Innate Pulmonary Phagocytes and Their Interactions with Pathogenic Cryptococcus Species
Abstract
1. Introduction
2. Dendritic Cells
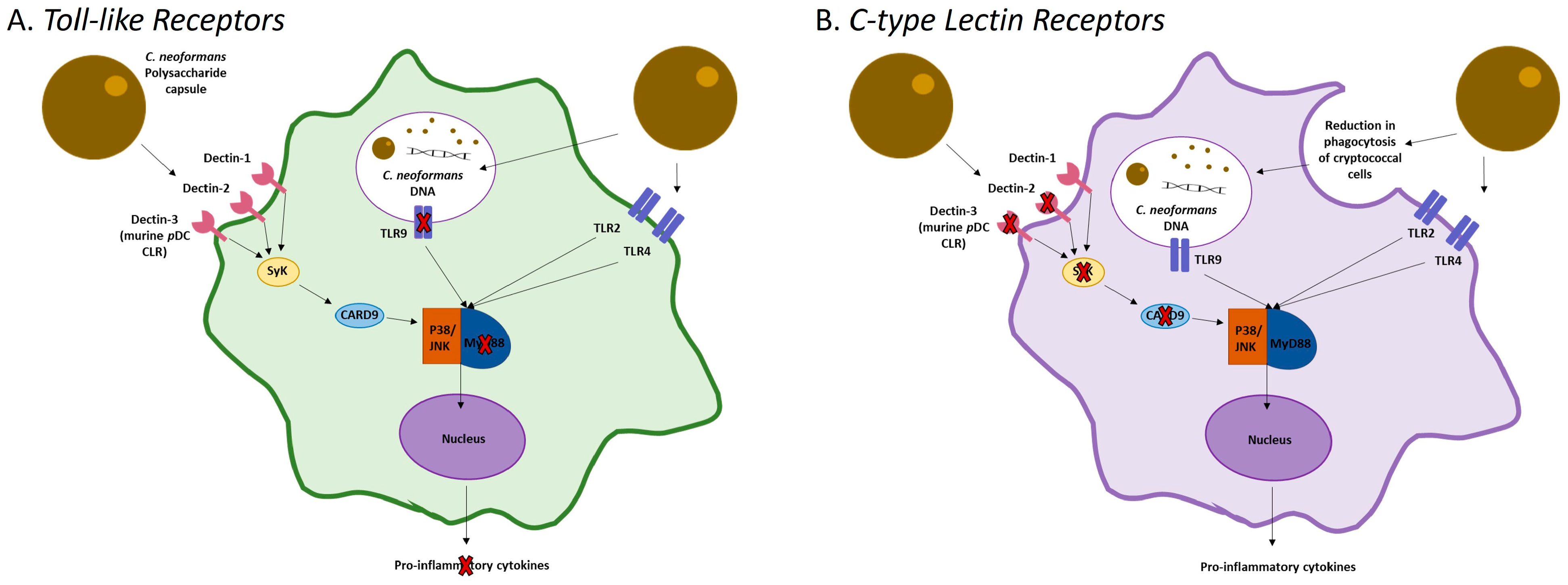
2.1. Recognition and Activation
2.2. Inhibition of DC Maturation
2.3. Pulmonary DC Interactions with C. neoformans
3. Macrophages
3.1. Activation
3.2. Trafficking of C. neoformans
3.3. Pulmonary Macrophage Interactions with C. neoformans
4. Neutrophils
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kwon-Chung, K.J.; Fraser, J.A.; Doering, T.L.; Wang, Z.; Janbon, G.; Idnurm, A.; Bahn, Y.S. Cryptococcus neoformans and Cryptococcus gattii, the Etiologic Agents of Cryptococcosis. Cold Spring Harb. Perspect. Med. 2014, 4, a019760. [Google Scholar] [CrossRef]
- Perfect, J.R.; Casadevall, A. Cryptococcosis. Infect. Dis. Clin. 2002, 16, 837–874. [Google Scholar] [CrossRef]
- Ellis, D.H.; Pfeiffer, T.J. Ecology, life cycle, and infectious propagule of Cryptococcus neoformans. Lancet 1990, 336, 923–925. [Google Scholar] [CrossRef]
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. N. Am. 2016, 30, 179–206. [Google Scholar] [CrossRef]
- Redlich, S.; Ribes, S.; Schütze, S.; Eiffert, H.; Nau, R. Toll-like receptor stimulation increases phagocytosis of Cryptococcus neoformans by microglial cells. J. Neuroinflamm. 2013, 10, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Chrisman, C.J.; Albuquerque, P.; Guimaraes, A.J.; Nieves, E.; Casadevall, A. Phospholipids Trigger Cryptococcus neoformans Capsular Enlargement during Interactions with Amoebae and Macrophages. PLoS Pathog. 2011, 7, e1002047. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.; Casadevall, A. Cell-to-cell spread and massive vacuole formation after Cryptococcus neoformans infection of murine macrophages. BMC Immunol. 2007, 8, 16. [Google Scholar] [CrossRef]
- Rohatgi, S.; Pirofski, L.-A. Host immunity to Cryptococcus neoformans. Futur. Microbiol. 2015, 10, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Mambula, S.S.; Simons, E.R.; Hastey, R.; Selsted, M.E.; Levitz, S.M. Human Neutrophil-Mediated Nonoxidative Antifungal Activity against Cryptococcus neoformans. Infect. Immun. 2000, 68, 6257–6264. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Vissers, M.C.; Kettle, A.J. Myeloperoxidase. Curr. Opin. Hematol. 2000, 7, 53–58. [Google Scholar] [CrossRef]
- Alcouloumre, M.S.; Ghannoum, M.A.; Ibrahim, A.S.; Selsted, M.E.; Edwards, J.E., Jr. Fungicidal properties of defensin NP-1 and activity against Cryptococcus neoformans in vitro. Antimicrob. Agents Chemother. 1993, 37, 2628–2632. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, K.L.; Levitz, S.M. Cryptococcus neoformans Enters the Endolysosomal Pathway of Dendritic Cells and Is Killed by Lysosomal Components. Infect. Immun. 2008, 76, 4764–4771. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Li, S.S.; Oykhman, P.; Timm-McCann, M.; Huston, S.M.; Stack, D.; Xiang, R.F.; Kelly, M.M.; Mody, C.H. An Acidic Microenvironment Increases NK Cell Killing of Cryptococcus neoformans and Cryptococcus gattii by Enhancing Perforin Degranulation. PLoS Pathog. 2013, 9, e1003439. [Google Scholar] [CrossRef]
- Marr, K.J.; Jones, G.J.; Zheng, C.; Huston, S.M.; Timm-McCann, M.; Islam, A.; Berenger, B.M.; Ma, L.L.; Wiseman, J.C.; Mody, C.H. Cryptococcus neoformans Directly Stimulates Perforin Production and Rearms NK Cells for Enhanced Anticryptococcal Microbicidal Activity. Infect. Immun. 2009, 77, 2436–2446. [Google Scholar] [CrossRef]
- Wozniak, K.L.; Vyas, J.M.; Levitz, S.M. In Vivo Role of Dendritic Cells in a Murine Model of Pulmonary Cryptococcosis. Infect. Immun. 2006, 74, 3817–3824. [Google Scholar] [CrossRef]
- Wozniak, K.L.; Kolls, J.K.; Wormley, F.L., Jr. Depletion of neutrophils in a protective model of pulmonary cryptococcosis results in increased IL-17A production by gamma/delta T cells. BMC Immunol. 2012, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Osterholzer, J.J.; Milam, J.E.; Chen, G.-H.; Toews, G.B.; Huffnagle, G.B.; Olszewski, M.A. Role of Dendritic Cells and Alveolar Macrophages in Regulating Early Host Defense against Pulmonary Infection with Cryptococcus neoformans. Infect. Immun. 2009, 77, 3749–3758. [Google Scholar] [CrossRef]
- Nelson, B.N.; Beakley, S.G.; Posey, S.; Conn, B.; Maritz, E.; Seshu, J.; Wozniak, K.L. Antifungal activity of dendritic cell lysosomal proteins against Cryptococcus neoformans. Sci. Rep. 2021, 11, 13619. [Google Scholar] [CrossRef]
- Coelho, C.; Bocca, A.L.; Casadevall, A. The Intracellular Life of Cryptococcus neoformans. Annu. Rev. Pathol. 2014, 9, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Leopold Wager, C.M.; Hole, C.R.; Wozniak, K.L.; Wormley, F.L., Jr. Cryptococcus and Phagocytes: Complex Interactions that Influence Disease Outcome. Front. Microbiol. 2016, 7, 105. [Google Scholar] [CrossRef]
- Mansour, M.K.; Reedy, J.L.; Tam, J.M.; Vyas, J.M. Macrophage–Cryptococcus Interactions: An Update. Curr. Fungal Infect. Rep. 2014, 8, 109–115. [Google Scholar] [CrossRef]
- Heung, L.J. Innate Immune Responses to Cryptococcus. J. Fungi 2017, 3, 35. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, D.; Liu, G.; Wu, H.; Zhou, H.; Shi, M. Real-time in vivo imaging reveals the ability of neutrophils to remove Cryptococcus neoformans directly from the brain vasculature. J. Leukoc. Biol. 2016, 99, 467–473. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Hu, F.; Chen, X.; Zhang, M. Nonlytic exocytosis of Cryptococcus neoformans from neutrophils in the brain vasculature. Cell Commun. Signal 2019, 17, 117. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, K.L. Interactions of Cryptococcus with Dendritic Cells. J. Fungi 2018, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Bauman, S.K.; Nichols, K.L.; Murphy, J.W. Dendritic Cells in the Induction of Protective and Nonprotective Anticryptococcal Cell-Mediated Immune Responses. J. Immunol. 2000, 165, 158–167. [Google Scholar] [CrossRef]
- Yauch, L.E.; Lam, J.S.; Levitz, S.M. Direct Inhibition of T-Cell Responses by the Cryptococcus Capsular Polysaccharide Glucuronoxylomannan. PLoS Pathog. 2006, 2, e120. [Google Scholar] [CrossRef]
- Zaragoza, O.; Rodrigues, M.L.; De Jesus, M.; Frases, S.; Dadachova, E.; Casadevall, A. The capsule of the fungal pathogen Cryptococcus neoformans. Adv. Appl. Microbiol. 2009, 68, 133–216. [Google Scholar] [CrossRef] [PubMed]
- Kozel, T.R.; Gotschlich, E.C. The capsule of cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J. Immunol. 1982, 129, 1675–1680. [Google Scholar] [CrossRef]
- Kozel, T.R.; Pfrommer, G.S.; Guerlain, A.S.; Highison, B.A.; Highison, G.J. Role of the capsule in phagocytosis of Cryptococcus neoformans. Rev. Infect. Dis. 1988, 10 (Suppl. 2), S436–S439. [Google Scholar] [CrossRef]
- Vecchiarelli, A.; Pericolini, E.; Gabrielli, E.; Kenno, S.; Perito, S.; Cenci, E.; Monari, C. Elucidating the immunological function of the Cryptococcus neoformans capsule. Futur. Microbiol. 2013, 8, 1107–1116. [Google Scholar] [CrossRef]
- Kelly, R.M.; Chen, J.; Yauch, L.E.; Levitz, S.M. Opsonic Requirements for Dendritic Cell-Mediated Responses to Cryptococcus neoformans. Infect. Immun. 2005, 73, 592–598. [Google Scholar] [CrossRef]
- Mukherjee, J.; Scharff, M.D.; Casadevall, A. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect. Immun. 1992, 60, 4534–4541. [Google Scholar] [CrossRef]
- Schmidt, B.; Fujimura, S.H.; Martin, J.N.; Levy, J.A. Variations in Plasmacytoid Dendritic Cell (PDC) and Myeloid Dendritic Cell (MDC) Levels in HIV-Infected Subjects on and off Antiretroviral Therapy. J. Clin. Immunol. 2006, 26, 55–64. [Google Scholar] [CrossRef]
- Hole, C.R.; Leopold Wager, C.M.; Mendiola, A.S.; Wozniak, K.L.; Campuzano, A.; Lin, X.; Wormley, F.L., Jr. Antifungal Activity of Plasmacytoid Dendritic Cells against Cryptococcus neoformans In Vitro Requires Expression of Dectin-3 (CLEC4D) and Reactive Oxygen Species. Infect. Immun. 2016, 84, 2493–2504. [Google Scholar] [CrossRef]
- Olszewski, M.A.; Zhang, Y.; Huffnagle, G.B. Mechanisms of cryptococcal virulence and persistence. Futur. Microbiol. 2010, 5, 1269–1288. [Google Scholar] [CrossRef] [PubMed]
- Huston, S.M.; Li, S.S.; Stack, D.; Timm-McCann, M.; Jones, G.J.; Islam, A.; Berenger, B.M.; Xiang, R.F.; Colarusso, P.; Mody, C.H. Cryptococcus gattii Is Killed by Dendritic Cells, but Evades Adaptive Immunity by Failing To Induce Dendritic Cell Maturation. J. Immunol. 2013, 191, 249–261. [Google Scholar] [CrossRef]
- Yauch, L.E.; Mansour, M.K.; Shoham, S.; Rottman, J.B.; Levitz, S.M. Involvement of CD14, Toll-Like Receptors 2 and 4, and MyD88 in the Host Response to the Fungal Pathogen Cryptococcus neoformans In Vivo. Infect. Immun. 2004, 72, 5373–5382. [Google Scholar] [CrossRef]
- Shoham, S.; Huang, C.; Chen, J.-M.; Golenbock, D.T.; Levitz, S.M. Toll-Like Receptor 4 Mediates Intracellular Signaling Without TNF-α Release in Response to Cryptococcus neoformans Polysaccharide Capsule. J. Immunol. 2001, 166, 4620–4626. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Miyagi, K.; Koguchi, Y.; Kinjo, Y.; Uezu, K.; Kinjo, T.; Akamine, M.; Fujita, J.; Kawamura, I.; Mitsuyama, M.; et al. Limited contribution of Toll-like receptor 2 and 4 to the host response to a fungal infectious pathogen, Cryptococcus neoformans. FEMS Immunol. Med. Microbiol. 2006, 47, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kinjo, T.; Saijo, S.; Miyazato, A.; Adachi, Y.; Ohno, N.; Fujita, J.; Kaku, M.; Iwakura, Y.; Kawakami, K. Dectin-1 Is Not Required for the Host Defense to Cryptococcus neoformans. Microbiol. Immunol. 2007, 51, 1115–1119. [Google Scholar] [CrossRef]
- Nakamura, Y.; Sato, K.; Yamamoto, H.; Matsumura, K.; Matsumoto, I.; Nomura, T.; Miyasaka, T.; Ishii, K.; Kanno, E.; Tachi, M.; et al. Dectin-2 Deficiency Promotes Th2 Response and Mucin Production in the Lungs after Pulmonary Infection with Cryptococcus neoformans. Infect. Immun. 2015, 83, 671–681. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Bhan, U.; Huffnagle, G.B.; Toews, G.B.; Standiford, T.J.; Olszewski, M.A. TLR9 Signaling Is Required for Generation of the Adaptive Immune Protection in Cryptococcus neoformans-Infected Lungs. Am. J. Pathol. 2010, 177, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, A.; Castro-Lopez, N.; Wozniak, K.L.; Leopold Wager, C.M.; Wormley, F.L., Jr. Dectin-3 Is Not Required for Protection against Cryptococcus neoformans Infection. PLoS ONE 2017, 12, e0169347. [Google Scholar] [CrossRef] [PubMed]
- Eastman, A.J.; Osterholzer, J.J.; Olszewski, M.A. Role of dendritic cell–pathogen interactions in the immune response to pulmonary cryptococcal infection. Futur. Microbiol. 2015, 10, 1837–1857. [Google Scholar] [CrossRef]
- Biondo, C.; Midiri, A.; Messina, L.; Tomasello, F.; Garufi, G.; Catania, M.R.; Bombaci, M.; Beninati, C.; Teti, G.; Mancuso, G. MyD88 and TLR2, but not TLR4, are required for host defense against Cryptococcus neoformans. Eur. J. Immunol. 2005, 35, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zeltzer, S.; Zhang, Y.; Wang, F.; Chen, G.-H.; Dayrit, J.; Murdock, B.J.; Bhan, U.; Toews, G.B.; Osterholzer, J.J.; et al. Early Induction of CCL7 Downstream of TLR9 Signaling Promotes the Development of Robust Immunity to Cryptococcal Infection. J. Immunol. 2012, 188, 3940–3948. [Google Scholar] [CrossRef]
- Edwards, L.; Williams, A.E.; Krieg, A.M.; Rae, A.J.; Snelgrove, R.J.; Hussell, T. Stimulation via Toll-like receptor 9 reduces Cryptococcus neoformans-induced pulmonary inflammation in an IL-12-dependent manner. Eur. J. Immunol. 2005, 35, 273–281. [Google Scholar] [CrossRef]
- Wang, J.P.; Lee, C.K.; Akalin, A.; Finberg, R.W.; Levitz, S.M. Contributions of the MyD88-Dependent Receptors IL-18R, IL-1R, and TLR9 to Host Defenses following Pulmonary Challenge with Cryptococcus neoformans. PLoS ONE 2011, 6, e26232. [Google Scholar] [CrossRef]
- Tanaka, M.; Ishii, K.; Nakamura, Y.; Miyazato, A.; Maki, A.; Abe, Y.; Miyasaka, T.; Yamamoto, H.; Akahori, Y.; Fue, M.; et al. Toll-Like Receptor 9-Dependent Activation of Bone Marrow-Derived Dendritic Cells by URA5 DNA from Cryptococcus neoformans. Infect. Immun. 2012, 80, 778–786. [Google Scholar] [CrossRef]
- Nakamura, K.; Miyazato, A.; Xiao, G.; Hatta, M.; Inden, K.; Aoyagi, T.; Shiratori, K.; Takeda, K.; Akira, S.; Saijo, S.; et al. Deoxynucleic Acids from Cryptococcus neoformans Activate Myeloid Dendritic Cells via a TLR9-Dependent Pathway. J. Immunol. 2008, 180, 4067–4074. [Google Scholar] [CrossRef] [PubMed]
- Hardison, S.E.; Brown, G.D. C-type lectin receptors orchestrate antifungal immunity. Nat. Immunol. 2012, 13, 817–822. [Google Scholar] [CrossRef]
- Kitai, Y.; Sato, K.; Tanno, D.; Yuan, X.; Umeki, A.; Kasamatsu, J.; Kanno, E.; Tanno, H.; Hara, H.; Yamasaki, S.; et al. Role of Dectin-2 in the Phagocytosis of Cryptococcus neoformans by Dendritic Cells. Infect. Immun. 2021, 89, e00330-21. [Google Scholar] [CrossRef] [PubMed]
- Willment, J.A.; Brown, G.D. C-type lectin receptors in antifungal immunity. Trends Microbiol. 2008, 16, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.J.; Osorio, F.; Rosas, M.; Freitas, R.P.; Schweighoffer, E.; Groß, O.; Verbeek, J.S.; Ruland, J.; Tybulewicz, V.; Brown, G.D.; et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J. Exp. Med. 2009, 206, 2037–2051. [Google Scholar] [CrossRef]
- Lee, M.J.; Yoshimoto, E.; Saijo, S.; Iwakura, Y.; Lin, X.; Katz, H.R.; Kanaoka, Y.; Barrett, N.A. Phosphoinositide 3-Kinase δ Regulates Dectin-2 Signaling and the Generation of Th2 and Th17 Immunity. J. Immunol. 2016, 197, 278–287. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, K.; Love, J.C.; Ploegh, H.L.; Vyas, J.M. Recruitment of CD63 to Cryptococcus neoformans phagosomes requires acidification. Proc. Natl. Acad. Sci. USA 2006, 103, 15945–15950. [Google Scholar] [CrossRef]
- Hole, C.R.; Bui, H.; Wormley, F.L., Jr.; Wozniak, K.L. Mechanisms of Dendritic Cell Lysosomal Killing of Cryptococcus. Sci. Rep. 2012, 2, 739. [Google Scholar] [CrossRef] [PubMed]
- Syme, R.M.; Spurrell, J.C.L.; Amankwah, E.K.; Green, F.H.Y.; Mody, C.H. Primary dendritic cells phagocytose Cryptococcus neoformans via mannose receptors and Fcgamma receptor II for presentation to T lymphocytes. Infect. Immun. 2002, 70, 5972–5981. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Vecchiarelli, A.; Pietrella, D.; Lupo, P.; Bistoni, F.; McFadden, D.C.; Casadevall, A. The polysaccharide capsule of Cryptococcus neoformans interferes with human dendritic cell maturation and activation. J. Leukoc. Biol. 2003, 74, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.W.; Truong, T.; Bickham, K.; Fonteneau, J.-F.; Larsson, M.; Da Silva, I.; Somersan, S.; Thomas, E.K.; Bhardwaj, N. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: Implications for immunotherapy. Vaccine 2002, 20 (Suppl. S4), A8–A22. [Google Scholar] [CrossRef]
- Grijpstra, J.; Tefsen, B.; van Die, I.; de Cock, H. The Cryptococcus neoformans cap10 and cap59 mutant strains, affected in glucuronoxylomannan synthesis, differentially activate human dendritic cells. FEMS Immunol. Med. Microbiol. 2009, 57, 142–150. [Google Scholar] [CrossRef]
- Nelson, B.N.; Hawkins, A.N.; Wozniak, K.L. Pulmonary Macrophage and Dendritic Cell Responses to Cryptococcus neoformans. Front. Cell. Infect. Microbiol. 2020, 10, 37. [Google Scholar] [CrossRef]
- Patel, V.I.; Booth, J.L.; Duggan, E.S.; Cate, S.; White, V.L.; Hutchings, D.; Kovats, S.; Burian, D.M.; Dozmorov, M.; Metcalf, J.P. Transcriptional Classification and Functional Characterization of Human Airway Macrophage and Dendritic Cell Subsets. J. Immunol. 2017, 198, 1183–1201. [Google Scholar] [CrossRef] [PubMed]
- Misharin, A.V.; Morales-Nebreda, L.; Mutlu, G.M.; Budinger, G.R.; Perlman, H. Flow Cytometric Analysis of Macrophages and Dendritic Cell Subsets in the Mouse Lung. Am. J. Respir. Cell Mol. Biol. 2013, 49, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Zaynagetdinov, R.; Sherrill, T.P.; Kendall, P.L.; Segal, B.H.; Weller, K.P.; Tighe, R.M.; Blackwell, T.S. Identification of Myeloid Cell Subsets in Murine Lungs Using Flow Cytometry. Am. J. Respir. Cell Mol. Biol. 2013, 49, 180–189. [Google Scholar] [CrossRef]
- Kopf, M.; Schneider, C.; Nobs, S.P. The development and function of lung-resident macrophages and dendritic cells. Nat. Immunol. 2014, 16, 36–44. [Google Scholar] [CrossRef]
- Satpathy, A.T.; Wu, X.; Albring, J.C.; Murphy, K.M. Re(de)fining the dendritic cell lineage. Nat. Immunol. 2012, 13, 1145–1154. [Google Scholar] [CrossRef]
- Vermaelen, K.; Pauwels, R. Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: Methodology and new insights. Cytometry A 2004, 61, 170–177. [Google Scholar] [CrossRef]
- Condon, T.V.; Sawyer, R.T.; Fenton, M.J.; Riches, D.W.H. Lung dendritic cells at the innate-adaptive immune interface. J. Leukoc. Biol. 2011, 90, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Shortman, K.; Liu, Y.-J. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2002, 2, 151–161. [Google Scholar] [CrossRef]
- Hawkins, A.N.; Determann, B.F., 2nd; Nelson, B.N.; Wozniak, K.L. Transcriptional Changes in Pulmonary Phagocyte Subsets Dictate the Outcome Following Interaction with the Fungal Pathogen Cryptococcus neoformans. Front. Immunol. 2021, 12, 722500. [Google Scholar] [CrossRef] [PubMed]
- Osterholzer, J.J.; Curtis, J.L.; Polak, T.; Ames, T.; Chen, G.-H.; McDonald, R.; Huffnagle, G.B.; Toews, G.B. CCR2 Mediates Conventional Dendritic Cell Recruitment and the Formation of Bronchovascular Mononuclear Cell Infiltrates in the Lungs of Mice Infected with Cryptococcus neoformans. J. Immunol. 2008, 181, 610–620. [Google Scholar] [CrossRef]
- Huston, S.M.; Ngamskulrungroj, P.; Xiang, R.F.; Ogbomo, H.; Stack, D.; Li, S.S.; Timm-McCann, M.; Kyei, S.K.; Oykhman, P.; Kwon-Chung, K.J.; et al. Cryptococcus gattii Capsule Blocks Surface Recognition Required for Dendritic Cell Maturation Independent of Internalization and Antigen Processing. J. Immunol. 2016, 196, 1259–1271. [Google Scholar] [CrossRef]
- Jablonski, K.A.; Amici, S.A.; Webb, L.M.; Ruiz-Rosado Jde, D.; Popovich, P.G.; Partida-Sanchez, S.; Guerau-de-Arellano, M. Novel Markers to Delineate Murine M1 and M2 Macrophages. PLoS ONE 2015, 10, e0145342. [Google Scholar] [CrossRef] [PubMed]
- Fels, A.O.; Cohn, Z.A. The alveolar macrophage. J. Appl. Physiol. 1986, 60, 353–369. [Google Scholar] [CrossRef]
- Porcheray, F.; Viaud, S.; Rimaniol, A.C.; Leone, C.; Samah, B.; Dereuddre-Bosquet, N.; Dormont, D.; Gras, G. Macrophage activation switching: An asset for the resolution of inflammation. Clin. Exp. Immunol. 2005, 142, 481–489. [Google Scholar] [CrossRef]
- Stout, R.D.; Jiang, C.; Matta, B.; Tietzel, I.; Watkins, S.K.; Suttles, J. Macrophages Sequentially Change Their Functional Phenotype in Response to Changes in Microenvironmental Influences. J. Immunol. 2005, 175, 342–349. [Google Scholar] [CrossRef]
- Gratchev, A.; Kzhyshkowska, J.; Köthe, K.; Muller-Molinet, I.; Kannookadan, S.; Utikal, J.; Goerdt, S. Mφ1 and Mφ2 can be re-polarized by Th2 or Th1 cytokines, respectively, and respond to exogenous danger signals. Immunobiology 2006, 211, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J.; Tsang, T.M.; Qiu, Y.; Dayrit, J.K.; Freij, J.B.; Huffnagle, G.B.; Olszewski, M.A. Macrophage M1/M2 Polarization Dynamically Adapts to Changes in Cytokine Microenvironments in Cryptococcus neoformans Infection. mBio 2013, 4, e00264-13. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Xu-Vanpala, S.; Deerhake, M.E.; Wheaton, J.D.; Parker, M.E.; Juvvadi, P.R.; MacIver, N.; Ciofani, M.; Shinohara, M.L. Functional heterogeneity of alveolar macrophage population based on expression of CXCL2. Sci. Immunol. 2020, 5, aba7350. [Google Scholar] [CrossRef]
- Stout, R.D.; Suttles, J. Functional plasticity of macrophages: Reversible adaptation to changing microenvironments. J. Leukoc. Biol. 2004, 76, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Davis, M.J.; Eastman, A.J.; Qiu, Y.; Gregorka, B.; Kozel, T.R.; Osterholzer, J.J.; Curtis, J.L.; Swanson, J.A.; Olszewski, M.A. Cryptococcus neoformans—Induced Macrophage Lysosome Damage Crucially Contributes to Fungal Virulence. J. Immunol. 2015, 194, 2219–2231. [Google Scholar] [CrossRef]
- Arora, S.; Hernandez, Y.; Erb-Downward, J.R.; McDonald, R.A.; Toews, G.B.; Huffnagle, G.B. Role of IFN-γ in Regulating T2 Immunity and the Development of Alternatively Activated Macrophages during Allergic Bronchopulmonary Mycosis. J. Immunol. 2005, 174, 6346–6356. [Google Scholar] [CrossRef]
- Müller, U.; Stenzel, W.; Köhler, G.; Werner, C.; Polte, T.; Hansen, G.; Schütze, N.; Straubinger, R.K.; Blessing, M.; McKenzie, A.N.J.; et al. IL-13 Induces Disease-Promoting Type 2 Cytokines, Alternatively Activated Macrophages and Allergic Inflammation during Pulmonary Infection of Mice with Cryptococcus neoformans. J. Immunol. 2007, 179, 5367–5377. [Google Scholar] [CrossRef]
- Voelz, K.; Lammas, D.A.; May, R.C. Cytokine Signaling Regulates the Outcome of Intracellular Macrophage Parasitism by Cryptococcus neoformans. Infect. Immun. 2009, 77, 3450–3457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, F.; Tompkins, K.C.; McNamara, A.; Jain, A.V.; Moore, B.B.; Toews, G.B.; Huffnagle, G.B.; Olszewski, M.A. Robust Th1 and Th17 Immunity Supports Pulmonary Clearance but Cannot Prevent Systemic Dissemination of Highly Virulent Cryptococcus neoformans H99. Am. J. Pathol. 2009, 175, 2489–2500. [Google Scholar] [CrossRef]
- Hardison, S.E.; Ravi, S.; Wozniak, K.L.; Young, M.L.; Olszewski, M.A.; Wormley, F.L., Jr. Pulmonary Infection with an Interferon-γ-Producing Cryptococcus neoformans Strain Results in Classical Macrophage Activation and Protection. Am. J. Pathol. 2010, 176, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Ruytinx, P.; Proost, P.; Van Damme, J.; Struyf, S. Chemokine-Induced Macrophage Polarization in Inflammatory Conditions. Front. Immunol. 2018, 9, 1930. [Google Scholar] [CrossRef] [PubMed]
- Hardison, S.E.; Herrera, G.; Young, M.L.; Hole, C.R.; Wozniak, K.L.; Wormley, F.L., Jr. Protective Immunity against Pulmonary Cryptococcosis Is Associated with STAT1-Mediated Classical Macrophage Activation. J. Immunol. 2012, 189, 4060–4068. [Google Scholar] [CrossRef]
- Leopold Wager, C.M.; Hole, C.R.; Wozniak, K.L.; Olszewski, M.A.; Wormley, F.L., Jr. STAT1 Signaling Is Essential for Protection against Cryptococcus neoformans Infection in Mice. J. Immunol. 2014, 193, 4060–4071. [Google Scholar] [CrossRef]
- Leopold Wager, C.M.; Hole, C.R.; Wozniak, K.L.; Olszewski, M.A.; Mueller, M.; Wormley, F.L., Jr. STAT1 Signaling within Macrophages Is Required for Antifungal Activity against Cryptococcus neoformans. Infect. Immun. 2015, 83, 4513–4527. [Google Scholar] [CrossRef]
- Leopold Wager, C.M.; Hole, C.R.; Campuzano, A.; Castro-Lopez, N.; Cai, H.; Caballero Van Dyke, M.C.; Wozniak, K.L.; Wang, Y.; Wormley, F.L., Jr. IFN-γ immune priming of macrophages in vivo induces prolonged STAT1 binding and protection against Cryptococcus neoformans. PLoS Pathog. 2018, 14, e1007358. [Google Scholar] [CrossRef]
- Arora, S.; Olszewski, M.A.; Tsang, T.M.; McDonald, R.A.; Toews, G.B.; Huffnagle, G.B. Effect of Cytokine Interplay on Macrophage Polarization during Chronic Pulmonary Infection with Cryptococcus neoformans. Infect. Immun. 2011, 79, 1915–1926. [Google Scholar] [CrossRef]
- Xiao, G.; Miyazato, A.; Inden, K.; Nakamura, K.; Shiratori, K.; Nakagawa, K.; Miyazawa, T.; Suzuki, K.; Kaku, M.; Kawakami, K. Cryptococcus neoformans inhibits nitric oxide synthesis caused by CpG-oligodeoxynucleotide-stimulated macrophages in a fashion independent of capsular polysaccharides. Microbiol. Immunol. 2008, 52, 171–179. [Google Scholar] [CrossRef]
- Naslund, P.K.; Miller, W.C.; Granger, D.L. Cryptococcus neoformans fails to induce nitric oxide synthase in primed murine macrophage-like cells. Infect. Immun. 1995, 63, 1298–1304. [Google Scholar] [CrossRef]
- Alspaugh, J.A.; Granger, D.L. Inhibition of Cryptococcus neoformans replication by nitrogen oxides supports the role of these molecules as effectors of macrophage-mediated cytostasis. Infect. Immun. 1991, 59, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, K.M.; Gibson, G.W. Differing requirement for inducible nitric oxide synthase activity in clearance of primary and secondary Cryptococcus neoformans infection. Med. Mycol. 2000, 38, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; Mukherjee, J.; Weiss, L.M.; Casadevall, A. Antibody Efficacy in Murine Pulmonary Cryptococcus neoformans Infection: A Role for Nitric Oxide. J. Immunol. 2002, 168, 3419–3427. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, W.; Müller, U.; Köhler, G.; Heppner, F.L.; Blessing, M.; McKenzie, A.N.; Brombacher, F.; Alber, G. IL-4/IL-13-Dependent Alternative Activation of Macrophages but Not Microglial Cells Is Associated with Uncontrolled Cerebral Cryptococcosis. Am. J. Pathol. 2009, 174, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Hardison, S.E.; Wozniak, K.L.; Kolls, J.K.; Wormley, F.L., Jr. Interleukin-17 Is Not Required for Classical Macrophage Activation in a Pulmonary Mouse Model of Cryptococcus neoformans Infection. Infect. Immun. 2010, 78, 5341–5351. [Google Scholar] [CrossRef]
- Subramani, A.; Griggs, P.; Frantzen, N.; Mendez, J.; Tucker, J.; Murriel, J.; Sircy, L.M.; Millican, G.E.; McClelland, E.E.; Seipelt-Thiemann, R.L.; et al. Intracellular Cryptococcus neoformans disrupts the transcriptome profile of M1- and M2-polarized host macrophages. PLoS ONE 2020, 15, e0233818. [Google Scholar] [CrossRef]
- Nelson, B.N.; Daugherty, C.S.; Sharp, R.R.; Booth, J.L.; Patel, V.I.; Metcalf, J.P.; Jones, K.L.; Wozniak, K.L. Protective interaction of human phagocytic APC subsets with Cryptococcus neoformans induces genes associated with metabolism and antigen presentation. Front. Immunol. 2022, 13, 1054477. [Google Scholar] [CrossRef]
- Hansakon, A.; Mutthakalin, P.; Ngamskulrungroj, P.; Chayakulkeeree, M.; Angkasekwinai, P. Cryptococcus neoformans and Cryptococcus gattii clinical isolates from Thailand display diverse phenotypic interactions with macrophages. Virulence 2019, 10, 26–36. [Google Scholar] [CrossRef]
- Feldmesser, M.; Kress, Y.; Novikoff, P.; Casadevall, A. Cryptococcus neoformans Is a Facultative Intracellular Pathogen in Murine Pulmonary Infection. Infect. Immun. 2000, 68, 4225–4237. [Google Scholar] [CrossRef]
- Tucker, S.C.; Casadevall, A. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc. Natl. Acad. Sci. USA 2002, 99, 3165–3170. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.A.; May, R.C. The Human Fungal Pathogen Cryptococcus neoformans Escapes Macrophages by a Phagosome Emptying Mechanism That Is Inhibited by Arp2/3 Complex-Mediated Actin Polymerisation. PLoS Pathog. 2010, 6, e1001041. [Google Scholar] [CrossRef] [PubMed]
- De Leon-Rodriguez, C.M.; Rossi, D.C.P.; Fu, M.S.; Dragotakes, Q.; Coelho, C.; Guerrero Ros, I.; Caballero, B.; Nolan, S.J.; Casadevall, A. The Outcome of the Cryptococcus neoformans—Macrophage Interaction Depends on Phagolysosomal Membrane Integrity. J. Immunol. 2018, 201, 583–603. [Google Scholar] [CrossRef]
- Boya, P.; Kroemer, G. Lysosomal membrane permeabilization in cell death. Oncogene 2008, 27, 6434–6451. [Google Scholar] [CrossRef]
- Davis, J.M.; Huang, M.; Botts, M.R.; Hull, C.M.; Huttenlocher, A. A Zebrafish Model of Cryptococcal Infection Reveals Roles for Macrophages, Endothelial Cells, and Neutrophils in the Establishment and Control of Sustained Fungemia. Infect. Immun. 2016, 84, 3047–3062. [Google Scholar] [CrossRef] [PubMed]
- Diamond, R.D.; Bennett, J.E. Growth of Cryptococcus neoformans Within Human Macrophages In Vitro. Infect. Immun. 1973, 7, 231–236. [Google Scholar] [CrossRef]
- Levitz, S.M.; Nong, S.-H.; Seetoo, K.F.; Harrison, T.S.; Speizer, R.A.; Simons, E.R. Cryptococcus neoformans Resides in an Acidic Phagolysosome of Human Macrophages. Infect. Immun. 1999, 67, 885–890. [Google Scholar] [CrossRef]
- Smith, L.M.; Dixon, E.F.; May, R.C. The fungal pathogen Cryptococcus neoformans manipulates macrophage phagosome maturation. Cell. Microbiol. 2014, 17, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Noverr, M.C.; Cox, G.M.; Perfect, J.R.; Huffnagle, G.B. Role of PLB1 in Pulmonary Inflammation and Cryptococcal Eicosanoid Production. Infect. Immun. 2003, 71, 1538–1547. [Google Scholar] [CrossRef]
- Evans, R.J.; Li, Z.; Hughes, W.S.; Djordjevic, J.T.; Nielsen, K.; May, R.C. Cryptococcal Phospholipase B1 Is Required for Intracellular Proliferation and Control of Titan Cell Morphology during Macrophage Infection. Infect. Immun. 2015, 83, 1296–1304. [Google Scholar] [CrossRef]
- Liu, T.-B.; Xue, C. Fbp1-Mediated Ubiquitin-Proteasome Pathway Controls Cryptococcus neoformans Virulence by Regulating Fungal Intracellular Growth in Macrophages. Infect. Immun. 2014, 82, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Croudace, J.E.; Lammas, D.A.; May, R.C. Expulsion of Live Pathogenic Yeast by Macrophages. Curr. Biol. 2006, 16, 2156–2160. [Google Scholar] [CrossRef] [PubMed]
- Nicola, A.M.; Robertson, E.J.; Albuquerque, P.; Derengowski, L.D.S.; Casadevall, A. Nonlytic Exocytosis of Cryptococcus neoformans from Macrophages Occurs In Vivo and Is Influenced by Phagosomal pH. mBio 2011, 2, e00167-11. [Google Scholar] [CrossRef] [PubMed]
- Chayakulkeeree, M.; Johnston, S.A.; Oei, J.B.; Lev, S.; Williamson, P.R.; Wilson, C.F.; Zuo, X.; Leal, A.L.; Vainstein, M.H.; Meyer, W.; et al. SEC14 is a specific requirement for secretion of phospholipase B1 and pathogenicity of Cryptococcus neoformans. Mol. Microbiol. 2011, 80, 1088–1101. [Google Scholar] [CrossRef]
- Santiago-Tirado, F.H.; Doering, T.L. False friends: Phagocytes as Trojan horses in microbial brain infections. PLoS Pathog. 2017, 13, e1006680. [Google Scholar] [CrossRef]
- Santiago-Tirado, F.H.; Onken, M.D.; Cooper, J.A.; Klein, R.S.; Doering, T.L. Trojan Horse Transit Contributes to Blood-Brain Barrier Crossing of a Eukaryotic Pathogen. mBio 2017, 8, e02183-16. [Google Scholar] [CrossRef]
- Gottfredsson, M.; Perfect, J.R. Fungal Meningitis. Semin. Neurol. 2000, 20, 307–322. [Google Scholar] [CrossRef]
- Chang, Y.C.; Stins, M.F.; McCaffery, M.J.; Miller, G.F.; Pare, D.R.; Dam, T.; Paul-Satyaseela, M.; Kim, K.S.; Kwon-Chung, K.J. Cryptococcal Yeast Cells Invade the Central Nervous System via Transcellular Penetration of the Blood-Brain Barrier. Infect. Immun. 2004, 72, 4985–4995. [Google Scholar] [CrossRef]
- Liu, T.-B.; Perlin, D.S.; Xue, C. Molecular mechanisms of cryptococcal meningitis. Virulence 2012, 3, 173–181. [Google Scholar] [CrossRef]
- Hawkins, T. Understanding and managing the adverse effects of antiretroviral therapy. Antivir. Res. 2010, 85, 201–209. [Google Scholar] [CrossRef]
- Montessori, V.; Press, N.; Harris, M.; Akagi, L.; Montaner, J.S.G. Adverse effects of antiretroviral therapy for HIV infection. Can. Med. Assoc. J. 2004, 170, 229–238. [Google Scholar]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.-K.; Noda, M.; Verkhratsky, A. Physiology of Microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Nau, R.; Ribes, S.; Djukic, M.; Eiffert, H. Strategies to increase the activity of microglia as efficient protectors of the brain against infections. Front. Cell. Neurosci. 2014, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Chhatbar, C.; Prinz, M. The roles of microglia in viral encephalitis: From sensome to therapeutic targeting. Cell. Mol. Immunol. 2021, 18, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Rock, R.B.; Gekker, G.; Hu, S.; Sheng, W.S.; Cheeran, M.; Lokensgard, J.R.; Peterson, P.K. Role of Microglia in Central Nervous System Infections. Clin. Microbiol. Rev. 2004, 17, 942–964. [Google Scholar] [CrossRef]
- Waltl, I.; Kalinke, U. Beneficial and detrimental functions of microglia during viral encephalitis. Trends Neurosci. 2022, 45, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, K.L.; Doyle, H.A. Requirement for CD4+ T Lymphocytes in Host Resistance against Cryptococcus neoformans in the Central Nervous System of Immunized Mice. Infect. Immun. 2000, 68, 456–462. [Google Scholar] [CrossRef]
- Chao, C.C.; Hu, S.; Molitor, T.W.; Shaskan, E.G.; Peterson, P.K. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J. Immunol. 1992, 149, 2736–2741. [Google Scholar] [CrossRef]
- Blasi, E.; Barluzzi, R.; Mazzolla, R.; Tancini, B.; Saleppico, S.; Puliti, M.; Pitzurra, L.; Bistoni, F. Role of nitric oxide and melanogenesis in the accomplishment of anticryptococcal activity by the BV-2 microglial cell line. J. Neuroimmunol. 1995, 58, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Adami, C.; Sorci, G.; Blasi, E.; Agneletti, A.L.; Bistoni, F.; Donato, R. S100B expression in and effects on microglia. Glia 2001, 33, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Sun, P.; Li, H.; Zhang, M.; Liu, G.; Strickland, A.B.; Chen, Y.; Fu, Y.; Xu, J.; Yosri, M.; et al. Fungal dissemination is limited by liver macrophage filtration of the blood. Nat. Commun. 2019, 10, 4566. [Google Scholar] [CrossRef] [PubMed]
- Heung, L.J.; Hohl, T.M. Inflammatory monocytes are detrimental to the host immune response during acute infection with Cryptococcus neoformans. PLoS Pathog. 2019, 15, e1007627. [Google Scholar] [CrossRef]
- Espinosa, V.; Jhingran, A.; Dutta, O.; Kasahara, S.; Donnelly, R.; Du, P.; Rosenfeld, J.; Leiner, I.; Chen, C.-C.; Ron, Y.; et al. Inflammatory Monocytes Orchestrate Innate Antifungal Immunity in the Lung. PLoS Pathog. 2014, 10, e1003940. [Google Scholar] [CrossRef]
- Hohl, T.M.; Rivera, A.; Lipuma, L.; Gallegos, A.; Shi, C.; Mack, M.; Pamer, E.G. Inflammatory Monocytes Facilitate Adaptive CD4 T Cell Responses during Respiratory Fungal Infection. Cell Host Microbe 2009, 6, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Wüthrich, M.; Ersland, K.; Sullivan, T.; Galles, K.; Klein, B.S. Fungi Subvert Vaccine T Cell Priming at the Respiratory Mucosa by Preventing Chemokine-Induced Influx of Inflammatory Monocytes. Immunity 2012, 36, 680–692. [Google Scholar] [CrossRef]
- Ngo, L.Y.; Kasahara, S.; Kumasaka, D.K.; Knoblaugh, S.E.; Jhingran, A.; Hohl, T.M. Inflammatory Monocytes Mediate Early and Organ-Specific Innate Defense During Systemic Candidiasis. J. Infect. Dis. 2014, 209, 109–119. [Google Scholar] [CrossRef]
- Szymczak, W.A.; Deepe, G.S., Jr. The CCL7-CCL2-CCR2 Axis Regulates IL-4 Production in Lungs and Fungal Immunity. J. Immunol. 2009, 183, 1964–1974. [Google Scholar] [CrossRef]
- Traynor, T.R.; Kuziel, W.A.; Toews, G.B.; Huffnagle, G.B. CCR2 Expression Determines T1 Versus T2 Polarization During Pulmonary Cryptococcus neoformans Infection. J. Immunol. 2000, 164, 2021–2027. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Gomez Perdiguero, E.; Klapproth, K.; Schulz, C.; Busch, K.; Azzoni, E.; Crozet, L.; Garner, H.; Trouillet, C.; de Bruijn, M.F.; Geissmann, F.; et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015, 518, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Van De Laar, L.; Saelens, W.; De Prijck, S.; Martens, L.; Scott, C.L.; Van Isterdael, G.; Hoffmann, E.; Beyaert, R.; Saeys, Y.; Lambrecht, B.N.; et al. Yolk Sac Macrophages, Fetal Liver, and Adult Monocytes Can Colonize an Empty Niche and Develop into Functional Tissue-Resident Macrophages. Immunity 2016, 44, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef]
- Hou, F.; Xiao, K.; Tang, L.; Xie, L. Diversity of Macrophages in Lung Homeostasis and Diseases. Front. Immunol. 2021, 12, 753940. [Google Scholar] [CrossRef] [PubMed]
- Morales-Nebreda, L.; Misharin, A.V.; Perlman, H.; Budinger, G.R. The heterogeneity of lung macrophages in the susceptibility to disease. Eur. Respir. Rev. 2015, 24, 505–509. [Google Scholar] [CrossRef]
- Guilliams, M.; De Kleer, I.; Henri, S.; Post, S.; Vanhoutte, L.; De Prijck, S.; Deswarte, K.; Malissen, B.; Hammad, H.; Lambrecht, B.N. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 2013, 210, 1977–1992. [Google Scholar] [CrossRef]
- Yona, S.; Kim, K.-W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate Mapping Reveals Origins and Dynamics of Monocytes and Tissue Macrophages under Homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef]
- Gibbings, S.L.; Thomas, S.M.; Atif, S.M.; McCubbrey, A.L.; Desch, A.N.; Danhorn, T.; Leach, S.M.; Bratton, D.L.; Henson, P.M.; Janssen, W.J.; et al. Three Unique Interstitial Macrophages in the Murine Lung at Steady State. Am. J. Respir. Cell Mol. Biol. 2017, 57, 66–76. [Google Scholar] [CrossRef]
- Gautier, E.L.; Shay, T.; Miller, J.; Greter, M.; Jakubzick, C.; Ivanov, S.; Helft, J.; Chow, A.; Elpek, K.G.; Gordonov, S.; et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012, 13, 1118–1128. [Google Scholar] [CrossRef]
- Tan, S.Y.S.; Krasnow, M.A. Developmental origin of lung macrophage diversity. Development 2016, 143, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Hey, Y.-Y.; Tan, J.K.; O’Neill, H.C. Redefining Myeloid Cell Subsets in Murine Spleen. Front. Immunol. 2015, 6, 652. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.S.; Huang, H.; Levitz, S.M. Effect of Differential N-linked and O-linked Mannosylation on Recognition of Fungal Antigens by Dendritic Cells. PLoS ONE 2007, 2, e1009. [Google Scholar] [CrossRef]
- Lam, J.S.; Mansour, M.K.; Specht, C.A.; Levitz, S.M. A Model Vaccine Exploiting Fungal Mannosylation to Increase Antigen Immunogenicity. J. Immunol. 2005, 175, 7496–7503. [Google Scholar] [CrossRef]
- Hewitt, E.W. The MHC class I antigen presentation pathway: Strategies for viral immune evasion. Immunology 2003, 110, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.T. Neutrophils and macrophages work in concert as inducers and effectors of adaptive immunity against extracellular and intracellular microbial pathogens. J. Leukoc. Biol. 2010, 87, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, A.; Wormley, F.L., Jr. Innate Immunity against Cryptococcus, from Recognition to Elimination. J. Fungi 2018, 4, 33. [Google Scholar] [CrossRef]
- Chiller, T.; Farrokhshad, K.; Brummer, E.; Stevens, D.A. Effect of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor on polymorphonuclear neutrophils, monocytes or monocyte-derived macrophages combined with voriconazole against Cryptococcus neoformans. Med. Mycol. 2002, 40, 21–26. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The Multifaceted Functions of Neutrophils. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 181–218. [Google Scholar] [CrossRef]
- Diamond, R.D.; Root, R.K.; Bennett, J.E. Factors Influencing Killing of Cryptococcus neoformans by Human Leukocytes In Vitro. J. Infect. Dis. 1972, 125, 367–376. [Google Scholar] [CrossRef]
- Miller, M.F.; Mitchell, T.G. Killing of Cryptococcus neoformans strains by human neutrophils and monocytes. Infect. Immun. 1991, 59, 24–28. [Google Scholar] [CrossRef]
- Lämmermann, T. In the eye of the neutrophil swarm—Navigation signals that bring neutrophils together in inflamed and infected tissues. J. Leukoc. Biol. 2015, 100, 55–63. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, M.; Liu, G.; Wu, H.; Zhu, X.; Zhou, H.; Shi, M. Real-Time Imaging of Interactions of Neutrophils with Cryptococcus neoformans Demonstrates a Crucial Role of Complement C5a-C5aR Signaling. Infect. Immun. 2016, 84, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Voelz, K.; May, R.C. Cryptococcal Interactions with the Host Immune System. Eukaryot. Cell 2010, 9, 835–846. [Google Scholar] [CrossRef]
- Sun, D.; Shi, M. Neutrophil swarming toward Cryptococcus neoformans is mediated by complement and leukotriene B4. Biochem. Biophys. Res. Commun. 2016, 477, 945–951. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, M.; Liu, G.; Wu, H.; Li, C.; Zhou, H.; Zhang, X.; Shi, M. Intravascular clearance of disseminating Cryptococcus neoformans in the brain can be improved by enhancing neutrophil recruitment in mice. Eur. J. Immunol. 2016, 46, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Roubey, R.A.; Ross, G.D.; Merrill, J.T.; Walton, F.; Reed, W.; Winchester, R.J.; Buyon, J.P. Staurosporine inhibits neutrophil phagocytosis but not iC3b binding mediated by CR3 (CD11b/CD18). J Immunol. 1991, 146, 3557–3562. [Google Scholar] [CrossRef]
- Podolnikova, N.P.; Kushchayeva, Y.S.; Wu, Y.; Faust, J.; Ugarova, T.P. The Role of Integrins αMβ2 (Mac-1, CD11b/CD18) and αDβ2 (CD11d/CD18) in Macrophage Fusion. Am. J. Pathol. 2016, 186, 2105–2116. [Google Scholar] [CrossRef]
- Filippi, M.-D. Neutrophil transendothelial migration: Updates and new perspectives. Blood 2019, 133, 2149–2158. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Xu, J.; Perkins, C.; Guo, F.; Snapper, S.; Finkelman, F.D.; Zheng, Y.; Filippi, M.-D. Cdc42 regulates neutrophil migration via crosstalk between WASp, CD11b, and microtubules. Blood 2012, 120, 3563–3574. [Google Scholar] [CrossRef] [PubMed]
- Cara, D.C.; Kaur, J.; Forster, M.; McCafferty, D.-M.; Kubes, P. Role of p38 Mitogen-Activated Protein Kinase in Chemokine-Induced Emigration and Chemotaxis In Vivo. J. Immunol. 2001, 167, 6552–6558. [Google Scholar] [CrossRef]
- Kim, D.; Haynes, C.L. The role of p38 MAPK in neutrophil functions: Single cell chemotaxis and surface marker expression. Analyst 2013, 138, 6826–6833. [Google Scholar] [CrossRef]
- Liu, X.; Ma, B.; Malik, A.B.; Tang, H.; Yang, T.; Sun, B.; Wang, G.; Minshall, R.D.; Li, Y.; Zhao, Y.; et al. Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat. Immunol. 2012, 13, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lam, B.K.; Kanaoka, Y.; Nigrovic, P.A.; Audoly, L.P.; Austen, K.F.; Lee, D.M. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J. Exp. Med. 2006, 203, 837–842. [Google Scholar] [CrossRef]
- Majumdar, R.; Tavakoli Tameh, A.; Arya, S.B.; Parent, C.A. Exosomes mediate LTB4 release during neutrophil chemotaxis. PLoS Biol. 2021, 19, e3001271. [Google Scholar] [CrossRef]
- Ng, L.G.; Qin, J.S.; Roediger, B.; Wang, Y.; Jain, R.; Cavanagh, L.L.; Smith, A.L.; Jones, C.A.; de Veer, M.; Grimbaldeston, M.A.; et al. Visualizing the Neutrophil Response to Sterile Tissue Injury in Mouse Dermis Reveals a Three-Phase Cascade of Events. J. Investig. Dermatol. 2011, 131, 2058–2068. [Google Scholar] [CrossRef]
- Rocha, J.D.B.; Nascimento, M.T.C.; Decote-Ricardo, D.; Côrte-Real, S.; Morrot, A.; Heise, N.; Nunes, M.P.; Previato, J.O.; Mendonça-Previato, L.; DosReis, G.A.; et al. Capsular polysaccharides from Cryptococcus neoformans modulate production of neutrophil extracellular traps (NETs) by human neutrophils. Sci. Rep. 2015, 5, 8008. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Ellerbroek, P.M.; Walenkamp, A.M.; Hoepelman, A.I.; Coenjaerts, F.E. Effects of the capsular polysaccharides of Cryptococcus neoformans on phagocyte migration and inflammatory mediators. Curr. Med. Chem. 2004, 11, 253–266. [Google Scholar] [CrossRef]
- Michaud, J.-P.; Bellavance, M.-A.; Préfontaine, P.; Rivest, S. Real-Time In Vivo Imaging Reveals the Ability of Monocytes to Clear Vascular Amyloid Beta. Cell Rep. 2013, 5, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Bryan, A.M.; Del Poeta, M.; Luberto, C. Sphingolipids as Regulators of the Phagocytic Response to Fungal Infections. Mediat. Inflamm. 2015, 2015, 640540. [Google Scholar] [CrossRef]
- Mednick, A.J.; Feldmesser, M.; Rivera, J.; Casadevall, A. Neutropenia alters lung cytokine production in mice and reduces their susceptibility to pulmonary cryptococcosis. Eur. J. Immunol. 2003, 33, 1744–1753. [Google Scholar] [CrossRef]
- Musubire, A.K.; Meya, D.B.; Rhein, J.; Meintjes, G.; Bohjanen, P.R.; Nuwagira, E.; Muzoora, C.; Boulware, D.R.; Hullsiek, K.H.; COAT and ASTRO Trial Teams. Blood neutrophil counts in HIV-infected patients with cryptococcal meningitis: Association with mortality. PLoS ONE 2018, 13, e0209337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, D.; Shi, M. Dancing cheek to cheek: Cryptococcus neoformans and phagocytes. Springerplus 2015, 4, 410. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, Z.W.; Strickland, A.B.; Shi, M. Cryptococcus neoformans Infection in the Central Nervous System: The Battle between Host and Pathogen. J. Fungi 2022, 8, 1069. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.A.; Andrews, A.; Wynn, C.; Barisch, C.; King, J.S.; Johnston, S.A. Cryptococcus neoformans Escape From Dictyostelium Amoeba by Both WASH-Mediated Constitutive Exocytosis and Vomocytosis. Front. Cell. Infect. Microbiol. 2018, 8, 108. [Google Scholar] [CrossRef]
- Chrisman, C.J.; Alvarez, M.; Casadevall, A. Phagocytosis of Cryptococcus neoformans by, and Nonlytic Exocytosis from, Acanthamoeba castellanii. Appl. Environ. Microbiol. 2010, 76, 6056–6062. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conn, B.N.; Wozniak, K.L. Innate Pulmonary Phagocytes and Their Interactions with Pathogenic Cryptococcus Species. J. Fungi 2023, 9, 617. https://doi.org/10.3390/jof9060617
Conn BN, Wozniak KL. Innate Pulmonary Phagocytes and Their Interactions with Pathogenic Cryptococcus Species. Journal of Fungi. 2023; 9(6):617. https://doi.org/10.3390/jof9060617
Chicago/Turabian StyleConn, Brittney N., and Karen L. Wozniak. 2023. "Innate Pulmonary Phagocytes and Their Interactions with Pathogenic Cryptococcus Species" Journal of Fungi 9, no. 6: 617. https://doi.org/10.3390/jof9060617
APA StyleConn, B. N., & Wozniak, K. L. (2023). Innate Pulmonary Phagocytes and Their Interactions with Pathogenic Cryptococcus Species. Journal of Fungi, 9(6), 617. https://doi.org/10.3390/jof9060617




