects of alternariols mixture on human colon carcinoma cells. Toxicol. Mech. Methods 2015, 25, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Blanco, C.; Font, G.; Ruiz, M.-J. Role of quercetin on Caco-2 cells against cytotoxic effects of alternariol and alternariol monomethyl ether. Food Chem. Toxicol. 2016, 89, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.N.; Viktorová, J.; Ruml, T. Mycotoxins: Biotransformation and bioavailability assessment using caco-2 cell monolayer. Toxins 2020, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Del Favero, G.; Zaharescu, R.; Marko, D. Functional impairment triggered by altertoxin II (ATXII) in intestinal cells in vitro: Cross-talk between cytotoxicity and mechanotransduction. Arch. Toxicol. 2018, 92, 3535–3547. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Lawrence, C.B. The Alternaria alternata mycotoxin alternariol suppresses lipopolysaccharide-induced inflammation. Int. J. Mol. Sci. 2017, 18, 1577. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, C.; Cenk, E.; Marko, D. The Alternaria mycotoxin alternariol triggers the immune response of IL-1β-stimulated, differentiated caco-2 cells. Mol. Nutr. Food Res. 2019, 63, 1900341. [Google Scholar] [CrossRef]
- Fehr, M.; Pahlke, G.; Fritz, J.; Christensen, M.O.; Boege, F.; Altemöller, M.; Podlech, J.; Marko, D. Alternariol acts as a topoisomerase poison, preferentially affecting the IIα isoform. Mol. Nutr. Food Res. 2009, 53, 441–451. [Google Scholar] [CrossRef]
- Pfeiffer, E.; Eschbach, S.; Metzler, M. Alternaria toxins: DNA strand-breaking activity in mammalian cellsin vitro. Mycotoxin Res. 2007, 23, 152–157. [Google Scholar] [CrossRef]
- Amuzie, C.J.; Shinozuka, J.; Pestka, J.J. Induction of suppressors of cytokine signaling by the trichothecene deoxynivalenol in the mouse. Toxicol. Sci. 2009, 111, 277–287. [Google Scholar] [CrossRef]
- Islam, Z.; Harkema, J.R.; Pestka, J.J. Satratoxin G from the black mold Stachybotrys chartarum evokes olfactory sensory neuron loss and inflammation in the murine nose and brain. Environ. Health Perspect. 2006, 114, 1099–1107. [Google Scholar] [CrossRef]
- Tang, X.; Chen, Y.; Zhu, X.; Miao, Y.; Wang, D.; Zhang, J.; Li, R.; Zhang, L.; Chen, J. Alternariol monomethyl ether toxicity and genotoxicity in male Sprague-Dawley rats: 28-Day in vivo multi-endpoint assessment. Mutat. Res. Genet. Toxicol. Environ. 2022, 873, 503435. [Google Scholar] [CrossRef]
- Hueza, I.M.; Raspantini, P.C.F.; Raspantini, L.E.R.; Latorre, A.O.; Górniak, S.L. Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins 2014, 6, 1080–1095. [Google Scholar] [CrossRef] [PubMed]
- Chandratre, G.A.; Telang, A.; Badgujar, P.C.; Raut, S.S.; Sharma, A. Toxicopathological alterations induced by high dose dietary T-2 mycotoxin and its residue detection in Wistar rats. Arch. Environ. Contam. Toxicol. 2014, 67, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi-Hattori, K.; Amuzie, C.J.; Flannery, B.M.; Pestka, J.J. Body composition and hormonal effects following exposure to mycotoxin deoxynivalenol in the high-fat diet-induced obese mouse. Mol. Nutr. Food Res. 2011, 55, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Shibutani, M.; Sugita-Konishi, Y.; Aihara, M.; Inoue, K.; Woo, G.-H.; Fujimoto, H.; Hirose, M. A 90-day subchronic toxicity study of nivalenol, a trichothecene mycotoxin, in F344 rats. Food Chem. Toxicol. 2008, 46, 125–135. [Google Scholar] [CrossRef]
- Wannemacher, R., Jr. Toxicity of trichothecenes and other related mycotoxins in laboratory animals. In Mycotoxins and Animal Foods; CRC Press: Boca Raton, FL, USA, 1991; pp. 499–552. [Google Scholar]
- Meloche, J.L.; Smith, T.K. Altered Tissue Amino Acid Metabolism in Acute T-2 Toxicosis. Proc. Soc. Exp. Biol. Med. 1995, 210, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Pathak, B.; Sethi, N.; Vora, V. Histopathology of Mycotoxicosis produced in Swiss albino mice by metabolites of some fungal isolates. Appl. Environ. Microbiol. 1981, 41, 752–757. [Google Scholar] [CrossRef]
- Xin, J.; Shang, Y. Alternariol alleviates breast carcinoma by inhibiting cellular proliferation correlated with increased apoptotic events in rats. Eur. J. Inflamm. 2022, 20, 1721727X221139485. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Y.; Tang, X.; Wang, D.; Miao, Y.; Zhang, J.; Li, R.; Zhang, L.; Chen, J. General toxicity and genotoxicity of altertoxin I: A novel 28-day multiendpoint assessment in male Sprague–Dawley rats. J. Appl. Toxicol. 2022, 42, 1310–1322. [Google Scholar] [CrossRef]
- Palanichamy, P.; Kannan, S.; Murugan, D.; Alagusundaram, P.; Marudhamuthu, M. Purification, crystallization and anticancer activity evaluation of the compound alternariol methyl ether from endophytic fungi Alternaria alternata. J. Appl. Microbiol. 2019, 127, 1468–1478. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, A.M.; Elshewy, E.S.; El-Ganainy, S.M.; Magistà, D.; Hamouda, A.F.; Alhudaib, K.A.; Ebrahim, W.; Almaghasla, M.I. Mycotoxins from Tomato Pathogenic Alternaria alternata and Their Combined Cytotoxic Effects on Human Cell Lines and Male Albino Rats. J. Fungi 2023, 9, 282. https://doi.org/10.3390/jof9030282
Ismail AM, Elshewy ES, El-Ganainy SM, Magistà D, Hamouda AF, Alhudaib KA, Ebrahim W, Almaghasla MI. Mycotoxins from Tomato Pathogenic Alternaria alternata and Their Combined Cytotoxic Effects on Human Cell Lines and Male Albino Rats. Journal of Fungi. 2023; 9(3):282. https://doi.org/10.3390/jof9030282
Chicago/Turabian StyleIsmail, Ahmed Mahmoud, Eman Said Elshewy, Sherif Mohamed El-Ganainy, Donato Magistà, Ahlam Farouk Hamouda, Khalid A. Alhudaib, Weaam Ebrahim, and Mustafa I. Almaghasla. 2023. "Mycotoxins from Tomato Pathogenic Alternaria alternata and Their Combined Cytotoxic Effects on Human Cell Lines and Male Albino Rats" Journal of Fungi 9, no. 3: 282. https://doi.org/10.3390/jof9030282
APA StyleIsmail, A. M., Elshewy, E. S., El-Ganainy, S. M., Magistà, D., Hamouda, A. F., Alhudaib, K. A., Ebrahim, W., & Almaghasla, M. I. (2023). Mycotoxins from Tomato Pathogenic Alternaria alternata and Their Combined Cytotoxic Effects on Human Cell Lines and Male Albino Rats. Journal of Fungi, 9(3), 282. https://doi.org/10.3390/jof9030282







