Abstract
Giant Stropharia (S. rugoso-annulata) is an edible mushroom recommended for consumption by the Food and Agriculture Organization of the United Nations. It possesses significant culinary and medicinal functionalities. The characteristics of this mushroom include high protein content, abundant bioactive compounds, delicious and sweet taste, and pleasant aroma. In recent years, the S. rugoso-annulata industry has seen strong growth, especially in China. This article presents the first comprehensive and systematic review of the nutritional, bioactive, and flavor components of S. rugoso-annulata, as well as their influencing factors. This article provides scientific evidence for the production of high-quality S. rugoso-annulata mushrooms, the extraction of bioactive components, post-harvest storage, and culinary processing, aiming to promote the consumption of S. rugoso-annulata and the health of consumers.
1. Introduction
Stropharia rugoso-annulata, also known as Giant Stropharia, Wine-cap mushroom, is a renowned edible fungus recommended for consumption by the Food and Agriculture Organization of the United Nations due to its potential to alleviate various illnesses associated with cancer [1]. Not only is it a high-protein, low-fat food rich in minerals, vitamins, and dietary fiber [2], but it is also a quality raw material for functional components such as fungal polysaccharides [3], sterols [4], and taste peptides [5], and can also be processed into various flavorful foods [6,7]. With particularly simple cultivation techniques and high yield [8], it holds significant application value in various fields such as dietary nutrition supply, bioactive compound extraction, functional food production, and flavorful food processing.
S. rugoso-annulata was first domesticated in Germany in 1969. Subsequently, it was introduced for cultivation in other European countries and the United States [8]. In 1989, the yearly production of S. rugoso-annulata reached approximately 1300 tons in Europe [9]. China imported a strain of S. rugoso-annulata from Poland in the 1980s and began widespread cultivation in the 1990s [10]. In recent years, S. rugoso-annulata has been rapidly promoted and widely cultivated throughout China. In 2021, China′s fresh S. rugoso-annulata mushroom production exceeded 210,000 tons, representing a surge of 43% compared to 2019 [11].
In recent years, numerous research has been reported on the food properties of S. rugoso-annulata, particularly its flavor components. Jing et al. reported on the nutritional components, some bioactive substances, and heavy metal content of S. rugoso-annulata [2]; Chen et al. reported on the flavor components of both fresh and dried S. rugoso-annulata [12]; Li et al. reported on the flavor substances produced by the liquid fermentation of S. rugoso-annulata mycelium [13]; and Lu et al. reported the flavor components of S. rugoso-annulata soup processed by different methods [14,15].
This article provides a comprehensive review of the research progress on the nutritional, bioactive, and flavor components of S. rugoso-annulata, as well as their influencing factors. It is a systematic theoretical reference for the production of high-quality S. rugoso-annulata mushrooms, the extraction of bioactive ingredients, post-harvest storage, and culinary processing. It can promote the consumption of S. rugoso-annulata and contribute to the health of consumers.
2. Nutritional Components of S. rugoso-annulata
As a food item, fresh mushrooms of S. rugoso-annulata have a high moisture content, with an average moisture content of 92% and an average dry matter content of 8% [8]. The subsequent content data are reported on a dry matter basis unless otherwise specified. The basic nutritional components of S. rugoso-annulata (dried by hot air drying, HAD) are ranked in order from highest to lowest content as follows: carbohydrates (45.17–54.60%), protein (25.75–34.17%), minerals (ash, 7.40–9.62%), dietary fiber (5.25–7.99%), fat (1.33–2.30%), and vitamins (<0.33%) [2,16,17]. S. rugoso-annulata contains 18 out of the 20 protein-composing amino acids, excluding Asn and Gln. It is rich in all eight essential amino acids for the human body including Leu, Ile, Val, Phe, Met, Trp, Thr, and Lys [2]. The total content of amino acids in this mushroom ranges from 18.89% to 31.01%, with essential amino acids accounting for 6.54% to 11.70%, and non-essential amino acids accounting for 7.19% to 19.97% [2,16,18,19]. According to most studies, Glu has the highest content (2.88–6.84%), followed by Asp (1.72–3.07%) [16,18,19,20]. However, some studies have suggested that Ile has the highest content (6.32%), followed by Tyr (1.86%) and Glu (1.82%) [2]. This difference may be related to different regions or the substrates used for cultivation.
S. rugoso-annulata mushrooms contain a variety of B-complex vitamins, notably riboflavin (B2), niacin (B3), pantothenic acid (B5), pyridoxine (B6), folic acid (B9), and cobalamin (B12) [2]. Additionally, they possess vitamin C [21], as well as ergosterol, a precursor to vitamin D2 [17]. The ergosterol content is abundant, up to 0.23% [17]. The contents of vitamin C (0.53‰) and B3 (0.39‰) are also relatively high [2,21].
S. rugoso-annulata mushrooms contain a variety of essential mineral elements, including K, P, Ca, Mn, Cu, Na, Fe, Zn, Mg, and Se, and can also accumulate heavy metal elements such as As, Cd, Hg, Pb, and Cr from the soil [2,16,21]. Among them, the content of necessary macro elements such as Na, K, Mg, P, and Ca exceeds 0.1%, with K having the highest amount (2.68–3.48%) [2,16] and P as the second highest (0.82–0.87%) [2,21].
S. rugoso-annulata accumulates Zn and Se from cultivation medium or substrate, serving as an excellent carrier of organic Zn and Se to the human body. The mycelium of S. rugoso-annulata cultured in a Zn-Se-rich liquid medium can produce organic Zn of up to 0.21 mg/g and organic Se of up to 0.82 mg/g [22]. The fruiting body of S. rugoso-annulata grown in the wild can contain up to 2.57 mg/kg of Se [2]. The addition of Se as fertilizer to cultivation substrates can increase the Se content of the S. rugoso-annulata fruiting body to 3.93 mg/kg [23].
The mycelium of S. rugoso-annulata exhibits a fatty acid content ranging from 3.64 to 7.52 mg/g, with unsaturated fatty acids constituting over 77% of the total fatty acids. The predominant unsaturated fatty acid is linoleic acid (C18:2), a polyunsaturated fatty acid, while the main saturated fatty acid is palmitic acid (C16:0). Linoleic acid has the highest content, comprising over 57% of the total fatty acids, followed by palmitic acid, accounting for over 13% of the total fatty acids [24]. Other fatty acids such as oleic acid (C18:1), palmitoleic acid (C16:1), stearic acid (C18:0), and lignoceric acid (C24:0) also have relatively high contents [24,25].
2.1. Nutritional Components of S. rugoso-annulata Processed by Different Drying Methods
Dried S. rugoso-annulata mushrooms are commonly produced using hot air drying (HAD) at temperatures ranging from 45 °C to 55 °C [12,26,27]. In addition to HAD, there are also other methods for drying such as vacuum freeze-drying (VFD), natural air drying (ND, NAD), microwave drying (MWD), and microwave vacuum drying (MVD) [27,28,29,30,31]. The dry matter and crude fat content of S. rugoso-annulata followed the order of VFD > HAD > ND, while the protein and polysaccharide content followed the order of VFD > ND > HAD. The differences between the three drying methods are significant. S. rugoso-annulata produced by VFD had a significantly higher content of protein (25.09%), polysaccharides (16.03%), and fat (6.05%) than those produced by HAD and ND. Additionally, VFD retained the fresh mushroom’s appearance and color effectively [29]. VFD is suitable for the production of high-quality S. rugoso-annulata mushrooms, while the method of ND followed by HAD is suitable for the production of regular products.
2.2. Nutritional Components of S. rugoso-annulata at Different Developmental Stages
As shown in Figure 1, the developmental process of the S. rugoso-annulata fruiting body can be divided into five stages, namely, S1 (the button stage), S2 (the gill formation stage), S3 (the early maturity stage), S4 (the maturity stage), and S5 (the parachute stage), in chronological order. The ash content reaches the highest level at the button stage and decreases significantly afterward. The total carbohydrate content increases continuously throughout the entire developmental process. The crude protein content remains relatively stable but declines during the parachute stage. The crude fiber and crude fat content did not vary much at each stage [32,33]. The crude protein content of S. rugoso-annulata was significantly higher in the unopened stage than in the opened stage [34]. S4 and S2 are the stages when S. rugoso-annulata exhibits a higher overall nutritional quality, providing a reference for the timely harvesting of S. rugoso-annulata [32].
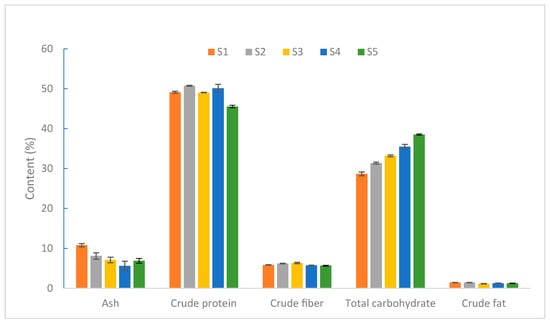
Figure 1.
The content of macronutrients of S. rugoso-annulata (HAD) at different developmental stages (data obtained from reference [34]).
2.3. Nutritional Components of Different Parts of S. rugoso-annulata
As shown in Table 1, the nutrient components of both the pileus and the stipe of S. rugoso-annulata are the same, but their contents are different. The pileus presents a significantly higher content of crude protein, total amino acids, crude fat, ash, Mn, and Zn than the stipe, resulting in a higher nutritional value [35].

Table 1.
Content of nutritional components in S. rugoso-annulata pileus and stipe.
3. Bioactive Components of S. rugoso-annulata
3.1. Soluble Polysaccharides
Soluble polysaccharides are the main bioactive components of S. rugoso-annulata [37]. They exhibit beneficial health properties like antioxidant [26,37,38,39], anti-tumor [40], anti-inflammatory [41], antimicrobial [37], immunomodulatory [42], hypoglycemic [43], hepatoprotective [44], mitochondrial swelling-inhibiting, and erythrocyte hemolysis-inhibiting activities [45], as well as plant growth promotion activity [46,47].
S. rugoso-annulata soluble polysaccharides are heteropolysaccharides, with glucose and galactose being the most common composing monosaccharides (Table 2) [3,28,41,48,49]. They may also contain mannose, arabinose [28], fucose [41], glucuronic acid, ribose [49], fructose, xylose, and rhamnose [3]. Glucose is the most abundant monosaccharide in the soluble polysaccharides of the fruiting body [3,28,41,48,49]. Based on the presence or absence of acidic groups such as glucuronic acid or sulfate groups in the composition of monosaccharides, S. rugoso-annulata polysaccharides can be divided into neutral polysaccharides and acidic polysaccharides [26,28,49]. Neutral polysaccharides mainly have a (1→6)-α-d-glucan or (1→6)-α-d-galactan as the main chain, while acidic polysaccharides mainly have a (1→6)-α-d-glucan or (1→3)-β-d-glucan as the main chain [28,49]. Both types of polysaccharides have α- and β-glycosidic bonds and a molecular weight of 13.28–8992 kDa [26,42]. The polysaccharides of S. rugoso-annulata mycelium are divided into intracellular polysaccharides and extracellular polysaccharides, composed of pyranose and connected by β-glycosidic bonds [40,48,50]. The content of soluble polysaccharides in the fruiting body ranges from 6.98% to 13.25% [37,49,51], while that in the mycelium ranges from 15.12% to 22.37% [50,52]. Studies have shown that appropriate chemical modifications (carboxymethylation, phosphorylation, and acetylation) can improve the physicochemical properties and biological activities of S. rugoso-annulata polysaccharides [41,44]. For example, carboxymethylated S. rugoso-annulata polysaccharides exhibit stable DPPH scavenging and reducing abilities, α-glucosidase inhibitory activity, and α-amylase inhibitory activity; phosphorylated S. rugoso-annulata polysaccharides have significant hydroxyl radical scavenging ability [41]; and acetylated S. rugoso-annulata polysaccharides have activity in alleviating non-alcoholic fatty liver disease and inhibiting fat synthesis [44]. No significant correlation was found between the antioxidant property and the molecular weight of the crude S. rugoso-annulata polysaccharides. Different extraction methods have no significant effect on the chemical structure of the crude polysaccharides [26]. Acidic polysaccharides exhibit stronger antioxidant activity than neutral polysaccharides [28,49].

Table 2.
Monosaccharide components, structure and optimum extraction conditions of soluble polysaccharides in S. rugoso-annulata.
Drying methods can affect the proportion of monosaccharides as well as the antioxidant activity of S. rugoso-annulata polysaccharides. The polysaccharides obtained through HAD have a higher proportion of glucose and a lower proportion of galactose compared to polysaccharides obtained through FVD, and they also exhibit stronger antioxidant activity [28].
Soluble polysaccharides of S. rugoso-annulata are traditionally extracted by the water extraction–alcohol precipitation method. Ultrasound or microwave assistance is often employed during water extraction to increase the yield and shorten the extraction time [26,28,37,49]. Recently, a new method, three-phase extraction, has been reported for the extraction of S. rugoso-annulata polysaccharides. This method utilizes a tert-butanol-(NH4)2SO4-water system, which avoids the need for an alcohol precipitation process and achieves the separation of polysaccharides faster [3]. The highest yield from the S. rugoso-annulata fruiting body is achieved by an ultrasound-assisted extraction process (62 °C, 1:30 w/v, ultrasound 62 min), with a polysaccharide yield of 13.25% [37]. The highest yield from the S. rugoso-annulata mycelium is achieved by an ultrasound-assisted extraction process (63.1 °C, 1:15 w/v, ultrasound 16.33 min), with a polysaccharide yield of 22.37% [52].
3.2. Other Bioactive Components
In addition to polysaccharides, S. rugoso-annulata contains many other bioactive components including protein [54], phenols [55], triterpenes [2], flavonoids [56], sterols [4,57,58], lectin [59], oligopeptides [60], and nucleosides [61]. The optimal extraction processes for these components are shown in Table 3.
Triterpenes have anti-tumor activity [62], and the content of triterpene in S. rugoso-annulata is 1.42% [2]. S. rugoso-annulata contains a variety of sterols, including ergosterol, seven sterols with unique carbon skeletons, four sterols named strophasterol A, B, C, and D, and two sterols with a unique ether ring structure (C28H44O4 and C30H50O3) [57,58,63,64,65]. Sterols in S. rugoso-annulata have bioactivities such as inhibiting osteoclast formation, inhibiting fungi growth, weakening endoplasmic reticulum stress to protect neuronal cells, and inhibiting toxic carotenoids [4,57,58]. S. rugoso-annulata also contains lectin (SRL), a 38 kD molecule of protein with a unique N-terminal sequence, which has activity in inhibiting the proliferation of Hep G2 liver cancer cells, L1210 leukemia cells, and HIV-1 reverse transcriptase [59]. Oligopeptides (octapeptides, nonapeptides, and decapeptides) in S. rugoso-annulata have activity in inhibiting angiotensin-converting enzymes [60]. S. rugoso-annulata’s petroleum ether extract and chloroform extract have anti-fatigue activity, with the common components being ergosterol and 5α-ergosta-7, 22-dien-3β-ol [65]. S. rugoso-annulata’s ethyl acetate extract has strong antioxidant activity, with nucleosides as the main active component [61].

Table 3.
Bioactivity and optimal extraction conditions of other bioactive components in the S. rugoso-annulata fruiting body.
Table 3.
Bioactivity and optimal extraction conditions of other bioactive components in the S. rugoso-annulata fruiting body.
| Components | Bioactivity | Optimal Extraction Conditions | Yield | References |
|---|---|---|---|---|
| Protein | Antioxidant activity and scavenging ability on DPPH radicals and hydroxyl radicals | Distilled water, pH = 12, 1:30 (w/v), 60 min, 45 °C | 37.54% | [54] |
| Oligopeptide | Antioxidant, ACE inhibitory activity | Pure water, 1:20 (w/v), ultrasound 120 w–400 w, 20 kHz, 10–35 min | 11.04–23.02% | [60] |
| Ergosterol | Precursor of Vitamin D2, anti-cancer, anti-aging | Ethanol 100%, 1:30 (w/v), 30 min, ultrasound, 3 repeats | 0.23% | [17] |
| Phenol | Antioxidant, antibacterial, anticancer, anti-aging, and inhibition of cholesterol elevation | Ethanol 30%, 1:20 (w/v), 70 °C, 1 h | 11.00% | [55] |
| Ethanol 30%, 1:20 (w/v), 60 °C, 6 min, ultrasound 240 w | 6.71% | |||
| Ethanol 35%, 1:20 (w/v), 2.5 min, microwave 640 w | 5.32% | |||
| Polyphenol | Antioxidation, antivirus, antibacterial | Ethanol 64.68%, 53.1 °C, ultrasound 39.3 min | 1.66% | [66] |
| Flavonoid | Antivirus, antioxidant, antibacterial, and protection of the cardiovascular and cerebrovascular systems. | Ethanol 30%, 1:15 (w/v), ultrasound 120 w, 1 h | 1.14% | [56] |
4. Flavor Components of S. rugoso-annulata
The flavor of edible mushrooms includes aroma and taste. The aroma is mainly produced by volatile compounds that are perceived by the nose, while the taste is produced by non-volatile compounds that are perceived by the tongue. The intensity of the flavor is determined by the concentration of flavor compounds and their perception thresholds [67]. The taste activity value (TAV) and odor activity value (OAV) are commonly used to evaluate the non-volatile or volatile compounds in edible mushrooms. TAV or OAV is the ratio of the concentration of a certain non-volatile or volatile compound to its taste or odor threshold concentration, reflecting the contribution of a single compound to the overall taste or odor. TAV or OAV > 1 indicates that the compound contributes to the taste or odor, and the larger the value, the greater the contribution [6,12]. The equivalent umami concentration (EUC) is often used to evaluate the umami taste compounds in mushrooms. EUC is the total amount of umami substances (umami amino acids and 5′-nucleotides) per 100 g of dry sample, usually expressed in terms of monosodium glutamate (MSG) content, also known as MSG equivalence, with units of g MSG·100 g−1. The higher the EUC value, the stronger the umami taste [12]. The relative odor activity value (ROAV) is another index commonly used to evaluate the key aroma compounds in mushrooms. A compound with an ROAV ≥ 1 is considered a key aroma compound, while substances with an ROAV 0.1–1 are considered to have a modifying effect on the aroma [68,69].
The summary of reports on the flavor components from multiple samples and processes of S. rugoso-annulata is shown in Table 4.

Table 4.
Research reports on the flavor components of S. rugoso-annulata.
As shown in Figure 2, the non-volatile flavor components of S. rugoso-annulata mainly include taste peptides, soluble sugars, free amino acids, organic acids, 5′-nucleotides, flavonoids, alkaloids, polyphenols, inorganic salts, etc., which are water-soluble substances [12,13,14,27,32,71]. The volatile flavor compounds of S. rugoso-annulata include aldehydes, alcohols and ketones, esters, alkanes, alkenes, acids, ethers, phenols, heterocycles (furans, pyrazines), and other classes. Among them, aldehydes, alcohols, and ketones are the main aromatic components with higher concentrations, which all contain octanoids (eight-carbon compounds) [6,12,31,68,72].
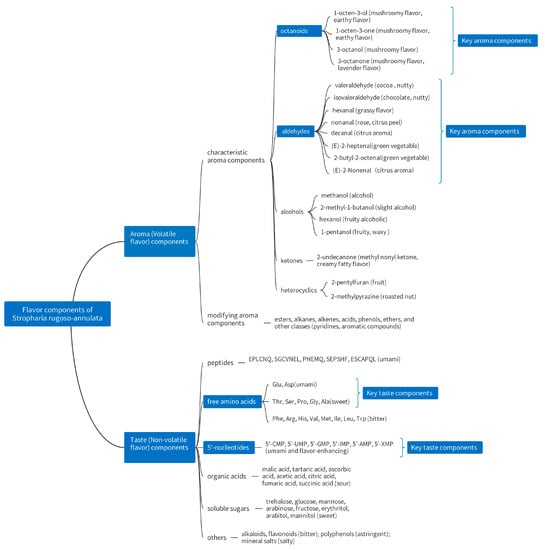
Figure 2.
Flavor components in S. rugoso-annulata (data obtained from references [12,13,14,27,30,32]).
The characteristic flavors and corresponding components of both fresh and dried S. rugoso-annulata mushrooms include umami taste (EUC, umami amino acids, and 5’-nucleotides), sweet taste (sweet amino acids), mushroomy aroma (octanoids), and green, grassy, and fruity aroma (aldehydes). The comprehensive flavor of S. rugoso-annulata mushrooms that combines both taste and aroma is VFD > HAD > ND > fresh > MWD [12]. The characteristic taste components produced by the fermentation mycelium of S. rugoso-annulata include glucose (sweet taste), arginine, leucine, flavonoids (bitter taste), acetic acid, citric acid (sour taste), and peptides (kokumi and rich taste) [13].
4.1. Taste (Non-Volatile Flavor) Components of S. rugoso-annulata
4.1.1. Taste Peptides
S. rugoso-annulata mushrooms are a premium source of umami peptides. Free peptides in the fruiting body of S. rugoso-annulata reach a content of 11.28–12.56% [73]. As shown in Table 5, the taste peptides of S. rugoso-annulata exhibit a strong umami taste, accompanied by saltiness, bitterness, richness, or aftertaste [5,74,75]. The taste peptides in the mature fruiting body are pentapeptides to undecapeptides and the taste threshold is relatively low (0.117–0.640 mmol·L−1) [5,74], while the peptide variety in the mycelium is particularly rich and the taste peptides are mostly heptapeptides to decapeptides [74].

Table 5.
Taste peptides in S. rugoso-annulata.
4.1.2. Soluble Sugars
Soluble sugars are sweet in taste. As shown in Table 6, the fruiting body of S. rugoso-annulata contains various soluble sugars or polyols such as trehalose, arabinose, glucose, mannose, fructose, galactose, xylose, ribose, mannitol, erythritol, and arabitol, with a total content range of 10.01–13.06% [12,27]. Trehalose (7.82–11.03%) or arabinose (5.46–26.54%) have the highest content [12,27], indicating that S. rugoso-annulata mushrooms, particularly the stipes, are excellent raw materials for extracting these two sugars. The mycelium of S. rugoso-annulata contains glucose, gluconic acid, galactose, rhamnose, and ribose, with a total content of 8.32–8.65%. Glucose has the highest content (7.92–8.25%) in the mycelium [13].

Table 6.
Soluble sugars in S. rugoso-annulata.
4.1.3. Free Amino Acids
In terms of free amino acids, Glu and Asp provide the umami taste while Thr, Ser, Pro, Gly, and Ala provide the sweet taste. Phe, Arg, His, Val, Met, Ile, Leu, and Trp provide the bitter taste, and other amino acids are tasteless [27]. As shown in Table 7, the fruiting body of S. rugoso-annulata contains 15–21 free amino acids, with a total content range of 3.07–9.38%. Thr or Glu have been reported to have the highest content [12,27,30,32]. The content of taste amino acids follows the order of sweet amino acids > bitter amino acids > umami amino acids [14,30,32]. In the fermented mycelium of S. rugoso-annulata, there are 20 free amino acids with a total content of 2.27–2.48%, and Arg has the highest content. The content of taste amino acids follows the order of bitter amino acids > sweet amino acids > umami amino acids [13].

Table 7.
Free amino acids in S. rugoso-annulata.
4.1.4. 5′-Nucleotides
5′-Nucleotides provide umami taste and can significantly reduce the perception threshold of umami amino acids at low concentrations, showing strong synergistic flavor-enhancing effects [30]. S. rugoso-annulata contains six 5′-nucleotides, including 5′-cytidylic acid (C), 5′-uridylic acid (U), 5′-guanylic acid (G), 5′-inosinic acid (I), 5′-xanthylic acid (X), and 5′-adenylic acid (A) (Table 8). The total content of these nucleotides ranges from 0.47% to 1.07% [12,14,27,32]. Four nucleotides, including G, I, X, and A, are referred to as umami nucleotides [27], which are also the purine component in S. rugoso-annulata and provide an intense umami taste.

Table 8.
5′-nucleotides in S. rugoso-annulata.
4.1.5. Organic Acids
Organic acids provide a sour taste. S. rugoso-annulata contains seven organic acids, including malic acid, tartaric acid, ascorbic acid, acetic acid, citric acid, fumaric acid, and succinic acid (Table 9). The total content of these organic acids ranges from 11.10 to 24.14% [13,14,27,30]. In the fruiting body, malic acid has the highest content (9.2–16.77%) [27,30], while in the fermented mycelium, acetic acid has the highest content (0.42%) [13].

Table 9.
Organic acids in S. rugoso-annulata.
4.1.6. Other Taste Components
Alkaloids and flavonoids provide a bitter taste, with contents of 0.016–0.020% and 0.35–0.41% respectively, in the mycelium of S. rugoso-annulata. Polyphenols produce astringency, with a content of 0.0063–0.014% in the mycelium [13]. S. rugoso-annulata mushrooms contain a relatively high amount of K (3.41%) and Na (0.12%) [2], which also contribute to their taste. K+ and Na+ provide a salty taste [76], and Na+ can also combine with glutamine or succinic acid to form MSG or sodium succinate, respectively. Both of these compounds are important umami substances [77].
4.2. Aroma (Volatile Flavor) Components of S. rugoso-annulata
The odor of fresh S. rugoso-annulata mushrooms is primarily characterized by mushroomy, earthy, and grassy aromas [12,70]. During the drying process of fresh mushrooms, the earthy and grassy aroma gradually decreases, while a burnt and malty odor emerges and becomes stronger. However, the mushroomy and earthy aroma remains the key characteristic aroma of both fresh and dried S. rugoso-annulata mushrooms.
The characteristic aroma components profile of S. rugoso-annulata fruiting body consists of 19 compounds common to fresh and dried mushrooms, which mainly belong to aldehydes, alcohols, ketones, and octanoids (Figure 3) [12]. The key aromatic components that contribute the most to the aroma of S. rugoso-annulata are aldehydes, including isovaleraldehyde, hexanal, etc., and octanoids, including 3-octanone, 3-octanol, 1-octene-3-one, 1-octen-3-ol, etc. Aldehydes were the most abundant both in number and contents [6,12,70]. Octanoids give S. rugoso-annulata its mushroomy and earthy smell. 1-octen-3-ol, also known as Fungusol, is a compound found in many mushrooms and is primarily responsible for the characteristic mushroomy smell [67]. The strong earthy odor in fresh S. rugoso-annulata mainly results from the odor of 3-octanone and 1-octen-3-one [70]. Hexanal in aldehydes imparts a grassy aroma and isovaleraldehyde imparts a malty and fruity aroma, while heterocyclic compounds such as furans, pyrazines, and pyridines give the burnt smell during the process of heating [6,7,12,31,70].
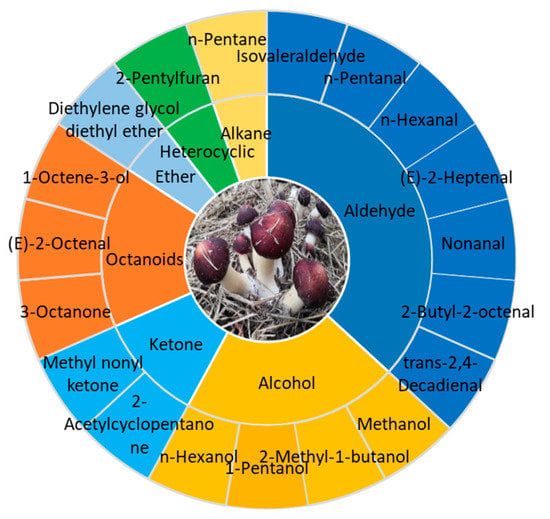
Figure 3.
Characteristic aroma components profile of S. rugoso-annulata (data obtained from reference [12]).
4.3. Factors Influencing Flavor Components of S. rugoso-annulata
4.3.1. Taste Components in Different Parts of S. rugoso-annulata
There was no difference in the variety of taste components between the pileus and stipe of S. rugoso-annulata, but there were significant differences in the content (Table 10). The pileus’s content of free amino acids, organic acids, 5′-nucleotides, and EUC were significantly higher than those in the stipe. The content of soluble sugars in the stipe was significantly higher than that in the pileus [27]. These indicate that the pileus of S. rugoso-annulata is more umami-rich, whereas the stipe tends to be sweeter in taste.

Table 10.
Content of taste components in S. rugoso-annulata pileus and stipe.
4.3.2. Aroma Components in Different Parts of S. rugoso-annulata
The fresh pileus and stipe of S. rugoso-annulata were found to contain 15 and 14 volatile components, respectively, with 3-octanone, schisterol, and hexanal being the same major ones. 3-octanone was the compound with the highest content (>70%) in both parts. Hexanal, 3-ethyl-2-methyl-1, 3-hexadiene, and pentadecane were found in the pileus but not in the stipe, while α-bisabolene and dehydrovomifoliol were detected in the stipe but not in the pileus. The pileus had a higher content of aldehydes, esters, alkenes, and alkanes, while the stipe had a higher content of alcohol [31].
The dried pileus and stipe of S. rugoso-annulata (first subjected to HAD and then FVD) were found to contain a total of 50 volatile components, respectively, with alcohols, esters, and alkanes being the main constituents in the pileus, and alcohols, esters, and ketones being the main constituents in the stipe. The dried pileus exhibited a higher content of alkanes, whereas the dried stipe showed a higher content of ketones [72].
4.3.3. Taste Components of S. rugoso-annulata at Different Developmental Stages
As shown in Figure 4, the content of free amino acids (2.48%) and 5′-nucleotides (0.0067%) in fermented mycelium is obviously lower than in the fruiting body [13], resulting in an almost negligible umami taste (EUC) in the fermented mycelium. The total content of free amino acids, 5′-nucleotides, EUC, and TAV in the fruiting body showed a trend of first increasing and then decreasing [32].
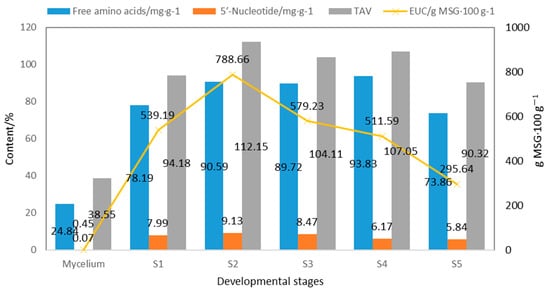
Figure 4.
Taste components and TAV at different developmental stages of S. rugoso-annulata (data obtained from references [13,32]).
S2 showed the highest 5′-nucleotide content (0.91%), TAV (112.15), and EUC (788.66 g MSG·100 g−1). S4 showed the highest content of total free amino acids (9.38%), umami amino acids (1.29%), and sweet amino acids (1.70%) [32]. These findings serve as a reference for determining the optimal timing for harvesting. S. rugoso-annulata. This study provides a reference for the timely harvesting of S. rugoso-annulata rich in nutrition and taste components.
4.3.4. Taste Components of S. rugoso-annulata with Different Processing Methods
In the preparation of S. rugoso-annulata soup (water extracts of fruiting body powder), processing methods present a significant influence on the content of taste components. The non-thermal treatment of HHP resulted in the highest levels of 5′-nucleotides (1.46–1.82%) and free amino acids (10.43–12.36%) and the highest EUC (827.44–1411.79 g MSG·100 g−1), while thermal treatments (70 °C and 90 °C heating) led to the highest concentration of organic acids (6.55–9.84%). Other non-thermal treatments, including UT and HG, led to higher levels of soluble sugars (1.56–3.05%) [14]. These findings are helpful in the cooking of highly flavorful S. rugoso-annulata soup.
Grinding the S. rugoso-annulata powder (particle size) also has a significant impact on the release of taste components into soup. Moderately ground powder (36.63 μm) leads to the highest concentration of free amino acids and soluble sugars in the soup. These findings suggest that HHP and moderately fine grinding are beneficial for the extraction of taste components from S. rugoso-annulata powder [14].
Different drying methods have been found to result in significant variations in the levels of taste components in S. rugoso-annulata [12,30,71]. HAD samples had the highest levels of total free amino acids (7.36%), sweet amino acids (2.85%), and bitter amino acids (1.91%). VFD samples had the highest contents of total organic acid (24.14%), umami amino acids (1.05%), and 5′-nucleotides (1.07%). The EUC values of different S. rugoso-annulata samples were ranked in the order of HAD (229.87 g MSG·100 g−1) > VFD (177.00 g MSG·100 g−1) > MWD (159.50 g MSG·100 g−1) [30].
HAD-dried S. rugoso-annulata exhibited the highest EUC value, while VFD-dried samples had the highest levels of taste components [27,30]. HAD and VFD have been found to be beneficial for preserving and enhancing the taste components in S. rugoso-annulata, making them suitable for the production of tasteful dried mushrooms [12,30]. Among them, HAD is the most economical and convenient drying method. On the other hand, MWD was shown to cause a significant decrease in the content of taste components and therefore is not suitable for drying.
4.3.5. Aroma Components of S. rugoso-annulata with Different Processing Methods
A total of 47 volatile components were identified in the S. rugoso-annulata soup, primarily consisting of alcohols, aldehydes, ketones, esters, hydrocarbons, and a small number of heterocyclic compounds (Figure 5). The main odor components (with higher concentrations) were hexanol, 1-octen-3-ol, and hexanal. The ranking of the processing methods for the aroma intensity of S. rugoso-annulata soup from high to low was UT > HHP > RT > HG > 70 °C > 90 °C. The non-thermal-treated soup displayed a more intense aroma than the thermal-treated soup. Thermal treatment reduced the volatile components of the soup, and the higher the processing temperature, the more severe the loss of volatile compounds. Grinding the mushroom powder (particle size) influenced the odor of the mushroom soup. Ultrafine grinding probably destroyed the volatile components of S. rugoso-annulata powder. The soup of non-ultrafine-ground mushroom powder (181.25 μm) had the strongest aroma [15].
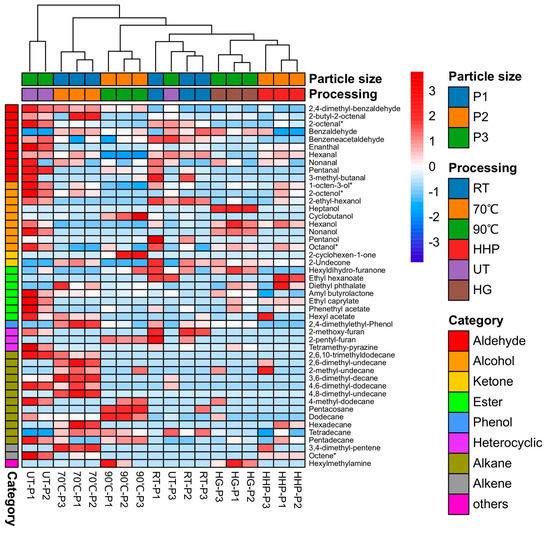
Figure 5.
Heatmap of the volatile components of S. rugoso-annulata soup processed by six different methods and 3 levels of particle sizes (data obtained from reference [15]). Notes: * means octanoids. P1—particle size 181.25 μm; P2—particle size 36.63 μm; P3—particle size 7.06 μm; RT—room temperature; HHP—high hydrostatic pressure; UT—ultrasound treatment; HG—homogenization.
Drying significantly affects the odor of S. rugoso-annulata; the variety of odor compounds in dried S. rugoso-annulata (59–68) is significantly higher than that of fresh S. rugoso-annulata (47). Different drying methods result in different aroma profiles—fresh mushrooms have a slightly grassy and earthy aroma, while HAD mushrooms have a mild nutty aroma with a decrease in grassy flavor. VFD mushrooms retain a more distinct grassy flavor; MWD mushrooms have a slightly nutty, burnt, and sulfurous smell; ND mushrooms have a slight sulfur odor. Among the drying methods, VFD can best preserve the odor quality of fresh mushrooms [12].
Deep frying significantly increased the richness of the aromas of S. rugoso-annulata stipe, adding roasted nut and chocolate aromas. In the unfried samples, the volatile flavor compounds were mainly alcohols and esters, and 1-octene-3-one contributed the most to the aroma. In the fried samples, aldehydes were found the most, mainly including n-hexanal, octanal, nonanal, and (E)-2-nonenal, with (E)-2-nonenal making the largest contribution to the aroma [7]. This finding provided a reference for the deep-frying processing of S. rugoso-annulata.
4.3.6. Flavor Components of S. rugoso-annulata at Different Temperatures
Rao et al. reported the effects of storage temperature (0 °C, 15 °C) on post-harvest quality and volatile flavor components [68]. The results revealed that fresh S. rugoso-annulata after harvest can be stored at 15 °C for 4–6 days and at 0 °C for 12 days while maintaining good quality without decaying. 3-octanol, 1-octene-3-ol, (E)-2-octenal, nonanal, decanal, 3-octanone, and 2-pentylfuran were the characteristic flavor substances in fresh S. rugoso-annulata. As the storage time extended, 3-octanol and hexanal were the key substances for the aroma deterioration of post-harvest S. rugoso-annulata. Compared to the samples stored at 15 °C, S. rugoso-annulata samples stored at 0 °C showed a lower ROVA of undesirable aroma components and a greater variety of aroma compounds [68]. This result indicated that 0 °C storage could effectively maintain the post-harvest quality of S. rugoso-annulata and inhibit its flavor deterioration, providing valuable references for maintaining the post-harvest storage of S. rugoso-annulata.
The processing temperature adopted for preparing S. rugoso-annulata soup led to significant differences in the content of 5’-nucleotides. When the soup is prepared at 70 °C or 90 °C, 5′-CMP and 5′-XMP are detected, unlike in soups prepared at room temperature. The total content of 5′-nucleotides in the soup follows a descending order of RT > 70 °C > 90 °C [14]. Additionally, the total concentration of volatile flavor components in S. rugoso-annulata soup follows a descending order of RT > 70 °C > 90 °C [15].
Qin et al. reported on the effect of various drying temperatures of HAD on the volatile flavor components of S. rugoso-annulata [38]. Within the range of 30–90 °C, as the HAD temperature increased, the diversity of volatile components decreased, and more alkanes were produced. A HAD temperature of 30 °C led to the most abundant volatile components (58) in S. rugoso-annulata [31].
Bao et al. reported the effects of roasting on the volatile flavor compounds of S. rugoso-annulata [6]. The result showed that roasting S. rugoso-annulata within the temperature range of 100–180 °C improved the richness of flavor, increased the malty, rose-like, nut-like, and cocoa-like aromas, and improved the overall flavor of S. rugoso-annulata. The largest number (63) of flavor components was found in S. rugoso-annulata roasted at 140 °C. With the increase in roasting temperature, the content of alcohols decreased significantly, while the content of aldehydes, alkanes, and pyrazines increased [6]. The results indicated that roasting enhanced the volatile flavor of S. rugoso-annulata.
The adsorption temperature (40–100 °C) in the microextraction step of headspace solid-phase microextraction gas chromatography–mass spectrometry (HS-SPME-GC-MS) significantly influenced the detection result of volatile components. The number of detected volatile compounds was positively correlated with the adsorption temperature, with only one for 40 °C and 18 for 100 °C. The optimal adsorption temperature was found to be 80 °C [31].
4.3.7. Comparison of Aroma Components between S. rugoso-annulata and Other Edible Mushrooms
The aroma components of S. rugoso-annulata are also related to its genetic characteristics. Different edible mushroom species have their own characteristic odor components, and there can be significant differences in aroma profiles between them (Table 11). The aroma components of S. rugoso-annulata do not contain sulfur compounds [12,70], whereas those of shiitake (Lentinula edodes) and straw mushroom (Volvariella volvacea) include sulfur compounds as the main and characteristic aroma components [78,79]. Sulfur compounds generally release a strong, unpleasant smell [80]. Therefore, unlike shiitake or straw mushrooms, S. rugoso-annulata mushrooms do not have an unpleasant odor, giving a mild earthy and grassy aroma.

Table 11.
Comparison of aroma components in S. rugoso-annulata and other edible mushrooms.
Generally, the flavor components of S. rugoso-annulata are influenced by various internal factors such as genetic characteristics, developmental stages, and parts of the mushroom, and external factors such as processing methods and temperature conditions.
5. Problems and Prospects
5.1. Zero Research Report on Chitin
As the main component of fungal cell walls, chitin is a structural polysaccharide composed of N-acetylglucosamine linked by β-1, 4 glycosidic linkages. It is widely present in fungi and crustaceans and was first discovered in mushrooms [81]. It is insoluble in water but can only be dissolved in strong acids [82]. The reason why mushrooms are not easily cooked to softness after prolonged cooking is because they contain a large amount of chitin [83].
The chitin contents of A. bisporus, P. ostreatus, L. edodes, F. velutipes, P. eryngii, and V. volvacea have been reported, ranging from 4.77% to 19.6% [84]. However, the chitin content of S. rugoso-annulata has not yet been reported and related studies are still lacking.
5.2. High Protein Content Distortion
S. rugoso-annulata mushrooms have a significantly high protein content (25.75–34.17%), being 1.8 times that of A. bisporus, 1.4 times that of L. edodes, and 1.3 times that of P. ostreatus [16,85]. However, in some studies, the protein content of S. rugoso-annulata has been reported to exceed 50% [32]. This could be due to the incorrect conversion factor when measuring the protein content using the Kjeldahl method.
The Kjeldahl method is a classic method for measuring the nitrogen and protein contents of samples and is included in the standard for determining protein content in food. This method determines the protein content by measuring the total nitrogen content and multiplying it by a conversion factor (nitrogen to protein factor, NPF), generally taken as 6.25 [86]. However, because edible mushrooms contain a large amount of non-protein nitrogen, in chitin, the content of non-protein nitrogen needs to be excluded when measuring the protein content of edible mushrooms using the Kjeldahl method.
To obtain a more accurate protein content, the NPF for edible mushrooms is generally modified to 4.38 [27,87]. After modifying the conversion factor, the protein content of S. rugoso-annulata, which was originally reported to exceed 50%, decreased to around 35%, which is a more realistic and accurate figure.
5.3. Little Research on the Bioactive Components in the Fermentation Mycelium and Liquid of S. rugoso-annulata
Mycelium and fermentation liquid produced through the liquid fermentation of edible mushrooms are important sources of pharmacologically active ingredients. Liquid fermentation has advantages such as a short cycle, year-round production, and controllable quality. Secondary metabolite production can also be increased intentionally by changing fermentation parameters and cultivation methods [88]. Currently, the bioactive ingredients of S. rugoso-annulata are mainly derived from its fruiting bodies, and there is little research on the bioactive ingredients and pharmacological activities of mycelium and fermentation liquids. Therefore, it is necessary to further conduct research on the liquid culture of S. rugoso-annulata mycelium and the bioactive components of its products.
5.4. Genes Related to the Metabolism of Nutritional and Flavoring Components Have Not Been Reported
Currently, research progress in the biosynthetic pathways and metabolic regulation mechanisms of key volatile components in S. rugoso-annulata has already been made. For example, the biosynthetic pathway of the main odor components (aldehydes, ketones, and alcohols) generated during the drying process of S. rugoso-annulata was found to be the lipoxygenase (LOX) metabolic pathway, and the key enzymes involved in regulation are lipoxygenase and alcohol dehydrogenase (ADH). LOX catalyzes the formation of aldehydes and ketones of C6, C8, and C9, while ADH catalyzes the reduction of aldehydes and ketones into corresponding alcohols [70], but the research findings are still in the preliminary stage. Research on nutritional and flavor components needs to delve into the genetic level in order to fully understand the mechanisms of component metabolism. So far, there have been no reports on the important functional genes of S. rugoso-annulata.
5.5. The Deep-Processed Products Are Almost Blank
In the current Chinese market, S. rugoso-annulata products are mainly limited to fresh mushrooms, with only a small quantity of dried and pickled mushrooms available [89]. The products are limited and quite primary. As an excellent raw material, S. rugoso-annulata mushroom can be used to extract various nutrients, bioactive ingredients, and flavor components.
Compared with other edible mushroom varieties, S. rugoso-annulata is not only rich in nutritional value such as protein and amino acids, but also abundant in bioactive and flavor substances such as polysaccharides, sterols, and taste peptides. There are great application prospects for S. rugoso-annulata in the fields of dietary nutrition supply, bioactive compound extraction, functional food production, flavor food processing, etc.
Author Contributions
Conceptualization, L.H. and C.H.; methodology, C.S.; software, H.S.; writing—original draft preparation, L.H.; writing—review and editing, J.D.; visualization, C.S. and H.S.; supervision, C.H.; funding acquisition, J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the 2022 Guangdong provincial rural revitalization strategy special funds for breeding industry revitalization project (2022-WPY-00-002) and Guangdong provincial forestry science and technology innovation project (2021KJCX016).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Diego, C.Z.; Pardo-Giménez, A. Edible and Medicinal Mushrooms: Technology and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Jing, B.-N.; Chang, X.; Wei, L.; Xie, X.-Y.; Zhou, Y.; Wang, Z.-Y.; Liu, Y.-Q.; Wang, W. Analysis and Evaluation of Nutrient Components, Bioactive Substances and Heavy Metal Content of Stropharia rugosoannulata in Bo’ai County. Sci. Technol. Food Ind. 2022, 43, 278–285. [Google Scholar] [CrossRef]
- Li, X.-X.; Zhang, Z.-Q.; Wang, L.; Zhao, H.-Q.; Jia, Y.-H.; Ma, X.; Li, J.-Z.; Wang, Y.; Ma, B.-J. Three-phase extraction of polysaccharide from Stropharia rugosoannulata: Process optimization, structural characterization and bioactivities. Front. Immunol. 2023, 13, 994706. [Google Scholar] [CrossRef]
- Wu, J.; Kobori, H.; Kawaide, M.; Suzuki, T.; Choi, J.; Yasuda, N.; Noguchi, K.; Matsumoto, T.; Hirai, H.; Kawagishi, H. Isolation of Bioactive Steroids from the Stropharia Rugosoannulata Mushroom and Absolute Configuration of Strophasterol. Biosci. Biotechnol. Biochem. 2013, 77, 1779–1781. [Google Scholar] [CrossRef]
- Li, W.; Chen, W.; Ma, H.; Wang, J.; Li, Z.; Wang, Q.; Zhang, Z.; Wu, D.; Zhang, J.; Yang, Y. Study on the Relationship Between Structure and Taste Activity of the Umami Peptide of Stropharia Rugosoannulata Prepared by Ultrasound. Ultrason. Sonochem. 2022, 90, 106206. [Google Scholar] [CrossRef]
- Bao, C.-L.-G.; Guan, C.-B.; Xin, M.-H.; Teng, X.; Liu, T.-T.; Wang, D.-W. To analyze the effects of roasting on volatile flavor compounds of Stropharia rugoso-annulate using HS-SPME-GC-MS and electronic nose. Food Sci. 2022, 43, 1–14. [Google Scholar]
- Li, X.-M.; Li, H.-J.; Xiao, X.; Liao, L.; Chen, R.; Xie, Z.-H.; He, Z.-F. Study on the change of volatile flavor substances during the processing of deep-fried and battered mushroom stalks of Stropharia rugoso-annulata. Food Ferment. Ind. 2022, 1–10. [Google Scholar] [CrossRef]
- Stamets, P.; Chilton, J.S. The Mushroom Cultivator-A Practical Guide to Growing Mushrooms at Home. 1983. Available online: http://library.uniteddiversity.coop/Permaculture/Mushroom_Cultivator-A_Practical_Guide_to_Growing_Mushrooms_at_Home.pdf (accessed on 8 October 2021).
- Domondon, D.; Poppe, J.; Griensven, L. Fruit optimization with wastes used for outdoor cultivation of king Stropharia. Neophilologus 2000, 79, 619–628. [Google Scholar]
- Yan, Q.-X.; Huang, M.-X.; Sun, P.; Cheng, S.-X.; Zhang, Q.; Dai, H. Steroids, fatty acids and ceramide from the mushroom Stropharia rugosoannulata Farlow apud Murrill. Biochem. Syst. Ecol. 2020, 88, 103963. [Google Scholar] [CrossRef]
- China Edible Fungi Association. Statistical Survey of Edible Fungi in China in 2021. Edible Fungi China 2023, 42, 118–127. [Google Scholar] [CrossRef]
- Chen, W.-C.; Li, W.; Wu, D.; Zhang, Z.; Chen, H.; Li, Z.-P.; Wang, C.-G.; Yang, Y. Multilevel analysis and evaluation of dried flavor quality of Stropharia rugoso-annulata based on component profile data. Food Sci. 2022, 44, 1–12. [Google Scholar]
- Li, W.; Feng, J.; Ma, H.-L.; Chen, W.-C.; Wu, D.; Zhang, Z.; Yang, Y. Analysis of the characteristic flavor components and flavor characteristics of the fermentation of Stropharia rugoso-annulata based on targeted metabolite assay. J. Food Saf. Qual. 2022, 13, 2736–2744. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, S.; Xue, S.; Yang, D.; Li, L. Comparison of Non-volatile Flavor Compounds in Stropharia rugosoannulata Soup Processed by Different Methods. J. Food Sci. Technol. 2022, 59, 1. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Xue, S.-J.; Yang, D.; Wang, S.-H.; Li, L. Effects of Different Processing Methods on the Volatile Components and Antioxidant Ability of the Water Extracts from Stropharia rugosoannulata. Sci. Technol. Food Ind. 2021, 42, 41–48. [Google Scholar] [CrossRef]
- Huang, J.-X.; Yuan, S.-N.; Pan, J.; Zheng, D.-H.; Chen, J.-M.; Li, J.; Gui, Q.; Zhou, L.-J. Difference of Primary Nutritional Ingredient between Lentinus edodes, Pleurotus ostreatus and Stropharia rugosoannulata Grown Mainly with Rubber Wood Dust. Chin. J. Trop. Crops 2018, 39, 1625–1629. [Google Scholar] [CrossRef]
- Yan, Q.-X. Study on Screening of Active Components and Quality Standard of Stropharia Rugosoannulata. Master’s Thesis, Guangxi University of Traditional Chinese Medicine, Nanning, China, 2019. Chinese Master’s Theses Full-Text Database. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbname=CMFD2022&filename=1021126383.nh&dbcode=CMFD (accessed on 9 October 2021).
- Li, S.-R.; Wang, L.; Ni, S.-J.; Wang, H.-H.; Liu, X.-F.; Liu, C.-X. The Amino Acids Content of Different Part of Stropharia rugoso-annulata and Their Nutrition Evaluation. Food Res. Dev. 2017, 38, 95–99. [Google Scholar] [CrossRef]
- Liu, M.-M.; Zhang, X.-L.; Xu, L.-L.; Jiang, P. Analysis of Amino Acids Content and Food Safety Assessment of Stropharia rugosoannulata Cultivated in the Imitated Wild Environment under Forest. Edible Fungi China 2021, 40, 67–70. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, Q.-Q.; Wang, X.-L.; Zhang, G.-Q.; Cheng, J.-H.; Chen, Q.-J. Effects of Different Formulations and Cultivation Techniques on Nutrient Quality of Stropharia rugoso-annulata. Vegetables 2022, 8, 51–54. [Google Scholar]
- Wang, X.-W.; Zhan, W.; Tao, M.-X.; Ji, Z.-S.; Liu, Y.; Wang, F.; Cheng, G.-Y. Analysis of the nutritional and antioxidant components of Stropharia rugoso-annulata. Edible Fungi 2007, 171, 62–63. [Google Scholar]
- Zeng, J.-Y.; Zhao, B.; Bi, W.-Y.; Liu, C.-Y.; Zhang, G.-C. Effects of Na2SeO3 and ZnSO4 on Liquid Fermentation of Stropharia rugoso-annulata Mycelia. J. Jilin Agric. Univ. 2018, 40, 171–177. [Google Scholar] [CrossRef]
- Wang, D. Study on the Accumulation of Heavy Metal Lead and Cadmium and the Interaction Effect of Selenium Supplementation in the Stropharia rugosoannulata. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2018. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbname=CMFD201901&filename=1018318645.NH&dbcode=CMFD (accessed on 10 October 2021).
- Zhao, Y.; Ren, Y.-F.; Chen, M.-J.; Zha, L.; Song, X.-X.; Guo, L.-G.; Wang, C.-G. Analysis of fatty acid composition and content in three strains of Stropharia rugoso-annulata by GC-MS. Food Mach. 2018, 34, 65–67+72. [Google Scholar] [CrossRef]
- Li, Z.-P.; Yu, C.-X.; Ren, Y.-F.; Chen, M.-J.; Zha, L.; Yang, H.-L.; Song, X.-X.; Zhao, Y. Effect of Heat Stress on Fatty Acids in Stropharia rugosoannulata Mycelia. Acta Edulis Fungi 2020, 27, 45–50. [Google Scholar] [CrossRef]
- Lu, Q.; Xue, S.-J.; Yang, D.; Wang, S.-H.; Li, L. Effects of Three Extraction Methods on Antioxidant Properties of Crude Polysaccharides from Stropharia Rugoso. Food Sci. Technol. 2021, 46, 171–178. [Google Scholar] [CrossRef]
- Hu, S.; Feng, X.; Huang, W.; Ibrahim, S.A.; Liu, Y. Effects of Drying Methods on Non-volatile Taste Components of Stropharia rugoso-annulata Mushrooms. LWT 2020, 127, 109428. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Y.; Feng, X.; Ibrahim, S.A.; Huang, W.; Liu, Y. Effects of Drying on the Structural Characteristics and Antioxidant Activities of Polysaccharides from Stropharia rugosoannulata. J. Food Sci. Technol. 2021, 58, 3622–3631. [Google Scholar] [CrossRef]
- Liu, L.-P.; Qian, W.-C.; Zhan, P.F.; Song, W.-M.; Wen, Y.; Wang, Q.-H. Effect of Different Substrates and Drying Methods on the Nutritional Composition of Stropharia rugosoannulata. J. Southwest Univ. Nat. Sci. Ed. 2018, 40, 8–13. [Google Scholar] [CrossRef]
- Yu, H.-P.; Hu, S.; Huang, W.; Wang, Y.; Liu, Y. Effects of Drying Process on the Tasty Components in Stropharia rugoso-annulata. Sci. Technol. Food Ind. 2021, 42, 251–256. [Google Scholar] [CrossRef]
- Qin, Y.-C.; Wu, D.-P.; Wang, L.-L.; Fang, R.; He, L.; Wang, Y.-B.; Qian, H.; Liu, B.-T. Effects of Various Drying Methods on Volatile Composition of Stropharia rugosoannulata by Headspace-Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry. Food Sci. 2022, 43, 273–280. [Google Scholar] [CrossRef]
- Chen, R.-R.; Li, W.; Wu, D.; Mao, C.-F.; Bao, D.-P.; Chen, W.-C.; Zhang, J.-S.; Yang, Y. Nutrients, Texture and Taste Characteristics of Stropharia rugosoannulata During Growth and Development. Acta Edulis Fungi 2022, 29, 42–54. [Google Scholar] [CrossRef]
- Brodzinska, Z.; Lasota, W. Chemical composition of cultivated mushrooms Part I. Stropharia rugoso-annulata Farlow ex. Murr. Bromatol. I Chem. Toksykol. 1981, 14, 229–238. [Google Scholar]
- Jiang, H. Studies on the Degradation and Cultivation under Forest of Stropharia rugoso-annulata. Master’s Thesis, Xinjiang Agricultural University, Ürümqi, China, 2020. Chinese Master’s Theses Full-Text Database. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbname=CMFDTEMP&filename=1022002887.nh&dbcode=CMFD (accessed on 11 October 2021).
- Yang, F.-T.; Long, S.-B.; Long, J.-Q.; Ma, X.-T.; Wang, K. A Culture Technique of Edible Fungi (Stropharia rugosoannulata Farl. ex Murrill)-paddy Rice Rotation in Guizhou Province. Tillage Cultiv. 2021, 41, 96–98. [Google Scholar] [CrossRef]
- Wang, L.; Ni, S.-J.; Li, S.-R.; Wang, H.-H.; Jiang, X.-X.; Liu, X.-F.; Pan, Y. Analysis of the Different Parts of Base Nutrition of Stropharia rugoso-annulata. Heilongjiang Agric. Sci. 2016, 269, 143–145. [Google Scholar]
- Wei, L.; Wang, W.; Xie, X.-Y.; Zhou, Y.; Liu, Y.-Q.; Ma, Y.-N.; Ning, E.-J.; Wang, T.; Li, N.-J.; Jing, B.-N. Optimization of Extraction Process of Polysaccharides from Stropharia rugosoannulata in Bo’ai County by Response Surface Method and Evalation of Their Antibacterial and Antioxidant Activity. Sci. Technol. Food Ind. 2023, 1–12. [Google Scholar] [CrossRef]
- Xie, X.-Y.; Chang, X.; Liu, Y.-Q.; Jing, B.-N.; Han, H.-Y.; Wei, L.; Zhou, Y.; Wang, W. Optimization of fermentation conditions and antioxidant activity of polysaccharides from fruiting body of Stropharia rugosoannulata. China Food Addit. 2022, 33, 55–61. [Google Scholar] [CrossRef]
- Jia, J.; Xie, X.-C.; Song, Y.; Sun, Y.; Xu, Y.-J.; Yao, S. Optimizing Culture Medium and Antioxidant Activity of Extracellular Polysaccharides in Liquid Fermentation of Stropharia rugosoannulata. North. Hortic. 2021, 495, 105–115. [Google Scholar]
- He, P.-X.; Geng, L.-J.; Wang, J.-Z.; Xu, C.-P. Production, purfication, molecular characterization and bioactivities of exopolysaccharides produced by the wine cap culinary-medicinal mushroom, Stropharia rugosoannulata 2# (higher Basidiomycetes). Int. J. Med. Mushrooms 2012, 14, 365–376. [Google Scholar]
- Jin, M.; Zhang, W.; Zhang, X.; Huang, Q.; Chen, H.; Ye, M. Characterization, Chemical Modification and Bioactivities of a Polysaccharide from Stropharia rugosoannulata. Process Biochem. 2023, 128, 30–39. [Google Scholar] [CrossRef]
- Jiang, L. The Research on Preparation, Structural Identification and Biological Activity of Polysaccharide from Stropharia rugosoannulata (SR-1) and Polysaccharide from Tricholoma lascivum (Fr.) Gillet (TLG-1). Master’s Thesis, China West Normal University, Nanchong, China, 2019. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbname=CMFD202001&filename=1019625385.NH&dbcode=CMFD (accessed on 12 October 2021).
- Zhai, X.; Zhao, A.; Geng, L.; Xu, C. Fermentation Characteristics and Hypoglycemic Activity of an Exopolysaccharide Produced by Submerged Culture of Stropharia rugosoannulata #2. Ann. Microbiol. 2012, 63, 1013–1020. [Google Scholar]
- Li, X.; Cui, W.; Cui, Y.; Song, X.; Jia, L.; Zhang, J. Stropharia rugoso-annulata Acetylated Polysaccharides Alleviate Nafld Via Nrf2/jnk1/ampk Signaling Pathways. Int. J. Biol. Macromol. 2022, 215, 560–570. [Google Scholar] [CrossRef]
- Wang, X.-W. Nutrition Components Analyse, Extraction and Antioxidant Properties of Polysaccharide of Stropharia rugoso-annulata. Master’s Thesis, Nanjing Normal University, Nanjing, China, 2007. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbname=CMFD2007&filename=2007107796.NH&dbcode=CMFD (accessed on 13 October 2021).
- Xiao, W.-F.; Chai, W.-G.; Qiu, J.-R.; Xin, Y.; Ruan, S.-L. Proteomic Analysis Reveals Mechanisms of Stropharia rugosoannulata Polysaccharides Promoting Kiwifruit Growth and High Temperature Resistance. J. Nucl. Agric. Sci. 2022, 36, 94–104. [Google Scholar] [CrossRef]
- Xiao, W.-F.; Ni, S.; Ruan, S.-L.; Chen, H.-Z.; Xin, Y.; Qiu, J.-R.; Chai, W.-G. Effects of Fruiting Body Polysaccharides from 9 Edible Fungi on Growth of Rice Seedling. Acta Edulis Fungi 2020, 27, 69–74. [Google Scholar] [CrossRef]
- Pozdnyakova, N.; Schlosser, D.; Dubrovskaya, E.; Balandina, S.; Sigida, E.; Grinev, V.; Turkovskaya, O. The Degradative Activity and Adaptation Potential of the Litter-decomposing Fungus Stropharia rugosoannulata. World J. Microbiol. Biotechnol. 2018, 34, 133. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, C.; Feng, X.; Cheng, L.; Ibrahim, S.A.; Wang, C.; Huang, W. Isolation, Characterization and Antioxidant of Polysaccharides from Stropharia rugosoannulata. Int. J. Biol. Macromol. 2019, 155, 883–889. [Google Scholar] [CrossRef]
- Wang, M.-X.; Sheng, Z.-C.; Chen, X.-L. Optimization of Extraction and Characterization of Polysaccharide from Stropharia rugosoannulata Fermentation Mycelium. North. Hortic. 2020, 01, 111–116. [Google Scholar] [CrossRef]
- Qian, C.-Q.; Lin, W.-F.; Wu, D.-P.; Liu, N.-Y. Study on the Extraction of Polysaccharides from Stropharia rugoso-annulata by Alkaline Extraction and Its Scavenging Oxygen Free Radical. Mod. Food 2021, 03, 99–102. [Google Scholar] [CrossRef]
- Miao, Y.-Z. Separation and Purification of Se-Polysaccharide from Stropharia rugoso-annulata and Its Antioxidant Activities. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2009. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbname=CMFD2010&filename=2009234859.NH&dbcode=CMFD (accessed on 14 October 2021).
- Chen, J.-C.; Weng, M.-J.; Lai, P.-F.; Li, Y.-B.; Zhou, X.-H.; Shen, H.-S. Distribution of Stropharia rugoso-annulata Polysaccharides Molecular Weight and Component Sugar. Sci. Agric. Sin. 2011, 44, 2109–2117. [Google Scholar]
- Cui, X.-R.; Wang, L.; Shi, F.-F.; Pan, Y.; Jia, H.-L.; Liu, X.-F.; Li, S.-R.; Song, H.-B. Extraction and antioxidant activity of Stropharia rugosoannulata protein. J. Food Saf. Qual. 2018, 9, 5949–5956. [Google Scholar]
- Huang, S. Study on the Extraction and Antioxidation of Polyphenol Compounds of Stropharia rugosoannulata Farlow. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2010. Chinese Master’s Thesis Full-Text Database. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbname=CMFD2012&filename=1011038174.nh&dbcode=CMFD (accessed on 15 October 2021).
- Chen, C.; Huang, J.; Ding, L. Study on Ultrasonic Wave Assisted Extraction of Flavonoids from Stropharia. Food Res. Dev. 2015, 36, 116–119. [Google Scholar] [CrossRef]
- Wu, J.; Tokuyama, S.; Nagai, K.; Yasuda, N.; Noguchi, K.; Matsumoto, T.; Hirai, H.; Kawagishi, H. Strophasterols a to D with an Unprecedented Steroid Skeleton: From the Mushroom Stropharia rugosoannulata. Angew. Chem. Int. Ed. 2012, 51, 10820–10822. [Google Scholar] [CrossRef]
- Sato, S.; Taguchi, Y.; Kuwahara, S. Synthesis and Stereochemistry of Glaucoposterol a and Strophasterol D. Tetrahedron 2020, 76, 131129. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, G.; Geng, X.; Zhao, Y.; Ng, T.; Zhao, L.; Wang, H. Isolation and Characterization of a Novel Lectin from the Edible Mushroom Stropharia rugosoannulata. Molecules 2014, 19, 19880–19891. [Google Scholar] [CrossRef]
- Li, W.; Chen, W.; Ma, H.; Wu, D.; Zhang, Z.; Yang, Y. Structural Characterization and Angiotensin-converting Enzyme (ace) Inhibitory Mechanism of Stropharia rugosoannulata Mushroom Peptides Prepared by Ultrasound. Ultrason. Sonochem. 2022, 88, 106074. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.-Y.; Song, T.-T.; He, W.-Q.; Zhang, Y.-Y.; Cai, W.-M.; Zhang, Z.-F. Comparison of antioxidant activities and constituent analysis of different polar extracts from Stropharia rugosoannulata. Mycosystema 2022, 41, 999–1007. [Google Scholar]
- Lei, P.; Zhang, W.; Men, X. Review on Anti-tumor Effect of Triterpene Acid Compounds. J. Cancer Res. Ther. 2014, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Suzuki, T.; Kawagishi, H. An unusual sterol from the mushroom Stropharia rugosoannulata. Tetrahedron Lett. 2013, 54, 4900–4902. [Google Scholar] [CrossRef]
- Wu, J.; Fushimi, K.; Tokuyama, S.; Ohno, M.; Miwa, T.; Koyama, T.; Yazawa, K.; Nagai, K.; Matsumoto, T.; Hirai, H.; et al. Functional-food Constituents in the Fruiting Bodies of Stropharia rugosoannulata. Biosci. Biotechnol. Biochem. 2011, 75, 1631–1634. [Google Scholar] [CrossRef]
- Yan, Q.-X.; Luo, X.-L.; Li, C.-Q.; Zhou, Y.-X.; Qin, X.-M.; Qin, J.; Dai, H. Anti-fatigue Effect of Stropharia rugosoannulata Fruiting Body Extracts Derived from Different Solvents. Acta Edulis Fungi 2018, 25, 65–70. [Google Scholar] [CrossRef]
- Hu, D.-S.; Li, J.; Zhang, Y.; Zhong, D.-M.; Fang, X.-F.; Liao, Y.-Z.; Su, G.-G. Optimization of Extraction Conditions of Polyphenols from Stropharia rugosoannulata Cultivated under Forest. Edible Fungi China 2022, 41, 65–70. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Z.; Xin, G.; Sun, B.; Bao, X.; Wei, Y.; Zhao, X.; Xu, H. Advances in umami taste and aroma of edible mushrooms. Trends Food Sci. Technol. 2020, 96, 176–187. [Google Scholar] [CrossRef]
- Rao, K.-C.; Huang, W.; Wang, Y.; Liu, Y. Effects of Storage Temperature on the Postharvest Quality and Volatile Flavor Components of Stropharia rugoso-annulata. Sci. Technol. Food Ind. 2023, 44, 369–378. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, J.; Chen, X.; Chen, D.; Deng, S. Use of Relative Odor Activity Value (roav) to Link Aroma Profiles to Volatile Compounds: Application to Fresh and Dried Eel (Muraenesox cinereus). Int. J. Food Prop. 2020, 23, 2257–2270. [Google Scholar] [CrossRef]
- Li, J.-L.; Yang, Y.; Li, W.; Chen, W.-C.; Liu, X.-F. Aroma Change and Its Relationship with Key Enzymatic Reactions in Drying Process of Stropharia rugosoannulata. J. Food Sci. Technol. 2023, 41, 30–42. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, F.; Tang, P.; Huang, D.; Li, Q.; Lin, M. Widely Targeted Metabolomics Analysis of the Changes to Key Non-volatile Taste Components in Stropharia rugosoannulata under Different Drying Methods. Front. Nutr. 2022, 9, 884400. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Shang, X.-D.; Wang, X.-Y.; Wang, C.-H.; Liu, J.-H.; Chen, W.-Z.; Wang, R.-J.; Xu, N. Comparison of Volatile Components in Different Parts of Fruiting Body of Stropharia rugoso-annulata. Mod. Food Sci. Technol. 2022, 38, 271–281+196. [Google Scholar] [CrossRef]
- Li, W.; Chen, W.-C.; Ma, H.-L.; Wu, D.; Zhang, Z.; Yang, Y. Ultrasonic Preparation of Stropharia rugosoannulata Peptides and Analysis of Their Taste Characteristics and Pharmacological Activities. Acta Edulis Fungi 2022, 29, 81–94. [Google Scholar] [CrossRef]
- Li, W.; Chen, W.-C.; Wu, D.; Zhang, Z.; Yang, Y. Taste peptides derived from Stropharia rugosoannulata fermentation mycelium and molecular docking to the taste receptor T1R1/T1R3. Front. Nutr. 2022, 9, 960218. [Google Scholar] [CrossRef]
- Chen, W.; Li, W.; Wu, D.; Zhang, Z.; Chen, H.; Zhang, J.; Wang, C.; Wu, T.; Yang, Y. Characterization of Novel Umami-active Peptides from Stropharia rugoso-annulata Mushroom and in Silico Study on Action Mechanism. J. Food Compos. Anal. 2022, 110, 104530. [Google Scholar] [CrossRef]
- Li, N.; Prescott, J.; Wu, Y.; Barzi, F.; Yu, X.; Zhao, L.; Neal, B. The Effects of a Reduced-sodium, High-potassium Salt Substitute on Food Taste and Acceptability in Rural Northern China. Br. J. Nutr. 2008, 101, 1088–1093. [Google Scholar] [CrossRef]
- Gu, Z.; Yang, Y. Research progress in flavor components of edible fungus. Sci. Technol. Food Ind. 2013, 34, 363–367. [Google Scholar] [CrossRef]
- Yin, C.-M.; Fan, X.-Z.; Shi, D.-F.; Fan, Z.; Cheng, W.; Gao, H. Flavor Compounds Analysis of 5 Fresh Mushrooms Using HS-SPME-GC-MS and HPLC. Sci. Technol. Food Ind. 2019, 40, 254–260. [Google Scholar] [CrossRef]
- Yu, C.-X.; Zhao, Y.; Chen, M.-J.; Wang, H.; Li, Z.-P.; Pan, G.-F.; Feng, A.-P. Analysis of volatile of flavor components in Volvariella volvacea fruiting bodies cultivated on different substrates. Acta Edulis Fungi 2019, 26, 37–44. [Google Scholar] [CrossRef]
- Anon. Biology of Sulfur; CRC Press: Boca Raton, FL, USA, 1996; pp. 30–51. [Google Scholar]
- Bowman, S.M.; Free, S.J. The Structure and Synthesis of the Fungal Cell Wall. Bioessays 2006, 28, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Deguchi, S.; Tsujii, K.; Horikoshi, K. In Situ Microscopic Observation of Chitin and Fungal Cells with Chitinous Cell Walls in Hydrothermal Conditions. Sci. Rep. 2015, 5, 11907. [Google Scholar] [CrossRef] [PubMed]
- Alimi, B.A.; Pathania, S.; Wilson, J.; Duffy, B.; Frias, J.M.C. Extraction, Quantification, Characterization, and Application in Food Packaging of Chitin and Chitosan from Mushrooms: A Review. Int. J. Biol. Macromol. 2023, 237, 124195. [Google Scholar] [CrossRef] [PubMed]
- Jedidi, I.K.; Ayoub, I.K.; Philippe, T.; Bouzouita, N. Chemical Composition and Nutritional Value of Three Tunisian Wild Edible Mushrooms; Springer Science and Business Media LLC: New York, NY, USA, 2017; Volume 11, pp. 2069–2075. [Google Scholar]
- DD ISO/TS 17837: 2008; Milk and Milk Products. Determination of Nitrogen Content and Crude Protein Calculation. Kjeldahl Method. BSI British Standards: London, UK, 2018.
- Cheung, P.C.K. The Nutritional and Health Benefits of Mushrooms. Nutr. Bull. 2010, 35, 292–299. [Google Scholar] [CrossRef]
- Elisashvili, V. Submerged Cultivation of M edicinal Mushrooms: Bioprocesses and Products (review). Int. J. Med. Mushrooms 2012, 14, 211–239. [Google Scholar] [CrossRef]
- Li, Y.-R.; Chen, Z.-L.; Wen, L.-H.; Sun, C.-Q.; Li, R.-J.; Lin, M.; Chen, X.-J.; Zhang, C.-R.; Meng, F.-B.; Huang, D.-M.; et al. Pollution-free cultivation technology of Stropharia rugosoannulata mushrooms in Guizhou. Agro-Tech. Serv. 2020, 37, 52–54+56. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).