Fungal–Bacterial Co-Infections and Super-Infections among Hospitalized COVID-19 Patients: A Systematic Review
Abstract
1. Introduction
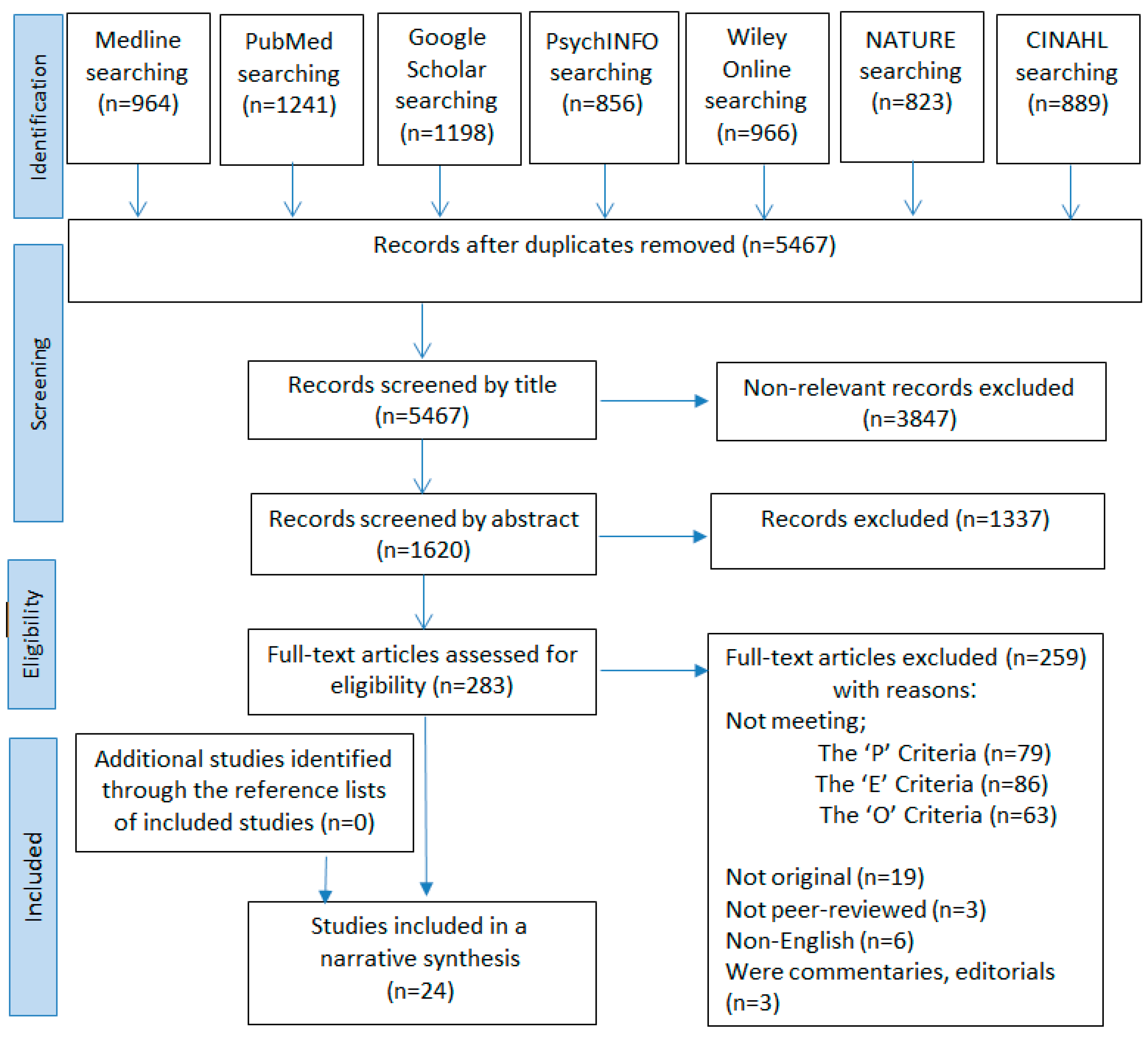
2. Methods
2.1. Protocol Registration
2.2. Eligibility Criteria
- Participants: patients of any age with a confirmed positive COVID-19 test who developed fungal–bacterial co-infections and super-infections during the hospital stay.
- Exposure: severe acute respiratory syndrome coronavirus 2.
- Outcome: fungal–bacterial co-infections and super-infections.
2.3. Search Strategy
2.4. Data Extraction
2.5. Assessment of Quality
2.6. Data Analysis
3. Results
3.1. Description of Articles
3.2. Quality Assessment
4. Discussion
4.1. Prevalence and Outcome
4.2. Antimicrobial Drugs
4.3. Detection Techniques
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acter, T.; Uddin, N.; Das, J.; Akhter, A.; Choudhury, T.R.; Kim, S. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: A global health emergency. Sci. Total Environ. 2020, 730, 138996. [Google Scholar] [CrossRef] [PubMed]
- Ienca, M.; Vayena, E. On the responsible use of digital data to tackle the COVID-19 pandemic. Nat. Med. 2020, 26, 463–464. [Google Scholar] [CrossRef] [PubMed]
- OCHA. Coronavirus Disease (COVID-19): Weekly Epidemiological Update. The United Nations Office for the Coordination of Humanitarian Affairs 2022. Available online: https://reliefweb.int/report/world/coronavirus-disease-covid-19-weekly-epidemiological-update-31-august-2022 (accessed on 31 August 2022).
- Yanes-Lane, M.; Winters, N.; Fregonese, F.; Bastos, M.; Perlman-Arrow, S.; Campbell, J.R.; Menzies, D. Proportion of asymptomatic infection among COVID-19 positive persons and their transmission potential: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0241536. [Google Scholar] [CrossRef]
- Desforges, M.; Gurdasani, D.; Hamdy, A.; Leonardi, A.J. uncertainty around the long-term implications of COVID-19. Pathogens 2021, 10, 1267. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, H.K.-A.; Mahmoud, M.A.; Aburahma, M.Z.; Elkhawaga, A.A.; El-Mokhtar, M.A.; Sayed, I.M.; Hosni, A.; Hassany, S.M.; Medhat, M.A. Predictors of severity and co-infection resistance profile in COVID-19 patients: First report from upper Egypt. Infect. Drug Resist. 2020, 13, 3409. [Google Scholar] [CrossRef]
- Randolph, H.E.; Barreiro, L.B. Herd immunity: Understanding COVID-19. Immunity 2020, 52, 737–741. [Google Scholar] [CrossRef]
- Viveiros-Rosa, S.G.; Mendes, C.D.; Farfán-Cano, G.G.; El-Shazly, M. The race for clinical trials on Omicron-based COVID-19 vaccine candidates: Updates from global databases. Narra J. 2022, 2, e88. [Google Scholar] [CrossRef]
- Masyeni, S.; Iqhrammullah, M.; Frediansyah, A.; Nainu, F.; Tallei, T.; Emran, T.B.; Ophinni, Y.; Dhama, K.; Harapan, H. Molnupiravir: A lethal mutagenic drug against rapidly mutating severe acute respiratory syndrome coronavirus 2—A narrative review. J. Med. Virol. 2022, 94, 3006–3016. [Google Scholar] [CrossRef]
- Chiari, W.; Damayanti, R.; Harapan, H.; Puspita, K.; Saiful, S.; Rahmi, R.; Rizki, D.R.; Iqhrammullah, M. Trend of polymer research related to COVID-19 pandemic: Bibliometric analysis. Polymers 2022, 14, 3297. [Google Scholar] [CrossRef]
- Hoque, M.N.; Akter, S.; Mishu, I.D.; Islam, M.R.; Rahman, M.S.; Akhter, M.; Islam, I.; Hasan, M.M.; Rahaman, M.M.; Sultana, M. Microbial co-infections in COVID-19: Associated microbiota and underlying mechanisms of pathogenesis. Microb. Pathog. 2021, 156, 104941. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M. Incidence of co-infections and super-infections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- Brandi, N.; Ciccarese, F.; Balacchi, C.; Rimondi, M.R.; Modolon, C.; Sportoletti, C.; Capozzi, C.; Renzulli, M.; Paccapelo, A.; Castelli, A. Co-Infections and Super-infections in COVID-19 Critically Ill Patients Are Associated with C.T. Imaging Abnormalities and the Worst Outcomes. Diagnostics 2022, 12, 1617. [Google Scholar] [CrossRef] [PubMed]
- Buehler, P.K.; Zinkernagel, A.S.; Hofmaenner, D.A.; Garcia, P.D.W.; Acevedo, C.T.; Gómez-Mejia, A.; Shambat, S.M.; Andreoni, F.; Maibach, M.A.; Bartussek, J. Bacterial pulmonary super-infections are associated with longer duration of ventilation in critically ill COVID-19 patients. Cell Rep. Med. 2021, 2, 100229. [Google Scholar] [CrossRef] [PubMed]
- SeyedAlinaghi, S.; Abbasian, L.; Solduzian, M.; Ayoobi Yazdi, N.; Jafari, F.; Adibimehr, A.; Farahani, A.; Salami Khaneshan, A.; Ebrahimi Alavijeh, P.; Jahani, Z. Predictors of the prolonged recovery period in COVID-19 patients: A cross-sectional study. Eur. J. Med. Res. 2021, 26, 41. [Google Scholar] [CrossRef]
- Bassetti, M.; Giacobbe, D.R.; Bruzzi, P.; Barisione, E.; Centanni, S.; Castaldo, N.; Corcione, S.; De Rosa, F.G.; Di Marco, F.; Gori, A. Clinical management of adult patients with COVID-19 outside intensive care units: Guidelines from the Italian Society of Anti-Infective Therapy (SITA) and the Italian Society of Pulmonology (SIP). Infect. Dis. Ther. 2021, 10, 1837–1885. [Google Scholar] [CrossRef]
- Silva, M.C.; Werlang, M.H.; Vandresen, D.F.; Fortes, P.C.; Pascotto, C.R.; Lúcio, L.C.; Ferreto, L.E. Genetic, antimicrobial resistance profile and mortality rates of Acinetobacter baumannii infection in Brazil: A systematic review. Narra J. 2022, 2, e68. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group*, T. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 769–777. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; Yu, T. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Zhu, X.; Ge, Y.; Wu, T.; Zhao, K.; Chen, Y.; Wu, B.; Zhu, F.; Zhu, B.; Cui, L. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020, 285, 198005. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Giordano, C.; Leonildi, A.; Menichini, M.; Vecchione, A.; Pistello, M.; Guarracino, F.; Ghiadoni, L.; Forfori, F. Predictors of hospital-acquired bacterial and fungal super-infections in COVID-19: A prospective observational study. J. Antimicrob. Chemother. 2021, 76, 1078–1084. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L. Bacterial and fungal co-infection among hospitalized patients with COVID-19: A retrospective cohort study in a U.K. secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Z.M.; Chen, L.D.; Zhan, Y.Q.; Li, S.Q.; Cheng, J.; Zhu, A.R.; Chen, L.Y.; Zhong, N.S.; Li, S.Y. Co-infection with SARS-CoV-2 and other respiratory pathogens in patients with COVID-19 in Guangzhou, China. J. Med. Virol. 2020, 92, 2381. [Google Scholar] [CrossRef] [PubMed]
- Intra, J.; Sarto, C.; Beck, E.; Tiberti, N.; Leoni, V.; Brambilla, P. Bacterial and fungal colonization of the respiratory tract in COVID-19 patients should not be neglected. Am. J. Infect. Control. 2020, 48, 1130–1131. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, M.A.; Tetaj, N.; Selleri, M.; Marchioni, L.; Capone, A.; Caraffa, E.; Di Caro, A.; Petrosillo, N. Incidence of bacterial and fungal bloodstream infections in COVID-19 patients in intensive care: An alarming ”collateral effect”. J. Glob. Antimicrob. Resist. 2020, 23, 290–291. [Google Scholar] [CrossRef]
- Nasir, N.; Farooqi, J.; Mahmood, S.F.; Jabeen, K. COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: An observational study from Pakistan. Mycoses 2020, 63, 766–770. [Google Scholar] [CrossRef]
- Sepulveda, J.; Westblade, L.F.; Whittier, S.; Satlin, M.J.; Greendyke, W.G.; Aaron, J.G.; Zucker, J.; Dietz, D.; Sobieszczyk, M.; Choi, J.J. Bacteremia and blood culture utilization during COVID-19 surge in New York City. J. Clin. Microbiol. 2020, 58, e00875-20. [Google Scholar] [CrossRef]
- Wang, L.; Amin, A.K.; Khanna, P.; Aali, A.; McGregor, A.; Bassett, P.; Gopal Rao, G. An observational cohort study of bacterial co-infection and implications for empirical antibiotic therapy in patients presenting with COVID-19 to hospitals in North West London. J. Antimicrob. Chemother. 2021, 76, 796–803. [Google Scholar] [CrossRef]
- May, A.; Swetenham, N.; Pandey, M.; Taylor, V.; Hughes, H.; Underwood, J. Bacterial and fungal respiratory co-infection among patients admitted to ICU with COVID-19: A retrospective cohort study in a U.K. hospital. Gut 2021, 70 (Suppl. 1), A196–A197. [Google Scholar]
- Yang, S.; Hua, M.; Liu, X.; Du, C.; Pu, L.; Xiang, P.; Wang, L.; Liu, J. Bacterial and fungal co-infections among COVID-19 patients in intensive care unit. Microbes Infect. 2021, 23, 104806. [Google Scholar] [CrossRef] [PubMed]
- Gerver, S.M.; Guy, R.; Wilson, K.; Thelwall, S.; Nsonwu, O.; Rooney, G.; Brown, C.S.; Muller-Pebody, B.; Hope, R.; Hall, V. National surveillance of bacterial and fungal co-infection and secondary infection in COVID-19 patients in England: Lessons from the first wave. Clin. Microbiol. Infect. 2021, 27, 1658–1665. [Google Scholar] [CrossRef]
- Nori, P.; Cowman, K.; Chen, V.; Bartash, R.; Szymczak, W.; Madaline, T.; Katiyar, C.P.; Jain, R.; Aldrich, M.; Weston, G. Bacterial and fungal co-infections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect. Control. Hosp. Epidemiol. 2021, 42, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Azzam Lopez, A.; Diez-Remesal, Y.; Martinez Castro, N.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Shafiekhani, M.; Shekari, Z.; Boorboor, A.; Zare, Z.; Arabsheybani, S.; Azadeh, N. Bacterial and fungal co-infections with SARS-CoV-2 in solid organ recipients: A retrospective study. Virol. J. 2022, 19, 35. [Google Scholar] [CrossRef]
- Ruiz-Rodriguez, A.; Lusarreta-Parga, P.; de Steenhuijsen Piters, W.A.; Koppensteiner, L.; Balcazar-Lopez, C.E.; Campbell, R.; Dewar, R.; McHugh, M.P.; Dockrell, D.; Templeton, K.E. Bacterial and fungal communities in tracheal aspirates of intubated COVID-19 patients: A pilot study. Sci. Rep. 2022, 12, 9896. [Google Scholar] [CrossRef]
- Alnimr, A.M.; Alshahrani, M.S.; Alwarthan, S.; AlQahtani, S.Y.; Hassan, A.A.; BuMurah, N.N.; Alhajiri, S.; Bukharie, H. Bacterial and Fungal Coinfection in Critically Ill COVID-19 Cases and Predictive Role of Procalcitonin During the First Wave at an Academic Health Center. J. Epidemiol. Glob. Health 2022, 12, 188–195. [Google Scholar] [CrossRef]
- Naseef, H.A.; Mohammad, U.; Al-Shami, N.; Sahoury, Y.; Abukhalil, A.D.; Dreidi, M.; Alsahouri, I.; Farraj, M. Bacterial and fungal co-infections among ICU COVID-19 hospitalized patients in a Palestinian hospital: A retrospective cross-sectional study. F1000Research 2022, 11, 30. [Google Scholar] [CrossRef]
- Nebreda-Mayoral, T.; Miguel-Gómez, M.A.; March-Rosselló, G.A.; Puente-Fuertes, L.; Cantón-Benito, E.; Martínez-García, A.M.; Muñoz-Martín, A.B.; Orduña-Domingo, A. Bacterial/fungal infection in hospitalized patients with COVID-19 in a tertiary hospital in the Community of Castilla y León, Spain. Enferm. Infecc. Y Microbiol. Clin. (Engl. Ed.) 2022, 40, 158–165. [Google Scholar] [CrossRef]
- NIH. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. National Institutes of Health: Bethesda, MD, USA, 2014. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 6 September 2022).
- Popay, J.; Roberts, H.; Sowden, A.; Petticrew, M.; Arai, L.; Rodgers, M.; Britten, N.; Roen, K.; Duffy, S. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. A Prod. ESRC Methods Programme Version 2006, 1, b92. [Google Scholar]
- Ramirez-Santana, M. Limitations and Biases in Cohort Studies; IntechOpen: London, UK, 2018. [Google Scholar]
- Naumenko, V.; Turk, M.; Jenne, C.N.; Kim, S.-J. Neutrophils in viral infection. Cell Tissue Res. 2018, 371, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.j.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.L.; Fu, W.; Li, W. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef]
- Lu, D.-E.; Hung, S.-H.; Su, Y.-S.; Lee, W.-S. Analysis of Fungal and Bacterial Co-Infections in Mortality Cases among Hospitalized Patients with COVID-19 in Taipei, Taiwan. J. Fungi 2022, 8, 91. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Q.; Mei, H.; Zheng, H.; Liang, G.; She, X.; Liu, W. Fungal co-infection in COVID-19 patients: Evidence from a systematic review and meta-analysis. Aging, 2021; 13, 7745. [Google Scholar]
- Gerriets, V.; Goyal, A.; Khaddour, K. Tumor Necrosis Factor Inhibitors. [Updated 2021 July 18]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lai, C.-C.; Wang, C.-Y.; Hsueh, P.-R. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 2020, 53, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Mughal, N.; Moore, L.S. Procalcitonin to Guide Antibacterial Prescribing in Patients Hospitalised with COVID-19. Antibiotics 2021, 10, 1119. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.; Poudel, A.N.; Alhusein, N.; Wang, H.; Yao, G.; Lambert, H. Antimicrobial use in COVID-19 patients in the first phase of the SARS-CoV-2 pandemic: A scoping review. Antibiotics 2021, 10, 745. [Google Scholar] [CrossRef]
- Zhou, D.; Dai, S.-M.; Tong, Q. COVID-19: A recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J. Antimicrob. Chemother. 2020, 75, 1667–1670. [Google Scholar] [CrossRef]
- Soni, S.; Namdeo Pudake, R.; Jain, U.; Chauhan, N. A systematic review on SARS-CoV-2-associated fungal co-infections. J. Med. Virol. 2022, 94, 99–109. [Google Scholar] [CrossRef]
| Participants | exp patients/or admitted* OR hospitalized* OR infected* OR positive COVID-19 |
| AND | |
| Exposure | Coronavirus infection* OR exp SARS coronavirus/or exp severe acute respiratory syndrome/OR COVID OR SARS |
| AND | |
| Outcomes | exp mixed infection/OR ‘bacterial infection’ OR exp fungal infection/or exp co-infection/or exp co-infection/or co-infect/or exp super-infection/or exp super-infection* or exp coinfect/or exp concomitant infect/OR concurrent infection* OR exp mixed infect/or exp anxiety disorders/OR exp stress, psychological/or ‘psychological distress*’ |
| Study | Country | Patients with SARS-CoV-2 Who Underwent Co-Pathogen Testing: n | Patients with Co-Infection, n (%) | ICU Admissions, n (%) | Mechanical Ventilation, n (%) | Deaths, n (%) | Bacterial Co-Infection,n (%) | Fungal Co-Infection,n (%) | Organisms | Antimicrobials Use, n |
|---|---|---|---|---|---|---|---|---|---|---|
| Wang et al. (2020) [19] | China | 57 | 9 (31.5) | NM | NM | 5 (7.5) | 5 (17.2) | 2 (6.9) | Bacteria Fungi | 39 F.Q. 8 Antifungal agents |
| Yang et al. (2020) [20] | China | 53 | 7 (13.5) | 52 (100.0) | 37 (71.0) | 32 (61.5) | 4 (7.7) | 3 (5.8) | Bacteria Fungi | NM |
| Zhu et al. (2020) [21] | China | 257 | 243 (94.5) | 3 (1.2) | 0 | 0 | 236 (91.8) | 60 (23.3) | Bacteria Fungi | NM |
| Falcone et al. (2020) [22] | Italy | 315 | 69 (21.9) | 56 (71.6) | 43 (62.4) | 13 (18.8) | 11 (15.6) | 5 (5.5) | Bacteria Fungi | 20 DOX 9 AZM 27 CRO 23 TZP 2 F.Q. |
| Chen et al. (2020) [23] | China | 99 | 5 (5) | 23 (23) | 17 (17) | 11 (11) | 1 (1) | 4 (4) | Bacteria Fungi | 7 CEP, CAR, TGC, and LZD 15 Antifungals |
| Hughes et al. (2020) [24] | UK | 836 | 51 (6.1) | 3 (5.9) | NM | NM | 51 (6.1) | 30 (3.6) | Bacteria Fungi | NM |
| Li et al. (2020) [25] | China | 32 | 14 (43.7) | 11 (78.6) | 4 (28.6) | N.M. | 10 (31.2) | 7 (21.9) | Bacteria Fungi | NM |
| Intra et al. (2020) [26] | Egypt | 260 | 28 (10.8) | 60 (23.0) | 8 (13.3) | 24 (40.0) | 28 (10.8) | 5 (1.9) | Bacteria Fungi | 28 CLR and AZM |
| Cataldo et al. (2020) [27] | UK | 77 | 39 (50.6) | 39 (100.0) | NM | NM | 28 (36.4) | 11 (14.3) | Bacteria Fungi | NM |
| Nasir et al. (2020) [28] | US | 140 | 57 (40.7) | 57 (100.0) | 56 (98.0) | 31 (54.0) | 51 (36.4) | 6 (4.3) | Bacteria Fungi | 53 CEP 53 CLR and AZM 47 Other antibiotics |
| Ramadan et al. (2020) [6] | Italy | 61 | 35 | 35 (100.0) | 35 (100.0) | N.M. | 13 (37.1) | 19 (54.3) | Bacteria Fungi | 17 β-Lactamase inhibitors, VAN, CAR, or MET |
| Sepulveda et al. (2020) [29] | Italy | NM | 57 | 57 (100.0) | 48 (84.0) | 18 (32.0) | 27 (47.4) | 28 (49.0) | Bacteria Fungi | 2 VAN 4 TZP 1 CAR |
| Wang et al. (2021) [30] | Pakistan | 23 | 9 (39.1) | 23 (100.0) | 2 (22.2) | 4 (17.4) | 9 (39.1) | 5 (21.7) | Bacteria Fungi | 7 CLR and AZM 5 Antifungals |
| May et al. (2021) [31] | US | 4185 | 159 (3.8) | NM | NM | NM | 156 (3.7) | 3 (0.07) | Bacteria Fungi | NM |
| Yang et al. (2021) [32] | UK | 1396 | 37 (2.7) | 11 (29.7) | N.M. | 10 (27.0) | 37 (2.7) | 4 (0.3) | Bacteria Fungi | NM |
| Gerver et al. (2021) [33] | China | NM | 20 | 20 (100.0) | 12 (60.0) | N.M. | 96 (100.0) | 3 (42.9) | Bacteria | NM |
| Nori et al. (2021) [34] | UK | 2279 | 879 (38.6) | NM | NM | 202 (23.0) | 404 (45.9) | 475 (54.0) | Bacteria | NM |
| Bardi et al. (2021) [35] | US | 4267 | 152 | 99 (65.0) | 112 (74.0) | 87 (57.0) | 112 (73.7) | 5 (3.6) | Bacteria Fungi | 4130 DOX, AZM, LVX, CIP, CRO, FEP, VAN, and TZP |
| Shafiekhani et al. (2022) [36] | Iran | 97 | 66 (68) | 97 (100.0) | 18 (18.6) | 6 (6.2) | 13 (13.4) | 9 (9.3) | Bacteria Fungi | 5 VAN 6 CRE |
| Ruiz-Rodriguez et al. (2022) [37] | Scotland | NM | 30 | 30 (100.0) | 25 (83.3) | 10 (33.3) | 9 (30.0) | 10 (33.0) | Bacteria Fungi | NM |
| Alnimr et al. (2022) [38] | Saudi Arabia | 1091 | 135 (12.4) | 182 (17.9) | 63 (92.6) | 70 (6.4) | 67 (6.6) | 67 (6.6) | Bacteria Fungi | NM |
| Naseef et al. (2022) [39] | Palestine | 458 | 321 (70.1) | 321 (100.0) | N.M. | 26 (8.1) | 164 (51.1) | 157 (48.9) | Bacteria Fungi | 138 MEM and VAN 114 TZP and LVX |
| Nebreda-Mayoral et al. (2022) [40] | Spain | 712 | 113 (15.9) | 50 (7.0) | 50 (44.0) | 43 (38.0) | 39 (5.0) | 80 (11.0) | Bacteria | 26 TZP 21 CAR 20 LZD 15 LVX |
| Shafiekhani et al. (2022) [36] | Iran | 66 | 14 (21.2) | 14 (100.0) | 5 (35.7) | 5 (35.7) | 8 (57.1) | 14 (100.0) | Bacteria Fungi | VAN MET CAR |
| Study | Quality Rating | Quality Appraisal Findings |
|---|---|---|
| Wang et al. (2020) [19] | Poor | Retrospective cohort Single-center study Small sample size and not justified (n = 57) |
| Yang et al. (2020) [20] | Poor | Retrospective cohort Single-center study Small sample size and not justified (n = 52) |
| Zhu et al. (2020) [21] | Fair | Retrospective cohort Single-center study Sample size was satisfactory and justified (n = 257) |
| Falcone et al. (2020) [22] | Poor | Retrospective cohort Single-center study Small sample size and not justified (n = 69) |
| Chen et al. (2020) [23] | Poor | Retrospective cohort Single-center study Small sample size and not justified (n = 99) |
| Hughes et al. (2020) [24] | Good | Retrospective cohort Multi-center study Sample size was satisfactory and justified (n = 836) |
| Li et al. (2020) [25] | Fair | Retrospective cohort Multi-center study Small sample size and not justified (n = 32) |
| Intra et al. (2020) [26] | Poor | Retrospective cohort Single-center study Small sample size and not justified (n = 35) |
| Cataldo et al. (2020) [27] | Poor | Retrospective cohort Single-center study Small sample size and not justified (n = 57) |
| Nasir et al. (2020) [28] | Poor | Retrospective cohort Single-center study Small sample size and not justified (n = 23) |
| Ramadan et al. (2020) [6] | Good | Prospective cohort Multi-center study Sample size was satisfactory and justified (n = 260) |
| Sepulveda et al. (2020) [29] | Good | Retrospective cohort Multi-center study Sample size was satisfactory and justified (n = 4185) |
| Wang et al. (2021) [30] | Good | Retrospective cohort Multi-center study Sample size was satisfactory and justified (n = 1396) |
| May et al. (2021) [31] | Poor | Retrospective cohort Single-center study Small sample size and not justified (n = 77) |
| Yang et al. (2021) [32] | Poor | Retrospective cohort Single-center study Small sample size and not justified (n = 77) |
| Gerver et al. (2021) [33] | Fair | Retrospective cohort Single-center study Sample size was satisfactory and justified (n = 879) |
| Nori et al. (2021) [34] | Poor | Retrospective cohort Single-center study Small sample size and not justified (n = 152) |
| Bardi et al. (2021) [35] | Poor | Retrospective cohort Single-center study Small sample size and not justified (n = 140) |
| Shafiekhani et al. (2022) [36] | Poor | Retrospective cohort Single-center study Small sample size and not justified (n = 97) |
| Ruiz-Rodriguez et al. (2022) [37] | Poor | Retrospective cohort Single-center study Small sample size and not justified (n = 30) |
| Alnimr et al. (2022) [38] | Fair | Retrospective cohort Single-center study Sample size was satisfactory and justified (n = 1091) |
| Naseef et al. (2022) [39] | Fair | Retrospective cohort Single-center study Sample size was satisfactory and justified (n = 321) |
| Nebreda-Mayoral et al. (2022) [40] | Fair | Retrospective cohort Single-center study Sample size was satisfactory and justified (n = 712) |
| Shafiekhani et al. (2022) [36] | Poor | Retrospective cohort Single-center study Small sample size and not justified (n = 30) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bantun, F. Fungal–Bacterial Co-Infections and Super-Infections among Hospitalized COVID-19 Patients: A Systematic Review. J. Fungi 2023, 9, 598. https://doi.org/10.3390/jof9060598
Bantun F. Fungal–Bacterial Co-Infections and Super-Infections among Hospitalized COVID-19 Patients: A Systematic Review. Journal of Fungi. 2023; 9(6):598. https://doi.org/10.3390/jof9060598
Chicago/Turabian StyleBantun, Farkad. 2023. "Fungal–Bacterial Co-Infections and Super-Infections among Hospitalized COVID-19 Patients: A Systematic Review" Journal of Fungi 9, no. 6: 598. https://doi.org/10.3390/jof9060598
APA StyleBantun, F. (2023). Fungal–Bacterial Co-Infections and Super-Infections among Hospitalized COVID-19 Patients: A Systematic Review. Journal of Fungi, 9(6), 598. https://doi.org/10.3390/jof9060598





