Erg4 Is Involved in Ergosterol Biosynthesis, Conidiation and Stress Response in Penicillium expansum
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains, Culture Conditions and Fruit
2.2. Sequence Alignments and Phylogenetic Analysis
2.3. Gene Knockout and Complementation
2.4. Gene expression Analysis
2.5. Determination of Ergosterol Content
2.6. Colony Diameter and Colony Morphology
2.7. Conidiophore Development and Spore Morphology
2.8. Determination of Sporulation and Spore Germination Rate
2.9. Exogenous Stress Susceptibility Test
2.10. Pathogenicity Test
2.11. Statistical Analysis
3. Results
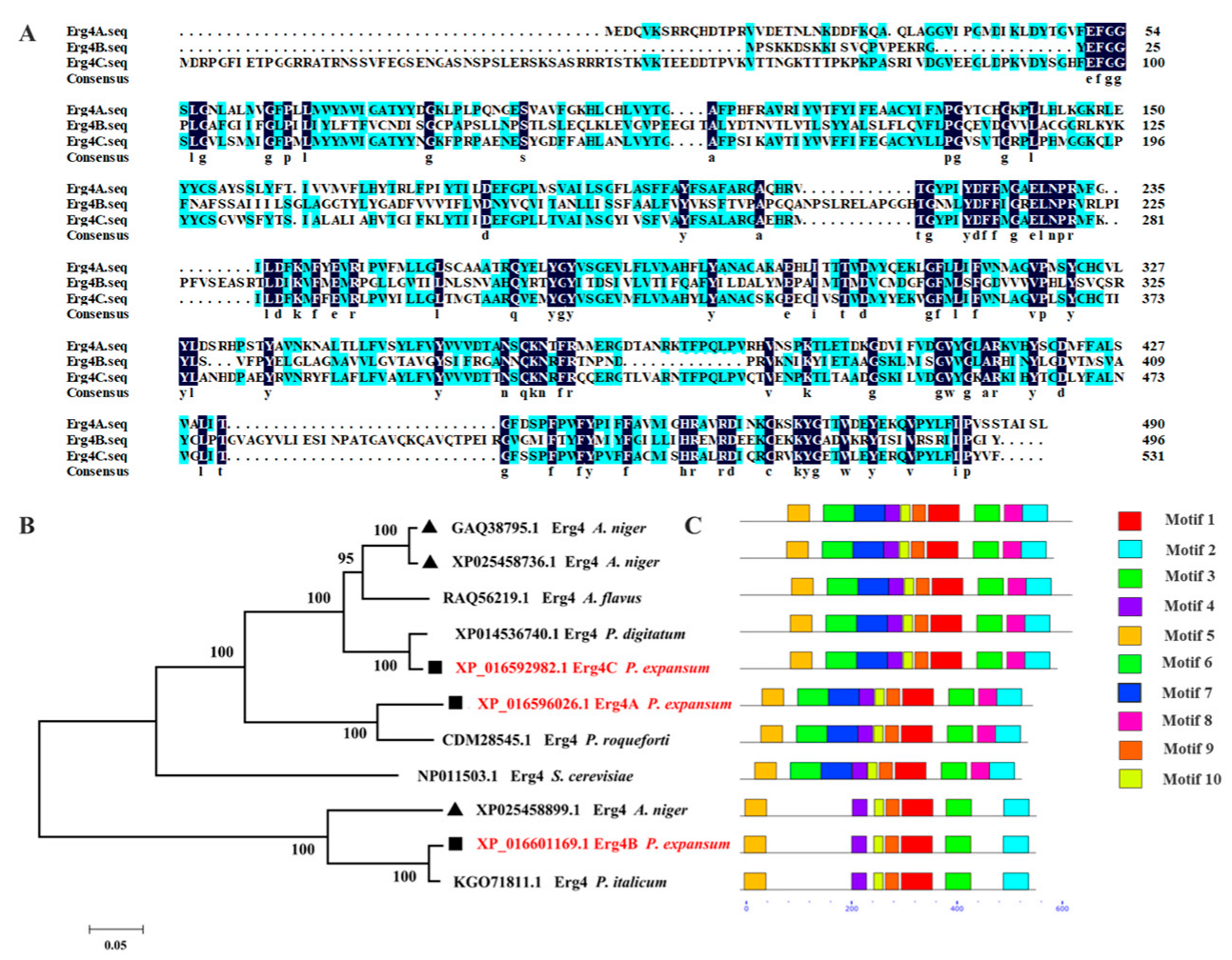
3.1. Sequence Alignment and Phylogenetic Analysis of Erg4A, Erg4B and Erg4C
3.2. Transcript Levels of erg4A, erg4B and erg4C in WT and Mutants
3.3. Effect of erg4A, erg4B and erg4C Deletion on Ergosterol Production
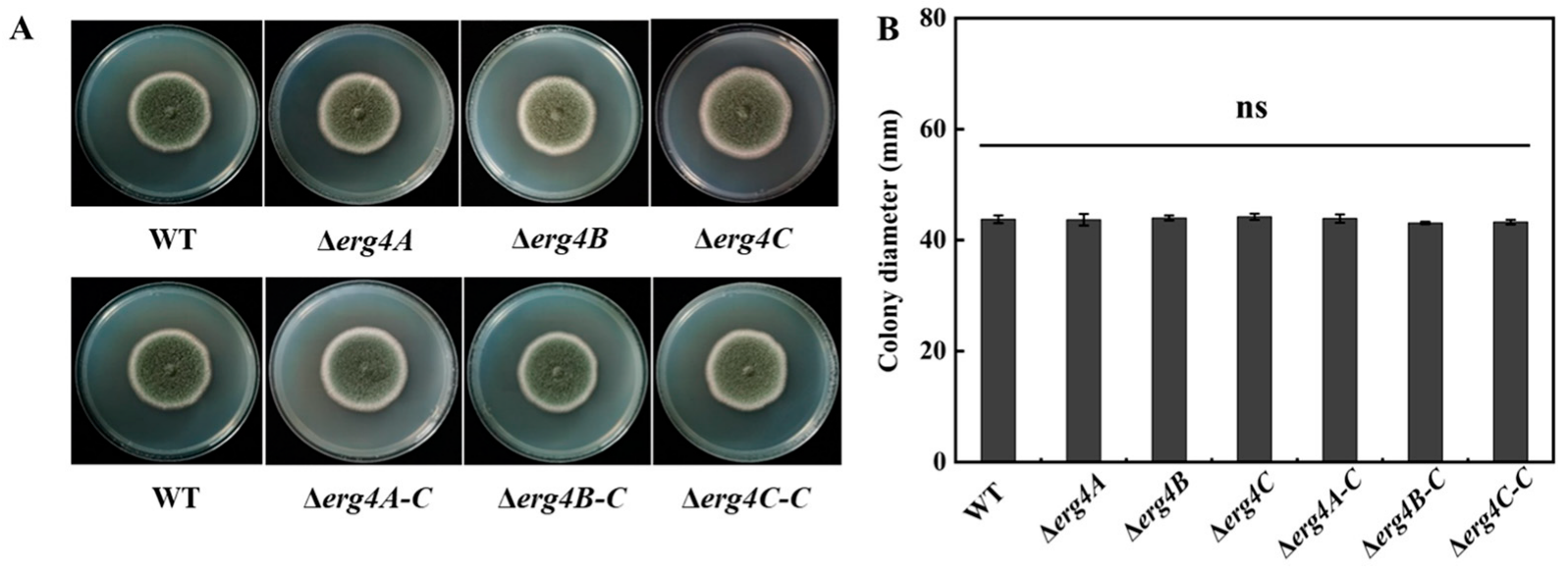
3.4. Effect of erg4A, erg4B and erg4C Deletion on Colony Morphology and Diameter
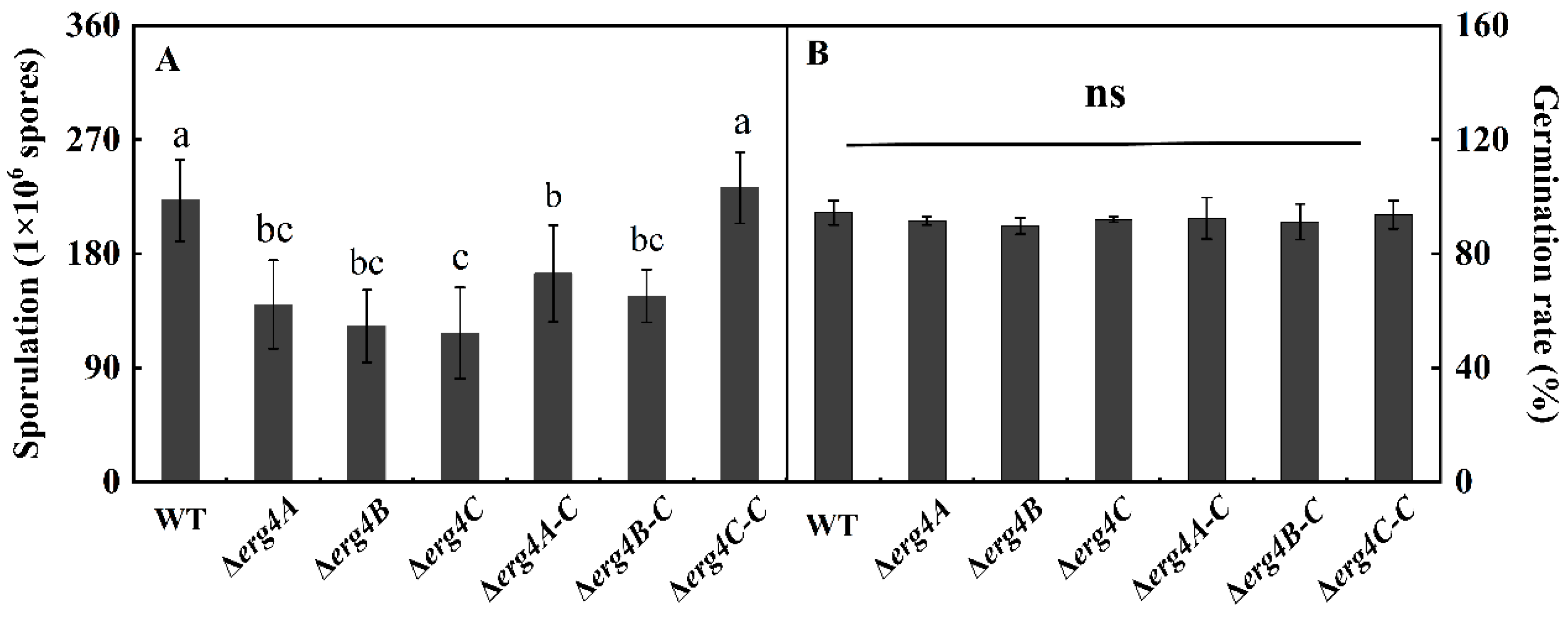
3.5. Effect of erg4A, erg4B and erg4C Deletion on Sporulation and Spore Germination Rate
3.6. Effect of erg4A, erg4B and erg4C Deletion on Conidiophore Development and Spore Morphology
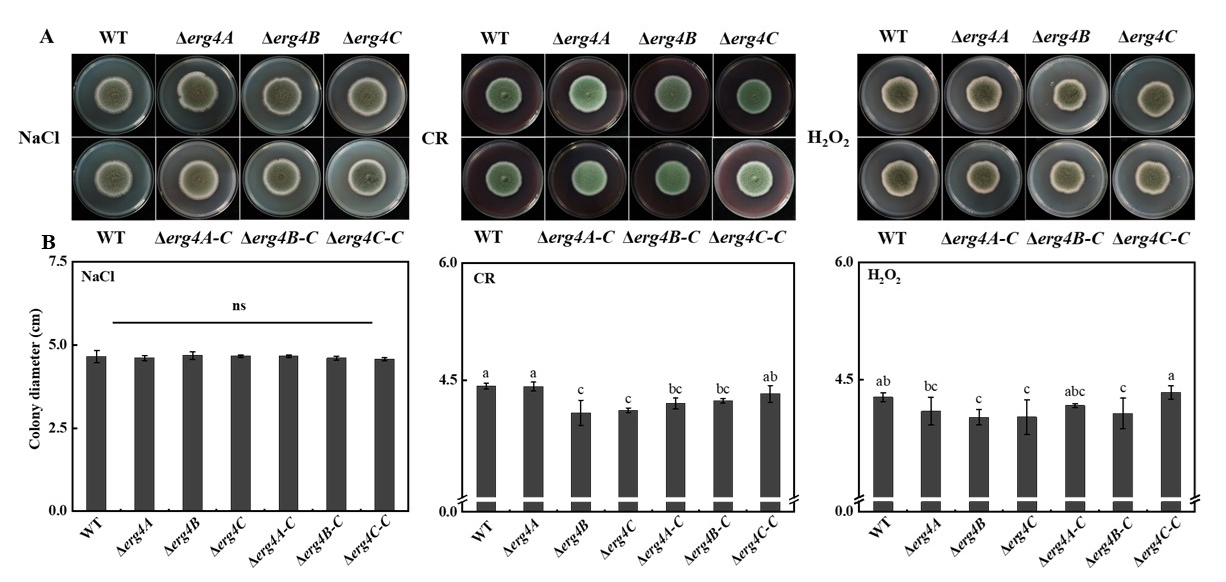
3.7. Effect of erg4A, erg4B and erg4C Deletion on Osmotic Stress, Cell Wall Integrity and Oxidative Stress
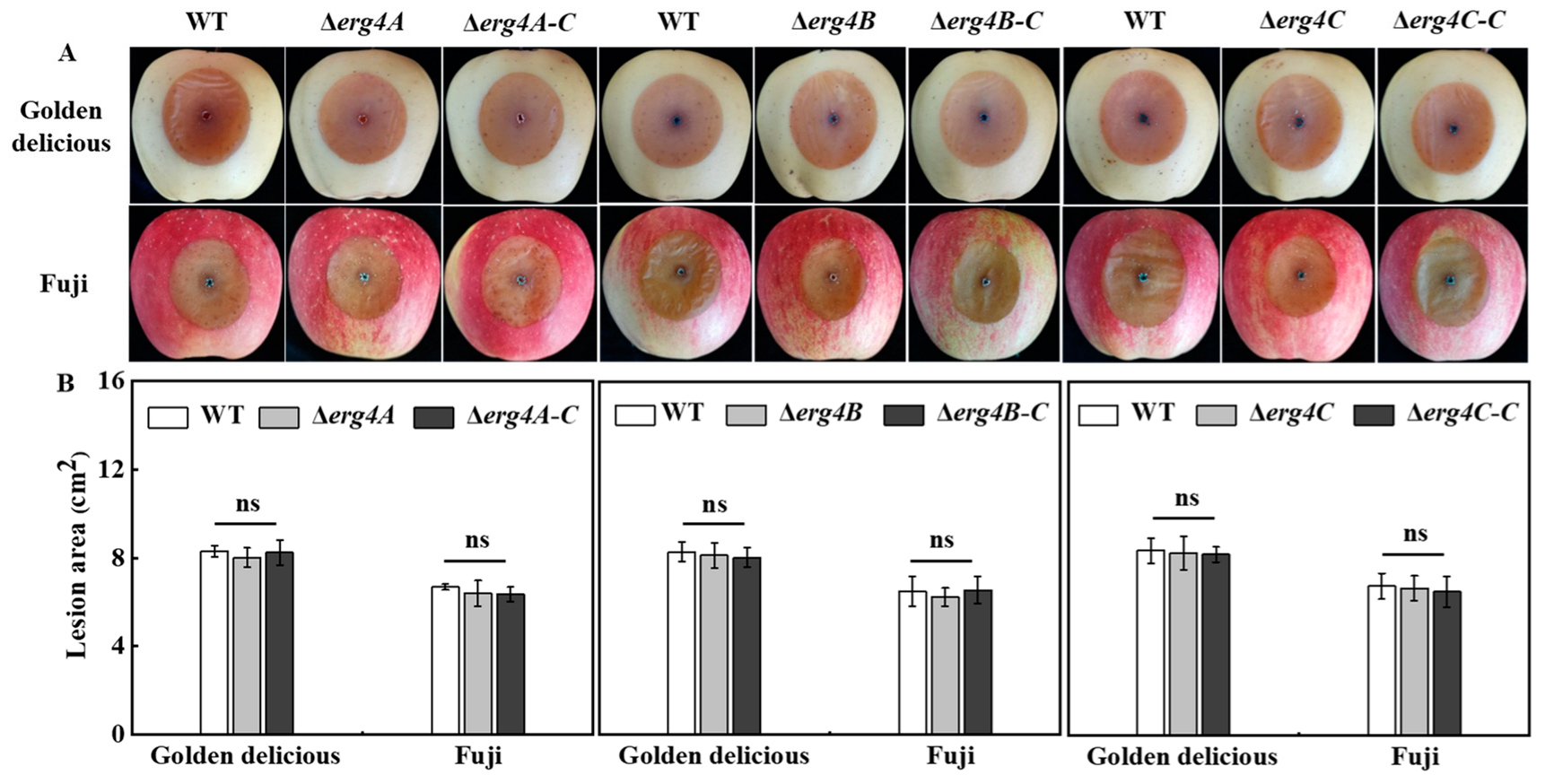
3.8. Effect of erg4A, erg4B and erg4C Deletion on Pathogenicity on Apple Fruit
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, K.L.; Ngea, G.L.N.; Godana, E.A.; Shi, Y.; Lanhuang, B.; Zhang, X.Y.; Zhao, L.N.; Yang, Q.Y.; Wang, S.Y.; Zhang, H.Y. Recent advances in Penicillium expansum infection mechanisms and current methods in controlling P. expansum in postharvest apples. Crit. Rev. Food Sci. Nutr. 2021, 20, 2598–2611. [Google Scholar] [CrossRef] [PubMed]
- Jordá, T.; Puig, S. Regulation of ergosterol biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef]
- Liu, J.F.; Xia, J.J.; Nie, K.L.; Wang, F.; Deng, L. Outline of the biosynthesis and regulation of ergosterol in yeast. World J. Microbiol. Biotechnol. 2019, 35, 98. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.H.; He, B.; Ma, L.; Sun, Y.L.; Niu, Y.L.; Zeng, B. Recent advances in ergosterol biosynthesis and regulation mechanisms in Saccharomyces cerevisiae. Indian J. Microbiol. 2017, 57, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Zweytick, D.; Hrastnik, C.; Kohlwein, S.D.; Daum, G. Biochemical characterization and subcellular localization of the sterol C-24(28) reductase, erg4p, from the yeast saccharomyces cerevisiae. FEBS Lett. 2000, 470, 83–87. [Google Scholar] [CrossRef] [PubMed]
- He, X.P.; Zhang, B.R.; Tan, H.R. Overexpression of a sterol C-24(28) reductase increases ergosterol production in Saccharomyces cerevisiae. Biotechnol. Lett. 2003, 25, 773–778. [Google Scholar] [CrossRef]
- Venegas, M.; Barahona, S.; González, A.M.; Sepúlveda, D.; Zúñiga, G.E.; Baeza, M.; Cifuentes, V.; Alcaíno, J. Phenotypic analysis of mutants of ergosterol biosynthesis genes (ERG3 and ERG4) in the red yeast Xanthophyllomyces dendrorhous. Front. Microbiol. 2020, 11, 1312. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.H.; Yin, Y.N.; Ma, Z.H. Involvement of FgERG4 in ergosterol biosynthesis, vegetative differentiation and virulence in Fusarium graminearum. Mol. Plant Pathol. 2013, 14, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Long, N.B.; Xu, X.L.; Zeng, Q.Q.; Sang, H.; Lu, L. Erg4A and Erg4B are required for conidiation and azole resistance via regulation of ergosterol biosynthesis in Aspergillus fumigatus. Appl. Environ. Microbiol. 2017, 83, e02924-16. [Google Scholar] [CrossRef]
- Han, Z.H.; Zong, Y.Y.; Zhang, X.M.; Wang, B.; Prusky, D.; Bi, Y. Bioinformatic, subcellular localization and expression analysis of erg4 in Penicillium expansum. Biotechnol. Bull. 2021, 37, 60–70. (In Chinese) [Google Scholar] [CrossRef]
- Li, B.Q.; Zong, Y.Y.; Du, Z.L.; Chen, Y.; Zhang, Z.Q.; Qin, G.Z.; Zhao, W.M.; Tian, S.P. Genomic characterization reveals insights into patulin biosynthesis and pathogenicity in Penicillium species. Mol. Plant Microbe Interact. 2015, 28, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, B.Q.; Xu, X.D.; Zhang, Z.Q.; Tian, S.P. The pH-responsive PacC transcription factor plays pivotal roles in virulence and patulin biosynthesis in Penicillium expansum. Environ. Microbiol. 2018, 20, 4063–4078. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.; Liu, Y.; Oketch, O.R.; Zhang, M.; Shao, X.; Tao, N. Citronellal exerts its antifungal activity by targeting ergosterol biosynthesis in Penicillium digitatum. J. Fungi 2021, 7, 432. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.Y.; Li, B.Q.; Tian, S.P. Effects of carbon, nitrogen and ambient pH on patulin production and related gene expression in Penicillium expansum. Int. J. Food Microbiol. 2015, 206, 102–108. [Google Scholar] [CrossRef]
- Prakash, P.Y.; Bhargava, K.A. A modified micro chamber agar spot slide culture technique for microscopic examination of filamentous fungi. J. Microbiol. Methods 2016, 123, 126–129. [Google Scholar] [CrossRef]
- Yahyazadeh, M.; Omidbaigi, R.; Zare, R.; Taheri, H. Effect of some essentialoils on mycelial growth of Penicillium digitatum Sacc. World J. Microbiol Biotechnol. 2008, 24, 1445–1450. [Google Scholar] [CrossRef]
- Xu, X.D.; Chen, Y.; Li, B.Q.; Tian, S.P. Arginine methyltransferase PeRmtC regulates development and pathogenicity of Penicillium expansum via mediating key genes in conidiation and secondary metabolism. J. Fungi 2021, 7, 807. [Google Scholar] [CrossRef]
- Ma, D.Y.; Ji, D.C.; Liu, J.L.; Xu, Y.; Chen, T.; Tian, S.P. Efficacy of methyl thujate in inhibiting Penicillium expansum growth and possible mechanism involved. Postharvest Biol. Technol. 2020, 3, 111070. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zong, Y.Y.; Gong, D.; Yu, L.R.; Sionov, E.; Bi, Y.; Prusky, D. NADPH oxidase regulates the growth and pathogenicity of Penicillium expansum. Front. Plant Sci. 2021, 12, 696210. [Google Scholar] [CrossRef]
- Chen, Y.; Li, B.Q.; Zhang, Z.Q.; Tian, S.P. Pathogenicity assay of Penicillium expansum on apple fruits. Bio-Protocol. 2017, 7, e2264. [Google Scholar] [CrossRef] [PubMed]
- Alcazar-Fuoli, L.; Mellado, E. Ergosterol biosynthesis in Aspergillus fumigatus: Its relevance as an antifungal target and role in antifungal drug resistance. Front. Microbiol. 2012, 3, 439. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, S.; Cramer, R.A. Regulation of sterol biosynthesis in the human fungal pathogen Aspergillus fumigatus: Opportunities for therapeutic development. Front. Microbiol. 2017, 8, 92. [Google Scholar] [CrossRef]
- Luciano-Rosario, D.; Keller, N.P.; Jurick, W.M. Penicillium expansum: Biology, omics, and management tools for a global postharvest pathogen causing blue mould of pome fruit. Mol. Plant Pathol. 2020, 21, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Mead, M.E.; Lee, M.K.; Ostrem Loss, E.M.; Kim, S.C.; Rokas, A.; Yu, J.H. Systematic dissection of the evolutionarily conserved wetA developmental regulator across a genus of filamentous fungi. mBio. 2018, 70, 317–343. [Google Scholar] [CrossRef]
- Chen, J.F.; Liu, Y.; Tang, G.R.; Jin, D.; Chen, X.; Pei, Y.; Fan, Y.H. The secondary metabolite regulator, BbSmr1, is a central regulator of conidiation via the BrlA-AbaA-WetA pathway in Beauveria bassiana. Environ. Microbiol. 2021, 23, 810–825. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.D.; Chai, Y.F.; Zhang, C.Y.; Qiao, W.R.; Sang, H.; Lu, L. The Gβ-like protein CpcB is required for hyphal growth, conidiophore morphology and pathogenicity in Aspergillus fumigatus. Fungal Genet. Biol. 2015, 81, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Long, N.B.; Zhong, G. The C-22 sterol desaturase Erg5 is responsible for ergosterol biosynthesis and conidiation in Aspergillus fumigatus. J. Microbiol. 2022, 60, 620–626. [Google Scholar] [CrossRef]
- Yin, Z.; Tang, W.; Wang, J.; Liu, X.; Yang, L.; Gao, C.; Zhang, J.; Zhang, H.; Zheng, X.; Wang, P.; et al. Phosphodiesterase MoPdeH targets MoMck1 of the conserved mitogen-activated protein (MAP) kinase signalling pathway to regulate cell wall integrity in rice blast fungus Magnaporthe oryzae. Mol. Plant Pathol. 2016, 17, 654–668. [Google Scholar] [CrossRef]
- Ram, A.F.; Klis, F.M. Identification of fungal cell wall mutants using susceptibility assays based on calcofluor white and congo red. Nat. Protoc. 2006, 1, 2253–2256. [Google Scholar] [CrossRef]
- Marisco, G.; Saito, S.T.; Ganda, I.S.; Brendel, M.; Pungartnik, C. Low ergosterol content in yeast adh1 mutant enhances chitin maldistribution and sensitivity to paraquat-induced oxidative stress. Yeast. 2011, 28, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Tiedje, C.; Holland, D.G.; Just, U.; Höfken, T. Proteins involved in sterol synthesis interact with Ste20 and regulate cell polarity. J. Cell Sci. 2007, 120, 3613–3624. [Google Scholar] [CrossRef] [PubMed]
- Lesage, G.; Bussey, H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2016, 70, 317–343. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Zong, Y.; Zhang, X.; Gong, D.; Wang, B.; Prusky, D.; Sionov, E.; Xue, H.; Bi, Y. Erg4 Is Involved in Ergosterol Biosynthesis, Conidiation and Stress Response in Penicillium expansum. J. Fungi 2023, 9, 568. https://doi.org/10.3390/jof9050568
Han Z, Zong Y, Zhang X, Gong D, Wang B, Prusky D, Sionov E, Xue H, Bi Y. Erg4 Is Involved in Ergosterol Biosynthesis, Conidiation and Stress Response in Penicillium expansum. Journal of Fungi. 2023; 9(5):568. https://doi.org/10.3390/jof9050568
Chicago/Turabian StyleHan, Zhanhong, Yuanyuan Zong, Xuemei Zhang, Di Gong, Bin Wang, Dov Prusky, Edward Sionov, Huali Xue, and Yang Bi. 2023. "Erg4 Is Involved in Ergosterol Biosynthesis, Conidiation and Stress Response in Penicillium expansum" Journal of Fungi 9, no. 5: 568. https://doi.org/10.3390/jof9050568
APA StyleHan, Z., Zong, Y., Zhang, X., Gong, D., Wang, B., Prusky, D., Sionov, E., Xue, H., & Bi, Y. (2023). Erg4 Is Involved in Ergosterol Biosynthesis, Conidiation and Stress Response in Penicillium expansum. Journal of Fungi, 9(5), 568. https://doi.org/10.3390/jof9050568










