Secondary Metabolites Produced by the Blue-Cheese Ripening Mold Penicillium roqueforti; Biosynthesis and Regulation Mechanisms
Abstract
1. Introduction
2. Brief Overview of Taxonomic and Biotechnological Aspects of P. roqueforti
3. Secondary Metabolites Produced by P. roqueforti
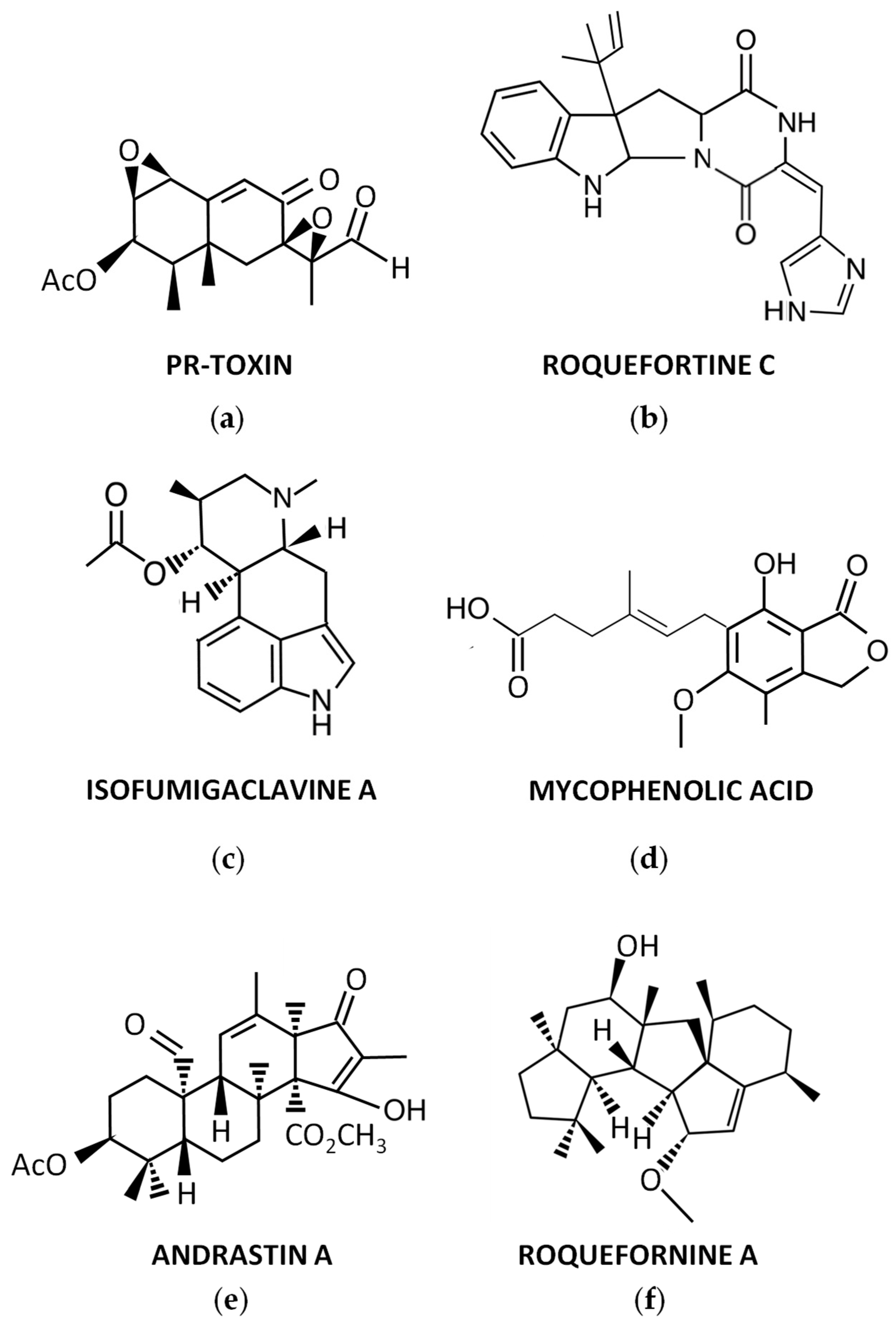
3.1. PR-Toxin and Related Compounds
3.2. Roquefortine C and Related Compounds
3.3. Other Secondary Metabolites Produced by P. roqueforti
4. Biosynthetic Gene Clusters and Pathways Functionally Characterized in P. roqueforti
4.1. Roquefortine C Biosynthetic Gene Cluster and Pathway
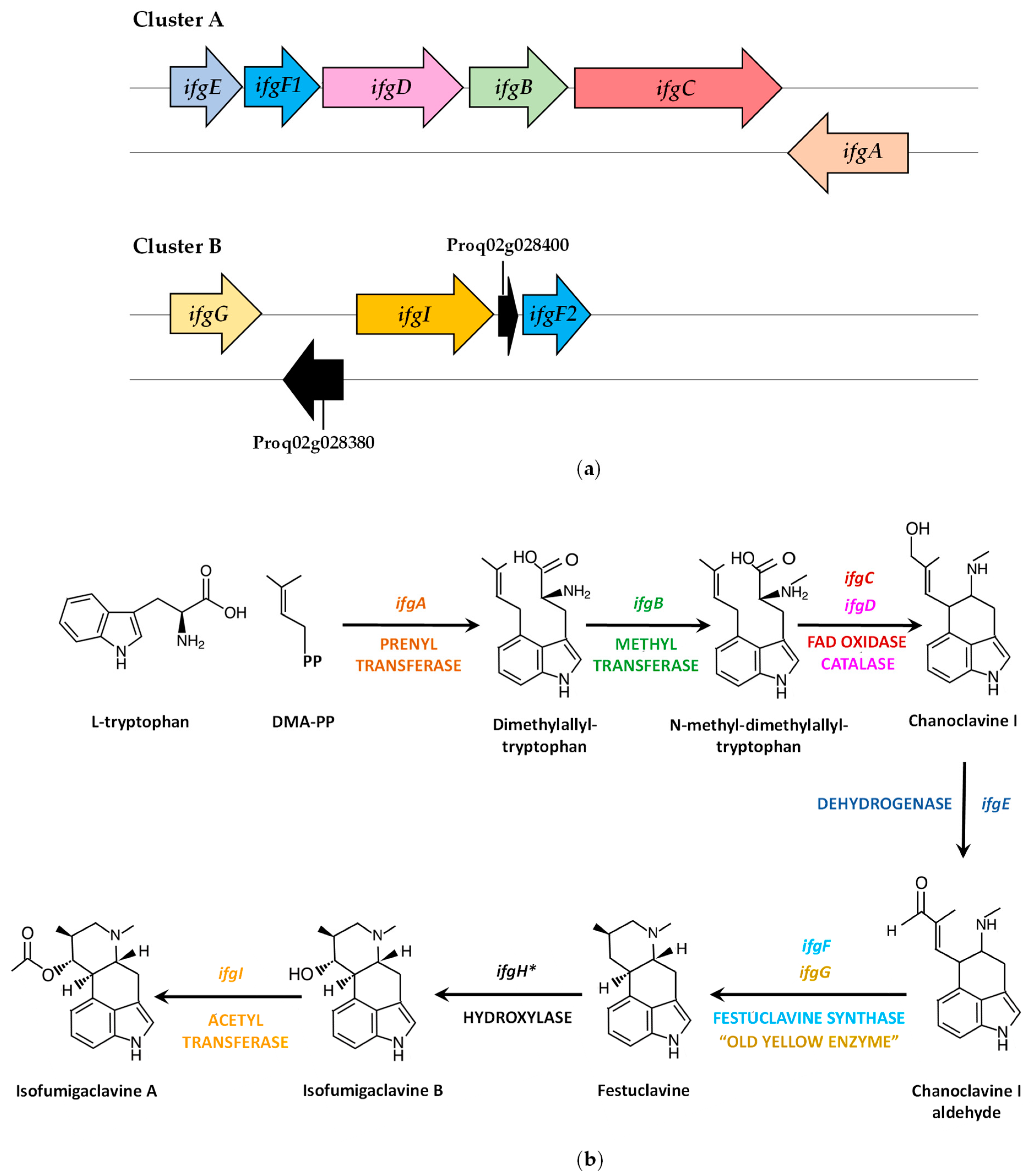
4.2. Isofumigaclavine A Biosynthetic Gene Cluster and Pathway
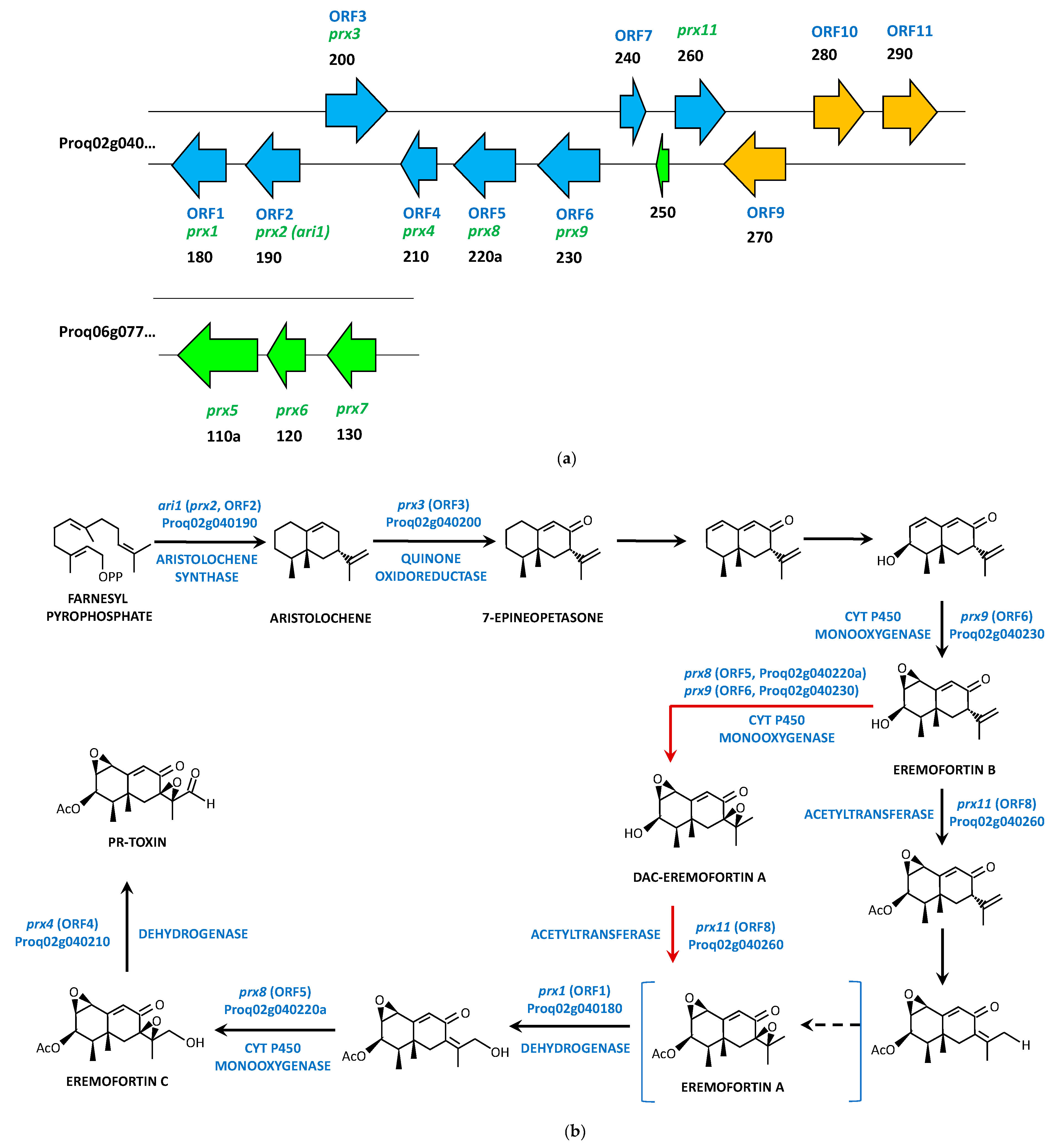
4.3. PR-Toxin Biosynthetic Gene Cluster and Pathway
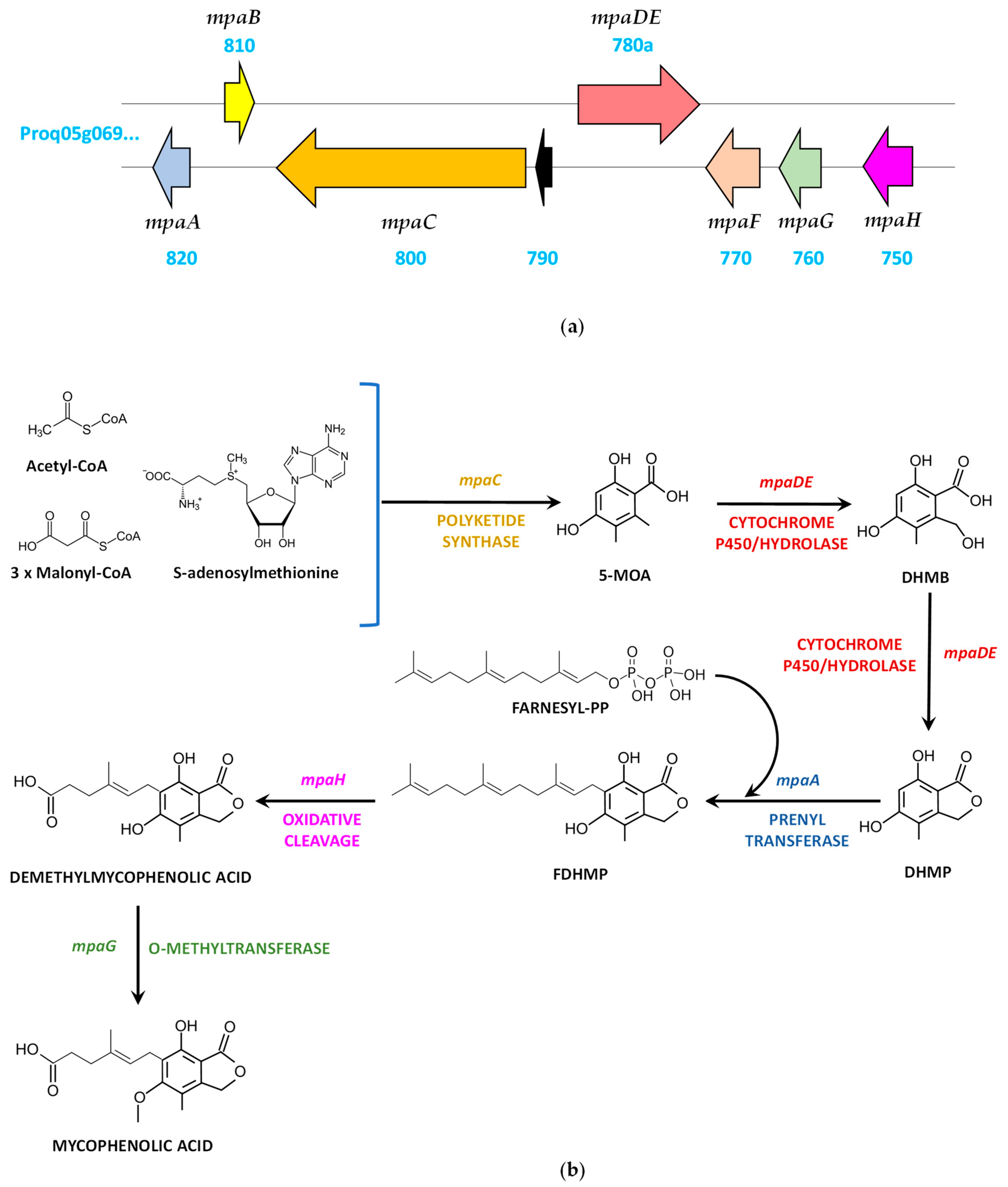
4.4. Mycophenolic Acid Biosynthetic Gene Cluster and Pathway
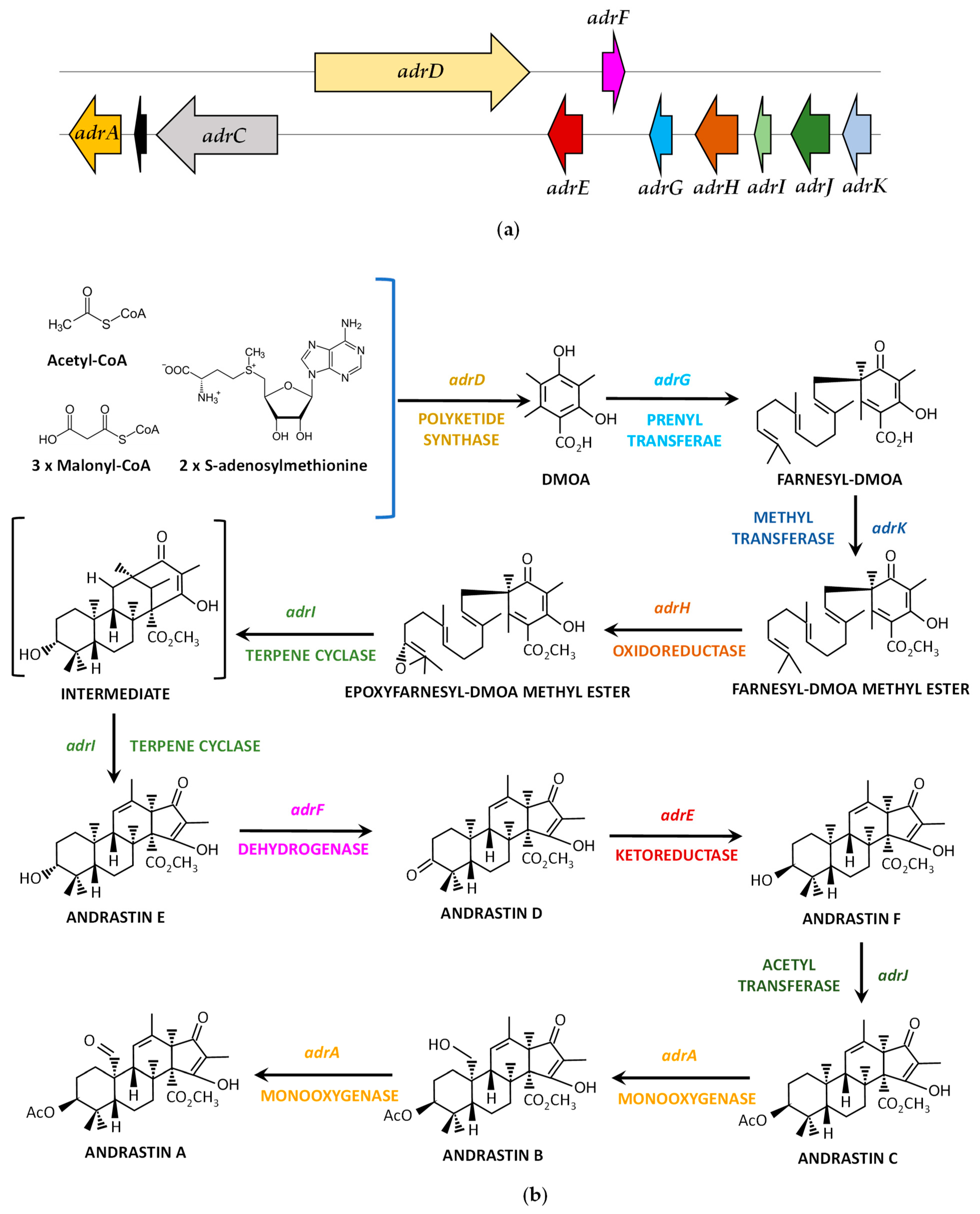
4.5. Andrastin A Biosynthetic Gene Cluster and Pathway
4.6. Annullatins D and F Biosynthetic Gene Cluster and Pathway
5. Control and Regulation of the Biosynthesis of Secondary Metabolites in P. roqueforti
5.1. Cluster-Specific Regulators in Biosynthetic Gene Clusters Functionally Characterized in P. roqueforti
5.2. Global Regulators of Biosynthetic Gene Clusters in P. roqueforti
5.2.1. The pga1 Gene Encoding for an α-Subunit of a Heterotrimeric G Protein
5.2.2. The sfk1 Gene Encoding for Suppressor of Four-Kinase 1 Protein
5.2.3. The pcz1 Gene Encoding a Protein with a Zn(II)2Cys6 Domain
5.3. Concluding Remarks on Regulation of Secondary Metabolism in P. roqueforti
6. Future Challenges and Perspectives in the Study of Secondary Metabolism in P. roqueforti
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Hautbergue, T.; Jamin, E.L.; Debrauwer, L.; Puel, O.; Oswald, I.P. From genomics to metabolomics, moving toward an integrated strategy for the discovery of fungal secondary metabolites. Nat. Prod. Rep. 2018, 35, 147–173. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razek, A.S.; El-Naggar, M.E.; Allam, A.; Morsy, O.M.; Othman, S.I. Microbial natural products in drug discovery. Processes 2020, 8, 470. [Google Scholar] [CrossRef]
- Fierro, F.; Vaca, I.; Castillo, N.I.; García-Rico, R.O.; Chávez, R. Penicillium chrysogenum, a vintage model with a cutting-edge profile in biotechnology. Microorganisms 2022, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- Sawant, A.M.; Vamkudoth, K.R. Biosynthetic process and strain improvement approaches for industrial penicillin production. Biotechnol. Lett. 2022, 44, 179–192. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Smedsgaard, J.; Larsen, T.O.; Samson, R.A. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud. Mycol. 2004, 49, 201–241. [Google Scholar]
- Grijseels, S.; Nielsen, J.C.; Randelovic, M.; Nielsen, J.; Nielsen, K.F.; Workman, M.; Frisvad, J.C. Penicillium arizonense, a new, genome sequenced fungal species, reveals a high chemical diversity in secreted metabolites. Sci. Rep. 2016, 6, 35112. [Google Scholar] [CrossRef]
- Nielsen, J.C.; Grijseels, S.; Prigent, S.; Ji, B.; Dainat, J.; Nielsen, K.F.; Frisvad, J.C.; Workman, M.; Nielsen, J. Global analysis of biosynthetic gene clusters reveals vast potential of secondary metabolite production in Penicillium species. Nat. Microbiol. 2017, 2, 17044. [Google Scholar] [CrossRef]
- Grijseels, S.; Nielsen, J.C.; Nielsen, J.; Larsen, T.O.; Frisvad, J.C.; Nielsen, K.F.; Frandsen, R.J.N.; Workman, M. Physiological characterization of secondary metabolite producing Penicillium cell factories. Fungal Biol. Biotechnol. 2017, 4, 8. [Google Scholar] [CrossRef]
- Coton, E.; Coton, M.; Hymery, N.; Mounier, J.; Jany, J.-L. Penicillium roqueforti: An overview of its genetics, physiology, metabolism and biotechnological applications. Fungal Biol. Rev. 2020, 34, 59–73. [Google Scholar] [CrossRef]
- Thom, C. Fungi in cheese ripening: Camembert and Roquefort. USDA Bureau Anim. Industry Bull. 1906, 82, 1–39. [Google Scholar]
- Boysen, M.; Skouboe, P.; Frisvad, J.; Rossen, L. Reclassification of the Penicillium roqueforti group into three species on the basis of molecular genetic and biochemical profiles. Microbiology 1996, 142, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of Penicillium subgenus Penicillium—A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud. Mycol. 2004, 49, 1–73. [Google Scholar]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): An overview of families, genera, subgenera, sections, series and species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef] [PubMed]
- Ropars, J.; López-Villavicencio, M.; Dupont, J.; Snirc, A.; Gillot, G.; Coton, M.; Jany, J.L.; Coton, E.; Giraud, T. Induction of sexual reproduction and genetic diversity in the cheese fungus Penicillium roqueforti. Evol. Appl. 2014, 7, 433–441. [Google Scholar] [CrossRef]
- Albillos, S.M.; García-Estrada, C.; Martín, J.-F. Spanish blue cheeses: Functional metabolites. In Cheese: Types, Nutrition and Consumption; Foster, R.D., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2011; pp. 89–105. [Google Scholar]
- Moreau, C. Le Penicillium roqueforti, morphologie, physiologie, interet en industrie fromagere, mycotoxines. Lait 1980, 60, 254–271. [Google Scholar] [CrossRef]
- Labbe, M.; Serres, J.-P. Chroniques du Roquefort: De la préhistoire à l′aube de l′ère industrielle; Graphi Imprimeur: La Primaube, France, 2004. [Google Scholar]
- Labbe, M.; Serres, J.-P. Chroniques du Roquefort: Des hommes, des entreprises, des marques, période modern; Graphi Imprimeur: La Primaube, France, 2009. [Google Scholar]
- McSweeney, P.L.H.; Sousa, M.J. Biochemical pathways for the production of flavour compounds in cheeses during ripening: A review. Lait 2000, 80, 293–324. [Google Scholar] [CrossRef]
- Collins, Y.F.; McSweeney, P.L.H.; Wilkinson, M.G. Lipolysis and free fatty acid catabolism in cheese: A review of current knowledge. Int. Dairy J. 2003, 13, 841–866. [Google Scholar] [CrossRef]
- Deetae, P.; Bonnarme, P.; Spinnler, H.E.; Helinck, S. Production of volatile aroma compounds by bacterial strains isolated from different surface-ripened French cheeses. Appl. Microbiol. Biotechnol. 2007, 76, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, A.A.; Ahmed, A.S.; El-Sayed, E.S.R. Optimization of submerged fermentation conditions for immunosuppressant mycophenolic acid production by Penicillium roqueforti isolated from blue-molded cheeses: Enhanced production by ultraviolet and gamma irradiation. World J. Microbiol. Biotechnol. 2014, 30, 2625–2638. [Google Scholar] [CrossRef]
- Silva, T.P.; Souza, L.O.; Reis, N.S.; Assis, S.A.; Ferreira, M.L.O.; Oliveira, J.R.; Aguiar-Oliveira, E.; Franco, M. Cultivation of Penicillium roqueforti in cocoa shell to produce and characterize its lipase extract. Rev. Mex. Ing. Quim. 2017, 16, 745–756. [Google Scholar]
- de Almeida Antunes Ferraz, J.L.; Souza, L.O.; Soares, G.A.; Coutinho, J.P.; de Oliveira, J.R.; Aguiar-Oliveira, E.; Franco, M. Enzymatic saccharification of lignocellulosic residues using cellulolytic enzyme extract produced by Penicillium roqueforti ATCC 10110 cultivated on residue of yellow mombin fruit. Bioresour. Technol. 2018, 248, 214–220. [Google Scholar] [CrossRef]
- Wei, R.D.; Still, P.E.; Smalley, E.B.; Schnoes, H.K.; Strong, F.M. Isolation and partial characterization of a mycotoxin from Penicillium roqueforti. Appl. Microbiol. 1973, 25, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.D.; Schnoes, H.K.; Hart, P.A.; Strong, F.M. The structure of PR toxin, a mycotoxin from Penicillium roqueforti. Tetrahedron 1975, 31, 109–114. [Google Scholar] [CrossRef]
- Dubey, M.K.; Aamir, M.; Kaushik, M.S.; Khare, S.; Meena, M.; Singh, S.; Upadhyay, R.S. PR toxin—Biosynthesis, genetic regulation, toxicological potential, prevention and control measures: Overview and challenges. Front. Pharmacol. 2018, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Lu, K.L.; Yeh, S.F. Secondary metabolites resulting from degradation of PR toxin by Penicillium roqueforti. Appl. Environ. Microbiol. 1993, 59, 981–986. [Google Scholar] [CrossRef]
- Chang, S.C.; Yeh, S.F.; Li, S.Y.; Lei, W.Y.; Chen, M.Y. A novel secondary metabolite relative to the degradation of PR toxin by Penicillium roqueforti. Curr. Microbiol. 1996, 32, 141–146. [Google Scholar] [CrossRef]
- Moreau, S.; Gaudemer, A.; Lablache-Combier, A.; Biguet, J. Metabolites de Penicillium roqueforti: PR toxine et metabolites associes. Tetrahedron Lett. 1976, 11, 833–834. [Google Scholar] [CrossRef]
- Moreau, S.; Cacan, M.; Eremofortin, C. A new metabolite obtained from Penicillium roqueforti cultures and from biotransformation of PR toxin. J. Org. Chem. 1977, 42, 2632–2634. [Google Scholar] [CrossRef] [PubMed]
- Moreau, S.; Biguet, J.; Lablache-Combier, A.; Baert, M.; Delfosse, C. Structures et stereochimie des sesquiterpenes de Penicillium roqueforti pr toxine et eremofortines a, b, c, d, e. Tetrahedron. 1980, 36, 2989–2997. [Google Scholar] [CrossRef]
- García-Estrada, C.; Martín, J.F. Biosynthetic gene clusters for relevant secondary metabolites produced by Penicillium roqueforti in blue cheeses. Appl. Microbiol. Biotechnol. 2016, 100, 8303–8313. [Google Scholar] [CrossRef]
- Ohmomo, S.; Sato, T.; Utagawa, T.; Abe, M. Isolation of festuclavine and three new indole alkaloids, roquefortine A, B and C from the cultures of Penicillium roqueforti. Agr. Biol. Chem. 1975, 39, 1333–1334. [Google Scholar] [CrossRef]
- Ohmomo, S.; Utagawa, T.; Abe, M. Identification of roquefortine C produced by Penicillium roqueforti. Agr. Biol. Chem. 1977, 41, 2097–2098. [Google Scholar] [CrossRef]
- Scott, P.M.; Merrien, M.A.; Polonsky, J. Roquefortine and isofumigaclavine A, metabolites from Penicillium roqueforti. Experientia 1976, 32, 140–142. [Google Scholar] [CrossRef]
- Ohmomo, S.; Oguma, K.; Ohashi, T.; Abe, M. Isolation of a new indole alkaloid, roquefortine D, from the cultures of Penicillium roqueforti. Agr. Biol. Chem. 1978, 42, 2387–2389. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Sumarah, M.W.; Frisvad, J.C.; Miller, J.D. Production of metabolites from the Penicillium roqueforti complex. J. Agric. Food Chem. 2006, 54, 3756–3763. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R. Mycophenolic acid: A one hundred year odyssey from antibiotic to immunosuppressant. Chem. Rev. 2000, 100, 3801–3826. [Google Scholar] [CrossRef] [PubMed]
- O′Brien, M.; Nielsen, K.F.; O′Kiely, P.; Forristal, P.D.; Fuller, H.T.; Frisvad, J.C. Mycotoxins and other secondary metabolites produced in vitro by Penicillium paneum Frisvad and Penicillium roqueforti Thom isolated from baled grass silage in Ireland. J. Agric. Food Chem. 2006, 54, 9268–9276. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.F.; Dalsgaard, P.W.; Smedsgaard, J.; Larsen, T.O. Andrastins A-D, Penicillium roqueforti metabolites consistently produced in blue-mold-ripened cheese. J. Agric. Food Chem. 2005, 53, 2908–2913. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Yu, J.; Shu, Y.; Shi, Y.X.; Luo, P.; Cai, L.; Ding, Z.T. Peniroquesines A-C: Sesterterpenoids possessing a 5-6-5-6-5-fused pentacyclic ring system from Penicillium roqueforti YJ-14. Org. Lett. 2018, 20, 5853–5856. [Google Scholar] [CrossRef]
- Wang, J.P.; Shu, Y.; Hu, J.T.; Liu, R.; Cai, X.Y.; Sun, C.T.; Gan, D.; Zhou, D.J.; Mei, R.F.; Ding, H.; et al. Roquefornine A, a sesterterpenoid with a 5/6/5/5/6-fused ring system from the fungus Penicillium roqueforti YJ-14. Org. Chem. Front. 2020, 7, 1463–1468. [Google Scholar] [CrossRef]
- Wang, J.P.; Shu, Y.; Liu, R.; Gan, J.L.; Deng, S.P.; Cai, X.Y.; Hu, J.T.; Cai, L.; Ding, Z.T. Bioactive sesterterpenoids from the fungus Penicillium roqueforti YJ-14. Phytochemistry 2021, 187, 112762. [Google Scholar] [CrossRef] [PubMed]
- Kosalková, K.; Domínguez-Santos, R.; Coton, M.; Coton, E.; García-Estrada, C.; Liras, P.; Martín, J.F. A natural short pathway synthesizes roquefortine C but not meleagrin in three different Penicillium roqueforti strains. Appl. Microbiol. Biotechnol. 2015, 99, 7601–7612. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Ries, M.I.; Nijland, J.G.; Lankhorst, P.P.; Hankemeier, T.; Bovenberg, R.A.; Vreeken, R.J.; Driessen, A.J. A branched biosynthetic pathway is involved in production of roquefortine and related compounds in Penicillium chrysogenum. PLoS ONE 2013, 8, e65328. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, K.; Ropars, J.; Renault, P.; Dupont, J.; Gouzy, J.; Branca, A.; Abraham, A.L.; Ceppi, M.; Conseiller, E.; Debuchy, R.; et al. Multiple recent horizontal transfers of a large genomic region in cheese making fungi. Nat. Commun. 2014, 5, 2876. [Google Scholar] [CrossRef]
- García-Estrada, C.; Ullán, R.V.; Albillos, S.M.; Fernández-Bodega, M.Á.; Durek, P.; von Döhren, H.; Martín, J.F. A single cluster of coregulated genes encodes the biosynthesis of the mycotoxins roquefortine C and meleagrin in Penicillium chrysogenum. Chem. Biol. 2011, 18, 1499–1512. [Google Scholar] [CrossRef]
- Ries, M.I.; Ali, H.; Lankhorst, P.P.; Hankemeier, T.; Bovenberg, R.A.; Driessen, A.J.; Vreeken, R.J. Novel key metabolites reveal further branching of the roquefortine/meleagrin biosynthetic pathway. J. Biol. Chem. 2013, 288, 37289–37295. [Google Scholar] [CrossRef]
- Barrow, K.D.; Colley, P.W.; Tribe, D.E. Biosynthesis of the neurotoxin alkaloid roquefortine. J. Chem. Soc. Chem. Commun. 1979, 5, 225–226. [Google Scholar] [CrossRef]
- Gorst-Allman, C.P.; Steyn, P.S.; Vleggaar, R. The biosynthesis of roquefortine. An investigation of acetate and mevalonate incorporation using high field n.m.r. spectroscopy. J. Chem. Soc. Chem. Commun. 1982, 12, 652–653. [Google Scholar] [CrossRef]
- Martín, J.F.; Liras, P. Evolutionary formation of gene clusters by reorganization: The meleagrin/roquefortine paradigm in different fungi. Appl. Microbiol. Biotechnol. 2016, 100, 1579–1587. [Google Scholar] [CrossRef]
- Fernández-Bodega, Á.; Álvarez-Álvarez, R.; Liras, P.; Martín, J.F. Silencing of a second dimethylallyltryptophan synthase of Penicillium roqueforti reveals a novel clavine alkaloid gene cluster. Appl. Microbiol. Biotechnol. 2017, 101, 6111–6121. [Google Scholar] [CrossRef]
- Martín, J.F.; Álvarez-Álvarez, R.; Liras, P. Clavine alkaloids gene clusters of Penicillium and related fungi: Evolutionary combination of prenyltransferases, monooxygenases and dioxygenases. Genes 2017, 8, 342. [Google Scholar] [CrossRef]
- Wallwey, C.; Li, S.M. Ergot alkaloids: Structure diversity, biosynthetic gene clusters and functional proof of biosynthetic genes. Nat. Prod. Rep. 2011, 28, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.L.; Panaccione, D.G. Heterologous expression of lysergic acid and novel ergot alkaloids in Aspergillus fumigatus. Appl. Environ. Microbiol. 2014, 80, 6465–6472. [Google Scholar] [CrossRef]
- Gerhards, N.; Li, S.M. A bifunctional old yellow enzyme from Penicillium roqueforti is involved in ergot alkaloid biosynthesis. Org. Biomol. Chem. 2017, 15, 8059–8071. [Google Scholar] [CrossRef]
- Hohn, T.M.; Plattner, R.D. Purification and characterization of the sesquiterpene cyclase aristolochene synthase from Penicillium roqueforti. Arch. Biochem. Biophys. 1989, 272, 137–143. [Google Scholar] [CrossRef]
- Proctor, R.H.; Hohn, T.M. Aristolochene synthase. isolation, characterization, and bacterial expression of a sesquiterpenoid biosynthetic gene (Ari1) from Penicillium roqueforti. J. Biol. Chem. 1993, 268, 4543–4548. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, P.I.; Ullán, R.V.; Albillos, S.M.; Montero, O.; Fernández-Bodega, M.Á.; García-Estrada, C.; Fernández-Aguado, M.; Martín, J.F. Molecular characterization of the PR-toxin gene cluster in Penicillium roqueforti and Penicillium chrysogenum: Cross talk of secondary metabolite pathways. Fungal Genet. Biol. 2014, 62, 11–24. [Google Scholar] [CrossRef]
- Hidalgo, P.I.; Poirier, E.; Ullán, R.V.; Piqueras, J.; Meslet-Cladière, L.; Coton, E.; Coton, M. Penicillium roqueforti PR toxin gene cluster characterization. Appl. Microbiol. Biotechnol. 2017, 101, 2043–2056. [Google Scholar] [CrossRef] [PubMed]
- Riclea, R.; Dickschat, J.S. Identification of intermediates in the biosynthesis of PR toxin by Penicillium roqueforti. Angew. Chem. Int. Ed. Engl. 2015, 54, 12167–12170. [Google Scholar] [CrossRef]
- Chalmers, A.A.; de Jesus, A.E.; Gorst-Allman, C.P.; Steyn, P.S. Biosynthesis of PR toxin by Penicillium roqueforti. J. Chem. Soc. Perkin. 1 1981, 10, 2899–2903. [Google Scholar] [CrossRef]
- Regueira, T.B.; Kildegaard, K.R.; Hansen, B.G.; Mortensen, U.H.; Hertweck, C.; Nielsen, J. Molecular basis for mycophenolic acid biosynthesis in Penicillium brevicompactum. Appl. Environ. Microbiol. 2011, 77, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.G.; Mnich, E.; Nielsen, K.F.; Nielsen, J.B.; Nielsen, M.T.; Mortensen, U.H.; Larsen, T.O.; Patil, K.R. Involvement of a natural fusion of a cytochrome P450 and a hydrolase in mycophenolic acid biosynthesis. Appl. Environ. Microbiol. 2012, 78, 4908–4913. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, S.; Qiu, L.; Qi, F.; Li, Z.; Yang, Y.; Huang, S.; Bai, F.; Liu, C.; Wan, X.; et al. Functional characterization of MpaG′, the O-methyltransferase involved in the biosynthesis of mycophenolic acid. Chembiochem 2015, 16, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Del-Cid, A.; Gil-Durán, C.; Vaca, I.; Rojas-Aedo, J.F.; García-Rico, R.O.; Levicán, G.; Chávez, R. Identification and functional analysis of the mycophenolic acid gene cluster of Penicillium roqueforti. PLoS ONE 2016, 11, e0147047. [Google Scholar] [CrossRef]
- Gillot, G.; Jany, J.L.; Dominguez-Santos, R.; Poirier, E.; Debaets, S.; Hidalgo, P.I.; Ullán, R.V.; Coton, E.; Coton, M. Genetic basis for mycophenolic acid production and strain-dependent production variability in Penicillium roqueforti. Food Microbiol. 2017, 62, 239–250. [Google Scholar] [CrossRef]
- Hansen, B.G.; Genee, H.J.; Kaas, C.S.; Nielsen, J.B.; Regueira, T.B.; Mortensen, U.H.; Frisvad, J.C.; Patil, K.R. A new class of IMP dehydrogenase with a role in self-resistance of mycophenolic acid producing fungi. BMC Microbiol. 2011, 11, 202. [Google Scholar] [CrossRef]
- Matsuda, Y.; Awakawa, T.; Abe, I. Reconstituted biosynthesis of fungal meroterpenoid andrastin A. Tetrahedron 2013, 69, 8199–8204. [Google Scholar] [CrossRef]
- Rojas-Aedo, J.F.; Gil-Durán, C.; Del-Cid, A.; Valdés, N.; Álamos, P.; Vaca, I.; García-Rico, R.O.; Levicán, G.; Tello, M.; Chávez, R. The biosynthetic gene cluster for andrastin A in Penicillium roqueforti. Front. Microbiol. 2017, 8, 813. [Google Scholar] [CrossRef]
- Ran, H.; Li, S.M. Fungal benzene carbaldehydes: Occurrence, structural diversity, activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 240–263. [Google Scholar] [CrossRef]
- Lin, L.B.; Gao, Y.Q.; Han, R.; Xiao, J.; Wang, Y.M.; Zhang, Q.; Zhai, Y.J.; Han, W.B.; Li, W.L.; Gao, J.M. Alkylated salicylaldehydes and prenylated indole alkaloids from the endolichenic fungus Aspergillus chevalieri and their bioactivities. J. Agric. Food Chem. 2021, 69, 6524–6534. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Chen, Y.; Wei, X.; Wang, J.; Zhang, W.; Wang, F.; Zhang, S. Salicylaldehyde derivatives from a marine-derived fungus Eurotium sp. SCSIO F452. J. Antibiot. 2021, 74, 273–279. [Google Scholar] [CrossRef]
- Li, D.L.; Li, X.M.; Li, T.G.; Dang, H.Y.; Proksch, P.; Wang, B.G. Benzaldehyde derivatives from Eurotium rubrum, an endophytic fungus derived from the mangrove plant Hibiscus tiliaceus. Chem. Pharm. Bull. 2008, 56, 1282–1285. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Morimoto, K.; Hamasaki, T. Flavoglaucin, a metabolite of Eurotium chevalieri, its antioxidation and synergism with tocopherol. J. Am. Oil Chem. Soc. 1984, 61, 1864–1868. [Google Scholar] [CrossRef]
- Gao, J.; León, F.; Radwan, M.M.; Dale, O.R.; Husni, A.S.; Manly, S.P.; Lupien, S.; Wang, X.; Hill, R.A.; Dugan, F.M.; et al. Benzyl derivatives with in vitro binding affinity for human opioid and cannabinoid receptors from the fungus Eurotium repens. J. Nat. Prod. 2011, 74, 1636–1639. [Google Scholar] [CrossRef] [PubMed]
- Nies, J.; Ran, H.; Wohlgemuth, V.; Yin, W.B.; Li, S.M. Biosynthesis of the prenylated salicylaldehyde flavoglaucin requires temporary reduction to salicyl alcohol for decoration before reoxidation to the final product. Org. Lett. 2020, 22, 2256–2260. [Google Scholar] [CrossRef]
- Xiang, P.; Kemmerich, B.; Yang, L.; Li, S.M. Biosynthesis of annullatin D in Penicillium roqueforti implies oxidative lactonization between two hydroxyl groups catalyzed by a BBE-like enzyme. Org. Lett. 2022, 24, 6072–6077. [Google Scholar] [CrossRef]
- Asai, T.; Luo, D.; Obara, Y.; Taniguchi, T.; Monde, K.; Yamashita, K.; Oshima, T. Dihydrobenzofurans as cannabinoid receptor ligands from Cordyceps annullata, an entomopathogenic fungus cultivated in the presence of an HDAC inhibitor. Tetrahedron Lett. 2012, 53, 2239–2243. [Google Scholar] [CrossRef]
- El Hajj Assaf, C.; Zetina-Serrano, C.; Tahtah, N.; Khoury, A.E.; Atoui, A.; Oswald, I.P.; Puel, O.; Lorber, S. Regulation of secondary metabolism in the Penicillium genus. Int. J. Mol. Sci. 2020, 21, 9462. [Google Scholar] [CrossRef]
- Chang, P.K.; Ehrlich, K.C. Genome-wide analysis of the Zn(II)2Cys6 zinc cluster-encoding gene family in Aspergillus flavus. Appl. Microbiol. Biotechnol. 2013, 97, 4289–4300. [Google Scholar] [CrossRef]
- García-Estrada, C.; Domínguez-Santos, R.; Kosalková, K.; Martín, J.-F. Transcription factors controlling primary and secondary metabolism in filamentous fungi: The β-lactam paradigm. Fermentation 2018, 4, 47. [Google Scholar] [CrossRef]
- Moon, H.; Han, K.H.; Yu, J.H. Upstream regulation of development and secondary metabolism in Aspergillus species. Cells 2022, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- García-Rico, R.O.; Martín, J.F.; Fierro, F. The pga1 gene of Penicillium chrysogenum NRRL 1951 encodes a heterotrimeric G protein alpha subunit that controls growth and development. Res. Microbiol. 2007, 158, 437–446. [Google Scholar] [CrossRef] [PubMed]
- García-Rico, R.O.; Chávez, R.; Fierro, F.; Martín, J.F. Effect of a heterotrimeric G protein alpha subunit on conidia germination, stress response, and roquefortine C production in Penicillium roqueforti. Int. Microbiol. 2009, 12, 123–129. [Google Scholar] [PubMed]
- García-Rico, R.O.; Martín, J.F.; Fierro, F. Heterotrimeric Gα protein Pga1 from Penicillium chrysogenum triggers germination in response to carbon sources and affects negatively resistance to different stress conditions. Fungal Genet. Biol. 2011, 48, 641–649. [Google Scholar] [CrossRef]
- Audhya, A.; Emr, S.D. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell. 2002, 2, 593–605. [Google Scholar] [CrossRef]
- Audhya, A.; Foti, M.; Emr, S.D. Distinct roles for the yeast phosphatidylinositol 4-kinases, Stt4p and Pik1p, in secretion, cell growth, and organelle membrane dynamics. Mol. Biol. Cell 2000, 11, 2673–2689. [Google Scholar] [CrossRef]
- Voelker, D.R. Protein and lipid motifs regulate phosphatidylserine traffic in yeast. Biochem. Soc. Trans. 2005, 33, 1141–1145. [Google Scholar] [CrossRef]
- Kishimoto, T.; Mioka, T.; Itoh, E.; Williams, D.E.; Andersen, R.J.; Tanaka, K. Phospholipid flippases and Sfk1 are essential for the retention of ergosterol in the plasma membrane. Mol. Biol. Cell. 2021, 32, 1374–1392. [Google Scholar] [CrossRef]
- Chung, J.; Nakatsu, F.; Baskin, J.M.; De Camilli, P. Plasticity of PI4KIIIα interactions at the plasma membrane. EMBO Rep. 2015, 16, 312–320. [Google Scholar] [CrossRef]
- Mioka, T.; Fujimura-Kamada, K.; Mizugaki, N.; Kishimoto, T.; Sano, T.; Nunome, H.; Williams, D.E.; Andersen, R.J.; Tanaka, K. Phospholipid flippases and Sfk1p, a novel regulator of phospholipid asymmetry, contribute to low permeability of the plasma membrane. Mol. Biol. Cell. 2018, 29, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Gil-Durán, C.; Rojas-Aedo, J.F.; Medina, E.; Vaca, I.; García-Rico, R.O.; Villagrán, S.; Levicán, G.; Chávez, R. The pcz1 gene, which encodes a Zn(II)2Cys6 protein, is involved in the control of growth, conidiation, and conidial germination in the filamentous fungus Penicillium roqueforti. PLoS ONE 2015, 10, e0120740. [Google Scholar] [CrossRef] [PubMed]
- Torrent, C.; Gil-Durán, C.; Rojas-Aedo, J.F.; Medina, E.; Vaca, I.; Castro, P.; García-Rico, R.O.; Cotoras, M.; Mendoza, L.; Levicán, G.; et al. Role of sfk1 Gene in the filamentous fungus Penicillium roqueforti. Front. Microbiol. 2017, 8, 2424. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Aedo, J.F.; Gil-Durán, C.; Goity, A.; Vaca, I.; Levicán, G.; Larrondo, L.F.; Chávez, R. The developmental regulator Pcz1 affects the production of secondary metabolites in the filamentous fungus Penicillium roqueforti. Microbiol. Res. 2018, 212–213, 67–74. [Google Scholar] [CrossRef]
- Ramamoorthy, V.; Dhingra, S.; Kincaid, A.; Shantappa, S.; Feng, X.; Calvo, A.M. The putative C2H2 transcription factor MtfA is a novel regulator of secondary metabolism and morphogenesis in Aspergillus nidulans. PLoS ONE 2013, 8, e74122. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Lohmar, J.M.; Satterlee, T.; Cary, J.W.; Calvo, A.M. The master transcription factor mtfA governs aflatoxin production, morphological development and pathogenicity in the fungus Aspergillus flavus. Toxins 2016, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, K.J.; Dolan, S.K.; Doyle, S. Endogenous cross-talk of fungal metabolites. Front. Microbiol. 2015, 5, 732. [Google Scholar] [CrossRef]
- Bergmann, S.; Funk, A.N.; Scherlach, K.; Schroeckh, V.; Shelest, E.; Horn, U.; Hertweck, C.; Brakhage, A.A. Activation of a silent fungal polyketide biosynthesis pathway through regulatory cross talk with a cryptic nonribosomal peptide synthetase gene cluster. Appl. Environ Microbiol. 2010, 76, 8143–8149. [Google Scholar] [CrossRef]
- Zhang, W.; Du, L.; Qu, Z.; Zhang, X.; Li, F.; Li, Z.; Qi, F.; Wang, X.; Jiang, Y.; Men, P.; et al. Compartmentalized biosynthesis of mycophenolic acid. Proc. Natl. Acad. Sci. USA 2019, 116, 13305–13310. [Google Scholar] [CrossRef]
- Cepeda-García, C.; Domínguez-Santos, R.; García-Rico, R.O.; García-Estrada, C.; Cajiao, A.; Fierro, F.; Martín, J.F. Direct involvement of the CreA transcription factor in penicillin biosynthesis and expression of the pcbAB gene in Penicillium chrysogenum. Appl. Microbiol. Biotechnol. 2014, 98, 7113–7124. [Google Scholar] [CrossRef]
- Chen, Y.; Li, B.; Xu, X.; Zhang, Z.; Tian, S. The pH-responsive PacC transcription factor plays pivotal roles in virulence and patulin biosynthesis in Penicillium expansum. Environ. Microbiol. 2018, 20, 4063–4078. [Google Scholar] [CrossRef] [PubMed]
- Gente, S.; Poussereau, N.; Fèvre, M. Isolation and expression of a nitrogen regulatory gene, nmc, of Penicillium roqueforti. FEMS Microbiol. Lett. 1999, 175, 291–297. [Google Scholar] [CrossRef]
- Chavez, R.; Fierro, F.; Rico, R.O.G.; Laich, F. Mold-fermented foods: Penicillium spp. as ripening agents in the elaboration of cheese and meat products. In Mycofactories; Leitão, A.L., Ed.; Bentham Science Publishers: Emirate of Sharjah, United Arab Emirates, 2011; pp. 73–98. [Google Scholar]
- Fontaine, K.; Passerò, E.; Vallone, L.; Hymery, N.; Coton, M.; Jany, J.L.; Mounier, J.; Coton, E. Occurrence of roquefortine C, mycophenolic acid and aflatoxin M1 mycotoxins in blue-veined cheeses. Food Control 2015, 47, 634–640. [Google Scholar] [CrossRef]
- Phukhamsakda, C.; Nilsson, R.H.; Bhunjun, C.S.; de Farias, A.R.G.; Sun, Y.R.; Wijesinghe, S.N.; Raza, M.; Bao, D.F.; Lu, L.; Tibpromma, S.; et al. The numbers of fungi: Contributions from traditional taxonomic studies and challenges of metabarcoding. Fungal Divers. 2022, 114, 327–386. [Google Scholar] [CrossRef]
- van der Nest, M.A.; Chávez, R.; De Vos, L.; Duong, T.A.; Gil-Durán, C.; Ferreira, M.A.; Lane, F.A.; Levicán, G.; Santana, Q.C.; Steenkamp, E.T.; et al. IMA genome—F14: Draft genome sequences of Penicillium roqueforti, Fusarium sororula, Chrysoporthe puriensis, and Chalaropsis populi. IMA Fungus 2021, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Kjærbølling, I.; Mortensen, U.H.; Vesth, T.; Andersen, M.R. Strategies to establish the link between biosynthetic gene clusters and secondary metabolites. Fungal Genet. Biol. 2019, 130, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.N.; Yen, M.R.; Chiang, C.Y.; Lin, H.C.; Chen, P.Y. Detecting and prioritizing biosynthetic gene clusters for bioactive compounds in bacteria and fungi. Appl. Microbiol. Biotechnol. 2019, 103, 3277–3287. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.N.; Liu, H.W.; Keller, N.P.; Yin, W.B. Harnessing diverse transcriptional regulators for natural product discovery in fungi. Nat. Prod. Rep. 2020, 37, 6–16. [Google Scholar] [CrossRef]
- Soldatou, S.; Eldjarn, G.H.; Huerta-Uribe, A.; Rogers, S.; Duncan, K.R. Linking biosynthetic and chemical space to accelerate microbial secondary metabolite discovery. FEMS Microbiol. Lett. 2019, 366, fnz142. [Google Scholar] [CrossRef]
- Qiao, Y.M.; Yu, R.L.; Zhu, P. Advances in targeting and heterologous expression of genes involved in the synthesis of fungal secondary metabolites. RSC Adv. 2019, 9, 35124–35134. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Tang, X.; Zhang, H.; Chen, W.; Chen, Y.Q. Molecular tools for gene manipulation in filamentous fungi. Appl. Microbiol. Biotechnol. 2017, 101, 8063–8075. [Google Scholar] [CrossRef] [PubMed]
- Kück, U.; Hoff, B. New tools for the genetic manipulation of filamentous fungi. Appl. Microbiol. Biotechnol. 2010, 86, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.Z.; Zhu, Y.L.; Huang, P.W.; Yang, Q.; Dai, C.C. Strategies for gene disruption and expression in filamentous fungi. Appl. Microbiol. Biotechnol. 2019, 103, 6041–6059. [Google Scholar] [CrossRef]
- Weld, R.J.; Plummer, K.M.; Carpenter, M.A.; Ridgway, H.J. Approaches to functional genomics in filamentous fungi. Cell Res. 2006, 16, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Goarin, A.; Silar, P.; Malagnac, F. Gene replacement in Penicillium roqueforti. Curr. Genet. 2015, 61, 203–210. [Google Scholar] [CrossRef]
- Chang, S.S.; Zhang, Z.; Liu, Y. RNA interference pathways in fungi: Mechanisms and functions. Annu. Rev. Microbiol. 2012, 66, 305–323. [Google Scholar] [CrossRef]
- Dang, Y.; Yang, Q.; Xue, Z.; Liu, Y. RNA interference in fungi: Pathways, functions, and applications. Eukaryot. Cell 2011, 10, 1148–1155. [Google Scholar] [CrossRef]
- Majumdar, R.; Rajasekaran, K.; Cary, J.W. RNA Interference (RNAi) as a potential tool for control of mycotoxin contamination in crop plants: Concepts and considerations. Front. Plant Sci. 2017, 8, 200. [Google Scholar] [CrossRef]
- Caribé dos Santos, A.C.; Sena, J.A.; Santos, S.C.; Dias, C.V.; Pirovani, C.P.; Pungartnik, C.; Valle, R.R.; Cascardo, J.C.; Vincentz, M. dsRNA-induced gene silencing in Moniliophthora perniciosa, the causal agent of witches′ broom disease of cacao. Fungal Genet. Biol. 2009, 46, 825–836. [Google Scholar] [CrossRef]
- Rappleye, C.A.; Engle, J.T.; Goldman, W.E. RNA interference in Histoplasma capsulatum demonstrates a role for alpha-(1,3)-glucan in virulence. Mol. Microbiol. 2004, 53, 153–165. [Google Scholar] [CrossRef]
- Wang, Q.; Coleman, J.J. Progress and challenges: Development and implementation of CRISPR/Cas9 technology in filamentous fungi. Comput. Struct. Biotechnol. J. 2019, 17, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Seekles, S.J.; Teunisse, P.P.P.; Punt, M.; van den Brule, T.; Dijksterhuis, J.; Houbraken, J.; Wösten, H.A.B.; Ram, A.F.J. Preservation stress resistance of melanin deficient conidia from Peritomies variotii and Penicillium roqueforti mutants generated via CRISPR/Cas9 genome editing. Fungal Biol. Biotechnol. 2021, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Punt, M.; Seekles, S.J.; van Dam, J.L.; de Adelhart Toorop, C.; Martina, R.R.; Houbraken, J.; Ram, A.F.J.; Wösten, H.A.B.; Ohm, R.A. High sorbic acid resistance of Penicillium roqueforti is mediated by the SORBUS gene cluster. PLoS Genet. 2022, 18, e1010086. [Google Scholar] [CrossRef] [PubMed]


| Gene | ORF Name | Size (bp) | Protein | Size (aa) | Proposed Function |
|---|---|---|---|---|---|
| gmt | Proq01g022760 | 1058 | Methyltransferase | 332 | Formation of methylated derivatives |
| Pseudogene | _ | _ | _ | _ | _ |
| rpt (dmaW) | Proq01g022770 | 1337 | Reverse prenyltransferase | 425 | Addition of an isopentenyl group to the cyclopeptide |
| rdh | Proq01g022780 | 1638 | Dehydrogenase | 517 | Dehydrogenation of the cyclopeptide |
| rds | Proq01g022790 | 7200 | Nonribosomal peptide synthetase | 2363 | Formation of the cyclopeptide from L-histidine and L-tryptophan |
| Gene (A) | ORF Name | Size (bp) | Protein | Size (aa) | Proposed Function |
| ifgE | Proq05g069260 | 848 | Short-chain dehydrogenase/reductase (CDM36673) | 261 | Formation of chanoclavine I aldehyde |
| ifgF1 | Proq05g069270 | 992 | Festuclavine synthase (CDM36674) | 287 | Contribution to the formation of festuclavine |
| ifgD | Proq05g069280 | 1464 | Catalase (CDM36675) | 466 | Contribution to the formation of chanoclavine I |
| ifgB | Proq05g069290 | 1087 | SAM-dependent methyltransferase (CDM36676) | 340 | Formation of N-methyl-dimethylallyltryptophan |
| ifgC | Proq05g069300 | 1953 | FAD oxidase (CDM36677) | 629 | Contribution to the formation of chanoclavine I |
| ifgA | Proq05g069310 | 1507 | Dimethylallyltryptophan synthase (CDM36678) | 462 | Formation of dimethylallyltryptophan |
| Gene (B) | ORF Name | Size (bp) | Protein | Size (aa) | Proposed Function |
| ifgG | Proq02g028370 | 1128 | FMN-containing “old yellow enzyme” aldolase-type protein (CDM30151) | 375 | Contribution to the formation of festuclavine |
| - | Proq02g028380 | 861 | Phytanoyl-CoA dioxygenase (CDM30152) | 286 | Unknown |
| ifgI | Proq02g028390 | 1455 | acetyltransferase (CDM30153) | 484 | Conversion of isofumigaclavine B to isofumigaclavine A |
| - | Proq02g028400 | 511 | Unnamed protein product (CDM30154) | 121 | Unknown |
| ifgF2 | Proq02g028410 | 996 | Festuclavine synthase (CDM30155) | 287 | Contribution to the formation of festuclavine |
| Gene | ORF Name | Size (bp) | Protein | Size (aa) | Proposed Function |
|---|---|---|---|---|---|
| prx1 (ORF1) | Proq02g040180 | 1072 | Short-chain dehydrogenase | 340 | Oxidation of eremofortin A |
| prx2 (ari1) (ORF2) | Proq02g040190 | 1129 | Aristolochene synthase | 342 | Biosynthesis of aristolochene |
| prx3 (ORF3) | Proq02g040200 | 1716 | Quinone oxidase | 521 | Formation of 7-epineopetasone |
| prx4 (ORF4) | Proq02g040210 | 990 | Short chain alcohol dehydrogenase | 329 | Dehydrogenation of eremofortin C |
| prx8 (ORF5) | Proq02g040220a | 1827 | Cytochrome P450 monooxygenase | 540 | Oxidation of intermediates |
| prx9 (ORF6) | Proq02g040230 | 1792 | Cytochrome P450 monooxygenase | 579 | Oxidation of intermediates |
| ORF7 | Proq02g040240 | 613 | Outer membrane protein, beta-barrel | 186 | Unknown |
| prx11 (ORF8) | Proq02g040260 | 1416 | Acetyltransferase | 471 | Acetylation of intermediates |
| ORF9 | Proq02g040270a | 1823 | Cytochrome P450 monooxygenase | 509 | Oxidation of non-activated hydrocarbons |
| ORF10 | Proq02g040280 | 1437 | Transcriptional regulator | 458 | Regulation of the gene cluster transcription |
| ORF11 | Proq02g040290 | 1650 | Cytochrome P450 monooxygenase | 480 | Oxidation of non-activated hydrocarbons |
| Gene | ORF Name | Size (bp) | Protein | Size (aa) | Proposed Function |
|---|---|---|---|---|---|
| mpaA | Proq05g069820 | 1126 | Prenyltransferase | 325 | Farnesylation of 5,7-dihydroxy-4-methylphthalide |
| mpaB | Proq05g069810 | 949 (1397) | Protein of unknown function | 298 (427) | Unknown |
| mpaC | Proq05g069800 | 7715 | Non-reducing polyketide synthase | 2477 | Formation of 5-methylorsellinic acid from acetyl-CoA, malonyl-CoA and S-adenosylmethionine |
| - | Proq05g069790 | 523 | Protein of unknown function | 116 | Unknown |
| mpaDE | Proq05g069780a | 3710 (2929) | Fusion protein (Cytochrome P450/hydrolase) | 932 (852) | Sequential hydroxylation/lactonization of 5-methylorsellinic acid |
| mpaF | Proq05g069770 | 1708 | Inosine-5′-monophosphate dehydrogenase like | 526 | Role in self-resistance to mycophenolic acid and in the production of this compound |
| mpaG | Proq05g069760 | 1250 | O-methyltransferase | 398 | Methylation of demethylmycophenolic acid to form mycophenolic acid |
| mpaH | Proq05g069750 | 1479 | Unnamed protein product | 433 | Oxidative cleavage of the farnesyl chain to form demethylmycophenolic acid |
| Gene | ORF Name | Size (bp) | Protein | Size (aa) | Proposed Function |
|---|---|---|---|---|---|
| adrA | Proq04g062820 | 1746 | Cytochrome P450 monooxygenase | 508 | Consecutive oxidations for the formation of Andrastin A from Andrastin C |
| adrC | Proq04g062830 | 4585 | ABC transporter | 1452 | Unknown (somehow involved in the production of Andrastin A) |
| adrD | Proq04g062840 | 7973 | Polyketide synthase | 2495 | Formation of 3,5-dimethylorsellinic acid from acetyl-CoA, malonyl-CoA and S-adenosylmethionine |
| adrE | Proq04g062850 | 1249 | Ketoreductase | 336 | Formation of Andrastin F from Andrastin D |
| adrF | Proq04g062860a | 952 | Short-chain dehydrogenase | 256 | Formation of Andrastin D from Andrastin E |
| adrG | Proq04g062870 | 951 | Prenyltransferase | 316 | Farnesylation of 3,5-dimethylorsellinic acid |
| adrH | Proq04g062880a | 1633 | FAD-dependent oxidoreductase | 476 | Formation of epoxyfarnesyl-3,5-dimethylorsellinic acid methyl ester |
| adrI | Proq04g062890 | 790 | Terpene cyclase | 245 | Formation of Andrastin E epoxyfarnesyl-3,5-dimethylorsellinic acid methyl ester |
| adrJ | Proq04g062900 | 1554 | Acetyltransferase | 496 | Formation of Andrastin C from Andrastin F |
| adrK | Proq04g062910 | 1050 | Methyltransferase | 278 | Methylation of farnesyl-3,5-dimethylorsellinic acid |
| Gene | ORF Name | Size (bp) | Protein | Size (aa) | Proposed Function |
|---|---|---|---|---|---|
| anuK | Proq03g054140 | 1246 | Transcription factor (77.4% identity with FogI) | 261 | Unknown (transcriptional activation of the anu genes) |
| anuA | Proq03g054150 | 6704 | Reducing polyketide synthase | 2130 | Formation of 2-hydroxymethyl-3-pentylphenol from acetyl-CoA and malonyl-CoA |
| anuB | Proq03g054160a | 1458 | Short-chain dehydrogenase/reductase | 283 | Formation of 2-hydroxymethyl-3-pentylphenol |
| anuC | Proq03g054170 | 276 | Protein with 44.3% identity with the short-chain dehydrogenase/reductase FogB | 91 | Unknown (Formation of 2-hydroxymethyl-3-pentylphenol) |
| anuD | Proq03g054180 | 1288 | Short-chain dehydrogenase | 381 | Unknown |
| anuE | Proq03g054190 | 1772 | Cytochrome P450 hydroxylase | 490 | Formation annullatin E from 2-hydroxymethyl-3-pentylphenol into |
| anuF | Proq03g054200 | 937 | Short-chain dehydrogenase/reductase | 276 | Formation of annullatin F |
| anuG | Proq03g054210 | 1797 | Berberine bridge enzyme-like protein | 509 | Formation of annullatin D |
| anuH | Proq03g054220 | 1512 | Prenyltransferase | 451 | Addition of a dimethylallyl group to annullatin E (formation of annullatin J) and formation of other prenylated derivatives |
| anuI | Proq03g054230 | 759 | Short-chain dehydrogenase/reductase | 252 | Unknown |
| anuJ | Proq03g054240 | 2587 | Aromatic ring hydroxylating dehydrogenase | 818 | Unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez, R.; Vaca, I.; García-Estrada, C. Secondary Metabolites Produced by the Blue-Cheese Ripening Mold Penicillium roqueforti; Biosynthesis and Regulation Mechanisms. J. Fungi 2023, 9, 459. https://doi.org/10.3390/jof9040459
Chávez R, Vaca I, García-Estrada C. Secondary Metabolites Produced by the Blue-Cheese Ripening Mold Penicillium roqueforti; Biosynthesis and Regulation Mechanisms. Journal of Fungi. 2023; 9(4):459. https://doi.org/10.3390/jof9040459
Chicago/Turabian StyleChávez, Renato, Inmaculada Vaca, and Carlos García-Estrada. 2023. "Secondary Metabolites Produced by the Blue-Cheese Ripening Mold Penicillium roqueforti; Biosynthesis and Regulation Mechanisms" Journal of Fungi 9, no. 4: 459. https://doi.org/10.3390/jof9040459
APA StyleChávez, R., Vaca, I., & García-Estrada, C. (2023). Secondary Metabolites Produced by the Blue-Cheese Ripening Mold Penicillium roqueforti; Biosynthesis and Regulation Mechanisms. Journal of Fungi, 9(4), 459. https://doi.org/10.3390/jof9040459







