Genome-Wide Association Study Reveals CLEC7A and PROM1 as Potential Regulators of Paracoccidioides brasiliensis-Induction of Cytokine Production in Peripheral Blood Mononuclear Cells
Abstract
1. Introduction
2. Material and Methods
2.1. Study Cohort and Ethical Approval
2.2. Microorganisms and Culture Conditions
2.3. Peripheral Blood Mononuclear Cells and Cytokine Measurement
2.4. Genotyping, Quality Control and Cytokine QTL Mapping
2.5. Functional Validation of Potential Causal Genes (CLEC7A, PROM1)
2.6. Statistical Analysis
3. Results
3.1. The Profile of P. brasiliensis-Induced IL-1β, IL17 and IL-22 Is Similar to Aspergillus fumigatus and Rhizopus oryzae
3.2. Genome-Wide Mapping Identifies Genetic Variations Associated with Innate- and Adaptive-Derived Cytokines Induced by P. brasiliensis
3.3. IL-1β Production Induced by Heat-Killed Yeasts Cells or Particulated P. brasiliensis Antigens Is Dependent on Dectin-1 Receptor
3.4. The PROM1 rs62290169 SNP Regulates the Surface Expression of CD38 in T-Helper Lymphocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Martinez, R. New Trends in Paracoccidioidomycosis Epidemiology. J. Fungi 2017, 3, 1. [Google Scholar] [CrossRef]
- Almeida, F.P. Comparative studies of coccidioic granuloma in the United States and Brazil. New genus for Brazilian parasite. Ann. Fac. Med. Univ. 1930, 5, 125–141. [Google Scholar]
- Ashraf, N.; Kubat, R.C.; Poplin, V.; Adenis, A.A.; Denning, D.W.; Wright, L.; McCotter, O.; Schwartz, I.S.; Jackson, B.R.; Chiller, T.; et al. Re-Drawing the Maps for Endemic Mycoses. Mycopathologia 2020, 185, 843–865. [Google Scholar] [CrossRef] [PubMed]
- Paniago, A.M.M.; Aguiar, J.I.A.; Aguiar, E.S.; da Cunha, R.V.; Pereira, G.R.D.O.L.; Londero, A.T.; Wanke, B. Paracoccidioidomicose: Estudo clínico e epidemiológico de 422 casos observados no Estado de Mato Grosso do Sul. Rev. Soc. Bras. Med. Trop. 2003, 36, 455–459. [Google Scholar] [CrossRef]
- Shikanai-Yasuda, M.A.; Mendes, R.P.; Colombo, A.L.; de Queiroz Telles, F.; Kono, A.; Paniago, A.M.M.; Nathan, A.; do Valle, A.C.F.; Bagagli, E.; Benard, G.; et al. II Consenso Brasileiro em Paracoccidioidomicose-2017. Epidemiol. Serviços Saúde 2018, 27, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.P.; Cavalcante, R.D.S.; Marques, S.A.; Marques, M.E.A.; Venturini, J.; Sylvestre, T.F.; Paniago, A.M.M.; Pereira, A.C.; da Silva, J.D.F.; Fabro, A.T.; et al. Paracoccidioidomycosis: Current Perspectives from Brazil. Open Microbiol. J. 2017, 11, 224–282. [Google Scholar] [CrossRef] [PubMed]
- Fortes, M.R.P.; Miot, H.A.; Kurokawa, C.S.; Marques, M.E.A.; Marques, S.A. Imunologia Da Paracoccidioidomicose. An. Bras. Dermatol. 2011, 86, 516–524. [Google Scholar] [CrossRef]
- Oliveira, S.J.; Mamoni, R.L.; Musatti, C.C.; Papaiordanou, P.M.O.; Blotta, M.H.S.L. Cytokines and Lymphocyte Proliferation in Juvenile and Adult Forms of Paracoccidioidomycosis: Comparison with Infected and Non-Infected Controls. Microbes Infect. 2002, 4, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Marques Mello, L.; Silva-Vergara, M.L.; Rodrigues, V. Patients with Active Infection with Paracoccidioides Brasiliensis Present a Th2 Immune Response Characterized by High Interleukin-4 and Interleukin-5 Production. Hum. Immunol. 2002, 63, 149–154. [Google Scholar] [CrossRef]
- De Castro, L.F.; Ferreira, M.C.; da Silva, R.M.; Blotta, M.H.D.S.L.; Longhi, L.N.A.; Mamoni, R.L. Characterization of the Immune Response in Human Paracoccidioidomycosis. J. Infect. 2013, 67, 470–485. [Google Scholar] [CrossRef]
- Pagliari, C.; Fernandes, E.R.; Stegun, F.W.; da Silva, W.L.F.; Seixas Duarte, M.I.; Sotto, M.N. Paracoccidioidomycosis: Cells Expressing IL17 and Foxp3 in Cutaneous and Mucosal Lesions. Microb. Pathog. 2011, 50, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Tristão, F.S.M.; Rocha, F.A.; Carlos, D.; Ketelut-Carneiro, N.; Souza, C.O.S.; Milanezi, C.M.; Silva, J.S. Th17-Inducing Cytokines IL-6 and IL-23 Are Crucial for Granuloma Formation during Experimental Paracoccidioidomycosis. Front. Immunol. 2017, 8, 949. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Awasthi, A.; Yosef, N.; Quintana, F.J.; Xiao, S.; Peters, A.; Wu, C.; Kleinewietfeld, M.; Kunder, S.; Hafler, D.A.; et al. Induction and Molecular Signature of Pathogenic TH17 Cells. Nat. Immunol. 2012, 13, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.H.; Magalhães, K.G.; Almeida, R.D.N.; Correa, R.; Burgel, P.H.; Bocca, A.L. NLRP3 Inflammasome Activation by Paracoccidioides Brasiliensis. PLoS Negl. Trop. Dis. 2013, 7, e2595. [Google Scholar] [CrossRef] [PubMed]
- Alexovič, M.; Lindner, J.R.; Bober, P.; Longuespée, R.; Sabo, J.; Davalieva, K. Human Peripheral Blood Mononuclear Cells: A Review of Recent Proteomic Applications. Proteomics 2022, 22, 2200026. [Google Scholar] [CrossRef]
- Moscardi-Bacchi, M.; Brummer, E.; Stevens, D.A. Support of Paracoccidioides Brasiliensis Multiplication by Human Monocytes or Macrophages: Inhibition by Activated Phagocytes. J. Med. Microbiol. 1994, 40, 159–164. [Google Scholar] [CrossRef]
- Calvi, S.A.; Peraçoli, M.T.S.; Mendes, R.P.; Marcondes-Machado, J.; Fecchio, D.; Marques, S.A.; Soares, A.M.V.C. Effect of Cytokines on the in Vitro Fungicidal Activity of Monocytes from Paracoccidioidomycosis Patients. Microbes Infect. 2003, 5, 107–113. [Google Scholar] [CrossRef]
- Parise-Fortes, M.R.; Marques, S.A.; Soares, A.M.V.C.; Kurokawa, C.S.; Marques, M.E.A.; Peracoli, M.T.S. Cytokines Released from Blood Monocytes and Expressed in Mucocutaneous Lesions of Patients with Paracoccidioidomycosis Evaluated before and during Trimethoprim-Sulfamethoxazole Treatment: Cytokines in Lesions of Paracoccidioidomycosis. Br. J. Dermatol. 2006, 154, 643–650. [Google Scholar] [CrossRef]
- Kurokawa, C.S.; Araujo, J.P.; Soares, A.M.V.C.; Sugizaki, M.F.; Peraçoli, M.T.S. Pro- and Anti-Inflammatory Cytokines Produced by Human Monocytes Challenged In Vitro with Paracoccidioides Brasiliensis. Microbiol. Immunol. 2007, 51, 421–428. [Google Scholar] [CrossRef]
- Guimarães de Matos, G.; Barroso de Figueiredo, A.M.; Diniz Gonçalves, P.H.; Luiz de Lima Silva, L.; Bastista, A.C.; Borges, C.L.; Maria de Almeida Soares, C.; Joosten, L.A.B.; Ribeiro-Dias, F. Paracoccidioides Brasiliensis Induces IL-32 and Is Controlled by IL-15/IL-32/Vitamin D Pathway in vitro. Microb. Pathog. 2021, 154, 104864. [Google Scholar] [CrossRef]
- Benard, G.; Romano, C.C.; Cacere, C.R.; Juvenale, M.; Mendes-Giannini, M.J.S.; Duarte, A.J.S. Imbalance of IL-2, IFN-γ and IL-10 Secretion in the Immunosuppression Associated with Human Paracoccidioidomycosis. Cytokine 2001, 13, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Prokunina, L.; Alarcón-Riquelme, M.E. Regulatory SNPs in Complex Diseases: Their Identification and Functional Validation. Expert Rev. Mol. Med. 2004, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bozzi, A.; Pereira, P.P.N.; Reis, B.S.; Goulart, M.I.; Pereira, M.C.N.; Pedroso, E.P.; Leite, M.F.; Goes, A.M. Interleukin-10 and Tumor Necrosis Factor–α Single Nucleotide Gene Polymorphism Frequency in Paracoccidioidomycosis. Hum. Immunol. 2006, 67, 931–939. [Google Scholar] [CrossRef]
- Bozzi, A.; Reis, B.S.; Pereira, P.P.; Pedroso, E.P.; Goes, A.M. Interferon-Gamma and Interleukin-4 Single Nucleotide Gene Polymorphisms in Paracoccidioidomycosis. Cytokine 2009, 48, 212–217. [Google Scholar] [CrossRef]
- Mendonça, M.S.; Peraçolli, T.S.; Silva-Vergara, M.L.; Ribeiro, S.C.; Oliveira, R.F.; Mendes, R.P.; Rodrigues, V., Jr. High Interleukin-4 Expression and Interleukin-4 Gene Polymorphisms Are Associated with Susceptibility to Human Paracoccidioidomycosis. Mem. Inst. Oswaldo Cruz 2015, 110, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Alves Pereira Neto, T.; Costa Pereira, A.A.; Costa Hanemann, J.A.; Coelho, L.F.L.; Malaquias, L.C.C. DC-SIGN and VDR Polymorphisms Are Associated with Chronic Form of Paracoccidioidomycosis with Oral Manifestations. Mycoses 2019, 62, 186–192. [Google Scholar] [CrossRef]
- Sato, P.K.; Busser, F.D.; Carvalho, F.M.D.C.; Gomes dos Santos, A.; Sadahiro, A.; Diogo, C.L.; Kono, A.S.G.; Moretti, M.L.; Luiz, O.D.C.; Shikanai-Yasuda, M.A. Polymorphism in the Promoter Region of the IL18 Gene and the Association With Severity on Paracoccidioidomycosis. Front. Immunol. 2020, 11, 542210. [Google Scholar] [CrossRef]
- Li, Y.; Oosting, M.; Deelen, P.; Ricaño-Ponce, I.; Smeekens, S.; Jaeger, M.; Matzaraki, V.; Swertz, M.A.; Xavier, R.J.; Franke, L.; et al. Erratum: Corrigendum: Inter-Individual Variability and Genetic Influences on Cytokine Responses to Bacteria and Fungi. Nat. Med. 2016, 22, 1192. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.B.; Li, Y.; Kumar, V.; Oosting, M.; Smeekens, S.; Jaeger, M.; ter Horst, R.; Schirmer, M.; Vlamakis, H.; et al. Understanding Human Immune Function Using the Resources from the Human Functional Genomics Project. Nat. Med. 2016, 22, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Bastos, K.P.; Bailão, A.M.; Borges, C.L.; Faria, F.P.; Felipe, M.S.; Silva, M.G.; Martins, W.S.; Fiúza, R.B.; Pereira, M.; Soares, C.M. The Transcriptome Analysis of Early Morphogenesis in Paracoccidioides Brasiliensis Mycelium Reveals Novel and Induced Genes Potentially Associated to the Dimorphic Process. BMC Microbiol. 2007, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.; Dewi, I.M.W.; Matzaraki, V.; ter Horst, R.; Pekmezovic, M.; Rösler, B.; Groh, L.; Röring, R.J.; Kumar, V.; Li, Y.; et al. Comparative Host Transcriptome in Response to Pathogenic Fungi Identifies Common and Species-Specific Transcriptional Antifungal Host Response Pathways. Comput. Struct. Biotechnol. J. 2021, 19, 647–663. [Google Scholar] [CrossRef]
- Shah, T.S.; Liu, J.Z.; Floyd, J.A.B.; Morris, J.A.; Wirth, N.; Barrett, J.C.; Anderson, C.A. OptiCall: A Robust Genotype-Calling Algorithm for Rare, Low-Frequency and Common Variants. Bioinformatics 2012, 28, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Pruim, R.J.; Welch, R.P.; Sanna, S.; Teslovich, T.M.; Chines, P.S.; Gliedt, T.P.; Boehnke, M.; Abecasis, G.R.; Willer, C.J. LocusZoom: Regional Visualization of Genome-Wide Association Scan Results. Bioinformatics 2010, 26, 2336–2337. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.-F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-Responsive Genes Involved in Oxidative Phosphorylation Are Coordinately Downregulated in Human Diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Shabalin, A.A. Matrix EQTL: Ultra Fast EQTL Analysis via Large Matrix Operations. Bioinformatics 2012, 28, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Gopisetty, G.; Xu, J.; Sampath, D.; Colman, H.; Puduvalli, V.K. Epigenetic Regulation of CD133/PROM1 Expression in Glioma Stem Cells by Sp1/Myc and Promoter Methylation. Oncogene 2013, 32, 3119–3129. [Google Scholar] [CrossRef]
- Wang, H.; Gong, P.; Li, J.; Fu, Y.; Zhou, Z.; Liu, L. Role of CD133 in Human Embryonic Stem Cell Proliferation and Teratoma Formation. Stem Cell Res. Ther. 2020, 11, 208. [Google Scholar] [CrossRef]
- Gringhuis, S.I.; den Dunnen, J.; Litjens, M.; van der Vlist, M.; Wevers, B.; Bruijns, S.C.M.; Geijtenbeek, T.B.H. Dectin-1 Directs T Helper Cell Differentiation by Controlling Noncanonical NF-ΚB Activation through Raf-1 and Syk. Nat. Immunol. 2009, 10, 203–213. [Google Scholar] [CrossRef]
- Jin, Y.; Li, P.; Wang, F. β-Glucans as Potential Immunoadjuvants: A Review on the Adjuvanticity, Structure-Activity Relationship and Receptor Recognition Properties. Vaccine 2018, 36, 5235–5244. [Google Scholar] [CrossRef]
- Savarino, A.; Bottarel, F.; Malavasi, F.; Dianzani, U. Role of CD38 in HIV-1 Infection: An Epiphenomenon of T-Cell Activation or an Active Player in Virus/Host Interactions? AIDS 2000, 14, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Fanucchi, S.; Mhlanga, M.M. Lnc-Ing Trained Immunity to Chromatin Architecture. Front. Cell Dev. Biol. 2019, 7, 2. [Google Scholar] [CrossRef]
- Ong, C.-T.; Corces, V.G. CTCF: An Architectural Protein Bridging Genome Topology and Function. Nat. Rev. Genet. 2014, 15, 234–246. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Spellberg, B.; Avanessian, V.; Fu, Y.; Edwards, J.E. Rhizopus Oryzae Adheres to, Is Phagocytosed by, and Damages Endothelial Cells In Vitro. Infect. Immun. 2005, 73, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, T.R.T.; Keller, N.P. Pathogenesis of Aspergillus Fumigatus in Invasive Aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef]
- Vasudevan, B.; Hazra, N.; Shijith, K.P.; Neema, S.; Vendhan, S. Mucormycosis: The Scathing Invader. Indian J Derm. 2021, 66, 393–400. [Google Scholar]
- Boahen, C.K.; Joosten, L.A.B.; Netea, M.G.; Kumar, V. Conceptualization of Population-Specific Human Functional Immune-Genomics Projects to Identify Factors That Contribute to Variability in Immune and Infectious Diseases. Heliyon 2021, 7, e06755. [Google Scholar] [CrossRef]
- Feriotti, C.; de Araújo, E.F.; Loures, F.V.; da Costa, T.A.; Galdino, N.A.D.L.; Zamboni, D.S.; Calich, V.L.G. NOD-Like Receptor P3 Inflammasome Controls Protective Th1/Th17 Immunity against Pulmonary Paracoccidioidomycosis. Front. Immunol. 2017, 8, 786. [Google Scholar] [CrossRef] [PubMed]
- de Castro, L.F.; Longhi, L.N.A.; Paião, M.R.; Justo-Júnior, A.D.S.; de Jesus, M.B.; Blotta, M.H. de S.L.; Mamoni, R.L. NLRP3 Inflammasome Is Involved in the Recognition of Paracoccidioides Brasiliensis by Human Dendritic Cells and in the Induction of Th17 Cells. J. Infect. 2018, 77, 137–144. [Google Scholar] [CrossRef]
- de Araújo, E.F.; Preite, N.W.; Veldhoen, M.; Loures, F.V.; Calich, V.L.G. Pulmonary Paracoccidioidomycosis in AhR Deficient Hosts Is Severe and Associated with Defective Treg and Th22 Responses. Sci. Rep. 2020, 10, 11312. [Google Scholar] [CrossRef]
- Feriotti, C.; Bazan, S.B.; Loures, F.V.; Araújo, E.F.; Costa, T.A.; Calich, V.L.G. Expression of Dectin-1 and Enhanced Activation of NALP3 Inflammasome Are Associated with Resistance to Paracoccidioidomycosis. Front. Microbiol. 2015, 6, 913. [Google Scholar] [CrossRef]
- Chaves, A.F.A.; Navarro, M.V.; de Barros, Y.N.; Silva, R.S.; Xander, P.; Batista, W.L. Updates in Paracoccidioides Biology and Genetic Advances in Fungus Manipulation. J. Fungi 2021, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Preite, N.W.; Feriotti, C.; Souza de Lima, D.; da Silva, B.B.; Condino-Neto, A.; Pontillo, A.; Calich, V.L.G.; Loures, F.V. The Syk-Coupled C-Type Lectin Receptors Dectin-2 and Dectin-3 Are Involved in Paracoccidioides Brasiliensis Recognition by Human Plasmacytoid Dendritic Cells. Front. Immunol. 2018, 9, 464. [Google Scholar] [CrossRef]
- Rogers, N.C.; Slack, E.C.; Edwards, A.D.; Nolte, M.A.; Schulz, O.; Schweighoffer, E.; Williams, D.L.; Gordon, S.; Tybulewicz, V.L.; Brown, G.D.; et al. Syk-Dependent Cytokine Induction by Dectin-1 Reveals a Novel Pattern Recognition Pathway for C Type Lectins. Immunity 2005, 22, 507–517. [Google Scholar] [CrossRef]
- Bonfim, C.V.; Mamoni, R.L.; Souza, M.H.; Blotta, L. TLR-2, TLR-4 and Dectin-1 Expression in Human Monocytes and Neutrophils Stimulated by Paracoccidioides Brasiliensis. Med. Mycol. 2009, 47, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Loures, F.V.; AraÃjo, E.F.; Feriotti, C.; Bazan, S.B.; Calich, V.L.G. TLR-4 Cooperates with Dectin-1 and Mannose Receptor to Expand Th17 and Tc17 Cells Induced by Paracoccidioides Brasiliensis Stimulated Dendritic Cells. Front. Microbiol. 2015, 6, 261. [Google Scholar] [CrossRef]
- Saïd-Sadier, N.; Padilla, E.; Langsley, G.; Ojcius, D.M. Aspergillus Fumigatus Stimulates the NLRP3 Inflammasome through a Pathway Requiring ROS Production and the Syk Tyrosine Kinase. PLoS ONE 2010, 5, e10008. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Man, S.M.; Malireddi, R.K.S.; Gurung, P.; Vogel, P.; Lamkanfi, M.; Kanneganti, T.-D. Concerted Activation of the AIM2 and NLRP3 Inflammasomes Orchestrates Host Protection against Aspergillus Infection. Cell Host Microbe 2015, 17, 357–368. [Google Scholar] [CrossRef]
- Briard, B.; Fontaine, T.; Samir, P.; Place, D.E.; Muszkieta, L.; Malireddi, R.K.S.; Karki, R.; Christgen, S.; Bomme, P.; Vogel, P.; et al. Galactosaminogalactan Activates the Inflammasome to Provide Host Protection. Nature 2020, 588, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Loures, F.V.; Araújo, E.F.; Feriotti, C.; Bazan, S.B.; Costa, T.A.; Brown, G.D.; Calich, V.L.G. Dectin-1 Induces M1 Macrophages and Prominent Expansion of CD8+IL-17+ Cells in Pulmonary Paracoccidioidomycosis. J. Infect. Dis. 2014, 210, 762–773. [Google Scholar] [CrossRef]
- de Quaglia e Silva, J.C.; Della Coletta, A.M.; Gardizani, T.P.; Romagnoli, G.G.; Kaneno, R.; Dias-Melicio, L.A. Involvement of the Dectin-1 Receptor upon the Effector Mechanisms of Human Phagocytic Cells against Paracoccidioides Brasiliensis. J. Immunol. Res. 2019, 2019, 1529189. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhou, Q.; Li, X.; Li, H.; Meng, T.; Zhong, Y.; Pu, J.; Zhu, M.; Xu, Y.; Gan, L.; et al. Differentiation and recruitment of IL-22-producing helper T cells in lgA nephropathy. Am. J. Transl. Res. 2016, 8, 3872–3882. [Google Scholar]
- Mayr, C. What Are 3′ UTRs Doing? Cold Spring Harb Perspect Biol 2019, 11, a034728. [Google Scholar] [CrossRef] [PubMed]
- Ketelut-Carneiro, N.; Ghosh, S.; Levitz, S.M.; Fitzgerald, K.A.; da Silva, J.S. A Dectin-1-Caspase-8 Pathway Licenses Canonical Caspase-1 Inflammasome Activation and Interleukin-1β Release in Response to a Pathogenic Fungus. J. Infect. Dis. 2018, 217, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E. Glutathione: An Overview of Biosynthesis and Modulation. Chem.-Biol. Interact. 1998, 111–112, 1–14. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The Importance of Glutathione in Human Disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Seegren, P.V.; Downs, T.K.; Stremska, M.E.; Harper, L.R.; Cao, R.; Olson, R.J.; Upchurch, C.M.; Doyle, C.A.; Kennedy, J.; Stipes, E.L.; et al. Mitochondrial Ca2+ Signaling Is an Electrometabolic Switch to Fuel Phagosome Killing. Cell Rep. 2020, 33, 108411. [Google Scholar] [CrossRef]
- Santos, L.A.; Rosalen, P.L.; Dias, N.A.; Grisolia, J.C.; Nascimento Gomes, B.J.; Blosfeld-Lopes, L.; Ikegaki, M.; Alencar, S.M.d.; Burger, E. Brazilian Red Propolis Shows Antifungal and Immunomodulatory Activities against Paracoccidioides Brasiliensis. J. Ethnopharmacol. 2021, 277, 114181. [Google Scholar] [CrossRef]
- Deaglio, S.; Vaisitti, T.; Serra, S.; Audrito, V.; Bologna, C.; D’Arena, G.; Laurenti, L.; Gottardi, D.; Malavasi, F. CD38 in chronic lymphocytic leukemia: From bench to bedside? Mini. Rev. Med. Chem. 2011, 11, 503–507. [Google Scholar] [CrossRef]
- Frasca, L.; Fedele, G.; Deaglio, S.; Capuano, C.; Palazzo, R.; Vaisitti, T.; Malavasi, F.; Ausiello, C.M. CD38 Orchestrates Migration, Survival, and Th1 Immune Response of Human Mature Dendritic Cells. Blood 2006, 107, 2392–2399. [Google Scholar] [CrossRef]
- Gangemi, S.; Allegra, A.; Alonci, A.; Pace, E.; Cannavò, A.; Ferraro, M.; Penna, G.; Saitta, S.; Gerace, D.; Musolino, C. Interleukin 22 Is Increased and Correlated with CD38 Expression in Patients with B-Chronic Lymphocytic Leukemia. Blood Cells Mol. Dis. 2013, 50, 39–40. [Google Scholar] [CrossRef]
- Wolk, K.; Kunz, S.; Asadullah, K.; Sabat, R. Cutting Edge: Immune Cells as Sources and Targets of the IL-10 Family Members? J. Immunol. 2002, 168, 5397–5402. [Google Scholar] [CrossRef]
- Rutz, S.; Eidenschenk, C.; Ouyang, W. IL-22, Not Simply a Th17 Cytokine. Immunol. Rev. 2013, 252, 116–132. [Google Scholar] [CrossRef]
- Gurney, A.L. IL-22, a Th1 Cytokine That Targets the Pancreas and Select Other Peripheral Tissues. Int. Immunopharmacol. 2004, 4, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Witte, E.; Witte, K.; Warszawska, K.; Sabat, R. Biology of Interleukin-22. Semin. Immunopathol. 2010, 32, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Maggini, S.; Wintergerst, E.S.; Beveridge, S.; Hornig, D.H. Selected Vitamins and Trace Elements Support Immune Function by Strengthening Epithelial Barriers and Cellular and Humoral Immune Responses. Br. J. Nutr. 2007, 98, S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.-R.; Chang, S.-Y.; Chang, J.-H.; Kim, J.-O.; Yang, J.-Y.; Kim, C.-H.; Kweon, M.-N. Downregulation of Th17 Cells in the Small Intestine by Disruption of Gut Flora in the Absence of Retinoic Acid. J. Immunol. 2010, 184, 6799–6806. [Google Scholar] [CrossRef] [PubMed]
- De Castro, L.F.; de Araújo Mathias, K.; Nunes, J.V.; Galastri, A.L.B.; da Silva, D.H.L.; Longhi, L.N.A.; de Souza Lima Blotta, M.H.; Mamoni, R.L. Ethanol Modulates the Effector Functions of Human Monocyte-Derived Macrophages in Response to Paracoccidioides Brasiliensis Yeast Cells. Med. Mycol. 2021, 59, 773–783. [Google Scholar] [CrossRef]
- Kurokawa, C.S.; Lopes, C.R.; Sugizaki, M.F.; Kuramae, E.E.; Franco, M.F.; Peraçoli, M.T.S. Virulence Profile of Ten Paracoccidioides Brasiliensis Isolates: Association with Morphologic and Genetic Patterns. Rev. Inst. Med. Trop. S. Paulo 2005, 47, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Singer-Vermes, L.M.; Vaz, C.A.; Calich, V.L. Comparative Studies on the Antibody Repertoire Produced by Susceptible and Resistant Mice to Virulent and Nonvirulent Paracoccidioides Brasiliensis Isolates. Am. J. Trop. Med. Hyg. 1998, 59, 971–977. [Google Scholar] [CrossRef]
- Ferwerda, B.; Ferwerda, G.; Plantinga, T.S.; Willment, J.A.; van Spriel, A.B.; Venselaar, H.; Elbers, C.C.; Johnson, M.D.; Cambi, A.; Huysamen, C.; et al. Human Dectin-1 Deficiency and Mucocutaneous Fungal Infections. N. Engl. J. Med. 2009, 361, 1760–1767. [Google Scholar] [CrossRef] [PubMed]
- Kalia, N.; Kaur, M.; Sharma, S.; Singh, J. A Comprehensive in Silico Analysis of Regulatory SNPs of Human CLEC7A Gene and Its Validation as Genotypic and Phenotypic Disease Marker in Recurrent Vulvovaginal Infections. Front. Cell. Infect. Microbiol. 2018, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Kalia, N.; Singh, J.; Kaur, M. The Role of Dectin-1 in Health and Disease. Immunobiology 2021, 226, 152071. [Google Scholar] [CrossRef]
- Parente, R.; Doni, A.; Bottazzi, B.; Garlanda, C.; Inforzato, A. The Complement System in Aspergillus Fumigatus Infections and Its Crosstalk with Pentraxins. FEBS Lett. 2020, 594, 2480–2501. [Google Scholar] [CrossRef]
- Li, D.; Dong, B.; Tong, Z.; Wang, Q.; Liu, W.; Wang, Y.; Liu, W.; Chen, J.; Xu, L.; Chen, L.; et al. MBL-Mediated Opsonophagocytosis of Candida Albicans by Human Neutrophils Is Coupled with Intracellular Dectin-1-Triggered ROS Production. PLoS ONE 2012, 7, e50589. [Google Scholar] [CrossRef]
- Jiménez, M.D.P.; Restrepo, A.; Radzioch, D.; Cano, L.E.; GarcÃa, L.F. Importance of Complement 3 and Mannose Receptors in Phagocytosis of Paracoccidioides Brasiliensis Conidia by Nramp1 Congenic Macrophages Lines. FEMS Immunol. Med. Microbiol. 2006, 47, 56–66. [Google Scholar] [CrossRef]
- Wu, W.-J.H.; Kim, M.; Chang, L.-C.; Assie, A.; Saldana-Morales, F.B.; Zegarra-Ruiz, D.F.; Norwood, K.; Samuel, B.S.; Diehl, G.E. Interleukin-1β Secretion Induced by Mucosa-Associated Gut Commensal Bacteria Promotes Intestinal Barrier Repair. Gut Microbes 2022, 14, 2014772. [Google Scholar] [CrossRef] [PubMed]
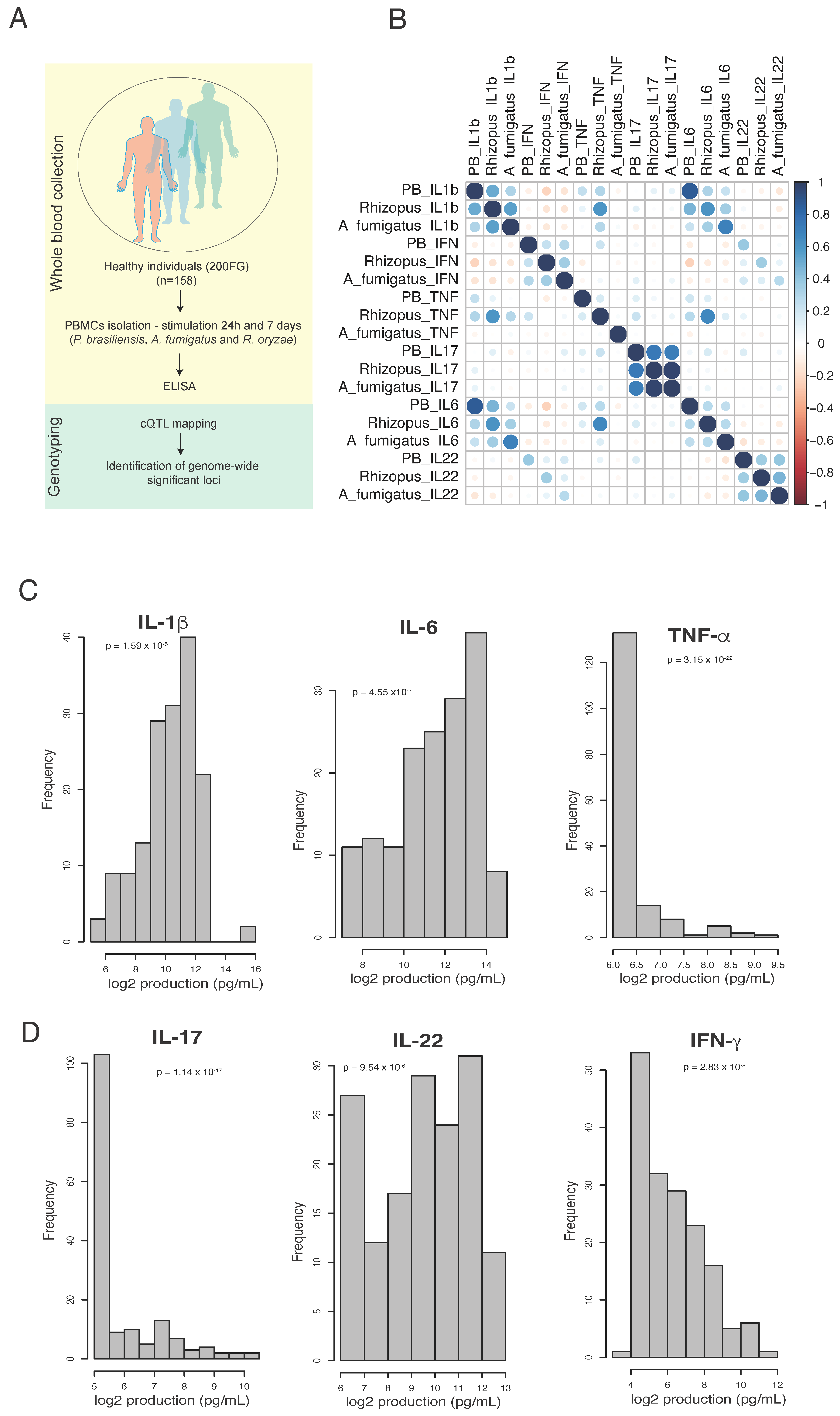
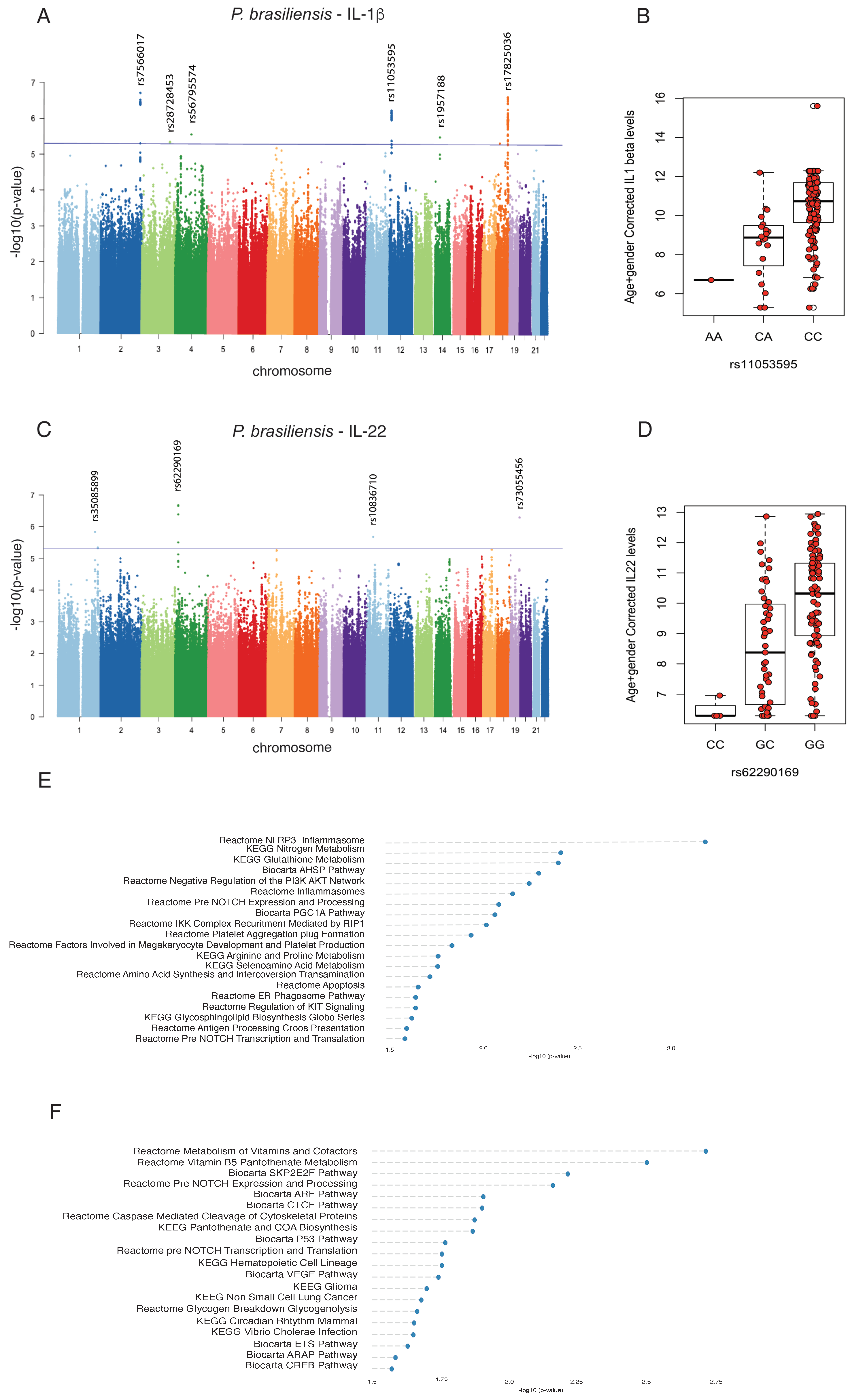

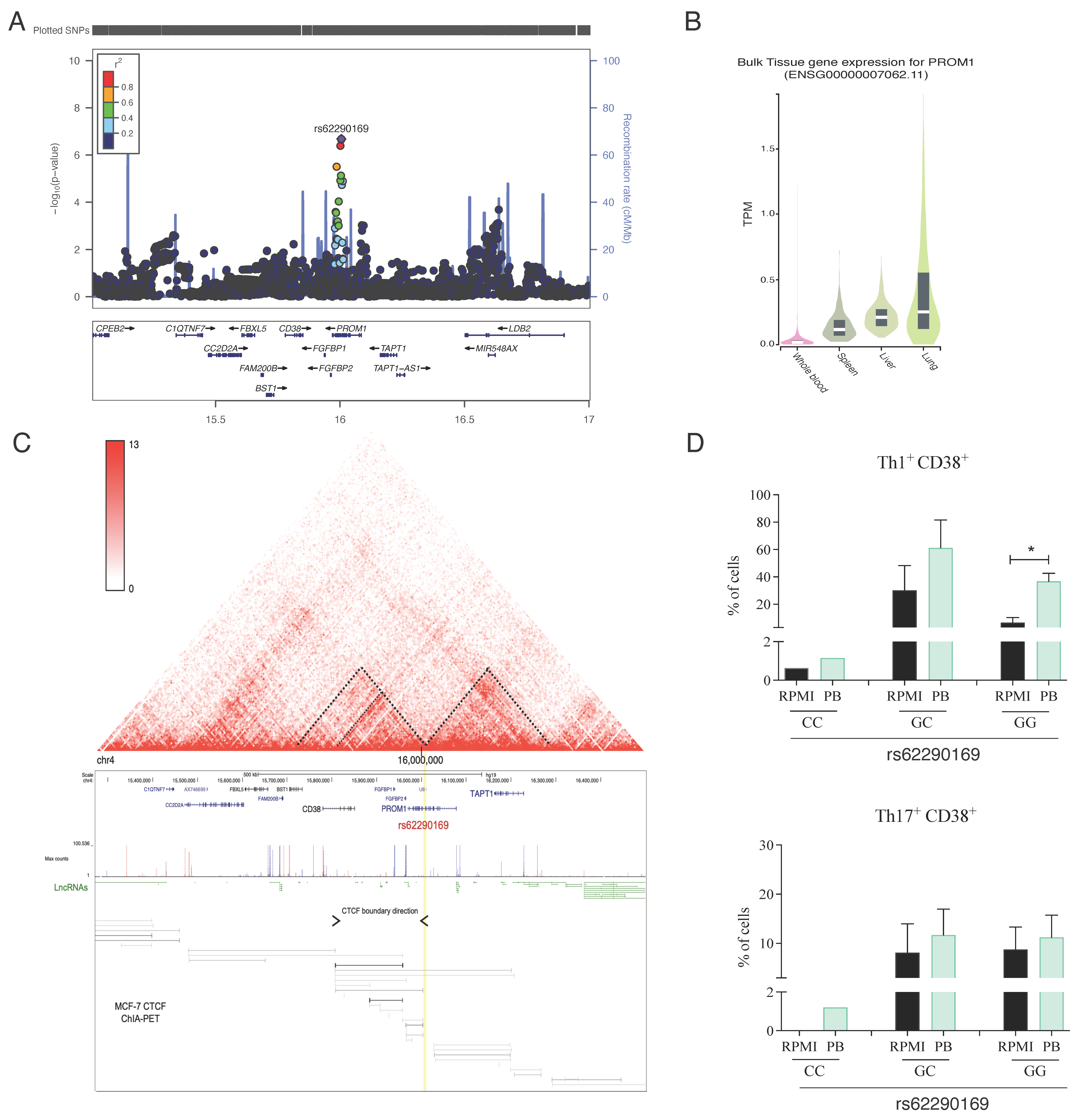
| SNP | Cytokine | p Value | CHROM | POS | REF | ALT | Gene |
|---|---|---|---|---|---|---|---|
| rs56795574 | IL-6 | 6.90 × 10−8 | 4 | 91745027 | A | G | CCSER1 |
| rs10850949 | IL-6 | 1.94 × 10−7 | 12 | 118442266 | G | T | RFC5 |
| rs17058173 | IL-6 | 2.20 × 10−7 | 18 | 73512093 | C | T | LOC339298 |
| rs35049004 | IL-6 | 9.03 × 10−7 | 16 | 76913364 | G | A | MIR4719 |
| rs76262774 | IL-6 | 3.04 × 10−6 | 17 | 33048609 | C | T | TMEM132E |
| rs7566017 | IL-1 beta | 1.92 × 10−7 | 2 | 231536848 | C | T | LOC151475 |
| rs17825036 | IL-1 beta | 2.39 × 10−7 | 18 | 65170712 | T | G | DSEL |
| rs11053595 | IL-1 beta | 5.82 × 10−7 | 12 | 10270822 | C | A | CLEC7A |
| rs56795574 | IL-1 beta | 2.75 × 10−6 | 4 | 91745027 | A | G | CCSER1 |
| rs28728453 | IL-1 beta | 4.42 × 10−6 | 3 | 163725033 | A | G | MIR1263 |
| rs1957188 | IL-1 beta | 9.59 × 10−6 | 14 | 46509206 | T | C | RPL10L |
| rs369304721 | IFN gamma | 1.34 × 10−6 | 22 | 51221190 | G | A | RABL2B |
| rs111342688 | IFN gamma | 1.58 × 10−6 | 5 | 78330613 | T | G | DMGDH |
| rs7185240 | IFN gamma | 2.29 × 10−6 | 16 | 8272911 | G | A | TMEM114 |
| rs2245968 | IFN gamma | 2.43 × 10−6 | 10 | 7528542 | T | G | TIH5 |
| rs74439139 | IFN gamma | 3.23 × 10−6 | 7 | 113757643 | T | G | FOXP2 |
| rs62290169 | IL-22 | 2.09 × 10−7 | 4 | 16005374 | G | C | PROM1 |
| rs73055456 | IL-22 | 5.10 × 10−7 | 19 | 54794580 | G | C | LILRA3 |
| rs35085899 | IL-22 | 1.47 × 10−6 | 1 | 216889197 | T | C | ESRRG |
| rs7252511 | IL-22 | 7.98 × 10−6 | 19 | 2396243 | C | T | TMPRSS9 |
| rs10836710 | IL-22 | 2.11 × 10−6 | 11 | 37256074 | T | G | SNORA31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Figueiredo, A.M.B.; dos Santos, J.C.; Kischkel, B.; Ardiansyah, E.; Oosting, M.; Guimarães Matos, G.; Barreto Neves Oliveira, I.; van de Veerdonk, F.; Netea, M.G.; Soares, C.M.d.A.; et al. Genome-Wide Association Study Reveals CLEC7A and PROM1 as Potential Regulators of Paracoccidioides brasiliensis-Induction of Cytokine Production in Peripheral Blood Mononuclear Cells. J. Fungi 2023, 9, 428. https://doi.org/10.3390/jof9040428
de Figueiredo AMB, dos Santos JC, Kischkel B, Ardiansyah E, Oosting M, Guimarães Matos G, Barreto Neves Oliveira I, van de Veerdonk F, Netea MG, Soares CMdA, et al. Genome-Wide Association Study Reveals CLEC7A and PROM1 as Potential Regulators of Paracoccidioides brasiliensis-Induction of Cytokine Production in Peripheral Blood Mononuclear Cells. Journal of Fungi. 2023; 9(4):428. https://doi.org/10.3390/jof9040428
Chicago/Turabian Stylede Figueiredo, Ana Marina B., Jéssica Cristina dos Santos, Brenda Kischkel, Edwin Ardiansyah, Marije Oosting, Grazzielle Guimarães Matos, Iara Barreto Neves Oliveira, Frank van de Veerdonk, Mihai G. Netea, Célia Maria de Almeida Soares, and et al. 2023. "Genome-Wide Association Study Reveals CLEC7A and PROM1 as Potential Regulators of Paracoccidioides brasiliensis-Induction of Cytokine Production in Peripheral Blood Mononuclear Cells" Journal of Fungi 9, no. 4: 428. https://doi.org/10.3390/jof9040428
APA Stylede Figueiredo, A. M. B., dos Santos, J. C., Kischkel, B., Ardiansyah, E., Oosting, M., Guimarães Matos, G., Barreto Neves Oliveira, I., van de Veerdonk, F., Netea, M. G., Soares, C. M. d. A., Ribeiro-Dias, F., & Joosten, L. A. B. (2023). Genome-Wide Association Study Reveals CLEC7A and PROM1 as Potential Regulators of Paracoccidioides brasiliensis-Induction of Cytokine Production in Peripheral Blood Mononuclear Cells. Journal of Fungi, 9(4), 428. https://doi.org/10.3390/jof9040428






