Although fungi have been known for a long time to pose a widespread threat to plants, the impact of fungal infections on animal health has been underestimated until recently, with major declines seen in wildlife due to fungal emergences [
1]. Indeed, in 1997, a major fungal infection of amphibians caused the largest biodiversity loss event in the world. The chytrid fungus
Batrachochytrium dendrobatidis, co-introduced with American bullfrogs (
Lithobates catesbeianus), was responsible for the extinction of at least 500 amphibian species in 54 countries [
2,
3]. In the Americas,
B. dendrobatidis has caused a loss of more than 40% of amphibian species [
4], also leading to major ecological changes [
5]. Globally, chytridiomycosis (the disease caused by
B. dendrobatidis) has led to the decline of nearly half of all amphibian species [
6]. Importantly, the fungal diseases causing the decline in global populations are emerging in a wide range of terrestrial, but also wild and farmed aquatic animal species, including, for example, soft corals (see-fan aspergillosis caused by
Aspergillus sydowii) and tilapia fish (epizootic ulcerative syndrome caused by
Aphanomyces invadans) [
1]. Similarly, the emerging fungus
Aphanomyces astaci has caused a dramatic decline in freshwater brown crayfish populations worldwide, through a disease called crayfish plague [
7]. Despite their impact on biodiversity, fungal diseases are also a major threat to the aquaculture sector worldwide [
8], i.e., they have rapidly expanded the range of species farmed, including crustaceans, mollusks, and finfish, notably tilapia [
9], and thus, food security. For example, fungal infections are among the most common diseases in fish in both temperate and tropical areas [
10]. The main fungal diseases reported in aquaculture include those caused by fungi of the genera
Aphanomyces,
Branchiomyces,
Lagenidium,
Saprolegnia sp.,
Sirolipidium,
Phoma,
Aphanomyces invaderis,
Leptolegnia, and
Dictyuchus, and infect a wide variety of farmed fish such as rainbow trout, yellowtail, mackerel, herring, flounder, cod, salmonids, tilapia, carps, etc. [
10]. These observations therefore highlight the need for improved surveillance systems, detection of emerging fungal pathogens, monitoring disease prevalence, and a sound knowledge of host–pathogen distribution (geographic range) and interaction (pathogen virulence vs host susceptibility).
In 2005, a global risk of disease emergence for freshwater fish biodiversity was identified in Europe and directly linked to the accidental introduction in the early 1960s of the highly invasive Asian gudgeon, also named topmouth gudgeon
Pseudorasbora parva. A healthy carrier of a newly described fungus, the rosette agent
Sphaerothecum destruens, in reference to the sphere containing spore-like structures (
Sphaerothecum),
destruens meaning destructive, once established in the host fish, the infection causes widespread destruction of various tissues [
11]. Since then, severe declines in fish populations have been confirmed across Europe, including wild and farmed fish, following the arrival of this invasive host–parasite complex [
12].
S. destruens is a unicellular eukaryotic fish parasite and an obligate intracellular parasite known as the “rosette agent”. This parasite was originally assigned to the category “Dermocystidium-like” because it shared similar morphological features with other enigmatic parasites of fish and crustaceans [
11]. Kerk et al. [
13] were the first to obtain the complete DNA sequence encoding the small rRNA subunit (18S rRNA gene) of
S. destruens and showed that it shared its most recent common ancestor with the choanoflagellates, a group of sister protists of multicellular animals. Further phylogenetic analyses of the 18S rRNA gene of two
Dermocystidium spp.,
S. destruens,
Ichthyophonus hoferi, and
Psorospermium haeckeli confirmed their relationship and divergence from the animal–fungal dichotomy [
14]. Based on these results, Ragan et al. [
14] assigned them to the clade of DRIPs (DRIP for the first letter of each of their names), also belonging to the class Ichthyosporea [
15]. Subsequently, Mendoza et al. modified this classification and created the class Mesomycetozoea [
16,
17], previously proposed by Herr et al. [
18] and notably composed of the Order Dermocystida and the Order Ichthyophonida [
15]. The class Mesomycetozoea includes 10 different parasitic and saprophytic micro-organisms belonging to the genera
Amoebidium,
Anurofeca,
Dermocystidium,
Ichthyophonus,
Pseudoperkinsus,
Psorospermium,
Rhinosporidium,
Sphaeroforma, and
Ichthyophonida sp. [
17] and the Order
Dermocystida includes
Dermocystidium spp.,
Rhinosporidium seeberi, and
S. destruens, which share the ability to cause infections in animals. For example, more than 20 species of
Dermocystidium spp. cause infections in carp, goldfish, salmonids, eels, newts, and frogs [
14]. However, the more recent phylogenetic classification grouped
S. destruens with animals, fungi, and choanoflagellates in the super-group Opisthokonta [
19].
1.1. Worldwide Distribution of Sphaerothecum destruens
The disease caused by
S. destruens first emerged in salmonids in the USA and was first described in 1984 [
23]. Between 1983 and 1984, 80% mortality occurred in a group of 2.5-years-old chinook salmon
Oncorhyncus tshawytscha brood stock, reared in seawater net-pens in Puget Sound, Washington, DC, USA. These mortalities could not be attributed to previously known pathogens. The diseased fish were anemic with marked lymphocytosis and had enlarged kidneys and spleens. Light and electron microscopic examinations of the spleen and kidney tissues revealed the presence of numerous intracellular spherical organisms of 3–7 µm in size with chemical and structural characteristics similar to marine algae or fungi. The infectious agent responsible has been described as a systemic protist and has been termed a “rosette” or “chinook rosette agent” because of the clustered organization of organisms observed in the stained tissues of infected fish [
23]. This first RA-1 isolate of
S. destruens was amplified on the CHSE-214 cell line developed from Chinook salmon embryos and held in the American Type Culture Collection (ATCC, Accession Number #50643) [
24]. A few years later, chronic mortalities in subadult Atlantic salmon
Salmo salar received as eyed eggs from Finland and reared in spring water on a private farm in northern California, USA, were reported [
25]. Although microscopic examinations suggested that the disease was caused by an organism similar to that described by Harrell et al. [
23], it was termed “Dermocystidium-like” and a second isolate of
S. destruens RA-2 was deposited at the ATCC (Accession Number #50644) [
25]. Finally, a third emergence of the disease was reported in the USA in captive subadult and winter-run adults of
O. tshawytscha broodstock from Sacramento River maintained at the Bodega Marine Laboratory (BML, Bodega Bay, CA, USA) in collaboration with the Coleman National Fish Hatchery (CNFH, CA, USA) and several state and federal agencies [
26]. Among the 1991–1994 brood years, disease prevalence peaked at 40.1% in the 1991 year class.
S. destruens was first detected in a few 1991 brood year salmon (14–16 months old) that had never been transferred to seawater since their arrival from the original freshwater hatchery (CNFH). Subsequently, the parasite was detected in wild adult late-fall-run
O. tshawytscha returning to freshwater to spawn in the upper Sacramento River. Since 1994, surveillance by the California–Nevada Fish Health Center has demonstrated the presence of
S. destruens in up to 32% of late-fall-run adult
O. tshawytscha returning to Battle Creek on the Upper Sacramento River, suggesting the persistence of the disease in the BML and a potential risk of disease emergence. A third isolate, named RA-3 or BML strain, was isolated from
O. tshawytscha-infected kidneys, amplified in fish cell lines, and also deposited at ATCC (Accession number #50615).
For several years, the disease caused by
S. destruens was only reported in salmonids reared on the northwest coast of the USA (Washington State and California). However, in 2005, Gozlan et al. [
27] were the first to describe the emergence of
S. destruens disease in invasive populations of
Pseudorasbora parva in Europe, but also in the non-native cyprinid
Leucaspius delineatus (sunbleak) in England [
27,
28]. As the local declines of
L. delineatus in Europe coincided with the initial introduction of
P. parva into Romanian ponds in 1960 near the Danube, followed by its rapid spread throughout Europe, they hypothesized that Asian populations of
P. parva might represent the asymptomatic carrier of
S. destruens. Their results not only provided the first occurrence of the disease outside North America, but also showed for the first time that several species of cyprinids were also susceptible to
S. destruens and could develop the disease, thus extending the potential host range of this parasite [
27,
28]. Furthermore, their experimental results confirmed that
P. parva populations could represent asymptomatic carriers of
S. destruens. Importantly, their work pioneered the use of targeted molecular tools to detect
S. destruens in infected fish [
22]. The RA-4 isolate of
S. destruens, also named UK-Cefas1, was extracted from tissues (kidney and liver) of wild-infected
L. delineatus following cohabitation experiments with
P. parva [
27]. Although no obvious morphological or pathological differences between
S. destruens infections in
L. delineatus and salmonids were noted, RA-4 isolate showed genetic variability compared with the three American isolates (RA-1, RA-2, RA-3) [
12,
28].
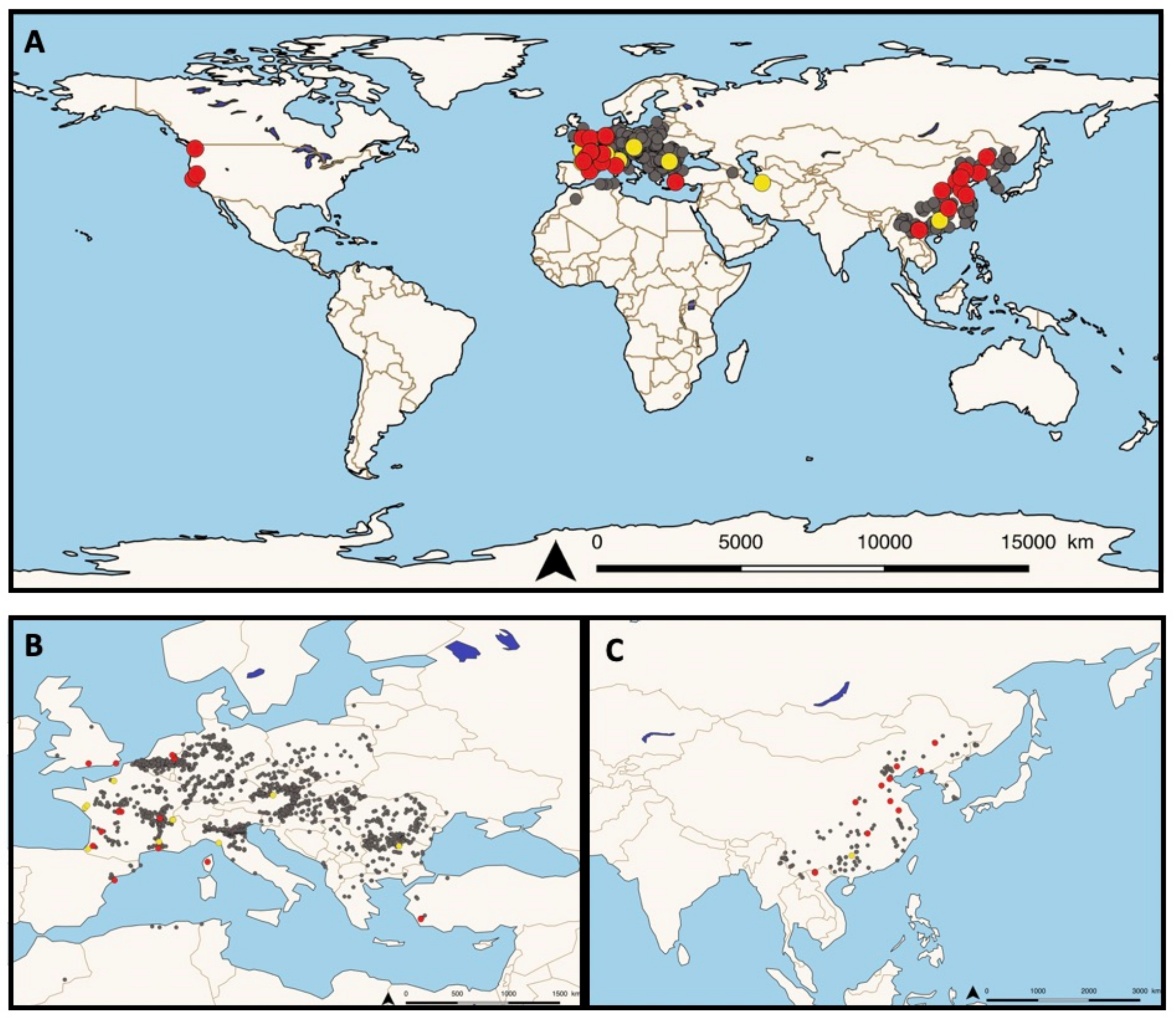
Following this work, based on the rapid spread of invasive
P. parva populations in freshwater ecosystems (see
Figure 1A–C), several research groups have been investigating the potential presence of the disease in European fish. In the Netherlands, populations of
P. parva were introduced in 1992 and rapidly colonized lakes via the Meuse river [
29].
Invasive
P. parva populations sampled in 2008 in the Everlose Beek floodplain and sampled in 2012 in the Teelebeek stream (floodplain of the Meuse river) were also found to be infected with
S. destruens [
30,
31]. In Turkey, from 2009 to 2013 in the Sariçay stream of Mugla, three species of native fish
Oxynoemacheilus sp. (Nemacheilidae),
Petroleuciscus smyrnaeus,
Squalius fellowesii, and the non-native
Lepomis gibbosus (Percidae) as well as farmed seabass
Dicentrarchus labrax (Moronidae) cohabiting with invasive wild populations of
P. parva were found to be infected by
S. destruens. Kidney, liver, spleen, and gonad tissues were all tested for the presence of the parasite using PCR assays and histological examinations, confirming its wide host range as well as its broad cellular tropism [
32]. In France, invasive populations of
P. parva were first reported in 1980 [
33] and a survey of fish populations conducted between 1990–2009 showed that this non-native species has spread dramatically [
34]. In 2016, Charrier et al. [
35] showed the presence of
S. destruens in 12 individuals of
P. parva caught in a small tributary stream of the Adour River near Dax, France [
35]. Just one year later, Boitard et al. [
36] reported the occurrence of two additional natural infections of salmonids in France. In November 2015, chronic mortality occurred in brown trout (
S. trutta) and rainbow trout (
O. mykiss) at an experimental facility, followed in 2016 by a second unusual episode of fish mortalities at a rainbow trout farm, both located in southwest France. In both outbreaks, kidneys, livers, and spleens were collected for histological and molecular examination and revealed the presence of foreign cells 2–4 µm in diameter with eosinophilic inclusions and were observed to be single or arranged in rosettes (spores aggregates) consistent with
S. destruens. Sequencing of PCR products revealed a disease prevalence of 80% and 100% in
O. mykiss and
S. trutta, respectively [
36]. In France, this pioneering work led to the deployment of a nationwide project involving seven French departmental angling associations and two research institutes. Sampling took place across the country between 2017–2019 and included 10 freshwater sites from which 50 invasive individuals of
P. parva and other native species were collected and screened by PCR for the presence and prevalence of
S. destruens [
22]. The results showed a wide distribution of
S. destruens in freshwater sites in France with at least five out of 10 of the sampling sites, such as Ain (central-eastern France), Indre (central France), Gironde (south-western France), Bouches-du-Rhône, and Corsica island (south-eastern France), where
S. destruens DNA was detected in five native fish species and one
P. parva population [
22]. Furthermore, it confirmed the wide range of climates (from temperate to oceanic and Mediterranean) and habitats (lotic and lentic) that
S. destruens can tolerate. Other invasive populations of
P. parva have been found to be infected and carrying
S. destruens in the UK and Spain with a prevalence of 5% in both populations [
37].
Finally, the third region of the world where
S. destruens has been reported to date is among native
P. parva populations in China [
37], and more recently, in an invasive population in Lào Cai Province, North Vietnam (Gozlan pers. Com.). Indeed, Sana et al. [
37] used Gozlan’s extensive 2010 sampling campaign of
P. parva populations in its native and invasive range to detect the presence of the parasite by PCR assays targeting the 18S rRNA gene. In the native range, among 10 Chinese populations collected in different locations [
20], they were able to detect the presence of
S. destruens in nine populations with a prevalence ranging from 0 to 10% depending on the population tested and an overall prevalence of 6%. By analyzing the ITS-1 region of geographically distinct
S. destruens isolates, [
37] showed a clustering of isolates according to their geographic origin. British and Chinese
S. destruens isolates clustered together, Turkish isolates were distinct, although more closely related to British and Chinese isolates, and American isolates formed another clade.
Taken together, these results show that the presence of
S. destruens is mainly linked to the presence and introduction of the healthy carrier
P. parva. It is therefore logical to assume that where
P. parva was introduced,
S. destruens has also been introduced, although not all local populations have been formally tested yet. Previous studies have suggested coevolution between
P. parva and
S. destruens lineages [
32,
37], indicating a true coexistence of several million years [
20]. Finally, Combe and Gozlan [
12] suggest that the origin of the US strains is to be sought among infected Asian O. tshawytscha (eastern Russia, Japan) living in sympatry with native
P. parva populations, which would have contaminated their American counterparts during their migratory movement in the North Pacific. Indeed, Arkush et al. [
26] reported that 33% of returning American Chinook stock from a Californian river were positive for
S. destruens. Therefore, it is expected that the American strains will be closely related to Japanese strains when the latter are tested.
1.2. Mortalities Associated with the Emergence of S. destruens
The parasite
Sphaerothecum destruens causes low-level mortalities, which makes it difficult to identify in wild fish populations. In many cases, whether in a pond, lake, or watershed setting, few will be aware of the mortalities that follow the arrival of the healthy
P. parva carrier [
12,
38]. First of all, the water is not transparent, which makes it difficult to see a dying fish that is not on the surface, and secondly, few people observe aquatic ecosystems on a daily basis [
38]. It is not as in the case of a virus or pollution where we would see mass mortalities on the surface that would sound the alarm for a reaction from competent authorities. In the case of the emergence of the agent, the mortalities are more insidious, with low-level mortalities adding up to a decline in the target populations [
27,
38,
39]. The observer interested in a target population will see fish that look healthy, but there will be fewer and fewer of them until there is a decline that may be total [
39]. This has been the case for sunbleak
Leucaspius delineatus populations, which have disappeared from the whole of Europe with a few residual pockets that would merit a targeted and rapid conservation plan [
27,
40]. Locally, populations of cyprinids and salmonids have declined significantly or even disappeared (refs). In this context, it is not surprising to see that the first identification and isolation of
S. destruens was observed in aquaculture systems [
24,
26], where it is indeed easier to monitor mortalities and therefore, to perform autopsies in order to identify the agent causing these mortalities [
11]. When
P. parva is introduced into an aquatic ecosystem where susceptible species are present, there will be an initial release of spores, probably in the feces or during reproduction. These spores in contact with fresh water will produce zoospores which will then contaminate other fish. Contamination by predation may also occur. In the first few weeks after introduction into a pond, no mortality will be observed and then, with time, the susceptible species will be less and less abundant without any mortality being observed from the edge of the pond. This can lead to complete extinction. In small aquaculture ponds, it is easier to observe mortalities, as was the case in the USA with salmonids.
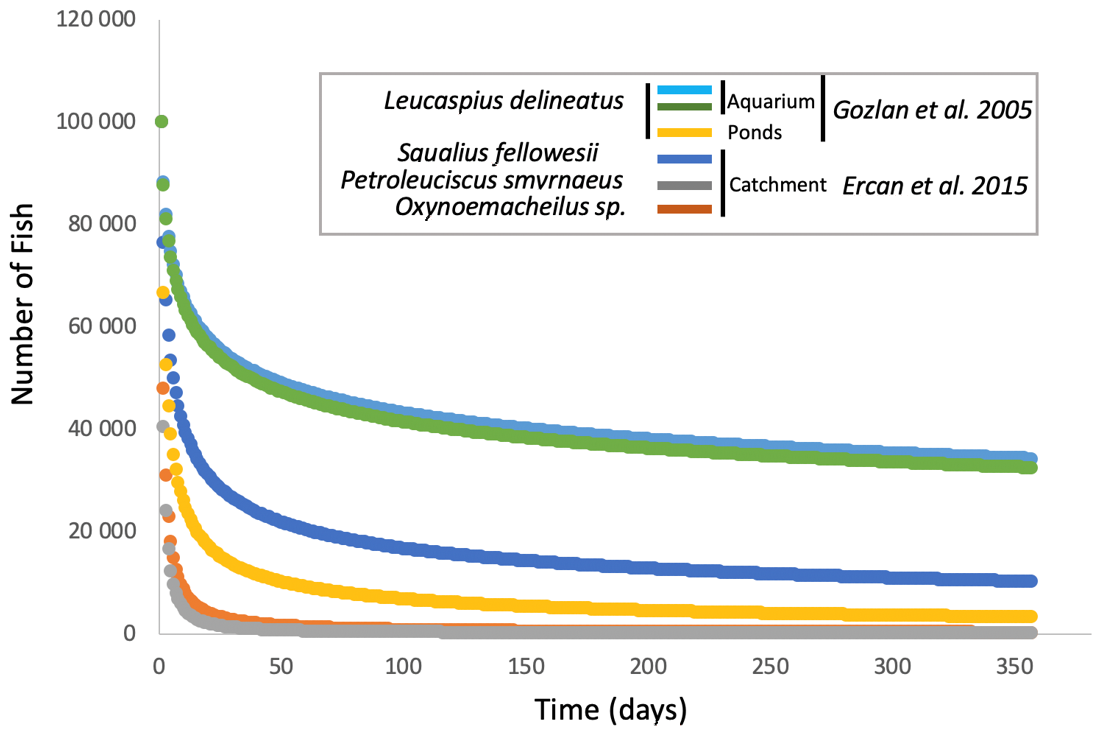
The emergence of
S. destruens in the different species tested, whether in aquariums, ponds, or at the level of a catchment area, shows a very similar mortality curve for susceptible species with the majority of mortalities occurring in the first two months and then spreading out over time towards a spiral of extinction [
27,
32,
39]. However, from what studies have been able to show ([
27,
39,
41],
Figure 2), in an artificial environment, mortality in the first month averages around 47% and reaches 54% in the second month, whereas in a natural environment or one subject to natural conditions, such as a pond, mortality averages 89% in the first month and climbs to 92% after two months (
Figure 2 [
32]). Therefore, there is probably an environmental effect on the kinetics of infection-related mortalities due to
S. destruens. Andreou et al. had shown the impact of temperature on the production and longevity of rosette agent zoospores, with zoospore production being more spread out at low temperatures (i.e., 4 °C) and faster at high temperatures (i.e., 30 °C). However, the number of zoospores produced remains the same overall, regardless of temperature. Additionally, in the natural environment, the physiological state of the hosts and their level of stress, which has an indirect impact on the efficiency of the immune system, can also explain these differences in the mortality curves (
Figure 2). Indeed, aquarium fish are fed ad libitum with little competition for resources and no risk of predation. However, we must bear in mind that the impact of the agent on a population of a sensitive species may eventually lead to a total decline in the population, as has been the case, for example, in salmon aquaculture in the USA, where
O. tshawytscha and
S. salar stock has been decimated, on the
L. delineatus in semi-natural basins [
27], in reservoirs with the disappearance of rudds following the introduction of the agent
S. destruens [
22], or even on a catchment scale [
32]. It is therefore extremely important, after the introduction of the healthy carrier
P. parva into an aquatic system, to test to confirm the presence of
S. destruens and to quickly take the necessary isolation measures in order to limit the extent of mortality that is induced to other populations. At the same time, some mortalities, such as those in USA aquaculture that were not related to the introduction of the healthy carrier reached mortality rates close to those related to the introduction of
P. parva, with the major difference being that the agent rapidly disappeared from these systems, which is the opposite of what happened when the healthy carrier was present since healthy carriers also allow for the persistence of the parasite in the system (role of reservoir). This indicates the crucial indirect role played by
P. parva on
S. destruens-related mortalities.
1.4. S. destruens Ultrastructure
In all histological analyses,
S. destruens spores appeared pink to red when stained with eosin (staining for basic and acidophilic proteins within and between cells), PAS-positive (Periodic Acid Schiff), argyrophilic (Warthin–Starry and Grocott’s), and basophilic (Giemsa staining), but not acid-fast (Ziehl–Neelsen) [
26].
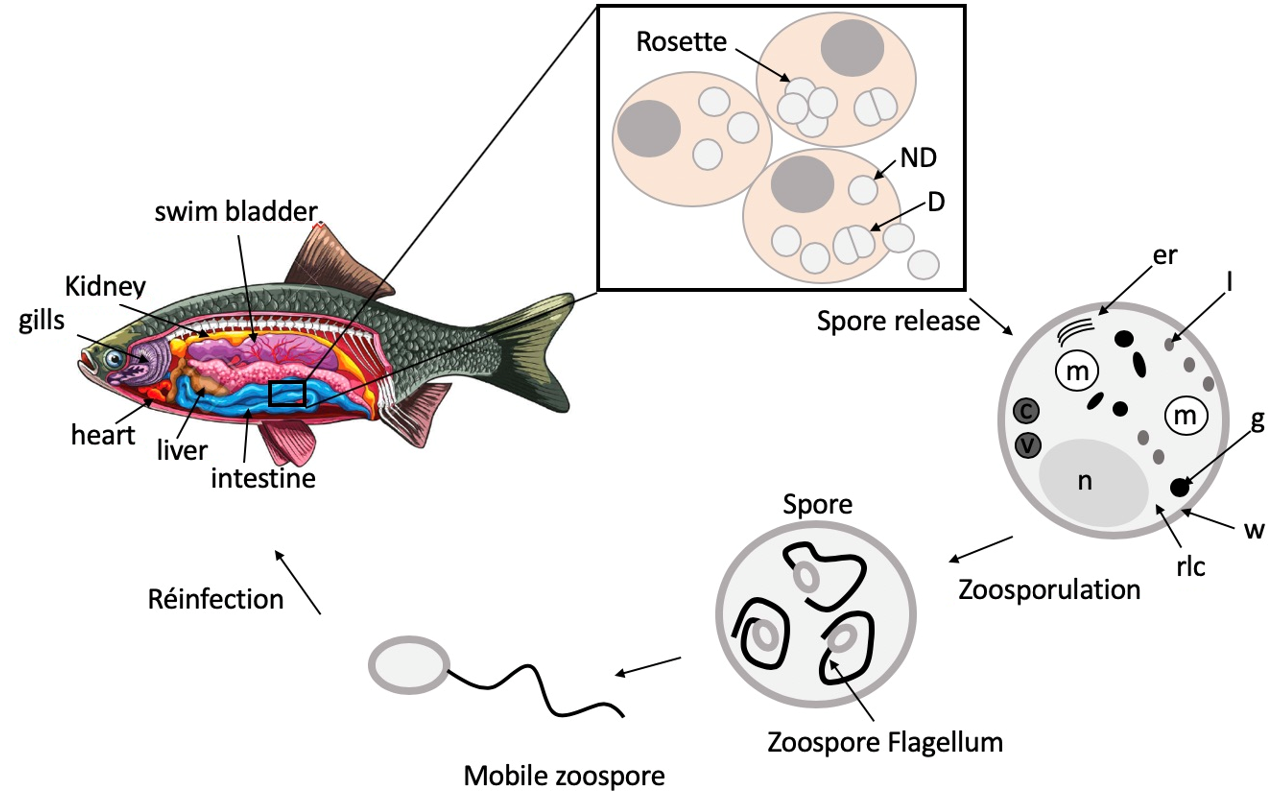
S. destruens spores are composed of a well delineated trilaminar cell wall which is coated by a dense fibrogranular layer that forms the partitions between dividing daughter cells [
25]. The
S. destruens cell wall is separated from the host cell cytoplasm by an intermediate amorphous region and an electron-dense layer with another membrane originating from the host cell [
45], potentially suggesting that
S. destruens enters the host cell by endocytosis or phagocytosis. Spores contain ribosome-laden cytoplasm with scattered segments of rough endoplasmic reticulum, numerous vesicular mitochondria, and a single nucleus (
Figure 3). Numerous vacuoles sometimes containing concentric bodies, electron-dense bodies ranging in shape from spheres to rods-like, and Gram-positive lipid droplets were also present in the cytoplasm [
25,
26,
45]. These intracytoplasmic materials and spore structures were observed for both stages, i.e., for dividing and non-dividing RA spores [
26]. Furthermore, these ultrastructural features of
S. destruens spores appear to be similar in salmonids and cyprinids, such as
S. salar [
25], winter-run
O. tshawytscha [
23,
24,
26], and
L. delineatus [
45].
Histopathology is associated with
S. destruens infection. Although Harrell et al. [
23] were the first to describe
S. destruens disease in
O. tshawytscha, Elston et al. [
24] were the first to isolate
S. destruens (using CHSE-214 cell line) from infected fish and validated the realization of Koch’s postulates. At that time, they were able to describe the intracellular localization of
S. destruens in macrophages and endothelial cells and confirmed the intracellular nature of the parasite by electron microscopy. They also observed focal areas of
S. destruens proliferation in the spleen and kidney with necrosis of adjacent tissues [
24], suggesting that intracellular replication of
S. destruens ultimately leads to host cell death [
26]. Internal examination of
S. salar revealed widely disseminated nodules in the kidney, spleen, liver, and gonads, with splenic and hepatic lesions characterized by granulomas surrounded by multiple layers of fibroblastic cells and macrophages containing numerous parasites [
25]. Although
S. destruens spores were mainly observed within macrophages (and to a lesser extent within host cells), cell-free
S. destruens spores have also been reported [
25]. Signs of disease in moribund fish were unremarkable, with the exception of some fish with advanced infection that were slightly emaciated, but did not appear to be anemic, as evidenced by the absence of gill and blood pallor despite the involvement of hematopoietic tissues [
25].
These preliminary histopathological examinations of infected tissues from naturally infected North American salmonids were later confirmed in captive broodstock of Sacramento River winter-run
O. tshawytscha [
26] and experimentally infected
S. salar [
46]. The authors reported two different forms of microscopy lesions in infected fish: (i) a nodular (focal) form of the disease characterized by distinct multifocal granulomas that replaced the normal parenchyma in the kidney, liver, and spleen. The granulomas were well delineated from the normal parenchyma and were characterized by central cores of necrotic material or closely apposed macrophages. Thin rims of fibroblasts sometimes surrounded the granulomas, while aggregates of
S. destruens spores were often seen in the central areas and in or between the macrophages of the granulomas. This nodular form, characteristic of fish with an immune response and therefore able to contain infections, was mainly observed in visceral organs such as the liver, kidney, and spleen, but also in the heart, the mesentery surrounding the intestinal tract, and between the pyloric cecae [
26]; (ii) a disseminated form of the disease with widely dispersed
S. destruens spores in various organs and cells, including kidney, liver, spleen, heart, gills, brain, ovary, testes, and hindgut, as well as hematopoietic, epithelial, and mesenchymal cells [
26,
46]. This form was characteristic of fish more susceptible to
S. destruens with the absence or low expression of host cell immune responses [
25,
26].
S. destruens spores were observed both in intra- and extracellular locations in tissues and as single rosettes or as aggregates of 4–5 rosettes. Disseminated infections included enlargement and pallor of the liver, kidney, and spleen and showed little macrophages or fibroblast proliferation around the lesions, while areas of oedema and focal necrosis were present in close proximity to
S. destruens spore proliferation [
26]. In the disseminated form of
S. destruens disease, kidney tissues were characterized by necrosis, loss of tubules, membranous glomerulonephritis, and necrotizing intestinal nephritis. Single or rosette structures of
S. destruens spores were observed in the cytoplasm of the biliary epithelium and renal tubules and in the lumen of bile ducts, suggesting that bile and urine might be routes of
S. destruens excretion. Necrosis of the renal tubular epithelium and multifocal hepatocellular necrosis were also reported. Spleen tissues contained numerous
S. destruens spores, single or in aggregates, in the pulp spaces of the spleen and in the cytoplasm of sinusoidal macrophages and reticuloendothelial cells. Rosettes of
S. destruens were found in the lumina and tunicae media of splenic arterioles where they were accompanied by segmental necrotizing vasculitis. In early infections (subadult fish),
S. destruens was sometimes observed in the gill vessels, while in advanced infections (adult fish), it was often found in subserosal aggregates in the swimbladder, mainly in macrophages, regardless of the type of lesions. It has sometimes been reported that
S. destruens was found in the epidermis, urine, seminal and ovarian fluids, and in the mucosa of the intestine, suggesting that the gut epithelium, skin, and gills could represent a second route of
S. destruens excretion ([
26]
Table 1).
The disease and pathology observed in wild populations of infected
L. delineatus (
Cyprinidae) in the UK (Stoneham Lakes) were similar to those reported in
O. tshawytscha from the USA, although some authors found slight differences, such as the presence of
S. destruens spores in giant cells and the observation of only the smallest (2–4 µm) spore morphology [
28,
45,
46]. The disseminated form of the disease was most frequently observed in infected
L. delineatus (80% of infected fish) [
28,
45]. Numerous stages of RA spores were observed with sizes ranging from 2–4 µm in diameter and most stages were intracellular. As in salmonids,
S. destruens infection induced hepatocellular necrosis [
28] and an inflammatory response in the testes and the liver, involving an influx of phagocytic cells with some lymphocytic infiltration of the liver parenchyma and necrosis [
45]. In ocular tissues,
S. destruens spores have been found in macrophages and giant cell formation has been reported. Various stages of granulomas have been described, ranging from enlarged macrophage aggregates surrounded by a single-cell layer of connective tissues to well demarcated lesions surrounded by a thick fibroblast layer [
45].
1.5. Comparisons with Other Closely Related or Fungal Parasites
Although members of the order Dermocystida share the ability to cause infections in animals, their phenotypic characteristics and the diseases they cause are very different [
11]. Their life cycles are not well known and evidence of sexual development has not yet been described [
11]. Members of the genus
Dermocystidium develop numerous spores 5–8 µm in diameter contained in cysts (a sac-like pocket) that average 0.5–1.1 mm in size and are localized between the epithelial (extracellular) tissues of the fish host without the expression of a host inflammatory response [
42]. In
Dermocystidium salmonis, once the spores mature, they differentiate into multiple flagellated zoospores that are about 1 µm in diameter, which are released from the cyst and are then able to reinfect the gill epithelium of a new fish host [
42].
Dermocystidium cyprini is also known to have a flagellated zoospore stage in its life cycle [
47]. The existence of a zoospore stage in other Dermocystidium species has not been reported. Interestingly, the ultrastructure of
S. destruens has been found to be quite similar to
Dermocystidium macrophagi infections in rainbow trout
Oncorhynchus mykiss [
48] and
Dermocystidium sp. described from
S. trutta and
S. salar cultured in Ireland [
49], although the “signet ring” appearance of a prominent vacuole previously described for
Dermocystidium sp. [
50] has not been observed in
S. destruens spores infecting winter-run
O. tshawytscha [
11] or
S. salar [
25]. Members of the genus
Rhinosporidium have a different life cycle to the genus
Dermocystidium and
S. destruens in that they have mature spherical sporangia (the enclosure in which spores are produced asexually) between 40 and 400 µm in diameter that release infective spores through a pore. The released spores then increase in size until they become mature sporangia containing hundreds of spores and the cycle begins again. Members of the genus
Rhinosporidium cause disease in humans, dogs, cattle, horses, and swans that is characterized by the formation of polyps, usually on the mucous membranes of the nose or nostrils, eyes, and mouth, with a chronic granulomatous inflammatory response consisting of mononuclear cells, polymorphic nuclear cells, and, in some cases, giant cells [
51]. Therefore, infection by
S. destruens differs from that of members of
Dermocystidium and
Rhinosporidium since the spore stages are found as intracellular parasites of host cells, infecting and replicating mainly in visceral organs where they cause a chronic granulomatous disease and only produce zoospores when released into freshwater [
26].
When compared with more phylogenetically distant fungal pathogens, such as the well known
Batrachochytrium dendrobatidis that causes chytridiomycosis in amphibians, the life cycle and mechanisms of the pathogenicity of
S. destruens appear to be very different, although they exhibit intracellular parasitism.
Batrachochytrium dendrobatidis compromises epidermal tissues of bullfrogs by colonizing keratin-containing cells [
2]. Pathological signs of chytridiomycosis include accumulation and erosion of corneal cells (epithelial and endothelial cells, keratocytes), swelling of the epidermis, damaged nuclei, and altered cytoplasm [
2]. Death of the animal is caused by inhibition of electrolyte transport across the epidermis, followed by disruption of cardiac electrical activity [
52]. The asexual life cycle of this fungus consists of a motile zoospore stage and a stationary thallus stage [
53]. The zoospores are flagellated and are not bound by a cell wall. Once exposed to a frog host, a zoospore encysts (attaches itself, retracts its flagellum, and forms a chitin wall around the spore body) on the host skin surface and produces a germination tube that penetrates the host epidermis. The content of the cyst then migrates into the host tissue via this tube and a single or colonial zoosporangium. A septum forms to separate the zoosporangium from the tube and maturation of the zoosporangium results in the cleavage of the zoosporangial contents into zoospores, which are then released from the host cell. Infected cells show, among other things, displacement of host organelles and clear zones around the zoosporangia [
2]. Although these examples are not exhaustive, they suggest that the life cycle of
S. destruens and the mechanisms of pathogenicity in infected fish hosts are specific to this parasite and also highlight the need for further study of its mechanisms of entry into cells, its modes of replication in infected cells, and the direct infectivity of zoospores.
1.6. All Species and Ontogenetic Stages Are Not Equally Susceptible to S. destruens
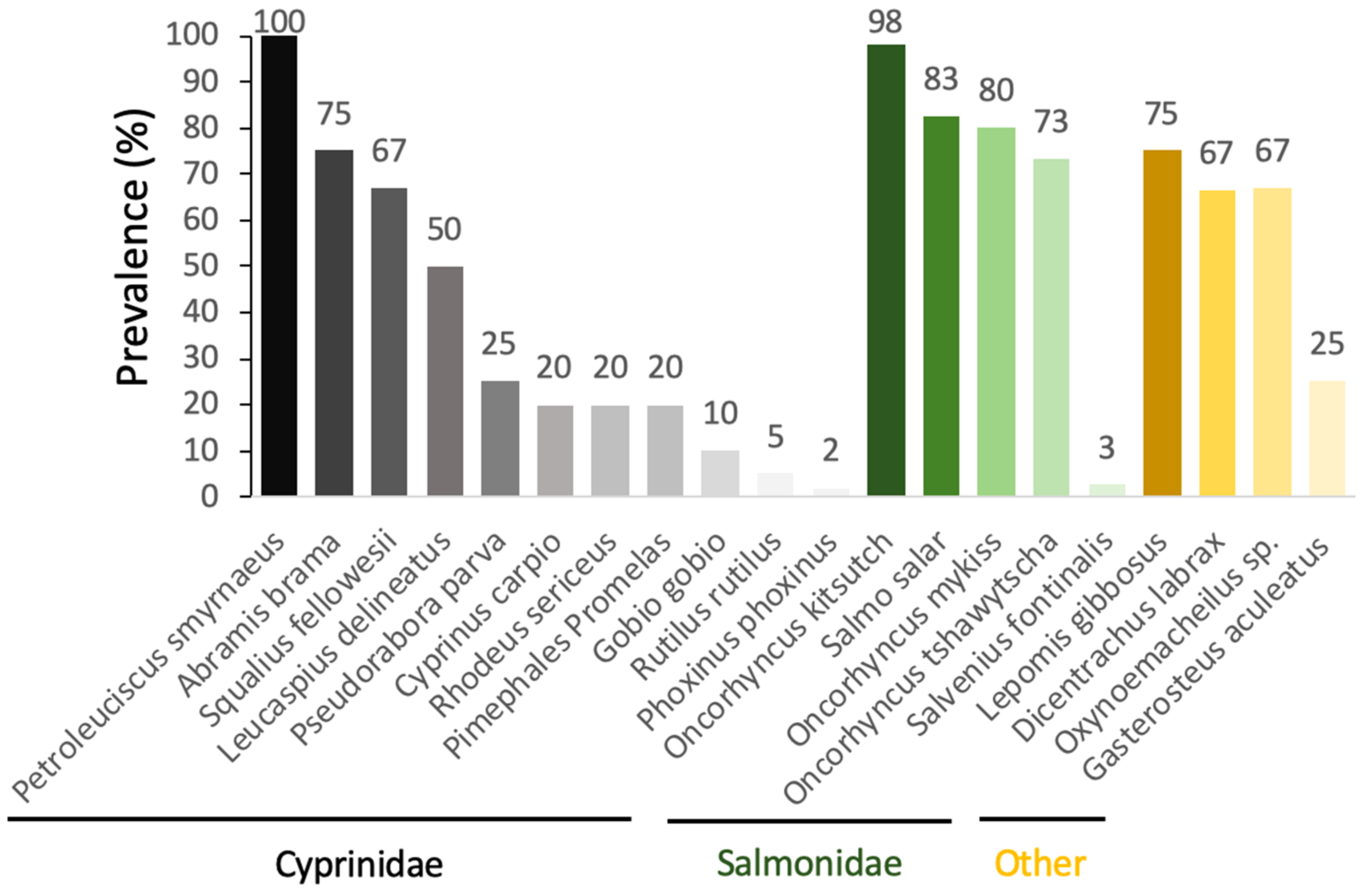
When looking at the prevalence of
S. destruens in populations of different susceptible host species, there is a large variability between species. Prevalence is measured here by the number of PCR-positive individuals with the rosette agent in all the fish tested within a population. We therefore observe prevalences that fluctuate from 100% to 2% depending on the species tested in the cyprinidae family, and from 98% to 3% in salmonids (
Figure 4).
Using the RA-3 isolate, Arkush et al. [
26] experimentally tested the susceptibility of a variety of juvenile salmonids. While their experimental infections led to a clinically identical disease to that previously described by Harrell et al. [
23] in
O. tshawytsch from Washington (USA), the authors found a trend towards host specificity, with
O. tshawytsch being the most susceptible species to
S. destruens, followed by coho salmon
Oncorhynchus kisutch, rainbow trout
Oncorhynchus mykiss, brown trout
Salmo trutta, and brook trout
Salvelinus fontinalis, the latter showing resistance to infection by
S. destruens [
26]. In addition, Gozlan et al. [
27,
28] conducted cohabitation experiments in both natural ponds and laboratory conditions using
P. parva captured from wild pond populations in Hampshire, UK, originally introduced in 1985 from German Danube populations. Their results showed up to 69% mortality of
L. delineatus under laboratory conditions and up to 96% mortality of fish in natural ponds. By implementing the first PCR-based DNA detection tool targeting a small fragment of ribosomal DNA (18S rRNA gene) [
27] and then the Internal Transcribed Spacer 1 (ITS-1) [
28] of
S. destruens, they were able to estimate the prevalence of the disease to be 67% in
L. delineatus and 20% in the fathead minnow
P. promelas. Conventional bacteriological, virological, parasitological, and histological examinations were carried out on moribund
L. delineatus fish and showed extensive infections of visceral organs by an intracellular parasite with characteristics similar to those of the previously described rosette agent in
O. tshawytscha [
23,
26] and
S. salar [
25]. Spikmans et al. [
30,
31] found that
P. parva populations sampled in the Meuse floodplain were infected with a disease prevalence of up to 67% at the Everlose Beek site and 74% and 25% for the Teelebeek site. In addition to the invasive
P. parva populations, a disease prevalence of 25% was found in the native
Gasterosteus aculeatus (
Gasterosteidae). In Turkey, in the Sariçay stream in Mugla,
S. destruens was found by PCR in
Oxynoemacheilus sp. (
Nemacheilidae, prevalence 67%),
P. smyrnaeus (prevalence 100%),
S. fellowesii (prevalence 67%),
D. labrax (
Moronidae, prevalence 33–100%), and
L. gibbosus (
Percidae, prevalence 75%). Based on these results, Ercan et al. [
32] highlighted the potential role of the emergence of
S. destruens in the extinction events observed in Turkey since 2009 in
Oxynoemacheilus sp.,
P. smyrnaeus, and
S. fellowesii. Finally, in France,
S. destruens was detected in populations of
P. parva (prevalence 2–4%), bleak
Alburnus alburnus (prevalence 9%), European bittern
Rhodeus amarus (prevalence 20%), roach
Rutilus rutilus (prevalence 4%), gudgeon
Gobio gobio (prevalence 10%), and minnow
Phoxinus phoxinus (prevalence 2%) [
22]. The
P. parva populations had a low parasite prevalence and did not develop the disease.
It is therefore very important in an infectious risk analysis following the arrival of a healthy
P. parva carrier to take into account the species present in the community. However, one hundred percent of the species tested or analyzed for mortalities related to
S. destruens were found to be susceptible to this infectious agent. This also applies to percids, such as pumkindseed
L. gibbosus, or even marine species, such as sea bass
D. labrax, as studies have shown that
S. destruens spores survive very well in both salt and freshwater [
11]. In view of the host spectrum within this new group of emerging infectious agents (i.e.,
Dermocystida), it would be useful to test the susceptibility of amphibians and invertebrates, such as certain crayfish, to the rosette agent to see if we are indeed dealing with an infectious agent of fish. Indeed, the pathogenic fungi of this group affect fish as well as amphibians and humans [
16,
17,
19] and therefore, the generalist aspect of
S. destruens should be tested beyond the simple fish group.
It is also important to note that host species with short life spans and rapid reproductive systems such as
L. delineatus,
R. amarus, or even
L. gibbosus that depend on high reproduction to maintain populations will show a more visible decline more quickly. In effect, the emergence and high mortalities of
S. destruens in
O. tshawytscha stocks have occurred in juvenile or smolting salmons and not in brood stocks [
26]. In contrast,
S. destruens was found in 30% of returning salmon, showing that they survived infection (asymptomatic infections, [
11]), which would suggest that species or stages with larger body mass hosts would be more resistant to infection in the short term.
1.7. What Is the Potential Economic Impact of the Emergence of S. destruens?
As mentioned, the first emergences of
S. destruens were observed in salmon farms in the USA with losses of up to 98% of juvenile salmon stocks. In such a situation, assessment of the economic cost is fairly straightforward as each fish has an economic value defined by aquaculture production in a specific national context. Where the economic impact is more complicated to assess is in the context of wild populations. In their recent assessments of the economic costs of invasive species, Diagne et al. [
54] show that the costs of introducing new pathogens are often assessed indirectly, either through the management costs of susceptible species or through the costs of eradicating the host. However, there is still very little data on the estimated costs of infectious agents per se and none on
S. destruens. However, environmental agencies in general assess that the primary risk of impact from the introduction of
P. parva on native fish populations is essentially the infectious risk from the emergence of
S. destruens. Based on the data on the emergence of
S. destruens in the absence of the healthy carrier
P. parva, it is reasonable to assume that the risk posed by
S. destruens would no longer be endemic, as has occurred in the USA. In this context, England has been a pioneer in managing this risk with the establishment of a program focused on rapid detection and eradication of populations of
P. parva carrying
S. destruens [
55]. Britton et al. [
56] accurately estimated the cost of a rotenone-based eradication and containment strategy for the management of
P. parva. The cost of eradicating a population of
P. parva averaged €80k over ten years, with 90% of the costs being for the first year of eradication and the remaining 10% being residual costs related to post-eradication monitoring. On this basis, which remains approximate but nonetheless quantitative and specific to the eradication program for populations of the healthy carrier
P. parva, it is therefore possible to evaluate the economic cost of controlling
S. destruens throughout the European continent based on the number of
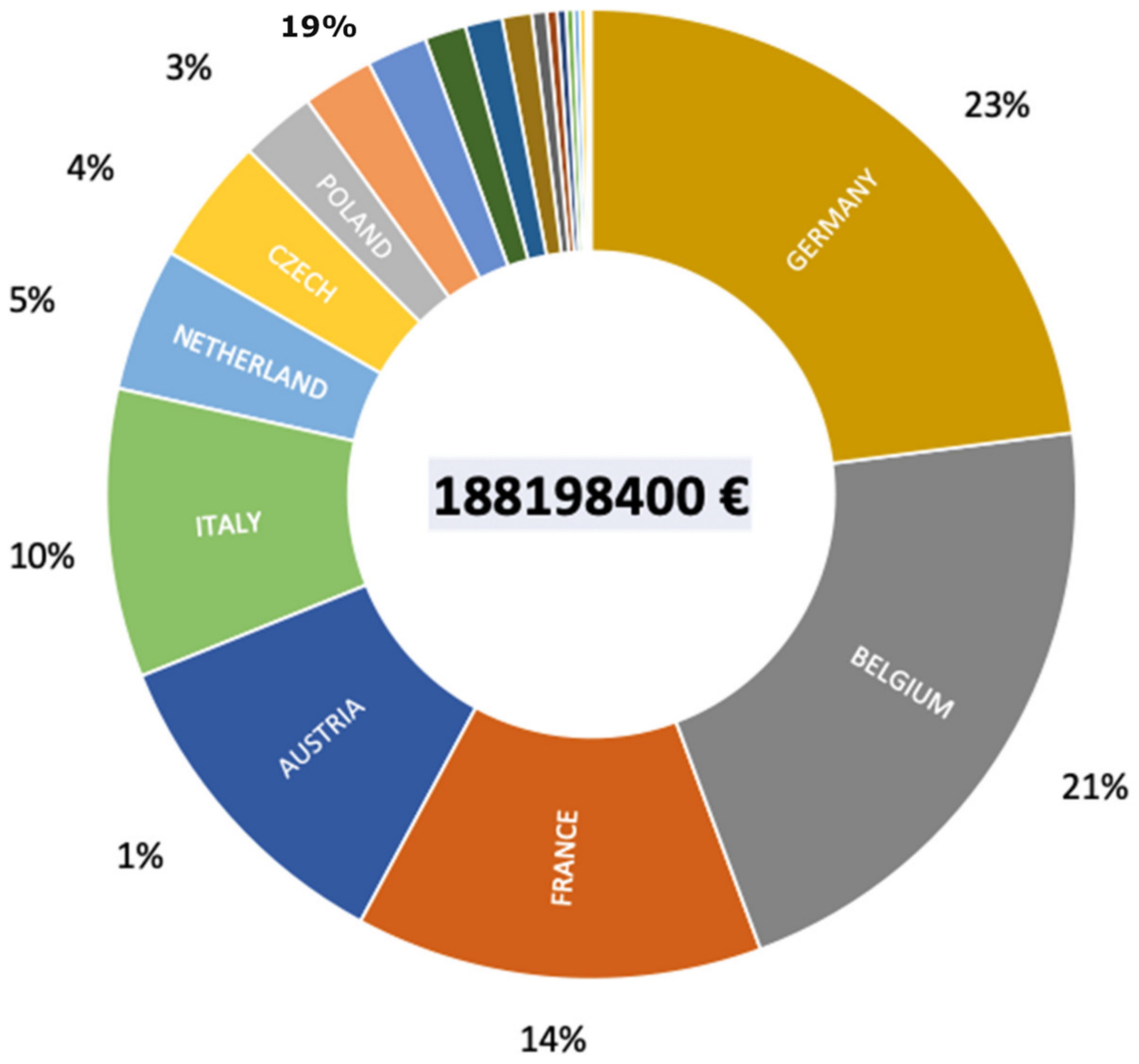
P. parva populations present in each country. This gives an average cost per country of just over 85M€, with a minimum cost of about 92k€ for Bosnia and Herzegovina and a maximum cost of about 43M€ for Germany. The total cost of eradicating the healthy carrier of
S. destruens in Europe would be around 188M€ (
Figure 5). Thus, it is quickly realized that, from an economic point of view, the introduction of
S. destruens into an ecosystem becomes very quickly prohibitive. The total cost of eradicating the healthy carrier in England was around €15 million as angling in that country represents a huge economic market; larger than the football Premier League, to put it mildly. Each country has its own situation and a cost-benefit analysis must be carried out before any eradication program can be implemented. Above all, we must learn from this case study that eradication is the last option and that other solutions for managing an
S. destruens epidemic are possible.
1.9. Recommendations
At this stage of our knowledge, it is important to take the infectious risk linked to S. destruens very seriously. All studies carried out to date point to rapid declines in fish populations following the introduction of S. destruens into fish farming communities. The first recommendation is the isolation of P. parva populations by controlling the transfer of fish from infected areas to non-infected areas. S. destruens can be transferred by free spores in freshwater, by transport of infected native fish, and also by transfer of infected P. parva. Of these three modes of contamination, the last is the most serious as it allows, in addition to introduction of the pathogen, the introduction of the healthy carrier which will serve as a reservoir for S. destruens and thus maintain a high level of virulence. These controls must be carried out for any transfer of fish to an area where P. parva is not yet present. There have been studies in England undertaken by the Environment Agency which tested the effectiveness of stocking controls and associated procedures against stocking levels of P. parva contamination ranging from 1, 5, 10, and 20% and the level of expertise of the auditors ranging from expert, intermediate, and novice. They showed that, with contamination rates of 10%, the probability of detection of P. parva by a trained team was above an 80% probability of detection. Such a study could be adapted to the French stocking management system.
The second recommendation is public risk communication. Indeed, the challenge of this approach is to include the maximum number of actors in the sector in this fight. This includes professional fish farmers, pond owners, and amateur fishermen. The greater the number of people informed of the risks linked to the introduction of P. parva and S. destruens, the more the authorities responsible for environmental protection will have allies in the field who will be involved in this fight. This will be done through communications within fishing federations, letters to fishing license holders, posters in fishing shops, and communications to fish farmers and other professionals in the sector. The use of social networks as a new means of communication is also highly recommended. The more information is shared, the more responsibility there will be on the part of the various actors. It is obvious that this will not be enough, but as many studies have shown, the alternative of not effectively communicating slows down the fight against biological invasions, such as P. parva. This recommendation must be part of a national coordination and implementation of a common strategy for the whole territory.
The third recommendation is to analyze the virulence of the strains found. Indeed, it is very likely that the strains found in a country are the result of their introduction history and are therefore not the same from a genetic point of view. More importantly, the virulence of these strains in relation to populations of native species is likely to be highly variable. The cultivation of these different strains would therefore be an important first step in testing their virulence. Their inoculation on different cell lines (e.g., Salmonidae, Cyprinidae) would be a simple and inexpensive indicator of virulence.
The fourth recommendation is the targeted eradication of P. parva populations. This is the option that was chosen by the UK government in 2005, which, through the use of rotenone on sites hosting P. parva populations, reduced the number of P. parva populations in the country from 37 to six in 2014 and targeted total eradication by 2017. According to the latest reports, they should have achieved their target. It is an expensive program but given the economic importance of freshwater recreational fishing in the UK, it has received the necessary budgetary support. In any country, a limited and targeted approach to the most economically or conservationally sensitive sites could therefore be an option for concerned organizations. A derogation request for the use of rotenone will nevertheless be mandatory at the European level.
The fifth recommendation is the search for new tools. Indeed, new scientific tools could be developed for both the detection and eradication of P. parva populations. First of all, the use of environmental DNA to monitor the presence of invasive species in a body of water or in a catchment area is already being studied in other countries. By sampling water, a list of present species can be established and thus allow for the routine and low-cost monitoring of communities and, above all, the rapid identification of the arrival of an invasive species. This would allow for early detection and eradication at the very beginning of invasion with a much greater chance of success and also, a reduction in the costs of eradication. Other tools such as the introduction of P. parva genetically modified to produce only males could be considered, at least in lakes or ponds. This kind of approach has already been initiated in Australia on carp populations and in Africa on mosquito populations. It would also be possible to work on similar approaches for S. destruens by introducing a deleterious gene with more limited ethical issues and environmental risks.
In summary, firstly, there is a priority in the implementation of recommendations and in the isolation of P. parva populations to minimize the risk of transfer from one body of water to another. Secondly, discussions will have to take place to evaluate the infectious risk linked to S. destruens in a wider context of other infectious risks on wild and farmed fish populations (i.e., inclusion on the OIE’s aquatic animal health code). We would therefore like to note that S. destruens is not specific to one species, but it always leads to mortality in a whole range of species and families of fish including Cyprinidae, Salmonidae, Percidae, and marine species, such as sea bass. We would also emphasize that it is the presence of the healthy carrier that maintains the high level of virulence.