Aspergillus Galactomannan Titer as a Diagnostic Marker of Invasive Pulmonary Aspergillosis in Lung Transplant Recipients: A Single-Center Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Proven IPA
2.3. Probable IPA
2.4. Pneumonia Unrelated to IPA
2.5. Antifungal Prophylaxis and Treatment
2.6. Statistical Analysis
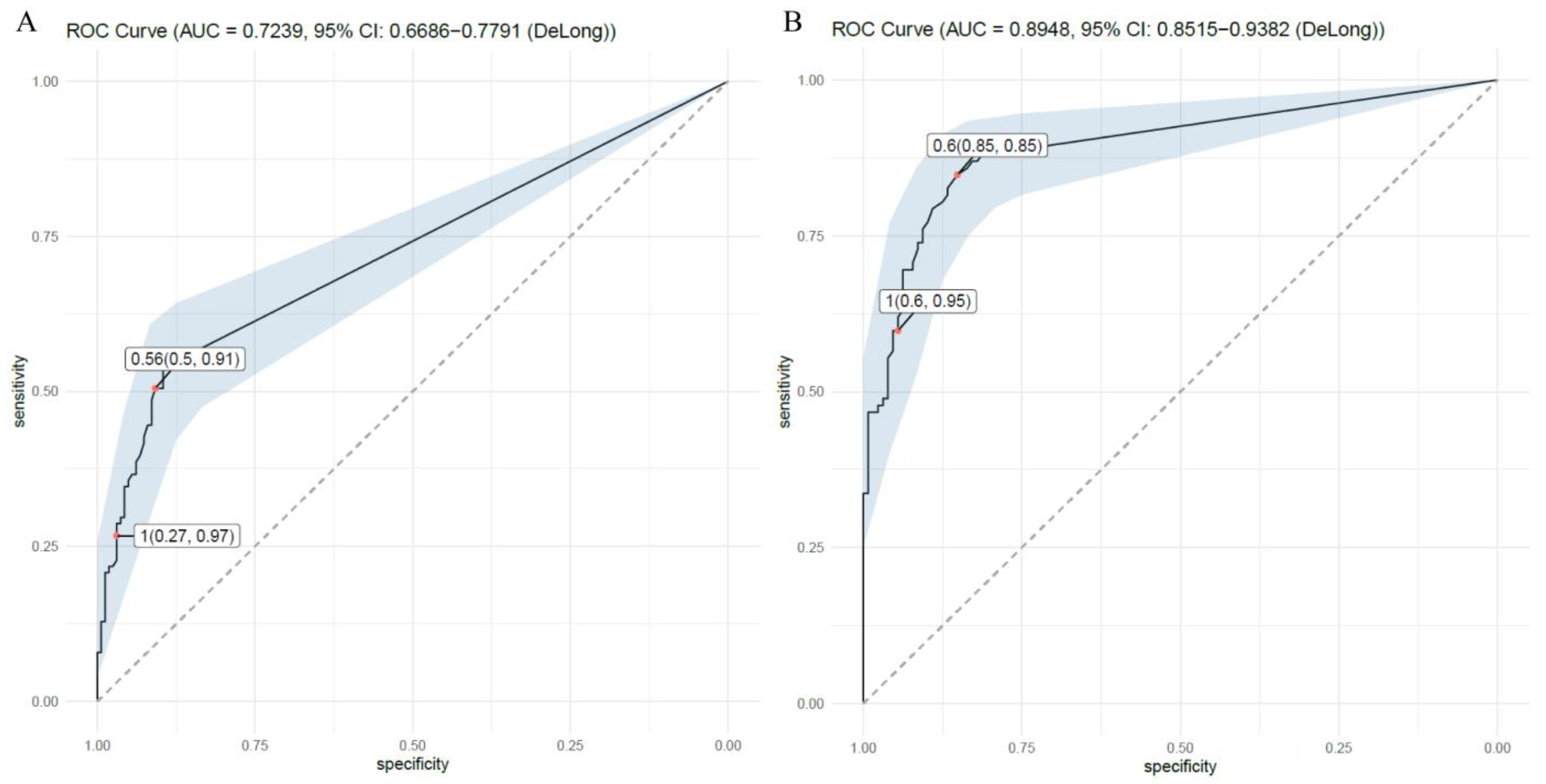
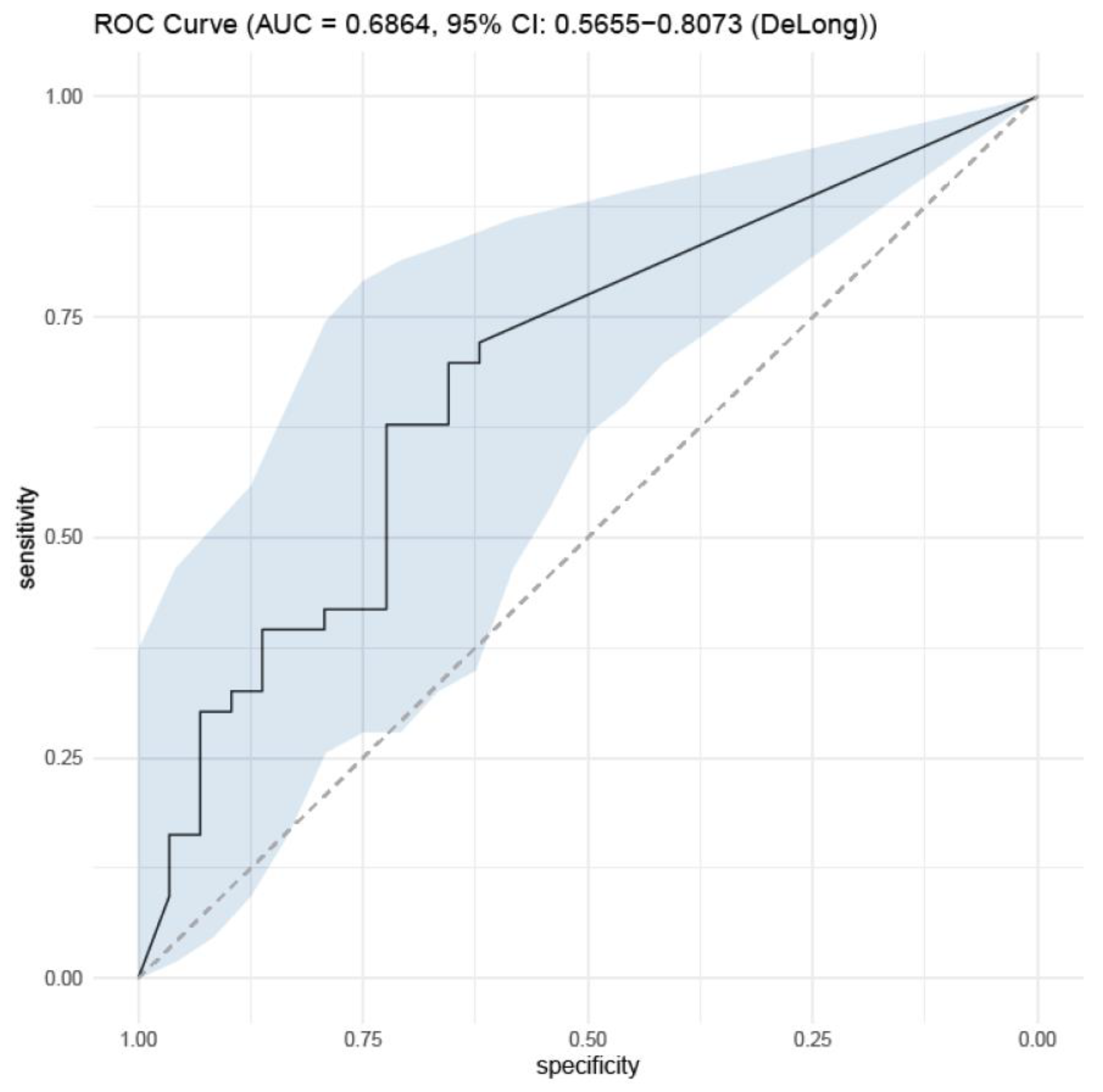
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miceli, M.H.; Kauffman, C.A. Aspergillus Galactomannan for Diagnosing Invasive Aspergillosis. JAMA 2017, 318, 1175–1176. [Google Scholar] [CrossRef] [PubMed]
- Arthurs, S.K.; Eid, A.J.; Deziel, P.J.; Marshall, W.F.; Cassivi, S.D.; Walker, R.C.; Razonable, R.R. The impact of invasive fungal diseases on survival after lung transplantation. Clin. Transplant. 2010, 24, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, M.P.; Guffroy, B.; Nivoix, Y.; Simand, C.; Herbrecht, R. Invasive Pulmonary Aspergillosis. Semin. Respir. Crit. Care Med. 2020, 41, 80–98. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Paterson, D.L. Aspergillus infections in transplant recipients. Clin. Microbiol. Rev. 2005, 18, 44–69. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Husain, S. Aspergillus infections after lung transplantation: Clinical differences in type of transplant and implications for management. J. Heart Lung Transplant. 2003, 22, 258–266. [Google Scholar] [CrossRef]
- Husain, S.; Sole, A.; Alexander, B.D.; Aslam, S.; Avery, R.; Benden, C.; Billaud, E.M.; Chambers, D.; Danziger-Isakov, L.; Fedson, S.; et al. The 2015 International Society for Heart and Lung Transplantation Guidelines for the management of fungal infections in mechanical circulatory support and cardiothoracic organ transplant recipients: Executive summary. J. Heart Lung Transplant. 2016, 35, 261–282. [Google Scholar] [CrossRef]
- Moura, S.; Cerqueira, L.; Almeida, A. Invasive pulmonary aspergillosis: Current diagnostic methodologies and a new molecular approach. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1393–1403. [Google Scholar] [CrossRef]
- Kono, Y.; Tsushima, K.; Yamaguchi, K.; Kurita, N.; Soeda, S.; Fujiwara, A.; Sugiyama, S.; Togashi, Y.; Kasagi, S.; To, M.; et al. The utility of galactomannan antigen in the bronchial washing and serum for diagnosing pulmonary aspergillosis. Respir. Med. 2013, 107, 1094–1100. [Google Scholar] [CrossRef]
- Sarwar, M.; Gardezi, S.A.H.; Zaman, G.; Ikram, A.; Satti, L.; Khadim, M.T. Evaluation of galactomannan and beta-d-glucan assays for the diagnosis of invasive aspergillosis in clinically suspected cases. J. Pak. Med. Assoc. 2020, 70, 442–446. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Haydour, Q.; Hage, C.A.; Carmona, E.M.; Epelbaum, O.; Evans, S.E.; Gabe, L.M.; Knox, K.S.; Kolls, J.K.; Wengenack, N.L.; Prokop, L.J.; et al. Diagnosis of Fungal Infections. A Systematic Review and Meta-Analysis Supporting American Thoracic Society Practice Guideline. Ann. Am. Thorac. Soc. 2019, 16, 1179–1188. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Herrera, S.; Husain, S. Early diagnosis of fungal infections in lung transplant recipients, colonization versus invasive disease? Curr. Opin. Organ. Transplant. 2018, 23, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Husain, S.; Camargo, J.F. Invasive Aspergillosis in solid-organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13544. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.A.; Raad, I.I.; Marr, K.A.; Patterson, T.F.; Kontoyiannis, D.P.; Cornely, O.A.; Bow, E.J.; Rahav, G.; Neofytos, D.; Aoun, M.; et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A phase 3, randomised-controlled, non-inferiority trial. Lancet 2016, 387, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Hachem, R.Y.; Langston, A.A.; Graybill, J.R.; Perfect, J.R.; Pedicone, L.D.; Patino, H.; Raad, I.I. Posaconazole as salvage treatment of invasive fungal infections in patients with underlying renal impairment. J. Antimicrob. Chemother. 2008, 62, 1386–1391. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jeong, S.J.; Lee, J.G.; Park, M.S.; Paik, H.C.; Na, S.; Kim, J. Critical Care after Lung Transplantation. Acute Crit. Care 2018, 33, 206–215. [Google Scholar] [CrossRef]
- Baek, Y.J.; Cho, Y.S.; Kim, M.H.; Hyun, J.H.; Sohn, Y.J.; Kim, S.Y.; Jeong, S.J.; Park, M.S.; Lee, J.G.; Paik, H.C. The Prediction and Prognosis of Fungal Infection in Lung Transplant Recipients-A Retrospective Cohort Study in South Korea. J. Fungi 2021, 7, 639. [Google Scholar] [CrossRef]
- Theel, E.S.; Doern, C.D. β-D-glucan testing is important for diagnosis of invasive fungal infections. J. Clin. Microbiol. 2013, 51, 3478–3483. [Google Scholar] [CrossRef] [PubMed]
- Herrera, S.; Husain, S. Current State of the Diagnosis of Invasive Pulmonary Aspergillosis in Lung Transplantation. Front. Microbiol. 2018, 9, 3273. [Google Scholar] [CrossRef] [PubMed]
- D’Haese, J.; Theunissen, K.; Vermeulen, E.; Schoemans, H.; De Vlieger, G.; Lammertijn, L.; Meersseman, P.; Meersseman, W.; Lagrou, K.; Maertens, J. Detection of galactomannan in bronchoalveolar lavage fluid samples of patients at risk for invasive pulmonary aspergillosis: Analytical and clinical validity. J. Clin. Microbiol. 2012, 50, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Geltner, C.; Lass-Flörl, C. Invasive pulmonary Aspergillosis in organ transplants—Focus on lung transplants. Respir. Investig. 2016, 54, 76–84. [Google Scholar] [CrossRef] [PubMed]


| Demographic Variables | Proven IPA | Probable IPA | Non-IPA Pneumonia | Total |
|---|---|---|---|---|
| Number of patients, n | 26 | 40 | 75 | 141 |
| Total cases, n | 39 | 61 | 164 | 264 |
| Age at transplantation, years median (IQR) | 55 (18–73) | 54 (17–71) | 55 (16–75) | 55 (16–75) |
| Male, n (%) | 17 (63.4) | 25 (62.5) | 47 (62.7) | 89 (63.1) |
| BMI (kg/m2), median (IQR) | 19.6 (11.6–25.2) | 21.0 (13.4–32.9) | 20.6 (12.9–29.5) | 20.6 (11.6–32.9) |
| Underlying cause of transplantation, n (%) | ||||
| IPF | 14 (53.8) | 18 (45.0) | 38 (50.2) | 70 (49.6) |
| Other lung disease * | 12 (46.2) | 22 (55.0) | 37 (49.8) | 71 (50.4) |
| Underlying disease, n (%) | ||||
| Hypertension | 6 (23.1) | 9 (22.5) | 17 (22.7) | 32 (22.7) |
| Diabetes mellitus | 11 (42.3) | 10 (25.0) | 12 (16.0) | 33 (23.4) |
| Chronic kidney disease | 0 (0.0) | 4 (10.0) | 4 (5.3) | 8 (5.7) |
| Liver cirrhosis | 0 (0.0) | 0 (0.0) | 1 (1.3) | 1 (0.7) |
| CVD | 3 (11.5) | 4 (10.0) | 8 (10.6) | 15 (10.6) |
| Level, mean ± SD | ||||
| Serum Aspergillus galactomannan antigen (index) | 1.12 ± 1.81 | 1.03 ± 171 | 0.13 ± 0.48 | 0.49 ± 1.22 |
| BALF Aspergillus galactomannan antigen (index) | 4.58 ± 3.31 | 2.24 ± 2.50 | 0.2 ± 0.55 | 1.46 ± 1.81 |
| Serum beta-D Glucan titer (pg/mL) | 204 ± 255 | 267 ± 320 | 107 ± 211 | 191 ± 275 |
| Combined fungal infection, n (%) | ||||
| Candida spp. | 10 (38.5) | 24 (60.0) | 40 (53.3) | 74 (52.5) |
| Mucormycosis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Saccharomyces spp. | 1 (3.9) | 4 (10.0) | 1 (1.3) | 6 (4.3) |
| Other fungal infection | 1 (3.9) | 2 (5.0) | 2 (2.6) | 5 (3.5) |
| Type of lung transplant, n (%) | ||||
| Double | 24 (92.3) | 40 (100) | 74 (98.7) | 138 (97.9) |
| Single | 2 (7.7) | 0 (0.0) | 1 (3.0) | 3 (2.1) |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variables | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Female sex | 1.04 (0.63–1.72) | 0.877 | 2.14 (0.91–5.16) | 0.084 |
| Age (<30) | ||||
| 31–40 | 0.26 (0.05–1.17) | 0.090 | 0.15 (0.01–2.08) | 0.169 |
| 41–50 | 0.38 (0.07–1.59) | 0.199 | 0.05 (0.00–0.82) | 0.042 |
| 51–60 | 0.22 (0.04–0.89) | 0.040 | 0.06 (0.00–0.84) | 0.042 |
| 61–70 | 0.37 (0.07–1.50) | 0.177 | 0.06 (0.00–0.86) | 0.044 |
| >70 | 0.30 (0.04–2.08) | 0.236 | 0.18 (0.00–5.76) | 0.348 |
| BMI (<15) | ||||
| 15 ≤ BMI < 20 | 0.42 (0.16–1.08) | 0.071 | ||
| 20 ≤ BMI < 25 | 0.73 (0.28–1.86) | 0.505 | ||
| 25 ≤ BMI < 30 | 0.38 (0.12–1.16) | 0.092 | ||
| Underlying cause of transplantation | ||||
| ILD except IPF and RA-ILD | 0.37 (0.15–0.80) | 0.017 | ||
| IPF | 1.10 (0.67–1.80) | 0.713 | ||
| COPD | 1.04 (0.37–2.72) | 0.944 | ||
| RA-ILD | 2.02 (0.94–4.39) | 0.072 | ||
| BO | 1.05 (0.42–2.49) | 0.916 | ||
| Others | 1.09 (0.46–2.51) | 0.838 | ||
| Lung transplantation type, double | 0.92 (0.15–7.09) | 0.930 | ||
| Underlying disease | ||||
| Hypertension | 0.65 (0.36–1.15) | 0.144 | ||
| Diabetes mellitus | 3.45 (1.98–6.07) | <0.001 | 3.73 (1.45–9.98) | 0.007 |
| CVD | 3.52 (1.76–7.33) | 0.001 | ||
| Combined fungal infection | ||||
| Candida spp. | 1.43 (0.87–2.37) | 0.164 | ||
| Other fungal infection | 7.24 (2.23–32.37) | 0.003 | ||
| Serum AGT | 4.04 (2.34–7.80) | <0.001 | 3.11 (1.54–8.26) | 0.011 |
| BALF AGT | 7.07 (3.64–15.22) | <0.001 | 4.80 (2.58–10.52) | <0.001 |
| Serum beta-D glucan | 1.00 (1.00–1.01) | 0.051 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.-Y.; Yong, S.-H.; Sung, M.-D.; Woo, A.-L.; Park, Y.-M.; Kim, H.-E.; Jung, S.-J.; Kim, S.-Y.; Lee, J.-G.; Kim, Y.-S.; et al. Aspergillus Galactomannan Titer as a Diagnostic Marker of Invasive Pulmonary Aspergillosis in Lung Transplant Recipients: A Single-Center Retrospective Cohort Study. J. Fungi 2023, 9, 527. https://doi.org/10.3390/jof9050527
Kim E-Y, Yong S-H, Sung M-D, Woo A-L, Park Y-M, Kim H-E, Jung S-J, Kim S-Y, Lee J-G, Kim Y-S, et al. Aspergillus Galactomannan Titer as a Diagnostic Marker of Invasive Pulmonary Aspergillosis in Lung Transplant Recipients: A Single-Center Retrospective Cohort Study. Journal of Fungi. 2023; 9(5):527. https://doi.org/10.3390/jof9050527
Chicago/Turabian StyleKim, Eun-Young, Seung-Hyun Yong, Min-Dong Sung, A-La Woo, Young-Mok Park, Ha-Eun Kim, Su-Jin Jung, Song-Yee Kim, Jin-Gu Lee, Young-Sam Kim, and et al. 2023. "Aspergillus Galactomannan Titer as a Diagnostic Marker of Invasive Pulmonary Aspergillosis in Lung Transplant Recipients: A Single-Center Retrospective Cohort Study" Journal of Fungi 9, no. 5: 527. https://doi.org/10.3390/jof9050527
APA StyleKim, E.-Y., Yong, S.-H., Sung, M.-D., Woo, A.-L., Park, Y.-M., Kim, H.-E., Jung, S.-J., Kim, S.-Y., Lee, J.-G., Kim, Y.-S., Paik, H.-C., & Park, M.-S. (2023). Aspergillus Galactomannan Titer as a Diagnostic Marker of Invasive Pulmonary Aspergillosis in Lung Transplant Recipients: A Single-Center Retrospective Cohort Study. Journal of Fungi, 9(5), 527. https://doi.org/10.3390/jof9050527






