Four Novel Species and Two New Records of Boletes from India
Abstract
1. Introduction
2. Materials and Methods
2.1. Macrofungal Survey and Morphological Study
2.2. Genomic DNA Extraction, PCR Amplification and Sequencing
2.3. Phylogenetic Analysis
3. Results
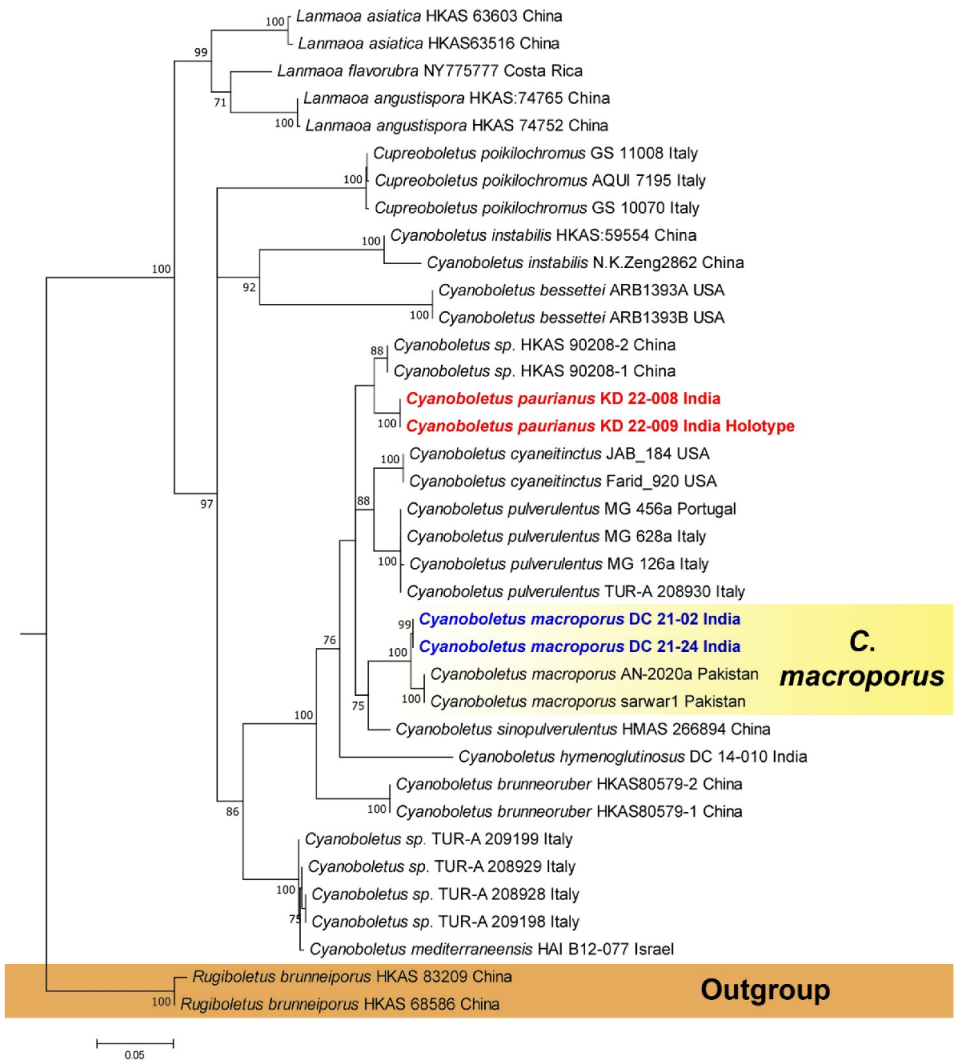
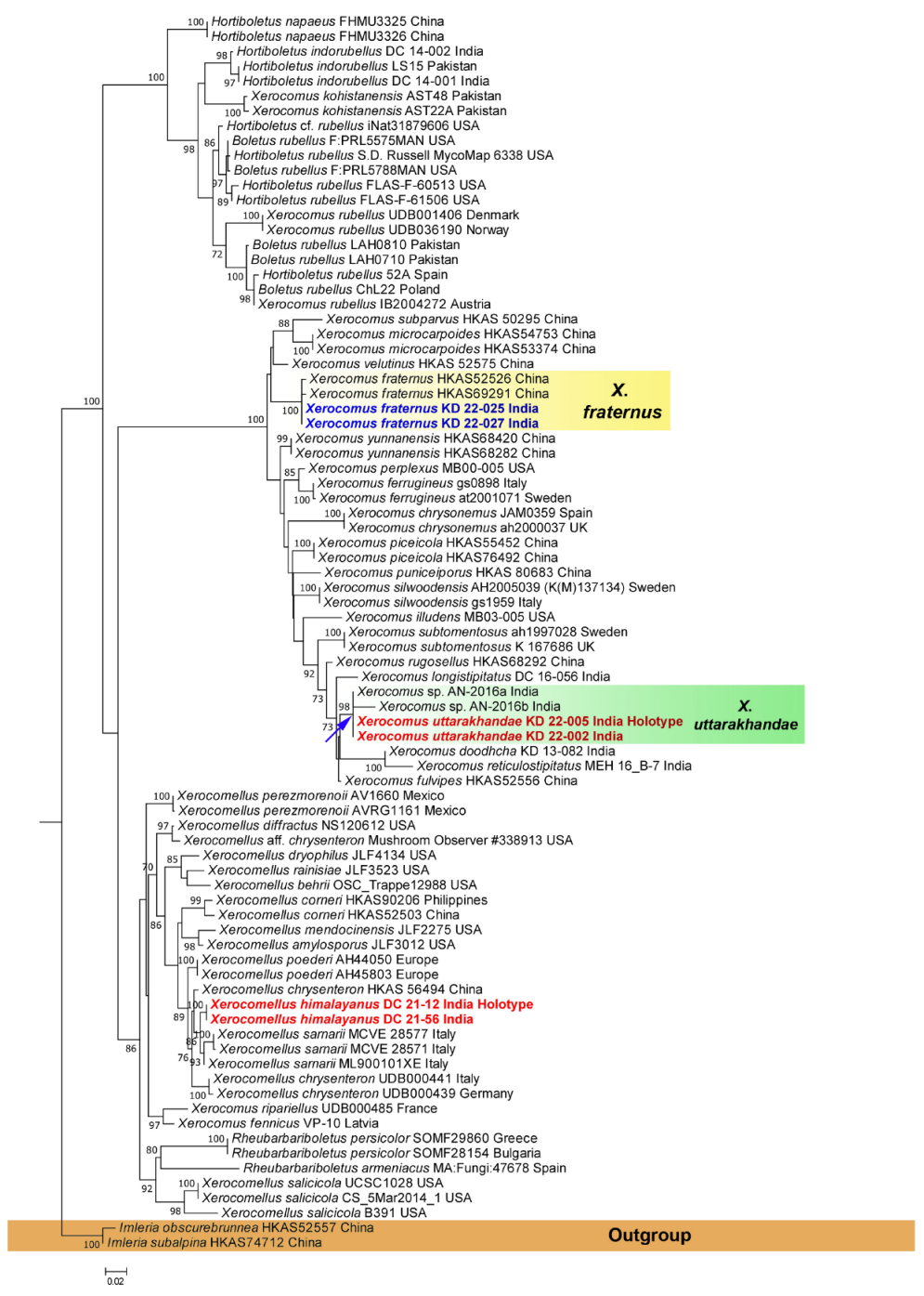
3.1. Phylogenetic Inferences
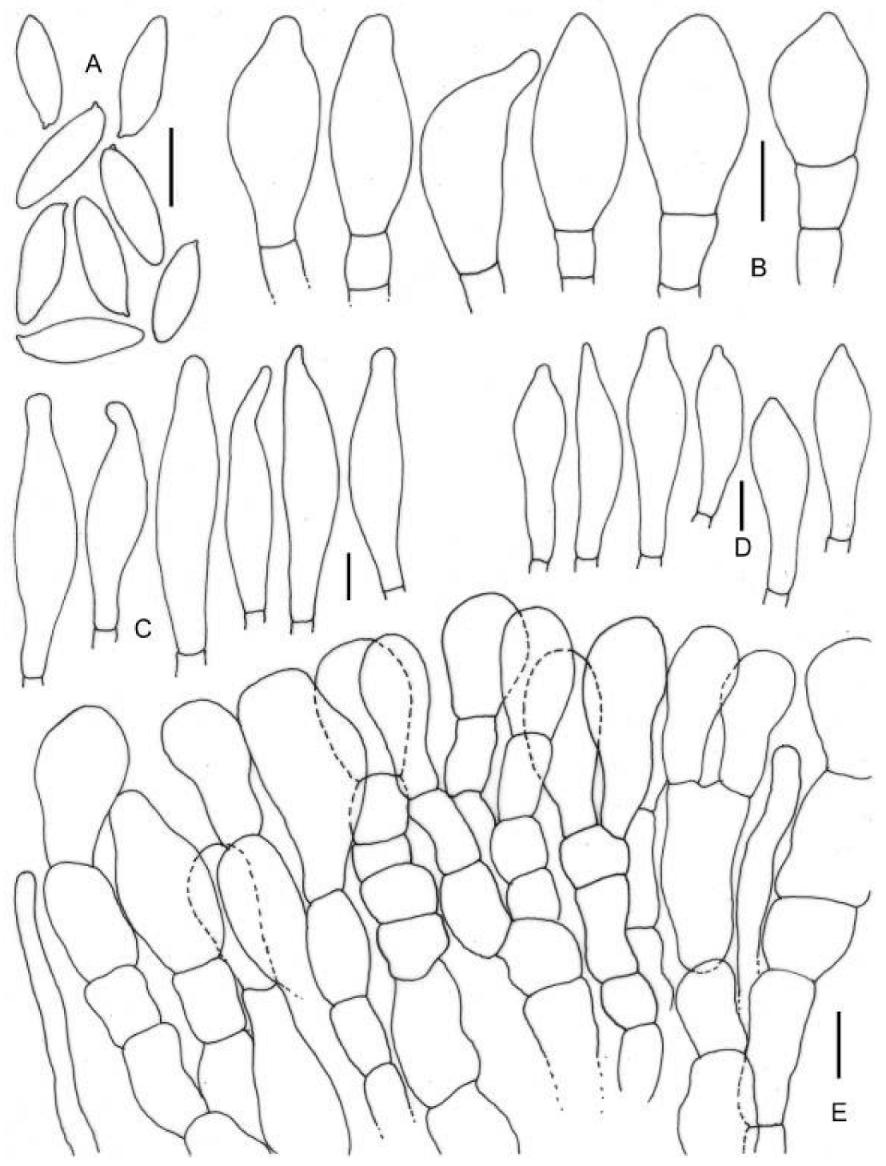
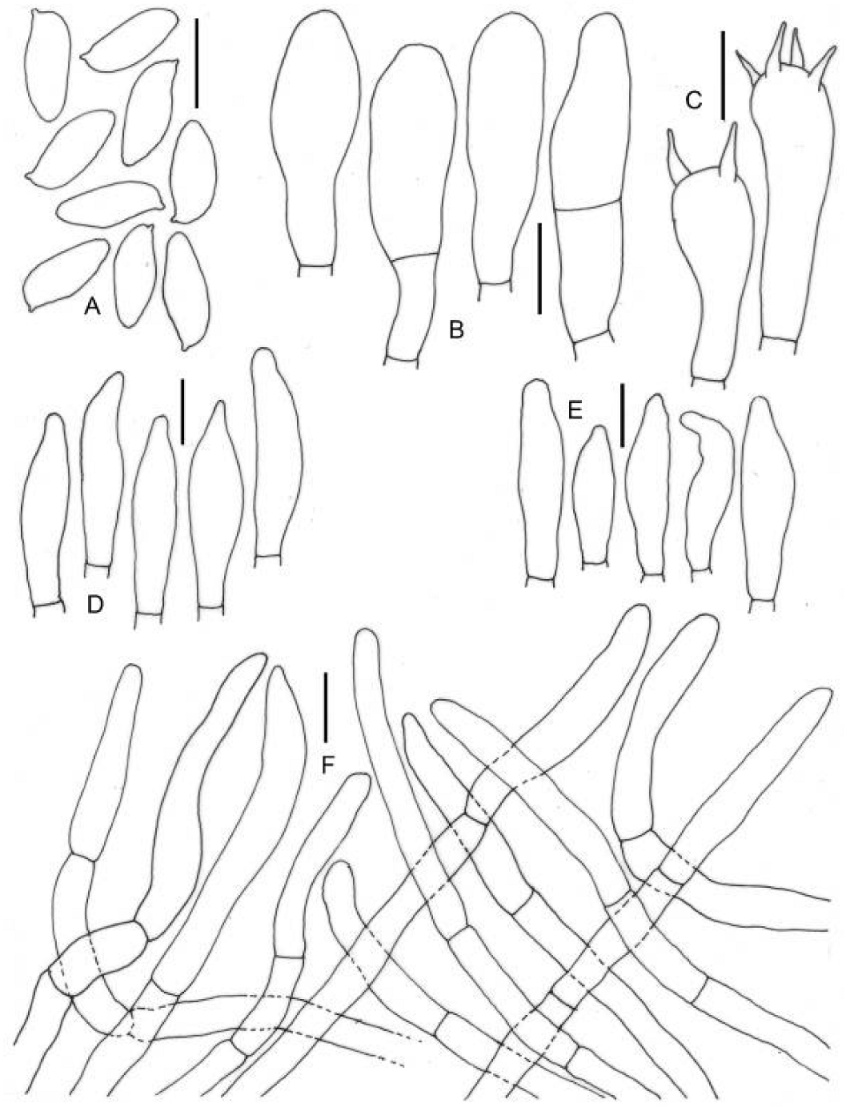
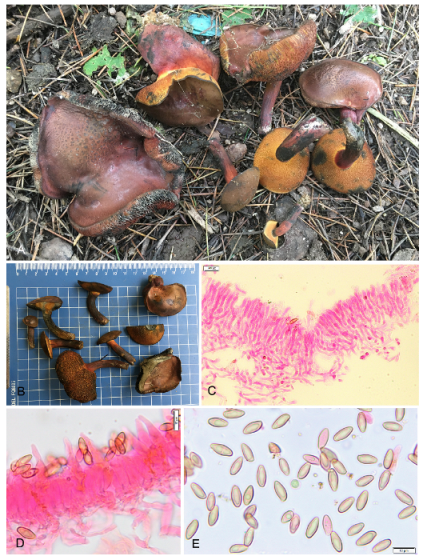
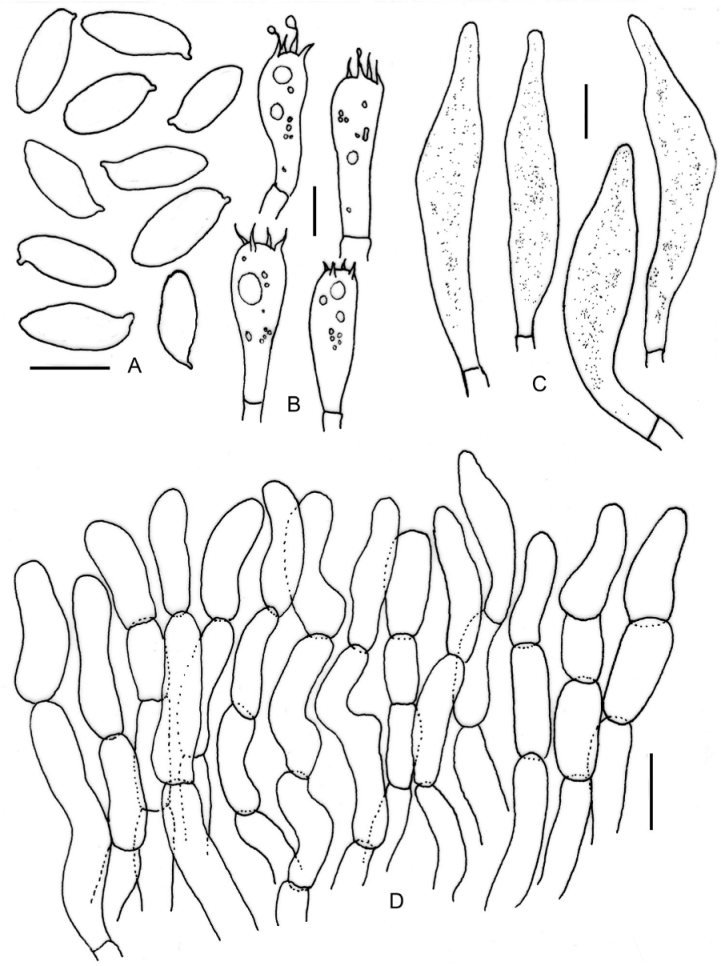
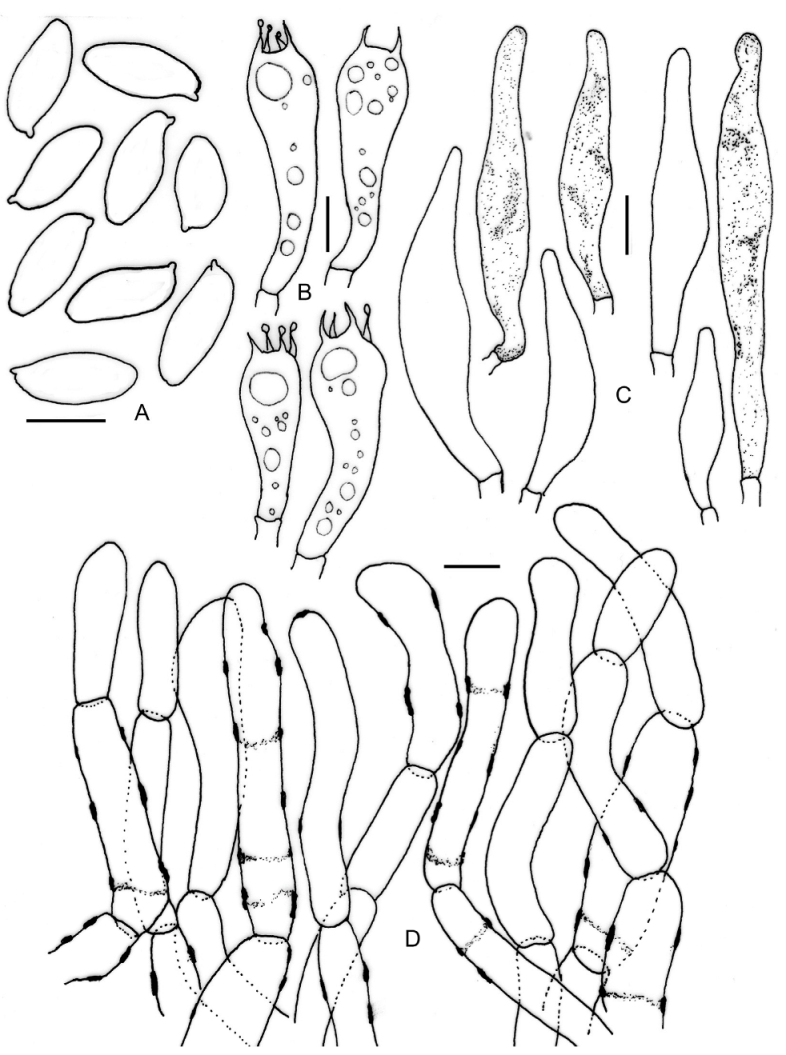
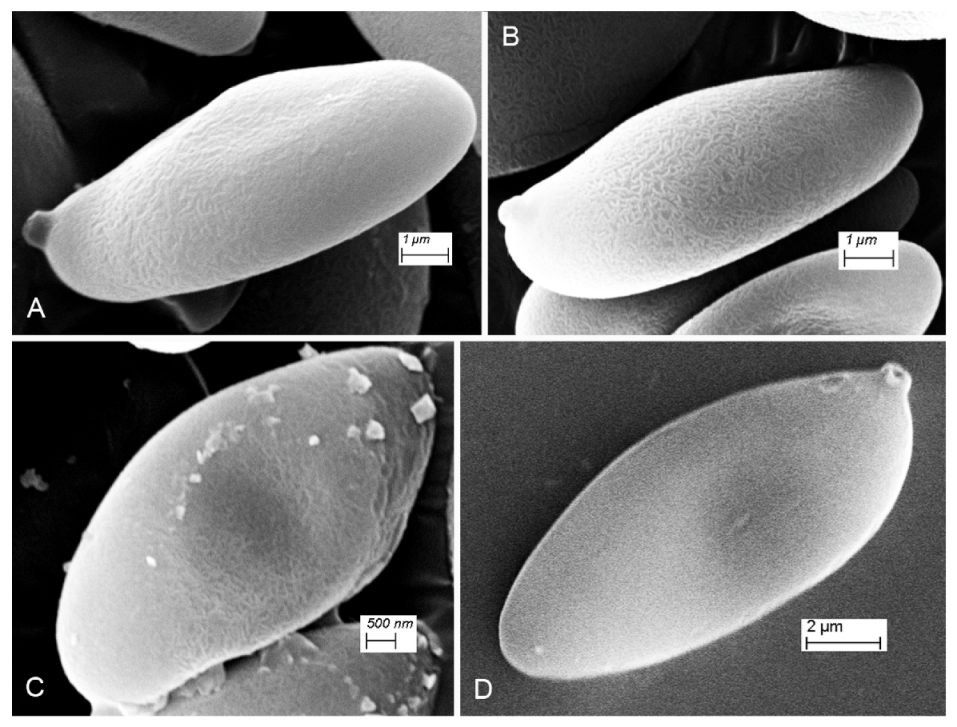
3.2. Taxonomy
4. Discussion
Key to the Studied Genera of Boletes
- Stipe surface with scabs that turns brownish black when bruised; stipe context is cream colored, changing brown-black when exposed ……………………Leccinellum
- 1a.
- Basidioma with different combinations of features ……………………………2
- 2
- Pileus surface is sticky; pileus surface, pore surface, stipe surface, and context turn dark blue instantly when bruised or exposed ……………………………Cyanoboletus
- 2a.
- Pileus surface velvety; only pore surface turns blue slowly when bruised …3
- 3
- Pileipellis as a trichodermium; spores with bacillate ornamentation (under SEM) …….………………………………………………………………………Xerocomus
- 3a.
- Pileipellis as a palisadoderm; spores smooth ………………………Xerocomellus.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nuhn, M.E.; Binder, M.; Taylor, A.F.; Halling, R.E.; Hibbett, D.S. Phylogenetic overview of the Boletineae. Fungal Biol. 2013, 117, 479–511. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Feng, B.; Xu, J.; Zhu, X.-T.; Li, Y.-C.; Zeng, N.-K.; Hosen, M.I.; Yang, Z.L. Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Divers. 2014, 69, 93–115. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, K.; Li, Y.-C.; Zeng, N.-K.; Feng, B.; Halling, R.E.; Yang, Z.L. Four new genera of the fungal family Boletaceae. Fungal Divers. 2015, 81, 1–24. [Google Scholar] [CrossRef]
- He, M.-Q.; Zhao, R.-L.; Hyde, K.D.; Begerow, D.; Kemler, M.; Yurkov, A.; McKenzie, E.H.C.; Raspé, O.; Kakishima, M.; Sánchez-Ramírez, S.; et al. Notes, outline and divergence times of Basidiomycota. Fungal Divers. 2019, 99, 105–367. [Google Scholar] [CrossRef]
- Vadthanarat, S.; Lumyong, S.; Raspé, O. Cacaoporus, a new Boletaceae genus, with two new species from Thailand. MycoKeys 2019, 54, 1–29. [Google Scholar] [CrossRef]
- Vadthanarat, S.; Raspé, O.; Lumyong, S. Rubinosporus auriporus gen. et sp. nov. (Boletaceae: Xerocomoideae) from Tropical Forests of Thailand, Producing Unusual Dark Ruby Spore Deposits. J. Fungi 2022, 8, 278. [Google Scholar] [CrossRef]
- Farid, A.; Bessette, A.E.; Bessette, A.R.; Bolin, J.A.; Kudzma, L.V.; Franck, A.R.; Garey, J.R. Investigations in the boletes (Boletaceae) of southeastern USA: Four novel species, and three novel combinations. Mycosphere 2021, 12, 1038–1076. [Google Scholar] [CrossRef]
- Hosen, M.I.; Yang, Z.L. Kaziboletus, a new boletoid genus of Boletaceae associated with Shorea robusta in Bangladesh. Mycol. Prog. 2021, 20, 1145–1156. [Google Scholar] [CrossRef]
- Magnago, A.C.; Alves-Silva, G.; Henkel, T.W.; Borges da Silveira, R.M. New genera, species, and combinations of Boletaceae from Brazil and Guyana. Mycologia 2022, 114, 607–625. [Google Scholar] [CrossRef]
- Mao, N.; Zhao, T.Y.; Xu, Y.Y.; Fan, L. Villoboletus persicinus, gen. et sp. nov. (Boletaceae), a bolete with flocculent-covered stipe from northern China. Mycologia 2023, 115, 255–262. [Google Scholar] [CrossRef]
- Lakhanpal, T.N. Mushrooms of India, Boletaceae Vol. I; A.P.H. Publishing Corporation: New Delhi, India, 1996; pp. 1–170. [Google Scholar]
- Das, K.; Sharma, J.R. Russulaceae of Kumaon Himalaya; Botanical Survey of India, Govt. of India: Kolkata, India, 2005; pp. 1–255. [Google Scholar]
- Joshi, S.; Bhatt, R.P.; Stephenson, S.L. The current status of the family Russulaceae in the Uttarakhand Himalaya, India. Mycosphere 2012, 3, 486–501. [Google Scholar] [CrossRef]
- Sharma, S.; Atri, N.S.; Saini, M.K.; Verma, B. Catalogue of Russulaceous Mushrooms of India. Nova Hedwig. 2018, 106, 357–401. [Google Scholar] [CrossRef]
- Kumar, A.; Mehmood, T.; Atri, N.; Sharma, Y.P. Revised and an updated checklist of the Amanitaceae from India with its specific distribution in the Indian States. Nova Hedwig. 2021, 12, 223–240. [Google Scholar] [CrossRef]
- Ghosh, A.; Das, K. Russula (Russulaceae) in western Himalaya 1: Two new species from subg. Russula. Phytotaxa 2017, 323, 237–252. [Google Scholar] [CrossRef]
- Hosen, M.I.; Mehmood, T.; Das, K.; Kudzma, L. Amanita tullossiana, a new species, and two new records of Amanita section Lepidella from north-western Himalaya, India. MycoKeys 2018, 37, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, T.; Das, K.; Hosen, M.I.; Bhatt, R.P.; Uniyal, P.; Rana, U. Two new species of Amanita (Amanitaceae) from North-western Himalaya, India. Phytotaxa 2018, 367, 219–232. [Google Scholar] [CrossRef]
- Uniyal, P.; Nuytinck, J.; Das, K. Lactarius subg. Lactarius (Russulaceae) in Indian Himalaya: Two New Species with Morphology and Phylogenetic Inferences. Cryptogam. Mycol. 2018, 39, 467–482. [Google Scholar] [CrossRef]
- Uniyal, P.; Das, K.; Bhatt, R.P. Lactarius pleuromacrocystidiatus (Russulaceae), a novel species from India. Kew Bull. 2019, 74, 8. [Google Scholar] [CrossRef]
- Ghosh, A.; Das, K.; Bhatt, R.P.; Hembrom, M.E. Two new species of the Genus Russula from western Himalaya with morphological details and phylogenetic estimations. Nova Hedwig. 2020, 111, 115–130. [Google Scholar] [CrossRef]
- Ghosh, A.; Das, K.; Chakraborty, D. Morphology and molecular approach reveal a new species of the genus Russula subsect. Lepidinae (Russulaceae) from India. Phytotaxa 2021, 483, 244–254. [Google Scholar] [CrossRef]
- Kukreti, S.; Vishwakarma, M.P.; Bhatt, R.P. Diversity, distribution and ecology of boletoid mushrooms from Garhwal Himalaya, Uttarakhand. J. Mt. Res. 2020, 15, 201–207. [Google Scholar] [CrossRef]
- Kornerup, A.; Wanscher, J.H. Methuen Handbook of Colour, 3rd ed.; Eyre Methuen: London, UK, 1967; pp. 1–252. [Google Scholar]
- Doyle, J. DNA protocols for plants. In Molecular Techniques in Taxonomy; Hewitt, G.M., Johnston, A.W.B., Young, J.P.W., Eds.; Springer: Berlin, Heidelberg, Gernamny, 1991; pp. 283–293. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Sninsky, J., White, T., Eds.; Academic Press Inc.: New York, NY, USA, 1990; p. 315. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Liu, Y.L.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Matheny, P.B. Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Mol. Phylogenet. Evol. 2005, 35, 1–20. [Google Scholar] [CrossRef]
- Rehner, S.A.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-a sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef]
- Drummond, A.J.; Ashton, B.; Buxton, S.; Cheung, M.; Cooper, A.; Heled, J.; Kearse, M.; Moir, R.; Stones-Havas, S.; Sturrock, S.; et al. Geneious v 5.1. 2010. Available online: https://www.geneious.com (accessed on 20 April 2023).
- Wu, G.; Li, Y.-C.; Zhu, X.-T.; Zhao, K.; Han, L.-H.; Cui, Y.-Y.; Li, F.; Xu, J.-P.; Yang, Z.L. One hundred noteworthy boletes from China. Fungal Divers. 2016, 81, 25–188. [Google Scholar] [CrossRef]
- Chakraborty, D.; Parihar, A.; Mehta, N.; Baghela, A.; Das, K. A new species of Xerocomus (Boletaceae) from India. Mycosphere 2017, 8, 44–50. [Google Scholar] [CrossRef]
- Das, K.; Chakraborty, D.; Baghela, A.; Singh, S.K.; Dentinger, B.T.M. New species of xerocomoid boletes (Boletaceae) from Himalayan India based on morphological and molecular evidence. Mycologia 2016, 108, 753–764. [Google Scholar] [CrossRef]
- Das, K.; Ghosh, A.; Chakraborty, D.; Li, J.; Qui, L.; Baghela, A.; Halama, M.; Hembrom, M.E.; Mehmood, T.; Parihar, A.; et al. Fungal Biodiversity Profiles 31–40. Cryptogam. Mycol. 2017, 38, 353–406. [Google Scholar] [CrossRef]
- Xue, R.; Wu, L.-L.; Jiang, S.; Hao, Y.-J.; Chai, H.; Liang, Z.-Q.; Zeng, N.-K.; Su, M.-S. Two new species of the genus Leccinellum (Boletaceae, Boletales) from the south of China. Phytotaxa 2019, 411, 93–104. [Google Scholar] [CrossRef]
- Meng, X.; Wang, G.-S.; Wu, G.; Wang, P.-M.; Yang, Z.L.; Li, Y.-C. The Genus Leccinum (Boletaceae, Boletales) from China Based on Morphological and Molecular Data. J. Fungi 2021, 7, 732. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, R.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]
- Bresinsky, A.; Besl, H. Schlüssel zur Gattungsbestimmung der Blätter-, Leisten-und Röhrenpilze mit Literaturhinweisen zur Artbestimmung. Regensbg. Mykol. Schriften 2003, 11, 5–236. [Google Scholar]
- Kuo, M.; Methven, A.S.; Minnis, A.M.; Halling, R.E. Studies of North American macrofungi, 1. Validation of Lactarius rubidus comb. nov. and Leccinellum quercophilum sp. nov. Mycotaxon 2013, 124, 323–332. [Google Scholar] [CrossRef]
- den Bakker, H.C.; Noordeloos, M.E. A revision of European species of Leccinum Gray and notes on extralimital species. Persoonia 2005, 18, 511–587. [Google Scholar]
- Della Maggiora, M. Nomenclatural novelties. Index Fungorum 2014, 246, 1. [Google Scholar]
- Li, F.; Zhao, K.; Deng, Q.L.; Zhang, M.; Staehelin, C.; Chen, X.X.; Chen, H.Y.; Wang, G.S.; Li, B.S. Three new species of Boletaceae from the Heishiding Nature Reserve in Guangdong Province, China. Mycol. Prog. 2016, 15, 1269–1283. [Google Scholar] [CrossRef]
- Terashima, Y.; Takahashi, H.; Taneyama, Y. The Fungal Flora in Southwestern Japan: Agarics and Boletes; Tokai University Press: Tokyo, Japan, 2016; p. 303. [Google Scholar]
- Mikšík, M. Nomenclatural novelties. Index Fungorum 2017, 338, 1. [Google Scholar]
- Blanco-Dios, J.B. Nomenclatural novelties. Index Fungorum 2018, 383, 1. [Google Scholar]
- Gelardi, M.; Vizzini, A.; Ercole, E.; Voyron, S.; Sun, J.-Z.; Liu, X.-Z. Boletus sinopulverulentus, a new species from Shaanxi Province (central China) and notes on Boletus and Xerocomus. Sydowia 2013, 65, 45–57. [Google Scholar]
- Sarwar, S.; Naseer, A.; Khalid, A.N. Cyanoboletus macroporus (Boletaceae), a new bolete species from Pakistani forests. Karstenia 2021, 59, 78–87. [Google Scholar] [CrossRef]
- Smith, A.H.; Thiers, H.D. The Boletes of Michigan; University of Michigan Press: Ann Arbor, MI, USA, 1971; p. 428. [Google Scholar]
- Ladurner, H.; Simonini, G. Xerocomus s.l. Fungi Europaei. Vol. 8; Edizioni Candusso: Alassio, Italy, 2003. [Google Scholar]
- Šutara, J. Xerocomus s. l. in the light of the present state of knowledge. Czech Mycol. 2008, 60, 29–62. [Google Scholar] [CrossRef]
- Ariyawansa, H.A.; Hyde, K.D.; Jayasiri, S.C.; Buyck, B. & al. Fungal diversity notes 111–252—Taxonomic and phylogenetic contributions to fungal taxa. Fungal Diver. 2015, 75, 178–182. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Richardson, D.M.; Leroux, J.J.; Strasberg, D.; Edwards, J.; Roets, F.; Hubka, V.; Taylor, P.W.J.; Heykoop, M.; et al. Fungal Planet description sheets: 400–468. Persoonia 2016, 36, 434–435. [Google Scholar] [CrossRef]
- Breitenbach, J.; Kränzlin, F. Fungi of Switzerland 3; Boletales and Agaricales Mykologia: Luzern, Switzerland, 1991; pp. 1–361. [Google Scholar]
- Frank, J.L.; Siegel, N.; Schwarz, C.F.; Araki, B.; Vellinga, E.C. Xerocomellus (Boletaceae) in western North America. Fungal Syst. Evol. 2020, 6, 265–288. [Google Scholar] [CrossRef]
- Verma, B.; Reddy, M.S. Suillus indicus sp. nov. (Boletales, Basidiomycota), a new boletoid fungus from northwestern Himalayas, India. Mycology 2015, 6, 35–41. [Google Scholar] [CrossRef]
- Das, K.; Chakraborty, D.; Cotter, H.V.T. Suillus adhikarii: A new species from the subalpine Himalaya of India and Nepal associated with Larix. Phytotaxa 2015, 219, 289–295. [Google Scholar] [CrossRef]
- Das, K.; Chakraborty, D.; Vizzini, A. Morphological and phylogenetic evidences unveil a novel species of Gyroporus (Gyroporaceae, Boletales) from Indian Himalaya. Nordic J. Bot. 2017, 35, 669–675. [Google Scholar] [CrossRef]
- Chakraborty, D.; Vizzini, A.; Das, K. Two new species and a new record of the genus Tylopilus (Boletaceae) from Indian Himalaya with morphological details and phylogenetic estimations. MycoKeys 2017, 33, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Das, K.; Lakhanpal, T.N. Reappraisal in the family Boletaceae in Indian Himalaya—Present scenario and future challenges. In Taxonomy: Theory and Practice; Maity, D., Ed.; Ruby Das: Hooghly, India, 2018. [Google Scholar]
- Chakraborty, D.; Gelardi, M.; Hembrom, M.E.; Ghosh, A. First records of Tylopilus glutinosus Iqbal Hosen (Boletaceae) from Shorea robusta dominated forests in tropical India: Morphological description and phylogenetic estimation. Check List 2022, 18, 553–562. [Google Scholar] [CrossRef]
- Parihar, A.; Hembrom, M.E.; Vizzini, A.; Das, K. Indoporus shoreae gen. et sp. nov. (Boletaceae) from tropical India. Cryptogam. Mycol. 2018, 39, 447–466. [Google Scholar] [CrossRef]
- Parihar, A.; Hembrom, M.E.; Vizzini, A.; Das, K. A new species of Boletellus (Boletaceae, Basidiomycota) from tropical India. Nordic J. Bot. 2018, 36. [Google Scholar] [CrossRef]
- Patil, P.B.; Gunasekaren, S.; Singh, S.K.; Vaidya, S. Parvixerocomus matheranensis (Boletaceae), a new species from India. Mycoscience 2021, 62, 244–249. [Google Scholar] [CrossRef]








| Species Name (as Reported in GenBank) | Voucher No. | GenBank Accession No. | ||
|---|---|---|---|---|
| nrLSU | rpb2 | tef1-α | ||
| Borofutus dhakanus | HKAS73789 | JQ928616 | JQ928597 | JQ928576 |
| Leccinellum albellum | MICH KUO-07241101 | MK601746 | MK766308 | MK721100 |
| Leccinellum alborufescens | FHMU1908 | MK816322 | MK816333 | MK816330 |
| Leccinellum alborufescens | FHMU1758 | MK816321 | MK816332 | MK816329 |
| Leccinellum binderi | KD 22-015 | OQ858379 | OQ914387 | OR102316 |
| Leccinellum binderi | KD 22-007 | OQ858380 | OQ914386 | OR102315 |
| Leccinellum corsicum | Buf 4507 | KF030347 | KF030389 | KF030435 |
| Leccinellum crocipodium | MICH KUO-07050707 | MK601749 | MK766311 | MK721103 |
| Leccinellum fujianense | FHMU2219 | MK816319 | MK816334 | MK816327 |
| Leccinellum fujianense | FHMU2223 | MK816320 | MK816336 | MK816328 |
| Leccinellum indoaurantiacum | DC 14-019 | KT860059 | — | — |
| Leccinellum lepidum | K(M)-142974 | MK601751 | MK766312 | MK721105 |
| Leccinellum pseudoscabrum | CFMR:DPL-11432 | MK601752 | MK766313 | MK721106 |
| Leccinellum sp. | OR0082 | — | MZ824749 | MZ803024 |
| Leccinum aff. griseum | KPM-NC-0017381 | JN378508 | — | JN378449 |
| Leccinum aff. scabrum | HKAS 57266 | KF112442 | KF112722 | KF112248 |
| Leccinum album | Li1072 | MW413907 | — | MW439267 |
| Leccinum aurantiacum | L:0342207 | MK601759 | MK766318 | MK721113 |
| Leccinum cerinum | MK11800 | AF139692 | — | — |
| Leccinum duriusculum | GL4676 | AF139699 | — | — |
| Leccinum duriusculum | Yang5971 | MZ675541 | MZ707779 | MZ707785 |
| Leccinum flavostipitatum | MENMB10801 | MH620342 | — | — |
| Leccinum holopus | Yang5972 | MW413906 | MW439258 | MW439266 |
| Leccinum holopus | 9109303 | AF139700 | — | — |
| Leccinum holopus | MICH: KUO-09150707 | MK601763 | MK766322 | MK721117 |
| Leccinum manzanitae | NY-14041 REH-6717 | MK601765 | MK766324 | MK721119 |
| Leccinum monticola | HKAS:76669 | KF112443 | KF112723 | KF112249 |
| Leccinum monticola | NY-00815448 REH-8591 | MK601767 | MK766326 | MK721121 |
| Leccinum monticola | NY-760388 REH-8288 | MK601766 | MK766325 | MK721120 |
| Leccinum palustre | MK11107 | AF139701 | — | — |
| Leccinum parascabrum | Li1700 | MW413912 | MW439265 | MW439272 |
| Leccinum parascabrum | Wu1784 | MW413911 | MW439264 | MW439271 |
| Leccinum pseudoborneense | WGS965 | — | MW439263 | — |
| Leccinum pseudoborneense | WGS960 | — | MW439262 | — |
| Leccinum pseudoborneense | WGS947 | MW413908 | MW439261 | MW439268 |
| Leccinum rugosiceps | CFMR BOS-866 | MK601770 | MK766329 | MK721124 |
| Leccinum scabrum | HKAS56371 | KT990587 | KT990423 | KT990782 |
| Leccinum scabrum | KPM-NC-0017840 | JN378515 | — | JN378455 |
| Leccinum variicolor | Lvar1 | AF139706 | — | — |
| Leccinum versipelle | FB27 | MZ675546 | MZ707782 | MZ707790 |
| Leccinum versipelle | LJW418 | MZ675545 | MZ707781 | MZ707789 |
| Leccinum versipelle | CFMR DLC2002-122 | MK601778 | MK766336 | MK721132 |
| Octaviania japonimontana | KPM-NC-0017812 | JN378486 | — | JN378428 |
| Octaviania tasmanica | NY-02449788 REH-10066 | MK601798 | MK766355 | MK721152 |
| Rossbeevera bispora | GDGM 45639 | MK036347 | MK350309 | — |
| Rossbeevera eucyanea | KPM-NC0023895 | KP222896 | — | KP222915 |
| Rossbeevera griseobrunnea | GDGM45913 | MH537793 | — | — |
| Rossbeevera griseovelutina | TNS-F-36991 | KC552032 | — | KC552077 |
| Rossbeevera vittatispora | MEL2321058 | KP222895 | — | KP222911 |
| Rossbeevera westraliensis | OSC61480 | JN378505 | — | JN378445 |
| Spongiforma thailandica | BBH:DED 7873 | NG_042464 | — | — |
| Species Name (as Reported in GenBank) | Voucher No. | GenBank Accession No. | ||
|---|---|---|---|---|
| nrITS | nrLSU | rpb2 | ||
| Cupreoboletus poikilochromus | GS 10070 | KT157051 | KT157060 | KT157068 |
| Cupreoboletus poikilochromus | GS 11008 | KT157050 | KT157059 | KT157067 |
| Cupreoboletus poikilochromus | AQUI 7195 | KT157052 | KT157061 | — |
| Cyanoboletus bessettei | ARB1393B | MW675738 | — | MW737458 |
| Cyanoboletus bessettei | ARB1393A | MW675737 | MW662571 | MW737457 |
| Cyanoboletus brunneoruber | HKAS80579-2 | — | KT990569 | KT990402 |
| Cyanoboletus brunneoruber | HKAS80579-1 | — | KT990568 | KT990401 |
| Cyanoboletus cyaneitinctus | JAB_184 | MW675731 | MW662584 | MW737467 |
| Cyanoboletus cyaneitinctus | Farid_920 | MW675744 | MW662579 | MW737465 |
| Cyanoboletus hymenoglutinosus | DC 14-010 | KT907355 | KT860060 | — |
| Cyanoboletus instabilis | N.K.Zeng2862 | MG030473 | MG030466 | — |
| Cyanoboletus instabilis | HKAS:59554 | — | KF112412 | KF112698 |
| Cyanoboletus macroporus | sarwar1 | MW369503 | — | — |
| Cyanoboletus macroporus | AN-2020a | MW045557 | — | — |
| Cyanoboletus macroporus | DC 21-02 | OQ860238 | OQ860239 | ON364552 |
| Cyanoboletus macroporus | DC 21-24 | OQ860240 | OQ860241 | OQ876894 |
| Cyanoboletus mediterraneensis | HAI B12-077 | OM801199 | OM801212 | — |
| Cyanoboletus paurianus | KD 22-008 | — | OQ859920 | OQ914389 |
| Cyanoboletus paurianus | KD 22-009 | — | OQ859919 | OQ914388 |
| Cyanoboletus pulverulentus | MG 126a | KT157053 | KT157062 | — |
| Cyanoboletus pulverulentus | MG 456a | KT157054 | KT157063 | — |
| Cyanoboletus pulverulentus | MG 628a | KT157055 | KT157064 | KT157069 |
| Cyanoboletus pulverulentus | TUR-A 208930 | MZ265186 | — | MZ265200 |
| Cyanoboletus sinopulvirulentus | HMAS266894 | KC579402 | — | — |
| Cyanoboletus sp. | HKAS90208-2 | — | — | KT990405 |
| Cyanoboletus sp. | HKAS90208-1 | — | KT990571 | KT990404 |
| Cyanoboletus sp. | TUR-A 209199 | MZ265183 | MZ265198 | — |
| Cyanoboletus sp. | TUR-A 208928 | MZ265179 | MZ265194 | — |
| Cyanoboletus sp. | TUR-A 209198 | MZ265182 | MZ265197 | — |
| Cyanoboletus sp. | TUR-A 208929 | MZ265181 | MZ265196 | — |
| Lanmaoa angustispora | HKAS:74765 | — | KF112322 | KF112680 |
| Lanmaoa angustispora | HKAS 74752 | — | KM605139 | KM605177 |
| Lanmaoa asiatica | HKAS63516 | — | KT990584 | KT990419 |
| Lanmaoa asiatica | HKAS 63603 | — | KM605143 | KM605176 |
| Lanmaoa flavorubra | NY775777 | — | JQ924339 | KF112681 |
| Rugiboletus brunneiporus | HKAS 83209 | — | KM605134 | KM605168 |
| Rugiboletus brunneiporus | HKAS 68586 | — | KF112402 | KF112719 |
| Species Name (as Reported in GenBank) | Voucher No. | GenBank Accession No. | |
|---|---|---|---|
| nrITS | nrLSU | ||
| Boletus rubellus | F:PRL5575MAN | GQ166888 | — |
| Boletus rubellus | F:PRL5788MAN | GQ166883 | — |
| Boletus rubellus | ChL22 | KX438318 | — |
| Boletus rubellus | LAH0710 | KJ802928 | — |
| Boletus rubellus | LAH0810 | KJ802929 | — |
| Hortiboletus cf. rubellus | iNat31879606 | MN498119 | — |
| Hortiboletus indorubellus | DC 14-002 | KT319647 | — |
| Hortiboletus indorubellus | DC 14-001 | — | KU566807 |
| Hortiboletus indorubellus | LS15 | MK002767 | MK002872 |
| Hortiboletus kohistanensis | AST48 | MG988192 | MG988187 |
| Hortiboletus kohistanensis | AST22A | MG988193 | — |
| Hortiboletus napaeus | FHMU3325 | MT646445 | MT646438 |
| Hortiboletus napaeus | FHMU3326 | MT646440 | MT646433 |
| Hortiboletus rubellus | FLAS-F-61506 | MH211937 | — |
| Hortiboletus rubellus | FLAS-F-60513 | MH211664 | — |
| Hortiboletus rubellus | 52A | MN652008 | — |
| Hortiboletus rubellus | S.D. Russell MycoMap 6338 | MK560106 | — |
| Imleria obscurebrunnea | HKAS52557 | KC215207 | KC215220 |
| Imleria subalpina | HKAS74712 | KC215208 | KC215218 |
| Rheubarbariboletus armeniacus | MA:Fungi:47678 | AJ419221 | — |
| Rheubarbariboletus persicolor | SOMF28154 | MH011932 | — |
| Rheubarbariboletus persicolor | SOMF29860 | MH011931 | — |
| Xerocomellus salicicola | UCSC1028 | KU144793 | KU144794 |
| Xerocomellus aff. chrysenteron | Mushroom Observer #338913 | ON705310 | — |
| Xerocomellus amylosporus | JLF3012 | KM213635 | KU144742 |
| Xerocomellus behrii | OSC_Trappe12988 | KJ882288 | — |
| Xerocomellus chrysenteron | HKAS:56494 | KF112357 | |
| Xerocomellus chrysenteron | — | UDB000441 | — |
| Xerocomellus chrysenteron | — | UDB000439 | — |
| Xerocomellus corneri | HKAS90206 | — | KT990669 |
| Xerocomellus corneri | HKAS52503 | — | KT990668 |
| Xerocomellus diffractus | NS120612 | KM213650 | KM213651 |
| Xerocomellus dryophilus | JLF4134 | KX534076 | KY659593 |
| Xerocomellus himalayanus | DC 21-56 | OQ847959 | OQ847979 |
| Xerocomellus himalayanus | DC 21-12 | OQ847832 | OQ847962 |
| Xerocomellus mendocinensis | JLF2275 | KM213653 | KM213654 |
| Xerocomellus perezmorenoi | AV1660 | OK350679 | OK350681 |
| Xerocomellus perezmorenoi | AVRG1161 | OK350680 | OK350682 |
| Xerocomellus poederi | AH44050 | KU355475 | KU355488 |
| Xerocomellus poederi | AH45803 | KU355480 | KU355491 |
| Xerocomellus rainisiae | JLF3523 | KU144789 | KU144790 |
| Xerocomellus salicicola | B391 | MW675727 | MW662569 |
| Xerocomellus salicicola | CS_5Mar2014_1 | KU144791 | KU144792 |
| Xerocomellus sarnarii | ML900101XE | MH011930 | — |
| Xerocomellus sarnarii | MCVE 28571 | KT271745 | — |
| Xerocomellus sarnarii | MCVE 28577 | KT271749 | — |
| Xerocomus chrysonemus | ah2000037 | DQ066381 | — |
| Xerocomus chrysonemus | JAM0359 | — | KF040544 |
| Xerocomus doodhcha | KD 13-082 | KR611867 | KU566806 |
| Xerocomus fennicus | VP-10 | KT692929 | — |
| Xerocomus ferrugineus | gs0898 | DQ066403 | — |
| Xerocomus ferrugineus | at2001071 | DQ066402 | — |
| Xerocomus fraternus | KD 22-025 | OQ776920 | OQ771932 |
| Xerocomus fraternus | KD 22-027 | OQ776919 | OQ771933 |
| Xerocomus fraternus | HKAS52526 | — | KT990682 |
| Xerocomus fraternus | HKAS69291 | — | KT990683 |
| Xerocomus fulvipes | HKAS52556 | — | KT990672 |
| Xerocomus illudens | MB03-005 | JQ003658 | — |
| Xerocomus longistipitatus | DC 16-056 | KY008398 | — |
| Xerocomus microcarpoides | HKAS54753 | — | KT990680 |
| Xerocomus microcarpoides | HKAS53374 | — | KT990679 |
| Xerocomus perplexus | MB00-005 | JQ003657 | JQ003702 |
| Xerocomus piceicola | HKAS55452 | — | KT990685 |
| Xerocomus piceicola | HKAS76492 | — | KT990684 |
| Xerocomus puniceiporus | HKAS 80683 | — | KU974141 |
| Xerocomus reticulostipitatus | MEH 16_B-7 | MF167353 | — |
| Xerocomus ripariellus | — | UDB000485 | |
| Xerocomus rubellus | — | UDB036190 | — |
| Xerocomus rubellus | — | UDB001406 | — |
| Xerocomus rubellus | IB2004272 | EF644119 | — |
| Xerocomus rugosellus | HKAS68292 | — | KT990686 |
| Xerocomus silwoodensis | AH2005039 (K(M)137134) | DQ438143 | — |
| Xerocomus silwoodensis | gs1959 | DQ066375 | — |
| Xerocomus sp. | AN-2016a | KU761593 | — |
| Xerocomus sp. | AN-2016b | KU761592 | — |
| Xerocomus subparvus | HKAS50295 | — | KT990667 |
| Xerocomus subtomentosus | ah1997028 | DQ066370 | — |
| Xerocomus subtomentosus | K 167686 | JQ967281 | JQ967238 |
| Xerocomus uttarakhandae | KD 22-002 | OQ748035 | OQ748038 |
| Xerocomus uttarakhandae | KD 22-005 | OQ748036 | OQ748037 |
| Xerocomus velutinus | HKAS 52575 | — | KF112393 |
| Xerocomus yunnanensis | HKAS68420 | — | KT990690 |
| Xerocomus yunnanensis | HKAS68282 | — | KT990691 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, K.; Ghosh, A.; Chakraborty, D.; Datta, S.; Bera, I.; Layola MR, R.; Banu, F.; Vizzini, A.; Wisitrassameewong, K. Four Novel Species and Two New Records of Boletes from India. J. Fungi 2023, 9, 754. https://doi.org/10.3390/jof9070754
Das K, Ghosh A, Chakraborty D, Datta S, Bera I, Layola MR R, Banu F, Vizzini A, Wisitrassameewong K. Four Novel Species and Two New Records of Boletes from India. Journal of Fungi. 2023; 9(7):754. https://doi.org/10.3390/jof9070754
Chicago/Turabian StyleDas, Kanad, Aniket Ghosh, Dyutiparna Chakraborty, Sudeshna Datta, Ishika Bera, Ranjith Layola MR, Farheen Banu, Alfredo Vizzini, and Komsit Wisitrassameewong. 2023. "Four Novel Species and Two New Records of Boletes from India" Journal of Fungi 9, no. 7: 754. https://doi.org/10.3390/jof9070754
APA StyleDas, K., Ghosh, A., Chakraborty, D., Datta, S., Bera, I., Layola MR, R., Banu, F., Vizzini, A., & Wisitrassameewong, K. (2023). Four Novel Species and Two New Records of Boletes from India. Journal of Fungi, 9(7), 754. https://doi.org/10.3390/jof9070754







