Genetic and Environmental Factors Influencing the Production of Select Fungal Colorants: Challenges and Opportunities in Industrial Applications
Abstract
1. Introduction
2. Carotenoids
2.1. Carotenoids and Carotenoid Synthesis Pathways
2.2. Production of Carotenoids
3. Melanins
3.1. Melanins and Melanin Synthesis Pathways
3.2. Production of Fungal Melanins
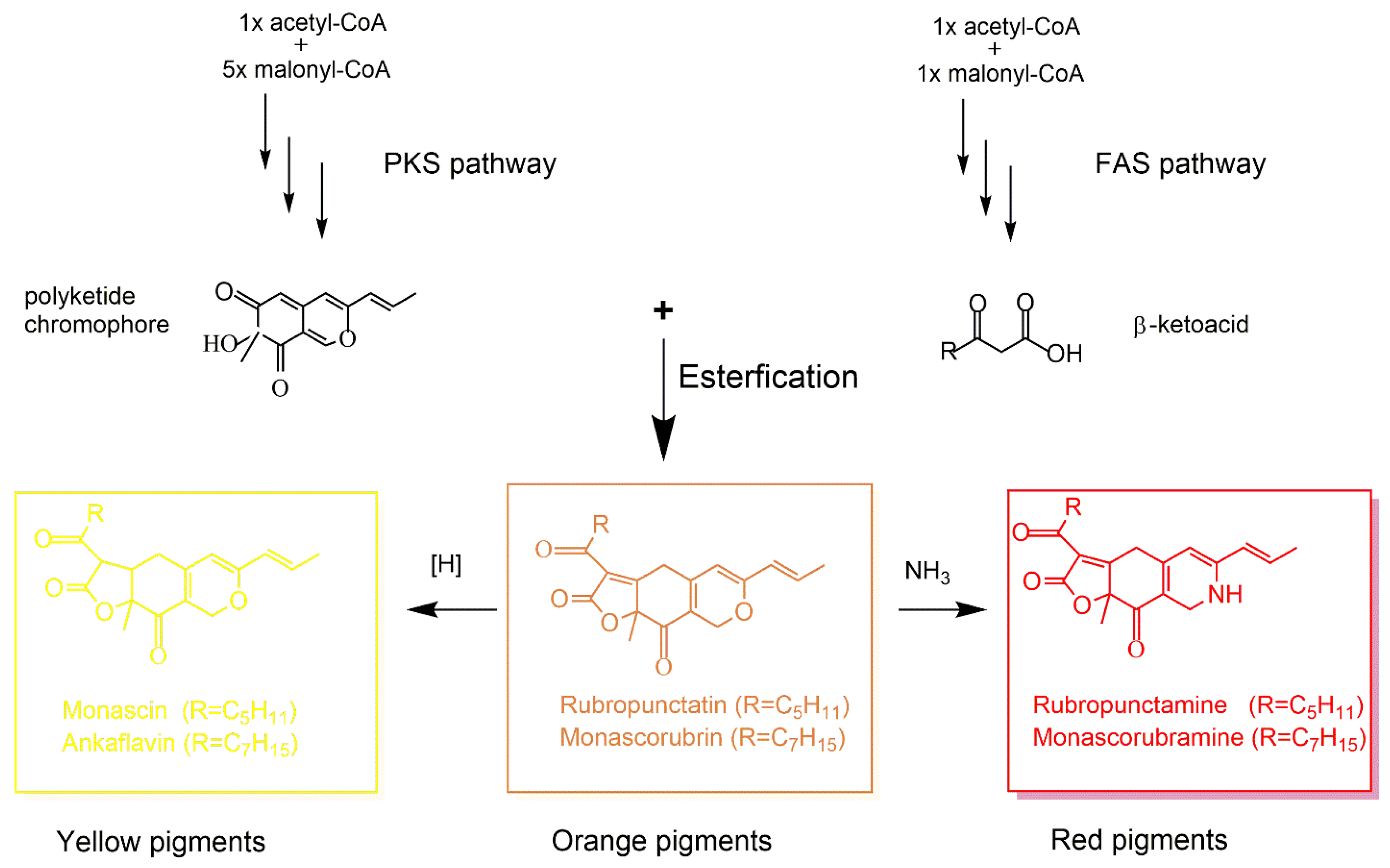
4. Fungal Polyketide-Derived Colorants
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demain, A.L.; Vandamme, E.J.; Collins, J.; BuchhoIz, K. History of industrial biotechnology. In Industrial Biotechnology: Microorganisms; Wittmann, C., Liao, J.C., Eds.; Wiley-VCH: Weinheim, Germany, 2017; pp. 3–84. [Google Scholar]
- Chatragadda, R.; Dufossé, L. Ecological and biotechnological aspects of pigmented microbes: A way forward in development of food and pharmaceutical grade pigments. Microorganisms 2021, 9, 637. [Google Scholar] [CrossRef]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial pigments in the food industry—Challenges and the way forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Oplatowska-Stachowiak, M.; Christopher, E.T. Food colours: Existing and emerging food safety concerns. Crit. Rev. Food Sci. Nutr. 2015, 57, 524–548. [Google Scholar] [CrossRef]
- Dikshit, R.; Tallapragada, P. Comparative study of natural and artificial flavoring agents and dyes. In Natural and Artificial Flavoring Agents and Food Dyes; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier: London, UK, 2018; Volume 7, pp. 83–111. [Google Scholar]
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: A randomised, double-blinded, placebo controlled trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, J.P. Fungal pigments and their roles associated with human health. J. Fungi 2020, 6, 280. [Google Scholar] [CrossRef]
- Venil, C.K.; Velmurugan, P.; Dufossé, L.; Devi, P.R.; Ravi, A.V. Fungal pigments: Potential coloring compounds for wide ranging applications in textile dyeing. J. Fungi 2020, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Dyes and pigments. In SpringerBriefs in Green Chemistry for Sustainability; Sharma, S.K., Ed.; Springer International Publishing AG: Cham, Switzerland, 2016; pp. 13–29. [Google Scholar]
- Urista, C.M.; Rodríguez, J.G.; Corona, A.A.; Cuenca, A.A.; Jurado, A.T. Pigments from fungi, an opportunity of production for diverse applications. Biologia 2016, 71, 1067–1079. [Google Scholar] [CrossRef]
- Sandmann, G. Carotenoids and their biosynthesis in fungi. Molecules 2022, 27, 1431. [Google Scholar] [CrossRef]
- Nanou, K.; Roukas, T. Waste cooking oil: A new substrate for carotene production by Blakeslea trispora in submerged fermentation. Bioresour. Technol. 2016, 203, 198–203. [Google Scholar] [CrossRef]
- Luo, W.; Xue, C.; Zhao, Y.; Zhang, H.; Rao, Z.; Yu, X. Blakeslea trispora photoreceptors: Identification and functional analysis. Appl. Environ. Microbiol. 2020, 86, e02962-19. [Google Scholar] [CrossRef]
- Bo, S.; Ni, X.; Guo, J.; Liu, Z.; Wang, X.; Sheng, Y.; Zhang, G.; Yang, J. Carotenoid biosynthesis: Genome-wide profiling, pathway identification in Rhodotorula glutinis X-20, and high-level production. Front. Nutr. 2022, 9, 918240. [Google Scholar] [CrossRef] [PubMed]
- Nutakor, C.; Kanwugu, O.N.; Kovaleva, E.G.; Glukhareva, T.V. Enhancing astaxanthin yield in Phaffia rhodozyma: Current trends and potential of phytohormones. Appl. Microbiol. Biotechnol. 2022, 106, 3531–3538. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sáiz, M.; Paz, B.; de la Fuente, J.L.; Lo´pez-Nieto, M.J.; Cabri, W.; Barredo, J.L. Blakeslea trispora genes for carotene biosynthesis. Appl. Eniviron. Microbiol. 2004, 70, 5589–5594. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Schmidt-Dannert, C. Metabolic engineering towards biotechnological production of carotenoids in microorganisms. Appl. Microbiol. Biotechnol. 2002, 60, 1–11. [Google Scholar] [PubMed]
- Mussagy, C.U.; Winterburn, J.; Santos-Ebinuma, V.C.; Pereira, J.F.B. Production and extraction of carotenoids produced by microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 1095–1114. [Google Scholar] [CrossRef]
- Sun, J.; Sun, X.X.; Tang, P.W.; Yuan, Q.P. Molecular cloning and functional expression of two key carotene synthetic genes derived from Blakeslea trispora into E. coli for increased β-carotene production. Biotechnol. Lett. 2012, 34, 2077–2082. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, C.; Bi, C.; Li, Q.; Zhang, X. Combinatory optimization of chromosomal integrated mevalonate pathway for β-carotene production in Escherichia coli. Microb. Cell Fact. 2016, 15, 202. [Google Scholar] [CrossRef]
- Verwaal, R.; Wang, J.; Meijnen, J.P.; Visser, H.; Sandmann, G.; van den Berg, J.A.; van Ooyen, A.J. High-level production of β-carotene in Saccharomyces cerevisiae by successive transformation with carotenogenic genes from Xanthophyllomyces dendrorhous. Appl. Environ. Microbiol. 2007, 73, 4342–4350. [Google Scholar] [CrossRef]
- Liu, L.; Qu, Y.L.; Dong, G.R.; Wang, J.; Hu, C.Y.; Meng, Y.H. Elevated β-carotene production using codon-adapted CarRA &B and metabolic balance in engineered Yarrowia lipolytica. Front. Microbiol. 2021, 12, 627150. [Google Scholar]
- Zhang, Y.; Navarro, E.; Cánovas-Márquez, J.T.; Almagro, L.; Chen, H.; Chen, Y.Q.; Zhang, H.; Torres-Martínez, S.; Chen, W.; Garre, V. A new regulatory mechanism controlling carotenogenesis in the fungus Mucor circinelloides as a target to generate β-carotene over-producing strains by genetic engineering. Microb. Cell Fact. 2016, 15, 99. [Google Scholar] [CrossRef]
- Dzurendova, S.; Zimmermann, B.; Kohler, A.; Reitzel, K.; Nielsen, U.G.; Dupuy-Galet, B.X.; Leivers, S.; Horn, S.J.; Shapaval, V. Calcium affects polyphosphate and lipid accumulation in Mucoromycota fungi. J. Fungi 2021, 7, 300. [Google Scholar] [CrossRef] [PubMed]
- Avalos, J.; Limon, M.C. Biological roles of fungal carotenoids. Curr. Genet. 2015, 61, 309–324. [Google Scholar] [CrossRef]
- Echavarri-Erasun, C.; Johnson, E.A. Stimulation of astaxanthin formation in the yeast Xanthophyllomyces dendrorhous by the fungus Epicoccum nigrum. FEMS Yeast Res. 2004, 4, 511–519. [Google Scholar] [CrossRef]
- Ge, X.; Li, R.; Zhang, X.; Zhao, J.; Zhang, Y.; Xin, Q. Transcriptome sequencing and global analysis of blue light-responsive genes provide clues for high carotenoid yields in Blakeslea trispora. Int. Microbiol. 2022, 25, 325–338. [Google Scholar] [CrossRef]
- Bindl, E.; Lang, W.; Rau, W. Light dependent carotenoid synthesis. 6. Time course of synthesis of various carotenoids in Fusarium aquaeductuum after various inductive treatments. Planta 1970, 94, 156–174. [Google Scholar] [CrossRef] [PubMed]
- Avalos, J.; Schrott, E.L. Photoinduction of carotenoid biosynthesis in Gibberella fujikuroi. FEMS Microbiol. Lett. 1990, 66, 295–298. [Google Scholar] [CrossRef]
- Rodríguez-Ortiz, R.; Michielse, C.; Rep, M.; Limón, M.C.; Avalos, J. Genetic basis of carotenoid overproduction in Fusarium oxysporum. Fungal Genet. Biol. 2012, 49, 684–696. [Google Scholar] [CrossRef]
- Rodríguez-Ortiz, R.; Limón, M.C.; Avalos, J. Functional analysis of the carS gene of Fusarium fujikuroi. Mol. Genet. Genomics 2013, 288, 157–173. [Google Scholar] [CrossRef]
- Castrillo, M.; Avalos, J. The flavoproteins CryD and VvdA cooperate with the white collar protein WcoA in the control of photocarotenogenesis in Fusarium fujikuroi. PLoS ONE 2015, 10, e0119785. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Zhan, W.; Li, Y.; Wang, X. Temperature influences β-carotene production in recombinant Saccharomyces cerevisiae expressing carotenogenic genes from Phaffia rhodozyma. World J. Microbiol. Biotechnol. 2014, 30, 125–133. [Google Scholar] [CrossRef]
- Kalra, R.; Conlan, X.A.; Goel, M. Fungi as a potential source of pigments: Harnessing filamentous fungi. Front. Chem. 2020, 8, 369. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Jackson, S.A.; Patry, S.; Dobson, A.D.W. Extending the “One Strain Many Compounds” (OSMAC) principle to marine microorganisms. Mar. Drugs 2018, 16, 244. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Park, H.M.; Kim, J.E.; Lee, S.H.; Choi, M.S.; Kim, J.Y.; Oh, D.K.; Keasling, J.D.; Kim, S.W. Increased β-carotene production in recombinant Escherichia coli harboring an engineered isoprenoid precursor pathway with mevalonate addition. Biotechnol. Prog. 2007, 23, 599–605. [Google Scholar] [CrossRef]
- Alper, H.; Miyaoku, K.; Stephanopoulos, G. Construction of lycopene-overproducing E. coli strains by combining systematic and combinatorial gene knockout targets. Nat. Biotechnol. 2005, 23, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Yamane, Y.; Higashida, K.; Nakashimada, Y.; Kakizono, T.; Nishio, N. Astaxanthin production by Phaffia rhodozyma enhanced in fed-batch culture with glucose and ethanol feeding. Biotechnol. Lett. 1997, 19, 1109–1111. [Google Scholar] [CrossRef]
- Choudhari, S.M.; Ananthanarayan, L.; Singhal, R.S. Use of metabolic stimulators and inhibitors for enhanced production of β-carotene and lycopene by Blakeslea trispora NRRL 2895 and 2896. Biores. Technol. 2008, 99, 3166–3173. [Google Scholar] [CrossRef] [PubMed]
- Naz, T.; Nazir, Y.; Nosheen, S.; Ullah, S.; Halim, H.; Fazili, A.B.A.; Li, S.; Mustafa, K.; Mohamed, H.; Yang, W.; et al. Redirecting metabolic flux towards the mevalonate pathway for enhanced β-carotene production in M. circinelloides CBS 277.49. BioMed Res. Intl. 2020, 2020, 8890269. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Yang, Q.; Zhao, J.; Feng, L.; Wang, M. SR5AL serves as a key regulatory gene in lycopene biosynthesis by Blakeslea trispora. Microb. Cell Fact. 2022, 21, 126. [Google Scholar] [CrossRef]
- Singh, S.; Nimse, S.B.; Mathew, D.E.; Dhimmar, A.; Sahastrabudhe, H.; Gajjar, A.; Ghadge, V.A.; Kumar, P.; Shinde, P.B. Microbial melanin: Recent advances in biosynthesis, extraction, characterization, and applications. Biotechnol. Adv. 2021, 53, 107773. [Google Scholar] [CrossRef]
- Pralea, I.-E.; Moldovan, R.-C.; Petrache, A.-M.; Ilieș, M.; Hegheș, S.-C.; Ielciu, I.; Nicoară, R.; Moldovan, M.; Ene, M.; Radu, M.; et al. From extraction to advanced analytical methods: The challenges of melanin analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef]
- Lu, Y.; Ye, M.; Song, S.; Li, L.; Shaikh, F.; Li, J. Isolation, purification, and antiaging activity of melanin from Lachnum singerianum. Appl. Biochem. Biotechnol. 2014, 174, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Schmaler-Ripcke, J.; Sugareva, V.; Gebhardt, P.; Winkler, R.; Kniemeyer, O.; Heinekamp, T.; Brakhage, A.A. Production of pyomelanin, a second type of melanin, via the tyrosine degradation pathway in Aspergillus fumigatus. Appl. Environ. Microbiol. 2009, 75, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Vasanthakumar, A.; DeAraujo, A.; Mazurek, J.; Schilling, M.; Mitchell, R. Pyomelanin production in Penicillium chrysogenum is stimulated by L-tyrosine. Microbiology 2015, 161, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, K.; Streibel, M.; Jahn, B.; Gerhard Haase, G.; Brakhage, A.A. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 2003, 38, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Valiante, V.; Baldin, C.; Hortschansky, P.; Jain, R.; Thywißen, A.; Straßburger, M.; Shelest, E.; Heinekamp, T.; Brakhage, A.A. The Aspergillus fumigatus conidial melanin production is regulated by the bifunctional bHLH DevR and MADS-box RlmA transcription factors. Mol. Microbiol. 2016, 102, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Xu, X.; Lowry, D.; Jackson, J.C.; Roberson, R.W.; Lin, X. Subcellular compartmentalization and trafficking of the biosynthetic machinery for fungal melanin. Cell Rep. 2016, 14, 2511–2518. [Google Scholar] [CrossRef]
- Strycker, B.D.; Han, Z.; Bahari, A.; Pham, T.; Lin, X.; Shaw, B.D.; Sokolov, A.V.; Scully, M.O. Raman characterization of fungal DHN and DOPA melanin biosynthesis pathways. J. Fungi 2021, 7, 841. [Google Scholar] [CrossRef]
- Eisenman, H.C.; Chow, S.; Tsé, K.K.; McClelland, E.; Casadevall, A. The effect of L-DOPA on Cryptococcus neoformans growth and gene expression. Virulence 2011, 2, 329–336. [Google Scholar] [CrossRef]
- Ribera, J.; Panzarasa, G.; Stobbe, A.; Osypova, A.; Rupper, P.; Klose, D.; Schwarze, F.W.M.R. Scalable biosynthesis of melanin by the Basidiomycete Armillaria cepistipes. J. Agric. Food Chem. 2019, 67, 132–139. [Google Scholar] [CrossRef]
- Ye, M.; Guo, G.; Lu, Y.; Song, S.; Wang, H.; Yang, L. Purification, structure and anti-radiation activity of melanin from Lachnum YM404. Int. J. Biol. Macromol. 2014, 63, 170–176. [Google Scholar] [CrossRef]
- Arun, G.; Eyini, M.; Gunasekaran, P. Characterization and biological activities of extracellular melanin produced by Schizophyllum commune (Fries). Indian J. Exp. Biol. 2015, 63, 380–387. [Google Scholar]
- Hu, H.L.; Dai, D.H.; Huang, G.R.; Zhang, Z.D. Isolation and characterization of extracellular melanin produced by Chroogomphus rutilus D447. Am. J. Food Technol. 2015, 10, 68–77. [Google Scholar] [CrossRef]
- Oh, J.-J.; Kim, Y.J.; Kim, J.Y.; Kwon, S.L.; Lee, C.; Lee, M.-E.; Kim, J.W.; Kim, G.-H. Genomic analysis and assessment of melanin synthesis in Amorphotheca resinae KUC3009. J. Fungi 2021, 7, 289. [Google Scholar] [CrossRef] [PubMed]
- Tudor, D.; Robinson, S.C.; Cooper, P.A. The influence of moisture content variation on fungal pigment formation in spalted wood. AMB Express 2012, 2, 69. [Google Scholar] [CrossRef]
- Duarte, A.W.F.; de Menezes, G.C.A.; e Silva, T.R.; Bicas, J.L.; Oliveira, V.M.; Rosa, L.H. Antarctic fungi as producers of pigments. In Fungi of Antarctica; Rosa, L.H., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 305–318. [Google Scholar]
- Sajjad, W.; Din, G.; Rafiq, M.; Iqbal, A.; Khan, S.; Zada, S.; Ali, B.; Kang, S. Pigment production by cold-adapted bacteria and fungi: Colorful tale of cryosphere with wide range applications. Extremophiles 2020, 24, 447–473. [Google Scholar] [CrossRef]
- Selbmann, L.; Isola, D.; Zucconi, L.; Onofri, S. Resistance to UV-B induced DNA damage in extreme-tolerant cryptoendolithic Antarctic fungi: Detection by PCR assays. Fungal Biol. 2011, 115, 937–944. [Google Scholar] [CrossRef]
- Rosa, L.H.; Vaz, A.B.M.; Caligiorne, R.B.; Campolina, S.; Rosa, C.A. Endophytic fungi associated with the Antarctic grass Deschampsia antarctica Desv. (Poaceae). Polar Biol. 2009, 32, 161–167. [Google Scholar] [CrossRef]
- Pacelli, C.; Cassaro, A.; Maturilli, A.; Timperio, A.M.; Gevi, F.; Cavalazzi, B.; Stefan, M.; Ghica, D.; Onofri, S. Multidisciplinary characterization of melanin pigments from the black fungus Cryomyces antarcticus. Appl. Microbiol. Biotechnol. 2020, 104, 6385–6395. [Google Scholar] [CrossRef]
- de Menezes, G.C.A.; Godinho, V.M.; Porto, B.A.; Gonçalves, V.N.; Rosa, L.H. Antarctomyces pellizariae sp. nov., a new, endemic, blue, snow resident psychrophilic ascomycete fungus from Antarctica. Extremophiles 2017, 21, 259–269. [Google Scholar] [CrossRef]
- Gamze, N.M. Natural melanin synthesized by Aureobasidium pullulans using food wastes and its characterization. Appl. Food Biotechnol. 2021, 8, 307–318. [Google Scholar]
- Rana, B.; Bhattacharyya, M.; Patni, B.; Arya, M.; Joshi, G.K. The realm of microbial pigments in the food color market. Front. Sustain. Food Syst. 2021, 5, 603892. [Google Scholar] [CrossRef]
- Saini, A.S.; Melo, J.S. Biosorption of uranium by melanin: Kinetic, equilibrium and thermodynamic studies. Bioresour. Technol. 2013, 149, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.; Reis, T.A.; Cotrim, M.; Mullan, T.K.; Correa, B. Resistant fungi isolated from contaminated uranium mine in Brazil shows a high capacity to uptake uranium from water. Chemosphere 2020, 248, 126068. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Kim, J.Y.; Kim, Y.J.; Kim, S.; Kim, G. Utilization of extracellular fungal melanin as an eco-friendly biosorbent for treatment of metal-contaminated effluents. Chemosphere 2021, 272, 129884. [Google Scholar] [CrossRef] [PubMed]
- Manirethan, V.; Raval, K.; Rajan, R.; Thaira, H.; Balakrishnan, R.M. Kinetic and thermodynamic studies on the adsorption of heavy metals from aqueous solution by melanin nanopigment obtained from marine source: Pseudomonas stutzeri. J. Environ. Manag. 2018, 214, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Tran-Ly, A.N.; Ribera, J.; Schwarze, F.W.M.; Brunelli, M.; Giuseppino, F. Fungal melanin-based electrospun membranes for heavy metal detoxification of water. Sustain. Mater. Technol. 2020, 23, e00146. [Google Scholar] [CrossRef]
- Song, S.; Yang, L.; Ye, M.; Chen, X.; Shi, F.; Shaikh, F. Antioxidant activity of a Lachnum YM226 melanin–iron complex and its influence on cytokine production in mice with iron deficiency anemia. Food Funct. 2016, 7, 1508. [Google Scholar] [CrossRef]
- Schweitzer, A.D.; Revskaya, E.; Chu, P.; Pazo, V.; Friedman, M.; Nosanchuk, J.D.; Cahill, S.; Frases, S.; Casadevall, A.; Dadachova, E. Melanin-covered nanoparticles for protection of bone marrow during radiation therapy of cancer. Int. J. Rad. Oncol. Biol. Phys. 2010, 78, 1494–1502. [Google Scholar] [CrossRef]
- Ju, K.-Y.; Lee, Y.; Lee, S.; Park, S.B.; Lee, J.K. Bioinspired polymerization of dopamine to generate melanin-like nanoparticles having an excellent free-radical-scavenging property. Biomacromolecules 2011, 12, 625–632. [Google Scholar] [CrossRef]
- Araújo, M.; Viveiros, R.; Correia, T.R.; Correia, I.J.; Bonifácio, V.D.B.; Casimiro, T.; Aguiar-Ricardo, A. Natural melanin: A potential pH-responsive drug release device. Int. J. Pharm. 2014, 469, 140–145. [Google Scholar] [CrossRef]
- Chyizhanska, N.; Beregova, T. Effect of melanin isolated from Antarctic yeasts on preservation of pig livestock after ablactation. Ukrainian Antarctic J. 2009, 8, 382–385. [Google Scholar] [CrossRef]
- Dufossé, L.; Caro, Y.; Fouillaud, M. Fungal Pigments; MDPI: Basel, Switzerland, 2018; ISBN 978-3-03842-787-2. [Google Scholar]
- Mund, N.K.; Čellárová, E. Recent advances in the identification of biosynthetic genes and gene clusters of the polyketide-derived pathways for anthraquinone biosynthesis and biotechnological applications. Biotechnol. Adv. 2023, 63, 108104. [Google Scholar] [CrossRef]
- Lin, L.; Xu, J.P. Production of fungal pigments: Molecular processes and their applications. J. Fungi 2023, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, Y.; Chen, M.; Fan, P.; Li, G.; Wang, C. Ammonium nitrate regulated the color characteristic changes of pigments in Monascus purpureus M9. AMB Expr. 2021, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Muangsin, N.; Wisetsakdakorn, W.; Chaichit, N.; Sihanonth, P.; Petsom, A.; Sangvanich, P. Austrocortinin: Crystal structure of a natural anthraquinone pigment from fungi. Dyes Pigments 2008, 77, 653–656. [Google Scholar] [CrossRef]
- Cui, Y.; Xia, C.-J.; Su, Y.-H.; Zhang, B.-Q.; Zhang, T.-T.; Liu, Y.; Hu, Z.-Y.; Tang, X.-J. Switching characteristics of anthraquinone molecular devices based on graphene electrodes. Acta Physica Sinica 2021, 70, 038501. [Google Scholar] [CrossRef]
- Giesbers, G.; Van Schenck, J.; Quinn, A.; Van Court, R.; Vega Gutierrez, S.M.; Robinson, S.C.; Ostroverkhova, O. Xylindein: Naturally produced fungal compound for sustainable (Opto)electronics. ACS Omega 2019, 4, 13309–13318. [Google Scholar] [CrossRef]
- Giesbers, G.; Krueger, T.; Schenck, J.V.; Court, R.V.; Moore, J.; Fang, C.; Robinson, S.C.; Ostroverkhova, O. Fungi-derived xylindein: Effect of purity on optical and electronic properties. MRS Adv. 2019, 4, 1769–1777. [Google Scholar] [CrossRef]
- Van Court, R.C.; Giesbers, G.; Ostroverkhova, O.; Robinson, S.C. Optimizing xylindein from Chlorociboria spp. for (Opto)electronic applications. Processes 2020, 8, 1477. [Google Scholar] [CrossRef]
- Khalid, S.; Keller, N.P. Chemical signals driving bacterial–fungal interactions. Environ. Microbiol. 2021, 23, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Spraker, J.E.; Wiemann, P.; Baccile, J.A.; Venkatesh, N.; Schumacher, J.; Schroeder, F.C. Conserved responses in a war of small molecules between a plant-pathogenic bacterium and fungi. MBio 2018, 9, e00820. [Google Scholar] [CrossRef] [PubMed]
- Moussa, M.; Ebrahim, W.; Bonus, M.; Gohlke, H.; Mandi, A.; Kurtan, T.; Hartmann, R.; Kalscheuer, R.; Lin, W.; Liu, Z.; et al. Co-culture of the fungus Fusarium tricinctum with Streptomyces lividans induces production of cryptic naphthoquinone dimers. RSC Adv. 2019, 9, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Medentsev, A.G.; Akimenko, V.K. Naphthoquinone metabolites of the fungi. Phytochemistry 1998, 47, 935–959. [Google Scholar] [CrossRef] [PubMed]
- Dufossé, L. Microbial production of food grade pigments. Food Technol. Biotechnol. 2006, 44, 313–321. [Google Scholar]
- Yang, Y.; Liu, B.; Du, X.; Li, P.; Liang, B.; Cheng, X.; Du, L.; Huang, D.; Wang, L.; Wang, S. Complete genome sequence and transcriptomics analyses reveal pigment biosynthesis and regulatory mechanisms in an industrial strain, Monascus purpureus YY-1. Sci. Rep. 2015, 5, 8331. [Google Scholar] [CrossRef]
- Hajjaj, H.; Klaebe, A.; Goma, G.; Blanc, P.J.; Barbier, E.; Francois, J. Medium-chain fatty acids affect citrinin production in the filamentous fungus Monascus ruber. Appl. Environ. Microbiol. 2000, 66, 1120–1125. [Google Scholar] [CrossRef]
- Shi, K.; Song, D.; Chen, G.; Pistolozzi, M.; Wu, Z.; Quan, L. Controlling composition and color characteristics of Monascus pigments by pH and nitrogen sources in submerged fermentation. J. Biosci. Bioeng. 2015, 120, 145–154. [Google Scholar] [CrossRef]
- Mapari, S.A.S.; Meyer, A.S.; Thrane, U.; Frisvad, J.C. Identification of potentially safe promising fungal cell factories for the production of polyketide natural food colorants using chemotaxonomic rationale. Microb. Cell Fact. 2009, 8, 24. [Google Scholar] [CrossRef]
- Pisareva, E.; Savov, V.; Kujumdzieva, A. Pigments and citrinin biosynthesis by fungi belonging to genus Monascus. Z. Naturforsch. 2005, 60C, 116–120. [Google Scholar] [CrossRef]
- Shimizu, T.; Kinoshita, H.; Ishihara, S.; Sakai, K.; Nagai, S.; Nihira, T. Polyketide synthase gene responsible for citrinin biosynthesis in Monascus purpureus. Appl. Environ. Microbiol. 2005, 71, 3453–3457. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Xu, Y.; Li, Y.; Tan, W. Construction of a replacement vector to disrupt pksCT gene for the mycotoxin citrinin biosynthesis in Monascus aurantiacus and maintain food red pigment production. Asia Pac. J. Clin. Nutr. 2007, 16, 137–142. [Google Scholar]
- Shimizu, T.; Kinoshita, H.; Nihira, T. Identification and in vivo functional analysis by gene disruption of ctnA, an activator gene involved in citrinin biosynthesis in Monascus purpureus. Appl. Environ. Microbiol. 2007, 73, 5097–5103. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, J.; Han, H.; Li, L.; Liu, Y.; Gao, M. Citrinin-producing capacity of Monascus purpureus in response to low-frequency magnetic fields. Process Biochem. 2017, 53, 25–29. [Google Scholar] [CrossRef]
- Koli, S.H.; Suryawanshi, R.K.; Patil, C.D.; Pati, S.V. Fluconazole treatment enhances extracellular release of red pigments in the fungus Monascus purpureus. FEMS Microbiol. Lett. 2017, 364, fnx058. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liu, X.; Wang, Y.; Xie, J.; Gao, H.; Li, X.; Huang, Z. Metabolomics analysis based on UHPLC-Q-TOF-MS/MS reveals effects of genistein on reducing mycotoxin citrinin production by Monascus aurantiacus Li AS3.4384. LWT-Food Sci. Technol. 2020, 130, 109613. [Google Scholar] [CrossRef]
- Liang, B.; Du, X.-J.; Li, P.; Sun, C.-C.; Wang, S. Investigation of citrinin and pigment biosynthesis mechanisms in Monascus purpureus by transcriptomic analysis. Front. Microbiol. 2018, 9, 1374. [Google Scholar] [CrossRef]
- Zhen, Z.; Xiong, X.; Liu, Y.; Zhang, J.; Wang, S.; Li, L.; Gao, M. NaCl inhibits citrinin and stimulates Monascus pigments and monacolin K production. Toxins 2019, 11, 118. [Google Scholar] [CrossRef]
- Chen, M.H.; Johns, M.R. Effect of pH and nitrogen source on pigment production by Monascus purpureus. Appl. Microbiol. Biotechnol. 1993, 40, 132–138. [Google Scholar] [CrossRef]
- Marcoleta, A.; Niklitschek, M.; Wozniak, A.; Lozano, C.; Alcaíno, J.; Baeza, M.; Cifuentes, V. Glucose and ethanol-dependent transcriptional regulation of the astaxanthin biosynthesis pathway in Xanthophyllomyces dendrorhous. BMC Microbiol. 2011, 11, 190. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Magalon, H.; Dufossé, L.; Fouillaud, M. Production of pigments from the tropical marine-derived fungi Talaromyces albobiverticillius: New resources for natural red-colored metabolites. J. Food Composit. Anal. 2018, 70, 35–48. [Google Scholar] [CrossRef]
- Egorova, A.S.; Gessler, N.N.; Belozerskaya, T.A. Melanin pigments in the fungus Paecilomyces lilacinus (thom) samson. Dokl Biochem. Biophys. 2011, 437, 84. [Google Scholar] [CrossRef] [PubMed]
- Xu, J. Assessing global fungal threats to humans. mLife 2022, 1, 223–240. [Google Scholar] [CrossRef]

| Class of Carotenoids | Species | Biosynthesis-Related Genes | References |
|---|---|---|---|
| β-Carotene | Blakeslea trispora | carRA, carB, carG | [12,13] |
| Xanthophyllomyces dendrorhous | crtE, crtI, crtYB | [11] | |
| Rhodotorula glutinis | crtI, crtYB | [14] | |
| Astaxanthin | Phaffia rhodozyma (syn. Xanthophyllomyces dendrorhous) | crtYB, crtS (also called Asy), crtI, crtE | [15] |
| Lycopene | Blakeslea trispora | carB, carRA | [16] |
| Type of Melanin Biosynthesis Pathway | Species | Biosynthesis -Related Genes | References |
|---|---|---|---|
| DHN–melanin | Aspergillus fumigatus | pksP/alb1, ayg1, arp1, arp2, abr1, abr2 | [47] |
| Aspergillus niger | alb1, ayg1, abr1, abr2 | [49] | |
| Aspergillus nidulans | ωA, yA | [50] | |
| Penicillium marneffei | alb1, ayg1, arp1, arp2, abr1, abr2 | [49] | |
| DOPA–melanin | Cryptococcus neoformans | Lac1, Lac2 | [51] |
| Pyomelanin | Aspergillus fumigatus | HppD, HmgA | [45] |
| Penicillium chrysogenum | HppD, HmgA | [46] |
| Class of Colorants/Paradigm | Fungal Species | Biosynthesis-Related Genes | References |
|---|---|---|---|
| Anthraquinones/endocrocin | Aspergillus fumigatus | encA, encB, encC | [77] |
| Naphthoquinone/bikaverin | Fusarium fujikuroi | Bik1, Bik2, Bik3, Bik4, Bik5, Bik6 | [78] |
| Azaphilone/Monascus pigments | Monascus purpureus | MppD, MpPKS5, MpFasB2, MpFasA2, MppF, MppA, MppB, MppE, Mpp7, MppC, MppG, MppR1, MppR2 | [79] |
| Categories of Colorants | Environmental Factors | References |
|---|---|---|
| Carotenoids | Light, temperature, oxygen, carbon source (i.e., glucose, ethanol), nitrogen, Ca++ availability | [24] |
| Melanins | UV irradiation, cold, desiccation, heavy-metal stress | [58,59] |
| Polyketide-derived pigments | Biotic stresses (i.e., other organisms), low-frequency magnetic field, NaCl, pH, NH4NO3 | [79,102,103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Zhang, T.; Xu, J. Genetic and Environmental Factors Influencing the Production of Select Fungal Colorants: Challenges and Opportunities in Industrial Applications. J. Fungi 2023, 9, 585. https://doi.org/10.3390/jof9050585
Lin L, Zhang T, Xu J. Genetic and Environmental Factors Influencing the Production of Select Fungal Colorants: Challenges and Opportunities in Industrial Applications. Journal of Fungi. 2023; 9(5):585. https://doi.org/10.3390/jof9050585
Chicago/Turabian StyleLin, Lan, Tong Zhang, and Jianping Xu. 2023. "Genetic and Environmental Factors Influencing the Production of Select Fungal Colorants: Challenges and Opportunities in Industrial Applications" Journal of Fungi 9, no. 5: 585. https://doi.org/10.3390/jof9050585
APA StyleLin, L., Zhang, T., & Xu, J. (2023). Genetic and Environmental Factors Influencing the Production of Select Fungal Colorants: Challenges and Opportunities in Industrial Applications. Journal of Fungi, 9(5), 585. https://doi.org/10.3390/jof9050585







