Studies on the Genus Pyrenopolyporus (Hypoxylaceae) in Thailand Using a Polyphasic Taxonomic Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey and Sample Collection
2.2. Morphological Characterization and HPLC Profiling
2.3. DNA Extraction, Polymerase Chain Reaction (PCR)
2.4. Sequencing Methods
2.5. Phylogenetic Analyses
2.6. Cultivation of Fungal Strains for MALDI-TOF MS Analyses
2.7. MALDI-TOF MS Analysis
3. Results
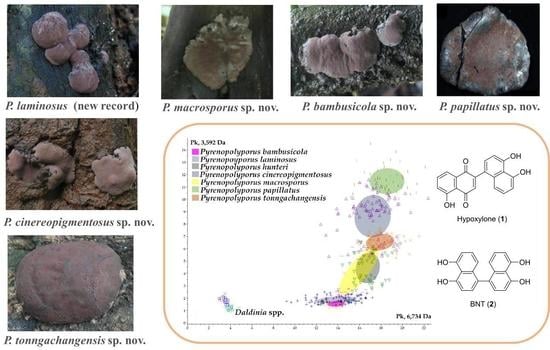
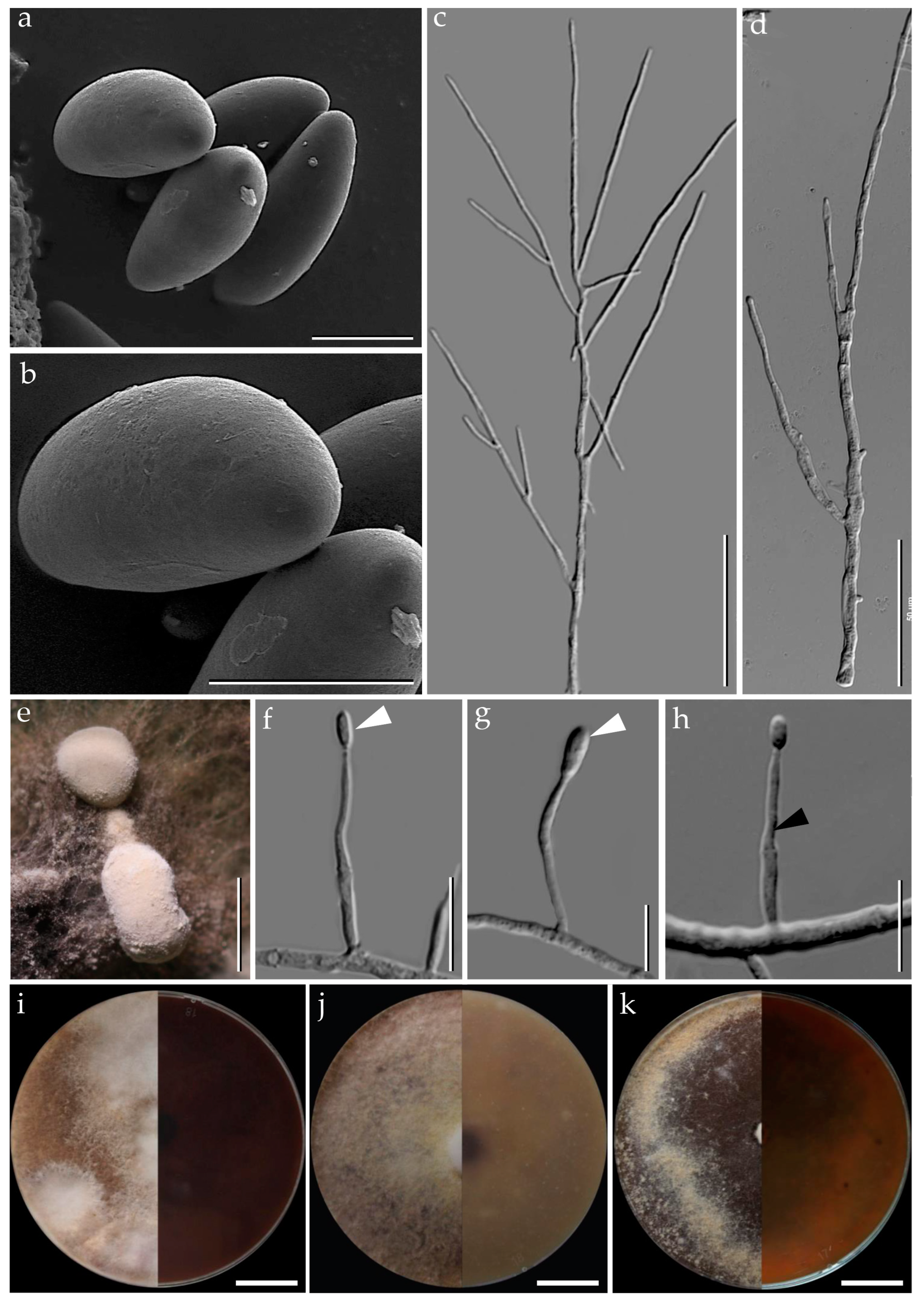
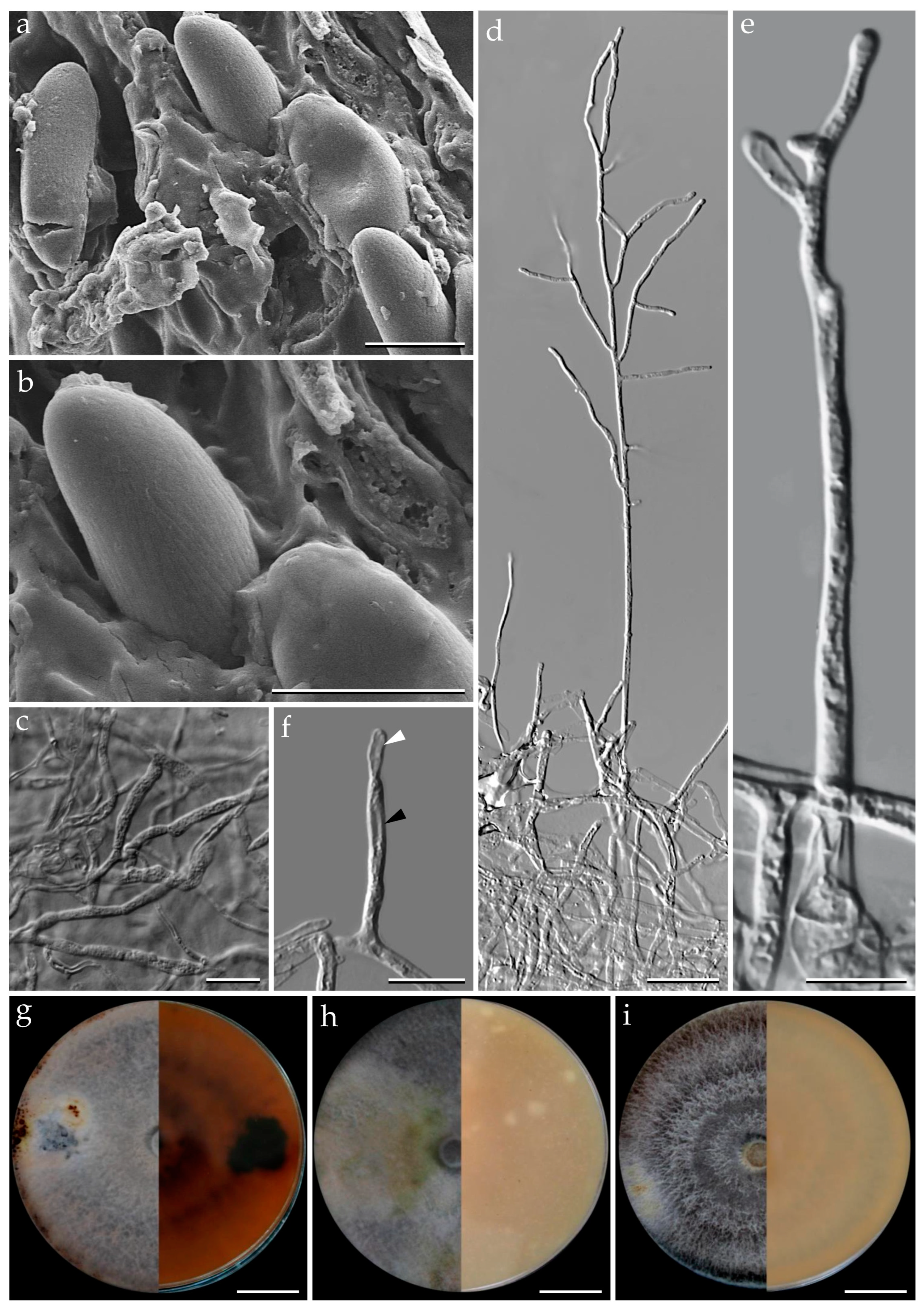
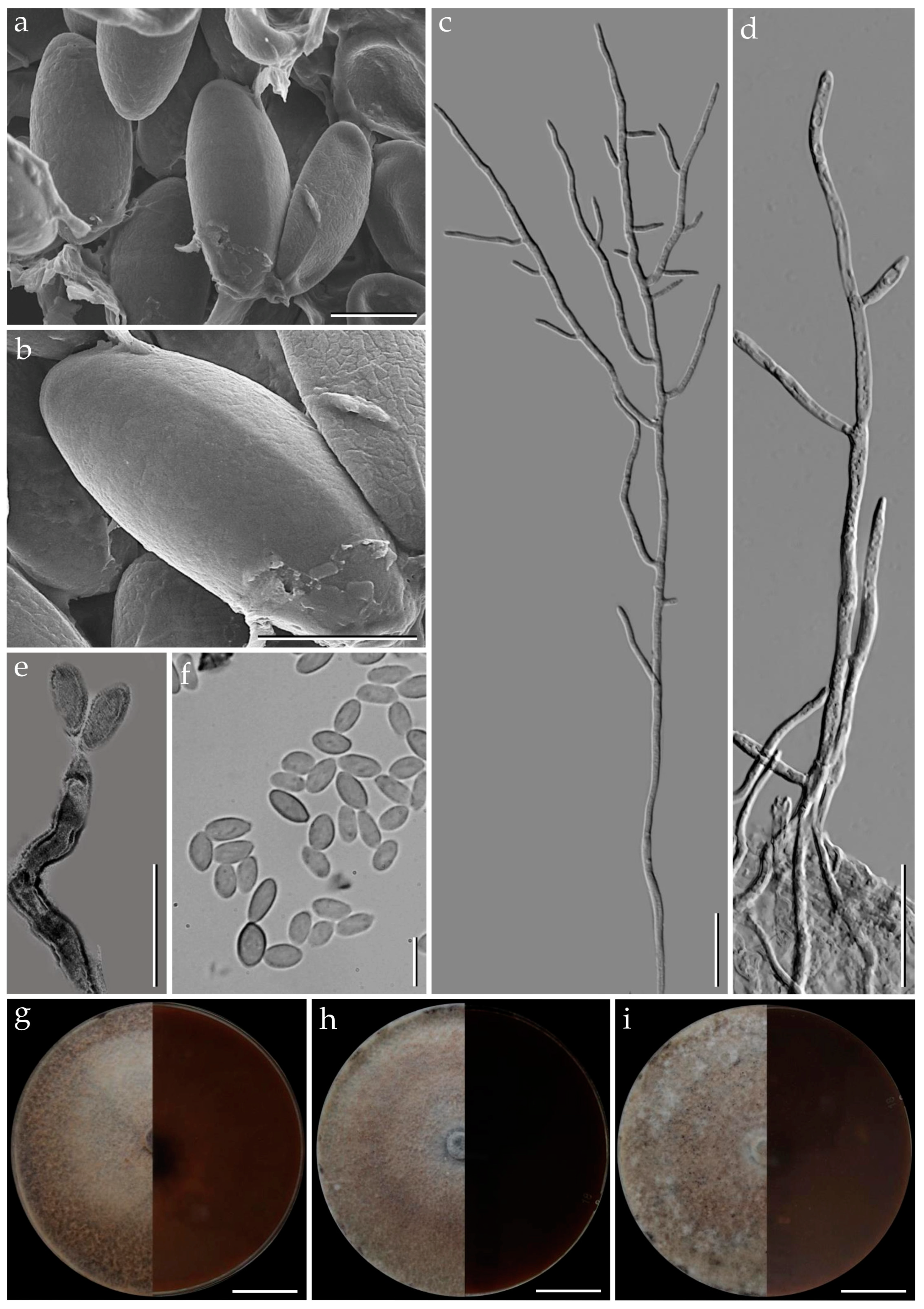
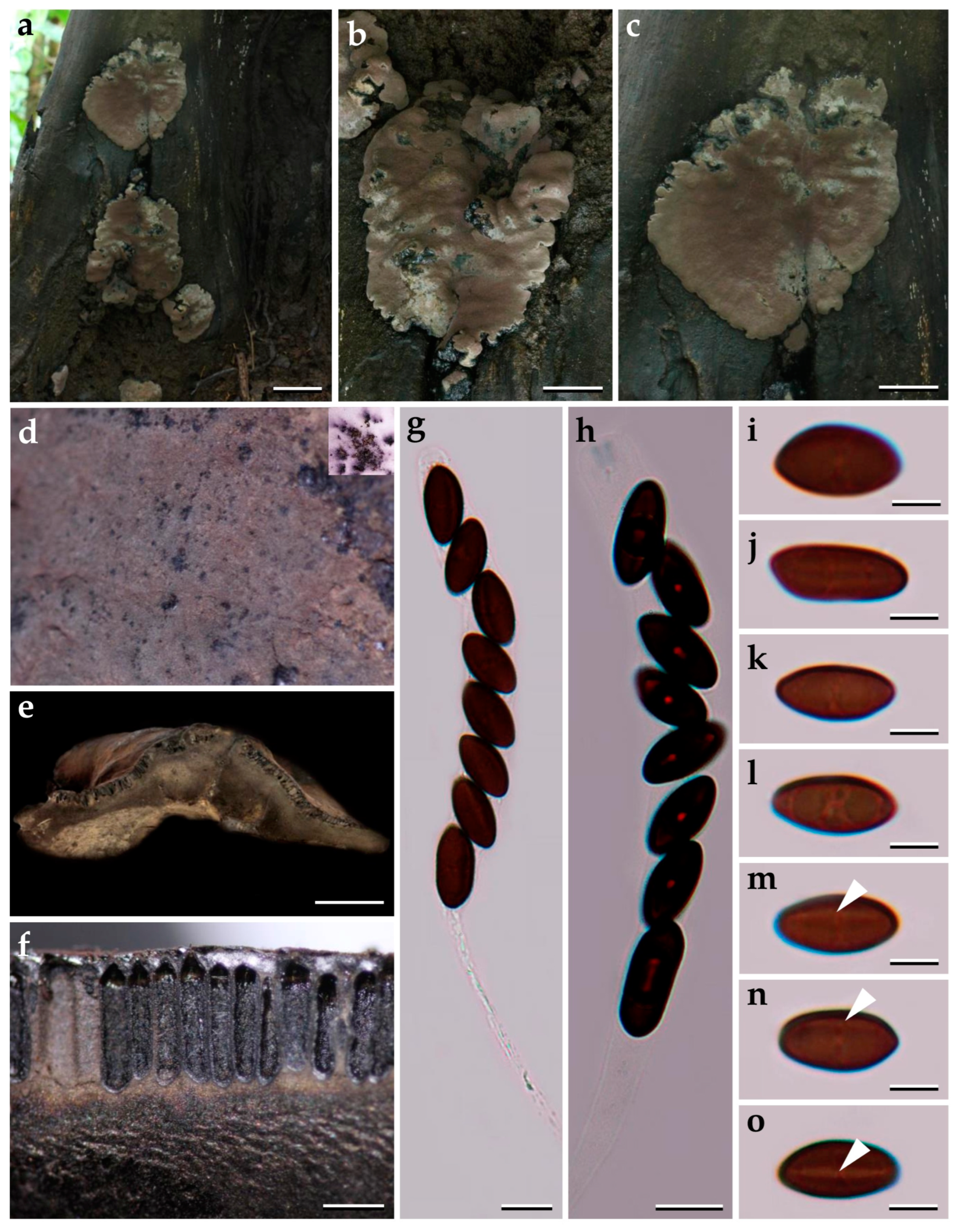
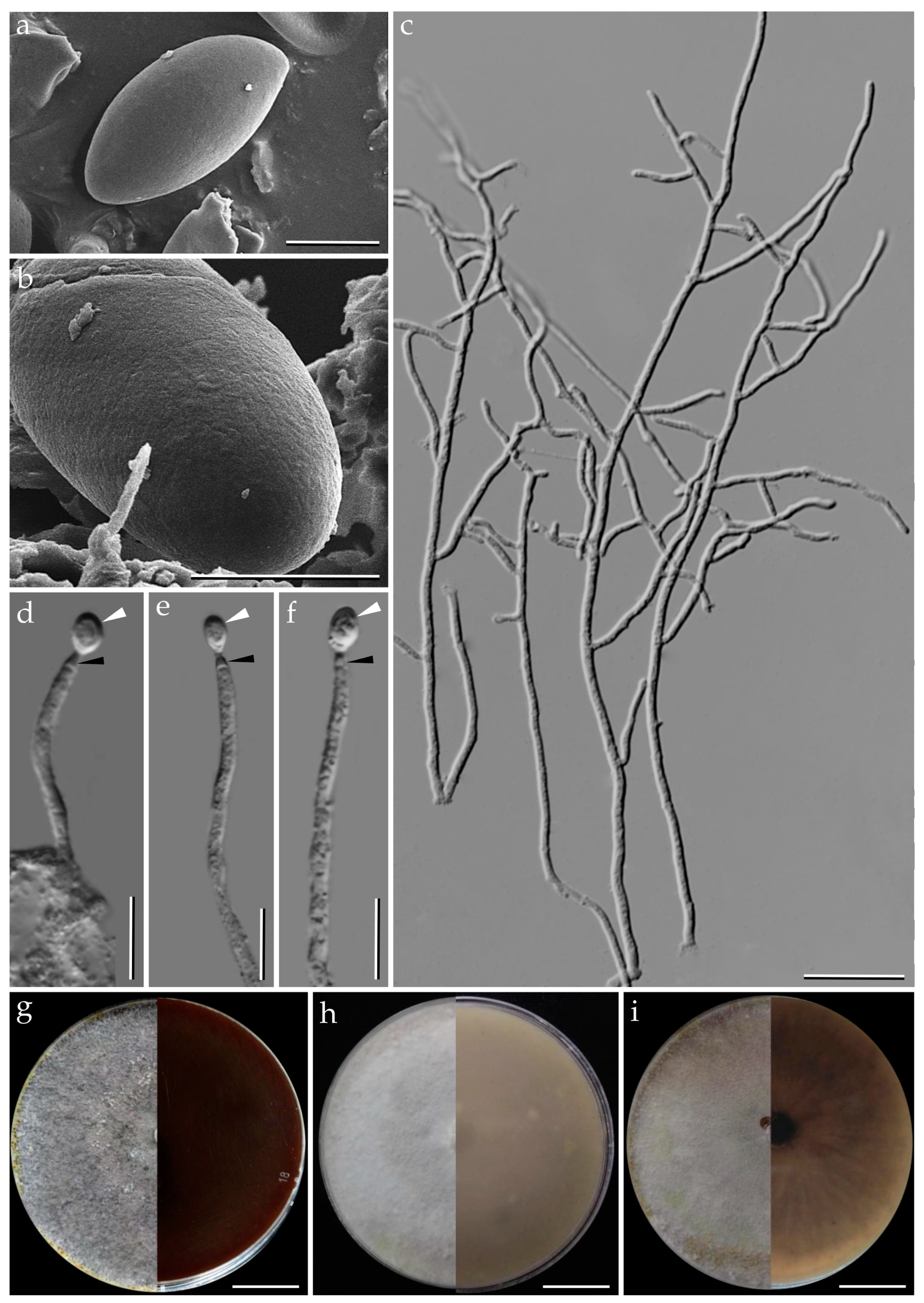
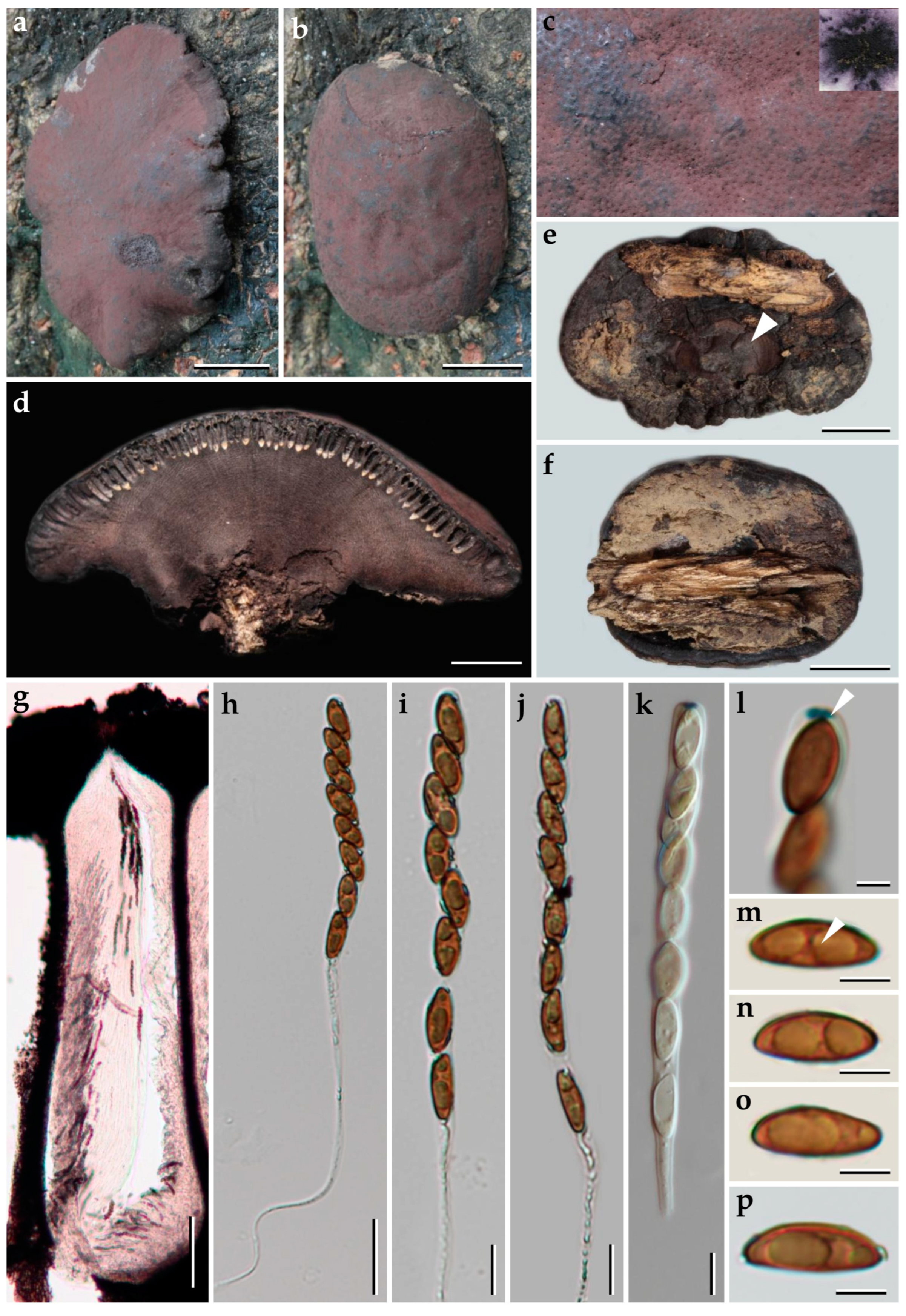
3.1. Morphological Characterization
3.2. Molecular Phylogeny (Figure 14)

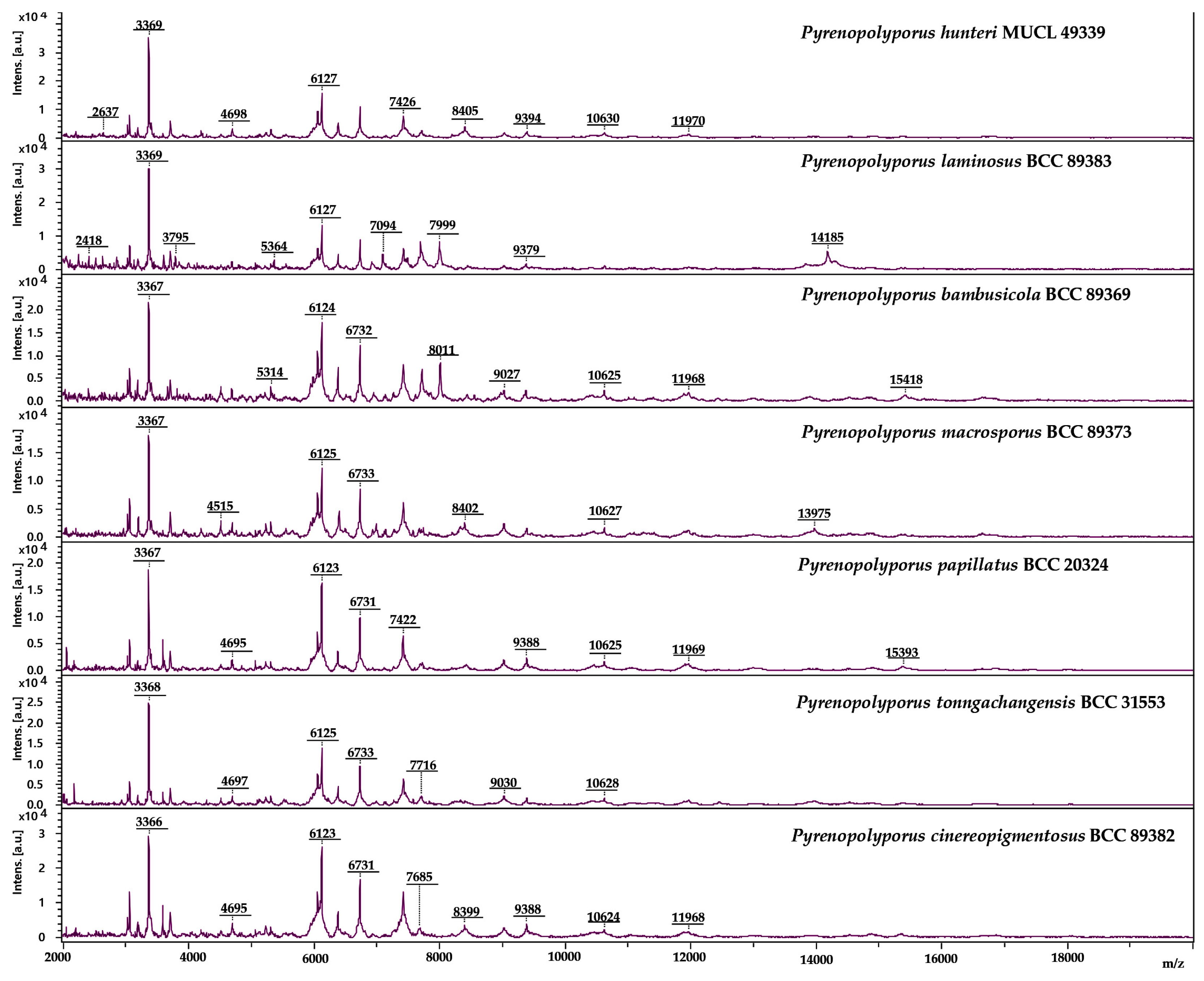
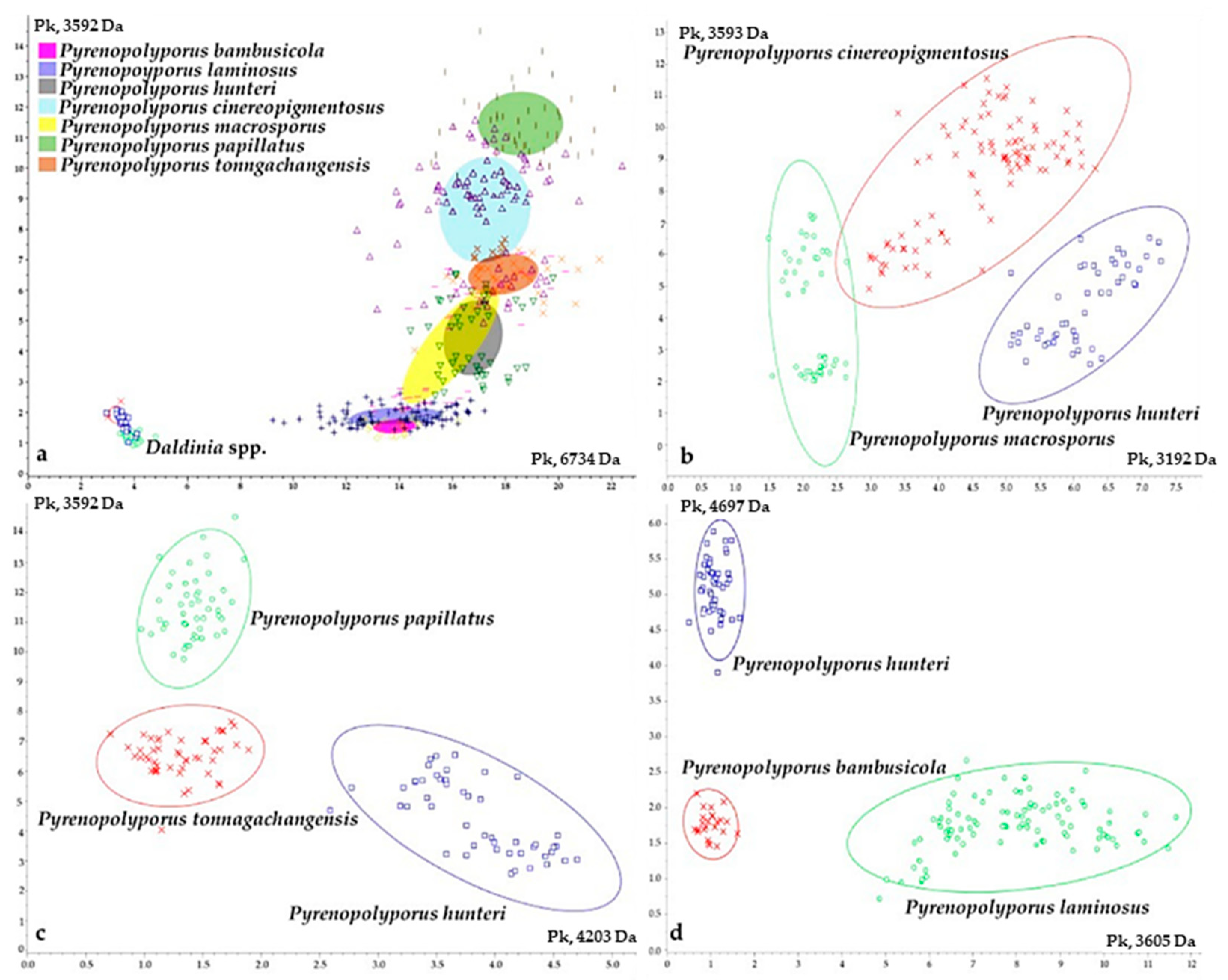
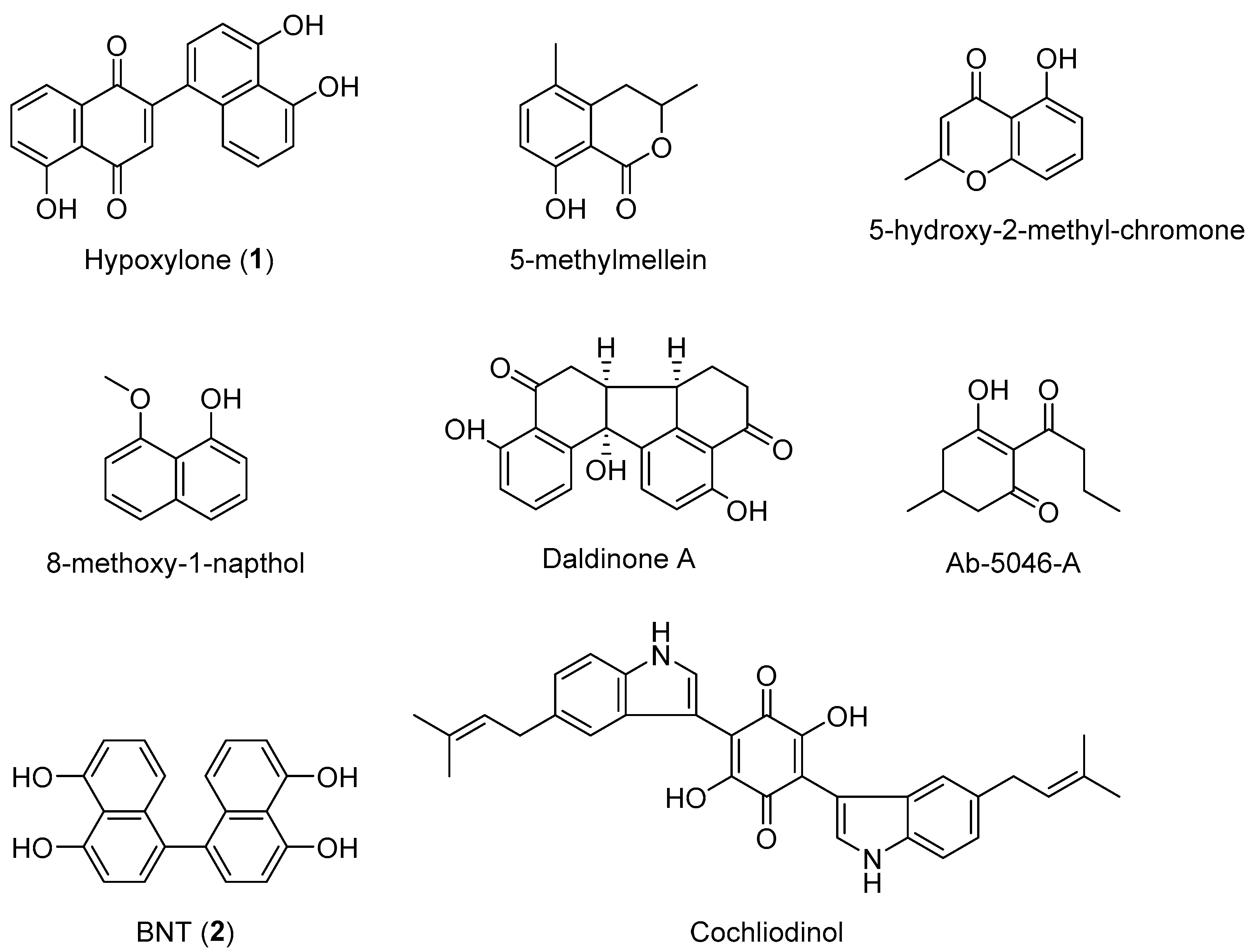
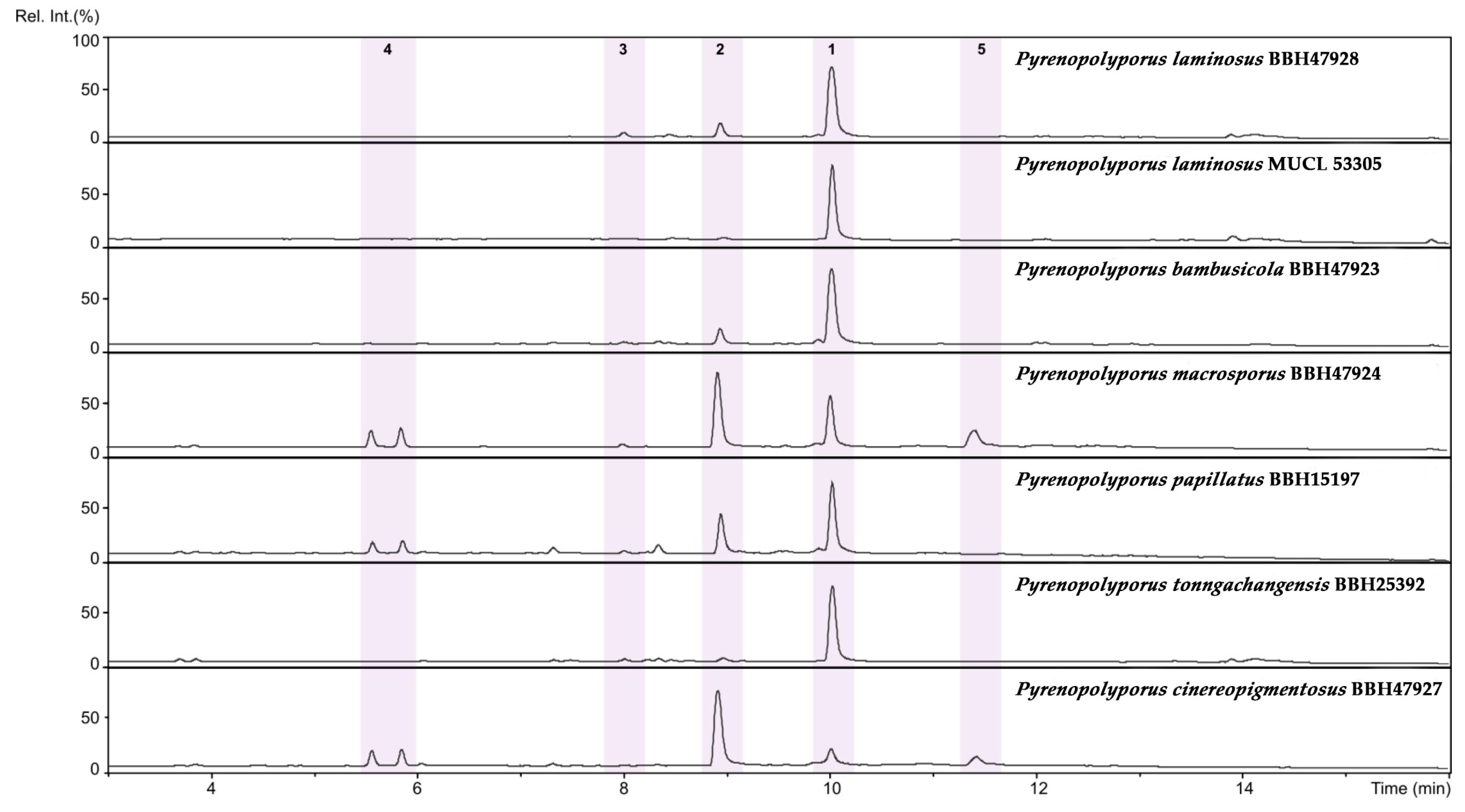
3.3. MALDI-TOF Mass Spectrometry (Figure 15, Figure 16 and Figure 17)

- Dichotomous key to the species of Pyrenopolyporus
- 1a. Ascospores highly variable in shape, ellipsoid to slightly ellipsoid-inequilateral……...2
- 1b. Ascospores less variable in shape, ellipsoid-inequilateral ….............................................4
- 2a. KOH-extractable pigments without purple shades; ascospores 9.5–14(–16) × 4–6 µm with straight to slightly sigmoid germ slit; much less than spore length on the more convex side ....................................................................................................................……P. tortisporus
- 2b. KOH-extractable pigments purple, ascospores with straight germ slit less than spore length………………………………...……………………………………………………………3
- 3a. Ascospores 9.5–12 (−13) × 4–5 µm with straight germ slit less than spore length frequently on the more flattened side..........................................................................P. symphyon
- 3b. Ascospores (14–) 16–17 × (6–) 7–8 µm with straight germ slit covering full spore length on convex side…………………………………………………………………... P. macrosporus
- 4a. Ascospores pale brown to light brown……………..………………………....…………....5
- 4b. Ascospores brown to dark brown…………………………………………..……...……….7
- 5a. Ostioles umbilicate; ascospores (12–) 13–14 (–16) × 4–5 µm with straight to rarely slightly sigmoid germ slit much less than spore-length or nearly spore-length on the convex side…...…………..…………………..……………...………..….….…P. tonngachangensis
- 5b. Ostioles lower than stromatal surface, punctiform, papillate ……………...…………….6
- 6a. Ostioles punctiform, slightly lower than the stromal surface, ascospores 11.5–14 × 5–5.5 µm with straight germ slit much less than spore-length……………..……………..P. hunteri
- 6b. Ostioles papillate; ascospores, (11–) 12–13 (–14) × 4–5 µm with straight germ slit much less than spore-length ……..……………………...………………………...…….. P. papillatus
- 7a. Species occurring on bamboos………………………….………...….……………..……….8
- 7b. Species on woody, dicot substrates…………………………………………………………9
- 8a. Stromata found in fire-damaged areas; ostioles conspicuous umbilicate; perithecia long tubular, 0.75–0.9 mm high; ascospores 10–11 (–12) × (3–) 4–5 µm ………..…P. bambusicola
- 8b. Stromata not found in fire-damaged area; ostioles umbilicate black to inconspicuous; perithecia long tubular 0.75–0.90 mm high, ascospores 11.0–13.5 × 4.2–4.5 µm...............................................................................................................................P. laminosus
- 9a. KOH-extractable pigment Dark Livid and Livid Purple; perithecia 0.8–1.5 mm high, ascospores (11.5–) 12–15(−16) × 5–6.5 μm…………………….……..………..P. nicaraguensis
- 9b. KOH-extractable pigment Dark Mouse Grey or Iron Grey; perithecia tubular 0.9–1.1 mm high ascospores (12–) 13–14 (–15) × 6–7 μm ……………………...P. cinereopigmentosus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lloyd, C.G. Synopsis of some genera of the large pyrenomycetes. Mycological Notes 50. Mycol. Writ. 1917, 5, 701–716. [Google Scholar]
- Ju, Y.M.; Rogers, J.D. Mycologia Memoir n° 20. In A Revision of the Genus Hypoxylon; APS Press: St. Paul, MN, USA, 1996; p. 365. [Google Scholar]
- Wendt, L.; Sir, E.B.; Kuhnert, E.; Heitkämper, S.; Lambert, C.; Hladki, A.I.; Romero, A.I.; Luangsa-ard, J.J.; Srikitikulchai, P.; Peršoh, D. Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycol. Prog. 2018, 17, 115–154. [Google Scholar] [CrossRef]
- Helaly, S.E.; Thongbai, B.; Stadler, M. Diversity of biologically active secondary metabolites from endophytic and saprotrophic fungi of the ascomycete order Xylariales. Nat. Prod. Rep. 2018, 35, 992–1014. [Google Scholar] [CrossRef] [PubMed]
- Sir, E.; Lambert, C.; Wendt, L.; Hladki, A.; AI, R.; Stadler, M. A New Species of Daldinia (Xylariaceae) from the Argentine Subtropical Montane Forest. Mycosphere 2016, 7, 1378–1388. [Google Scholar] [CrossRef]
- Bitzer, J.; Laessoe, T.; Fournier, J.; Kummer, V.; Decock, C.; Tichy, H.V.; Piepenbring, M.; Persoh, D.; Stadler, M. Affinities of Phylacia and the daldinoid Xylariaceae, inferred from chemotypes of cultures and ribosomal DNA sequences. Mycol. Res. 2008, 112, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Triebel, D.; Peršoh, D.; Wollweber, H.; Stadler, M. Phylogenetic relationships among Daldinia, Entonaema and Hypoxylon as inferred from ITS nrDNA sequences. Nova Hedwig. 2005, 80, 25–43. [Google Scholar] [CrossRef]
- Daranagama, D.A.; Hyde, K.D.; Sir, E.B.; Thambugala, K.M.; Tian, Q.; Samarakoon, M.C.; McKenzie, E.H.C.; Jayasiri, S.C.; Tibpromma, S.; Bhat, J.D.; et al. Towards a natural classification and back-bone tree for Graphostromataceae, Hypoxylaceae, Lopadostomataceae and Xylariaceae. Fungal Divers. 2018, 88, 1–165. [Google Scholar] [CrossRef]
- Wibberg, D.; Stadler, M.; Lambert, C.; Bunk, B.; Spröer, C.; Rückert, C.; Kalinowski, J.; Cox, R.J.; Kuhnert, E. High quality genome sequences of thirteen Hypoxylaceae (Ascomycota) strengthen the phylogenetic family backbone and enable the discovery of new taxa. Fungal Divers. 2021, 106, 7–28. [Google Scholar] [CrossRef]
- Kuhnert, E.; Navarro-Muñoz, J.; Becker, K.; Stadler, M.; Collemare, J.; Cox, R. Secondary metabolite biosynthetic diversity in the fungal family Hypoxylaceae and Xylaria hypoxylon. Stud. Mycol. 2021, 99, 100118. [Google Scholar] [CrossRef]
- Čmoková, A.; Kolařík, M.; Dobiáš, R.; Hoyer, L.L.; Janouškovcová, H.; Kano, R.; Kuklová, I.; Lysková, P.; Machová, L.; Maier, T.; et al. Resolving the taxonomy of emerging zoonotic pathogens in the Trichophyton benhamiae complex. Fungal Divers. 2020, 104, 333–387. [Google Scholar] [CrossRef]
- Patel, R. A Moldy Application of MALDI: MALDI-ToF Mass Spectrometry for Fungal Identification. J. Fungi 2019, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, E.; Surup, F.; Sir, E.; Lambert, C.; Hyde, K.; Hladki, A.; Romero, A.; Stadler, M. Lenormandins A–G, new azaphilones from Hypoxylon lenormandii and Hypoxylon jaklitschii sp. nov., recognised by chemotaxonomic data. Fungal Divers. 2015, 71, 165–184. [Google Scholar] [CrossRef]
- Rayner, R.W. Commonwealth Mycological Institute (Great Britain). In A Mycological Colour Chart; British Mycological Society: Manchester, UK, 1970. [Google Scholar]
- Cedeño-Sanchez, M.; Charria-Girón, E.; Lambert, C.; Luangsa-ard, J.J.; Decock, C.; Franke, R.; Brönstrup, M.; Stadler, M. Segregation of the genus Parahypoxylon (Hypoxylaceae, Xylariales) from Hypoxylon by a polyphasic taxonomic approach. Mycokeys 2023, 95, 131–162. [Google Scholar] [CrossRef]
- Mongkolsamrit, S.; Luangsa-ard, J.J.; Spatafora, J.W.; Sung, G.H.; Hywel-Jones, N.L. A combined ITS RDNA and β-tubulin phylogeny of Thai species of Hypocrella with non-fragmenting ascospores. Mycol. Res. 2009, 113, 684–699. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Rehner, S.A.; Samuels, G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA Sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 2013, 41, 95–98. [Google Scholar]
- Kuhnert, E.; Sir, E.B.; Lambert, C.; Hyde, K.D.; Hladki, A.I.; Romero, A.I.; Rohde, M.; Stadler, M. Phylogenetic and chemotaxonomic resolution of the genus Annulohypoxylon (Xylariaceae) including four new species. Fungal Divers. 2017, 85, 1–43. [Google Scholar] [CrossRef]
- Daranagama, D.A.; Camporesi, E.; Tian, Q.; Liu, X.; Chamyuang, S.; Stadler, M.; Hyde, K.D. Anthostomella is polyphyletic comprising several genera in Xylariaceae. Fungal Divers. 2015, 73, 203–238. [Google Scholar] [CrossRef]
- Hsieh, H.M.; Ju, Y.M.; Rogers, J.D. Molecular phylogeny of Hypoxylon and closely related genera. Mycologia 2005, 97, 844–865. [Google Scholar] [CrossRef] [PubMed]
- Wongkanoun, S.; Becker, K.; Boonmee, K.; Srikitikulchai, P.; Boonyuen, N.; Chainuwong, B.; Luangsa-ard, J.; Stadler, M. Three novel species and a new record of Daldinia (Hypoxylaceae) from Thailand. Mycol. Prog. 2020, 19, 1113–1132. [Google Scholar] [CrossRef]
- U’Ren, J.M.; Miadlikowska, J.; Zimmerman, N.B.; Lutzoni, F.; Stajich, J.E.; Arnold, A.E. Contributions of North American endophytes to the phylogeny, ecology, and taxonomy of Xylariaceae (Sordariomycetes, Ascomycota). Mol. Phylogenetics Evol. 2016, 98, 210–232. [Google Scholar] [CrossRef]
- Kuhnert, E.; Fournier, J.; Peršoh, D.; Luangsa-ard, J.J.D.; Stadler, M. New Hypoxylon species from Martinique and new evidence on the molecular phylogeny of Hypoxylon based on ITS rDNA and β-tubulin data. Fungal Divers. 2014, 64, 181–203. [Google Scholar] [CrossRef]
- Stadler, M.; Laessoe, T.; Fournier, J.; Decock, C.; Schmieschek, B.; Tichy, H.V.; Persoh, D. A polyphasic taxonomy of Daldinia (Xylariaceae). Stud. Mycol. 2014, 77, 1–143. [Google Scholar] [CrossRef]
- Wongkanoun, S.; Wendt, L.; Stadler, M.; Luangsa-Ard, J.; Srikitikulchai, P. A novel species and a new combination of Daldinia from Ban Hua Thung community forest in the northern part of Thailand. Mycol. Prog. 2019, 18, 553–564. [Google Scholar] [CrossRef]
- Johannesson, H.; Laessøe, T.; Stenlid, J. Molecular and morphological investigation of the genus Daldinia in northern Europe. Mycol. Res. 2000, 104, 275–280. [Google Scholar] [CrossRef]
- Zhang, N.; Castlebury, L.A.; Miller, A.N.; Huhndorf, S.M.; Schoch, C.L.; Seifert, K.A.; Rossman, A.Y.; Rogers, J.D.; Kohlmeyer, J.; Volkmann-Kohlmeyer, B.; et al. An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia 2006, 98, 1076–1087. [Google Scholar] [CrossRef]
- Koukol, O.; Kelnarová, I.; Černý, K. Recent observations of sooty bark disease of sycamore maple in Prague (Czech Republic) and the phylogenetic placement of Cryptostroma corticale. For. Pathol. 2015, 45, 21–27. [Google Scholar] [CrossRef]
- Lambert, C.; Wendt, L.; Hladki, A.I.; Romero, A.I.; Stadler, M.; Sir, E.B. Hypomontagnella (Hypoxylaceae): A new genus segregated from Hypoxylon by a polyphasic taxonomic approach. Mycol. Prog. 2019, 18, 187–201. [Google Scholar] [CrossRef]
- Stadler, M.; Kuhnert, E.; Persoh, D.; Fournier, J. The Xylariaceae as model example for a unified nomenclature following the “One Fungus-One Name” (1F1N) Concept. Mycology 2013, 4, 5–21. [Google Scholar] [CrossRef]
- Sir, E.B.; Kuhnert, E.; Lambert, C.; Hladki, A.I.; Romero, A.I.; Stadler, M. New species and reports of Hypoxylon from Argentina recognized by a polyphasic approach. Mycol. Prog. 2016, 15, 42. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP* 4.0b10: Phylogenetic Analysis Using Parsimony (*and other Methods); Sinauer Associates: Sunderland, UK, 2002. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Nylander, J. MrModeltest V2; Uppsala Universitit: Uppsala, Sweden, 2004. [Google Scholar]
- Möller, A. Phycomyceten und Ascomyceten. Untersuchungen aus Brasilien; G. Fischer: Schaffhausen, Swiss, 1901; Volume 9, pp. 1–319. [Google Scholar]
- Fournier, J.; Lechat, C.; Courtecuisse, R. The genus Hypoxylon (Xylariaceae) in Guadeloupe and Martinique (French West Indies). Ascomycete Org. 2016, 7, 145–212. [Google Scholar]
- Miller, J.H. A Monograph of the World Species of Hypoxylon; Univ. Georgia Press: Athens, Georgia, USA, 1961; p. 158. [Google Scholar]
- Stadler, M.; Lambert, C.; Wibberg, D.; Kalinowski, J.; Cox, R.J.; Kolařík, M.; Kuhnert, E. Intragenomic polymorphisms in the ITS region of high-quality genomes of the Hypoxylaceae (Xylariales, Ascomycota). Mycol. Prog. 2020, 19, 235–245. [Google Scholar] [CrossRef]






| Taxa | Strain/Status | Origin | GenBank Accession Number | Reference | |||
|---|---|---|---|---|---|---|---|
| ITS | LSU | RPB2 | TUB2 | ||||
| Annulohypoxylon annulatum | CBS 140775/ET | Texas | KY610418 | KY610418 | KY624263 | KX376353 | ITS, LSU, RPB2: [3]; TUB2: [23] |
| A. moriforme | CBS 123579 | Martinique | KX376321 | KY610425 | KY624289 | KX271261 | ITS, TUB2: [23]; RPB2, LSU: [3] |
| A. nitens | MFLUCC 12.0823 | Thailand | KJ934991 | KJ934992 | KJ934994 | KJ934993 | [24] |
| A. stygium | MUCL 54601 | French Guiana | KY610409 | KY610475 | KY624292 | KX271263 | [3] |
| A. truncatum | CBS 140778/ET | Texas | KY610419 | KY610419 | KY624277 | KX376352 | TUB2: [23]; ITS, LSU, RBP2: [3] |
| Daldinia andina | CBS 114736/HT | Ecuador | AM749918 | KY610430 | KY624239 | KC977259 | ITS: [5]; TUB2: [23]; LSU, RPB2: [3] |
| D. bambusicola | CBS 122872/HT | Thailand | KY610385 | KY610431 | KY624241 | AY951688 | TUB2: [25]; ITS, LSU, RPB2: [3] |
| D. brachysperma | BCC33676 | Thailand | MN153854 | MN153871 | N/a | MN172205 | [26] |
| D. caldariorum | CBS122874 | USA | KU683756 | KU683756 | KU684289 | KU684128 | [27] |
| D. chiangdaoensis | BCC88220/HT | Thailand | MN153850 | MN153867 | MN172208 | MN172197 | [26] |
| D. concentrica | CBS 113277 | Germany | AY616683 | KY610434 | KY624243 | KC977274 | ITS: [7]; TUB2: [28]; LSU, RPB2: [3] |
| D. dennisii | CBS 114741/HT | Australia | JX658477 | KY610435 | KY624244 | KC977262 | ITS: [29]; TUB2: [28]; LSU, RPB2: [3] |
| D. eschscholtzii | MUCL 45435 | Benin | JX658484 | KY610437 | KY624246 | KC977266 | ITS: [29]; TUB2: [28]; LSU, RPB2: [3] |
| D. flavogranulata | BCC89363/HT | Thailand | MN153856 | MN153873 | MN172211 | MN172200 | [26] |
| D. korfii | EBS 067 | Argentina | KY204018 | N/A | N/A | KY204014 | [5] |
| D. kretzschmarioides | TBRC 8875/ET | Thailand | MH938531 | MH938540 | MK165425 | MK165416 | [30] |
| D. loculatoides | CBS 113279/ET | UK | AF176982 | KY610438 | KY624247 | KX271246 | ITS: [31]; LSU, RPB2, TUB2: [3] |
| D. macaronesica | CBS 113040/PT | Spain | KY610398 | KY610477 | KY624294 | KX271266 | [3] |
| D. padaengensis | BCC89349/HT | Thailand | MN153852 | MN153869 | MN172206 | MN172195 | [26] |
| D. petriniae | MUCL 49214/ET | Austria | AM749937 | KY610439 | KY624248 | KC977261 | ITS: [5]; TUB2: [28]; LSU, RPB2: [3] |
| D. placentiformis | MUCL 47603 | Mexico | AM749921 | KY610440 | KY624249 | KC977278 | ITS: [5]; TUB2: [28]; LSU, RPB2: [3] |
| D. pyrenaica | MUCL 53969 | France | KY610413 | KY610413 | KY624274 | KY624312 | [3] |
| D. steglichii | MUCL 43512 | Papua New Guinea | KY610399 | KY610479 | KY624250 | KX271269 | [3] |
| D. subvernicosa | TBRC 8877/HT | Thailand | MH938533 | MH938542 | MK165430 | MK165421 | [30] |
| D. theissenii | CBS 113044/PT | Argentina | KY610388 | KY610441 | KY624251 | KX271247 | [3] |
| D. vernicosa | CBS 119316/ET | Germany | KY610395 | KY610442 | KY624252 | KC977260 | TUB2: [28]; ITS, LSU, RPB2: [3] |
| Graphostroma platystomum | CBS 270.87/ET | France | JX658535 | DQ836906 | KY624296 | HG934108 | ITS: [29]; LSU: [32]; TUB2: [33], RPB2: [3] |
| Hypomontagnella barbarensis | STMA 14081/HT | Argentina | MK131720 | MK131718 | MK135891 | MK135893 | [34] |
| Hy. monticulosa | MUCL 54604/ET | French Guiana | KY610404 | KY610487 | KY624305 | KX271273 | [34] |
| Hy. submonticulosa | CBS 115280 | France | KC968923 | KY610457 | KY624226 | KC977267 | ITS, TUB2: [28]; LSU, RPB2: [3] |
| Hypoxylon crocopeplum | CBS 119004 | France | KC968907 | KY610445 | KY624255 | KC977268 | ITS, TUB2: [28]; LSU, RPB2: [3] |
| H. fragiforme | MUCL 51264/ET | Germany | KC477229 | KM186295 | KM186296 | KX271282 | ITS: [35]; LSU, RPB2: [13]; TUB2: [3] |
| H. fuscum | CBS 113049/ET | France | KY610401 | KY610482 | KY624299 | KX271271 | [3] |
| H. haematostroma | MUCL 53301/ET | Martinique | KC968911 | KY610484 | KY624301 | KC977291 | ITS, TUB2: [28]; LSU, RPB2: [3] |
| H. haematostroma | BCC50533 | Thailand | MN153866 | MN153883 | MN172221 | MN172204 | [30] |
| H. investiens | CBS 118183/ET | Malaysia | KC968925 | KY610450 | KY624259 | KC977270 | ITS, TUB2: [28]; LSU, RPB2: [3] |
| H. lateripigmentum | MUCL 53304/HT | Martinique | KC968933 | KY610486 | KY624304 | KC977290 | ITS, TUB2: [28]; LSU, RPB2: [3] |
| H. lenormandii | CBS 119003 | Ecuador | KC968943 | KY610452 | KY624261 | KC977273 | ITS, TUB2: [28]; LSU, RPB2: [3] |
| H. petriniae | CBS 114746/HT | France | KY610405 | KY610491 | KY624279 | KX271274 | TUB2: [28]; ITS, LSU, RPB2, TUB2: [3] |
| H. rickii | MUCL 53309/ET | Martinique | KC968932 | KY610416 | KY624281 | KC977288 | ITS, TUB2: [28]; LSU, RPB2: [3] |
| H.rubiginosum | MUCL 52887/ET | Germany | KC477232 | KY610469 | KY624266 | KY624311 | ITS: [35]; LSU, RPB2, TUB2: [3] |
| H. samuelsii | MUCL 51843/ET | Guadeloupe | KC968916 | KY610466 | KY624269 | KC977286 | ITS, TUB2: [28]; LSU, RPB2: [3] |
| J. cohaerens | CBS 119126 | Germany | KY610396 | KY610497 | KY624270 | KY624314 | [3] |
| J. minutella | CBS 119015 | Portugal | KY610381 | KY610424 | KY624235 | KX271240 | TUB2: [28]; ITS, LSU, RPB2: [3] |
| J. multiformis | CBS 119016/ET | Germany | KC477234 | KY610473 | KY624290 | KX271262 | ITS: [28]; TUB2: [23]; LSU, RPB2: [3] |
| Pyrenopolyporus bambusicola | BCC89355/HT | Thailand | OP304856 | OP304876 | OP981624 | OQ101839 | This study |
| P. bambusicola | BCC89369 | Thailand | OP304858 | OP304878 | OP981623 | OQ101840 | This study |
| P. cinereopigmentosus | BCC89362 | Thailand | OP304857 | OP304877 | OP981625 | OQ101841 | This study |
| P. cinereopigmentosus | BCC89375 | Thailand | OP304859 | OP304881 | OP981626 | OQ101842 | This study |
| P. cinereopigmentosus | BCC89382/HT | Thailand | OP304860 | OP304882 | OP981627 | OQ101843 | This study |
| P. cinereopigmentosus | BCC33615 | Thailand | OP304867 | OP304889 | OP981628 | OQ101839 | This study |
| P. cinereopigmentosus | BCC82690 | Thailand | OP304868 | OP304890 | OP981629 | OQ101840 | This study |
| P. hunteri | MUCL 52673/ET | Ivory Coast | KY610421 | KY610472 | KY624309 | KU159530 | TUB2: [20]; ITS, LSU, RPB2: [3] |
| P. laminosus | MUCL 53305 | Martinique | KC968934 | KY610485 | KY624303 | KC977292 | ITS, TUB2: [28]; LSU, RPB2: [3] |
| P. laminosus | NBTF1892 | Thailand | OP304864 | OQ123731 | N/A | OQ032514 | This study |
| P. laminosus | BCC89383 | Thailand | MN153855 | MN153872 | MN172210 | MN172199 | [26] |
| P. laminosus | BCC89388 | Thailand | OP304861 | OP304883 | OP981634 | OQ032513 | This study |
| P. laminosus | BCC82043 | Thailand | OP304855 | OP304875 | OP981633 | OQ032515 | This study |
| P. laminosus | BCC83642 | Thailand | OP304863 | OP304885 | OP981635 | OQ032516 | This study |
| P. macrosporus | BCC89373/HT | Thailand | OP304870 | OP304879 | OP981621 | OQ101844 | This study |
| P. macrosporus | BCC89374 | Thailand | OP304871 | OP304880 | OP981622 | OQ101845 | This study |
| P. nicaraguensis | CBS 117739/HT | Burkina Faso | AM749922 | KY610489 | KY624307 | KC977272 | ITS: [5]; TUB: [28]; LSU, RPB2: [3] |
| P. papillatus | BCC20324/HT | Thailand | OP304854 | OP304874 | OP981619 | OQ101846 | This study |
| P. papillatus | BCC33622 | Thailand | OP304869 | OP304891 | OP981620 | N/A | This study |
| P. tonngachangensis | BCC31553/HT | Thailand | OP304865 | OP304887 | OP981632 | OQ101847 | This study |
| P. tonngachangensis | BCC31555 | Thailand | OP304866 | OP304888 | OP981630 | OQ101848 | This study |
| P. tonngachangensis | BCC91226 | Thailand | OP304862 | OP304884 | OP981631 | OQ101849 | This study |
| Xylaria hypoxylon | CBS12260/HT | Sweden | KY610407 | KY610495 | KY624231 | KX271279 | TUB2: [36]; ITS, LSU, RPB2: [3] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongkanoun, S.; Chainuwong, B.; Kobmoo, N.; Roytrakul, S.; Somrithipol, S.; Luangsa-ard, J.; Charria-Girón, E.; Srikitikulchai, P.; Stadler, M. Studies on the Genus Pyrenopolyporus (Hypoxylaceae) in Thailand Using a Polyphasic Taxonomic Approach. J. Fungi 2023, 9, 429. https://doi.org/10.3390/jof9040429
Wongkanoun S, Chainuwong B, Kobmoo N, Roytrakul S, Somrithipol S, Luangsa-ard J, Charria-Girón E, Srikitikulchai P, Stadler M. Studies on the Genus Pyrenopolyporus (Hypoxylaceae) in Thailand Using a Polyphasic Taxonomic Approach. Journal of Fungi. 2023; 9(4):429. https://doi.org/10.3390/jof9040429
Chicago/Turabian StyleWongkanoun, Sarunyou, Boonchuai Chainuwong, Noppol Kobmoo, Sittiruk Roytrakul, Sayanh Somrithipol, Jennifer Luangsa-ard, Esteban Charria-Girón, Prasert Srikitikulchai, and Marc Stadler. 2023. "Studies on the Genus Pyrenopolyporus (Hypoxylaceae) in Thailand Using a Polyphasic Taxonomic Approach" Journal of Fungi 9, no. 4: 429. https://doi.org/10.3390/jof9040429
APA StyleWongkanoun, S., Chainuwong, B., Kobmoo, N., Roytrakul, S., Somrithipol, S., Luangsa-ard, J., Charria-Girón, E., Srikitikulchai, P., & Stadler, M. (2023). Studies on the Genus Pyrenopolyporus (Hypoxylaceae) in Thailand Using a Polyphasic Taxonomic Approach. Journal of Fungi, 9(4), 429. https://doi.org/10.3390/jof9040429