Mucorales and Mucormycosis: Recent Insights and Future Prospects
Abstract
1. Introduction
2. Genes Involved in the Pathogenic Potential of Mucorales
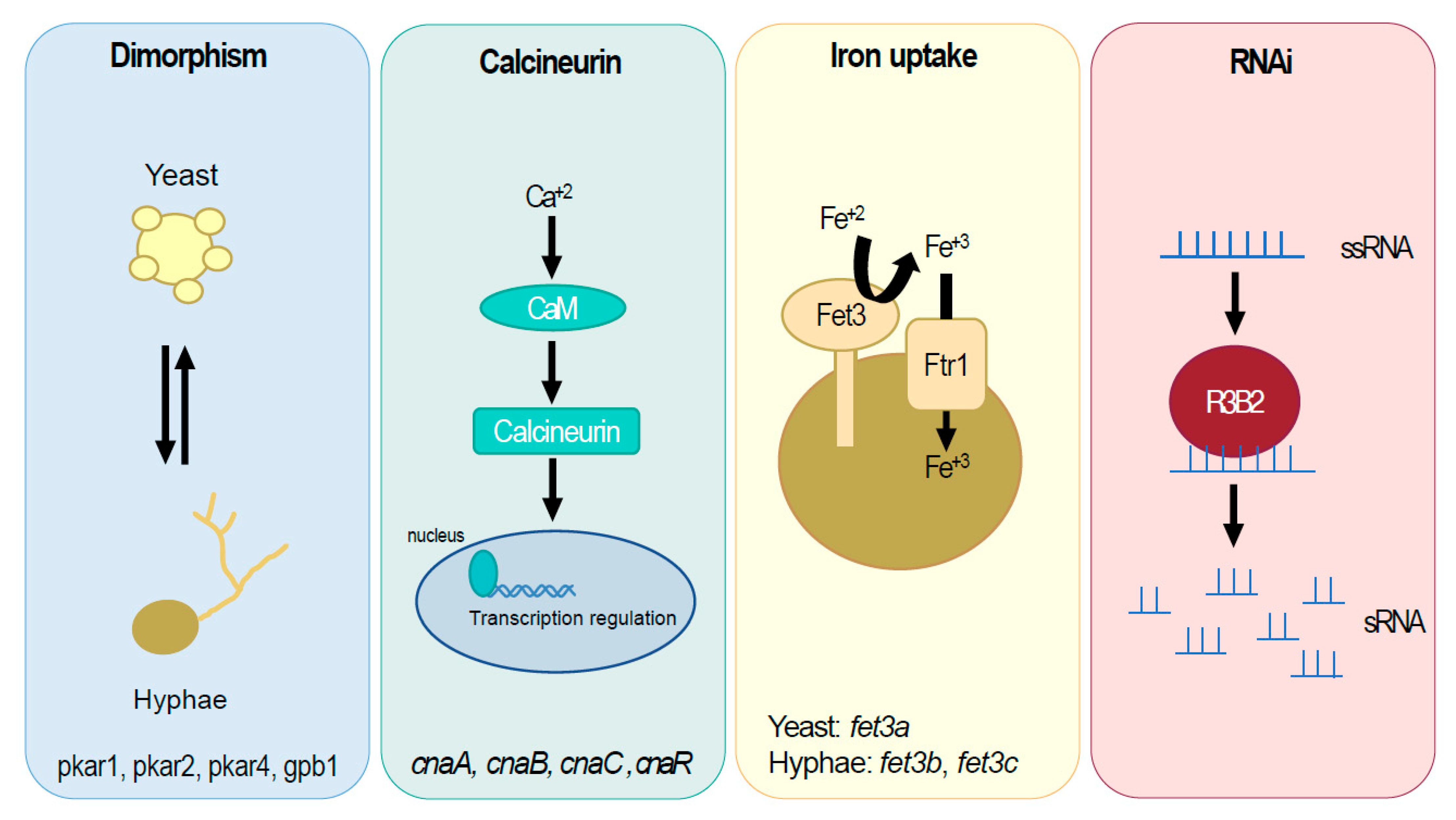
2.1. The High-Affinity Iron Uptake System in Mucorales
2.2. Azole Resistance in Mucorales: The Cytochrome P450
2.3. The cotH Gene Family, an Important Source of Virulence Factors in Mucorales
2.4. Genomic Approaches to Identify New Virulence-Related Genes
3. Gene Pathways with Pleiotropic Effects on the Pathogenic Potential of Mucorales
3.1. Dimorphism Controls Virulence in M. lusitanicus
3.2. The Epimutant RNAi Pathway and Its Role in Antifungal Resistance
3.3. The Non-Canonical RNAi Pathway and Its Role in the Pathogenic Potential of Mucorales
4. Genetic Manipulation in Mucorales and New Study Models
4.1. In Vitro Models to Study the Host–Pathogen Interaction
4.2. M. lusitanicus, the Traditional Model for Genetic Manipulation in Mucorales
Homologous Recombination and Related Genetic Tools in M. lusitanicus
4.3. Rhizopus Microsporus, the First High-Virulent Mucoral Available for Genetic Analysis in Mucorales
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dannaoui, E. Antifungal resistance in Mucorales. Int. J. Antimicrob. Agents 2017, 50, 617–621. [Google Scholar] [CrossRef]
- Petrikkos, G.; Skiada, A.; Lortholary, O.; Roilides, E.; Walsh, T.J.; Kontoyiannis, D.P. Epidemiology and Clinical Manifestations of Mucormycosis. Clin. Infect. Dis. 2012, 54, S23–S34. [Google Scholar] [CrossRef]
- García-Carnero, L.C.; Mora-Montes, H.M. Mucormycosis and COVID-19-Associated Mucormycosis: Insights of a Deadly but Neglected Mycosis. J. Fungi 2022, 8, 445. [Google Scholar] [CrossRef]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Kong, D.C.M.; Chen, S.C.-A. The epidemiology and clinical manifestations of mucormycosis: A systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019, 25, 26–34. [Google Scholar] [CrossRef]
- Hassan, M.I.A.; Voigt, K. Pathogenicity patterns of mucormycosis: Epidemiology, interaction with immune cells and virulence factors. Med. Mycol. 2019, 57, S245–S256. [Google Scholar] [CrossRef]
- Sridhara, S.R.; Paragache, G.; Panda, N.K.; Chakrabarti, A. Mucormycosis in immunocompetent individuals: An increasing trend. J. Otolaryngol. 2005, 34, 402–406. [Google Scholar] [CrossRef]
- Gutiérrez, A.; López-García, S.; Garre, V. High reliability transformation of the basal fungus Mucor circinelloides by electroporation. J. Microbiol. Methods 2011, 84, 442–446. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, Z.; Du, G.; Zhou, J.; Chen, J. Efficient transformation of Rhizopus delemar by electroporation of germinated spores. J. Microbiol. Methods 2014, 103, 58–63. [Google Scholar] [CrossRef]
- Lax, C.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; Navarro, E.; Nicolás, F.E.; Garre, V. Stable and reproducible homologous recombination enables CRISPR-based engineering in the fungus Rhizopus microsporus. Cell Rep. Methods 2021, 1, 100124. [Google Scholar] [CrossRef]
- Calo, S.; Shertz-Wall, C.; Lee, S.C.; Bastidas, R.J.; Nicolás, F.E.; Granek, J.A.; Mieczkowski, P.; Torres-Martínez, S.; Ruiz-Vázquez, R.M.; Cardenas, M.E.; et al. Antifungal drug resistance evoked via RNAi-dependent epimutations. Nature 2014, 513, 555–558. [Google Scholar] [CrossRef]
- Bullen, J.J. Natural resistance, iron and infection: A challenge for clinical medicine. J. Med. Microbiol. 2006, 55, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, T.; Lin, L.; Liu, M.; Kontoyiannis, D.P.; French, S.; Edwards, J.E.; Filler, S.G.; Ibrahim, A.S. Bicarbonate correction of ketoacidosis alters host-pathogen interactions and alleviates mucormycosis. J. Clin. Investig. 2016, 126, 2280–2294. [Google Scholar] [CrossRef]
- Alejandre-Castañeda, V.; Patiño-Medina, J.A.; Valle-Maldonado, M.I.; Nuñez-Anita, R.E.; Santoyo, G.; Castro-Cerritos, K.V.; Ortiz-Alvarado, R.; Corrales-Escobosa, A.R.; Ramírez-Díaz, M.I.; Gutiérrez-Corona, J.F.; et al. Secretion of the siderophore rhizoferrin is regulated by the cAMP-PKA pathway and is involved in the virulence of Mucor lusitanicus. Sci. Rep. 2022, 12, 10649. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.S.; Grieve, C.L.; Murugathasan, I.; Bennet, A.J.; Czekster, C.M.; Liu, H.; Naismith, J.; Moore, M.M. The rhizoferrin biosynthetic gene in the fungal pathogen Rhizopus delemar is a novel member of the NIS gene family. Int. J. Biochem. Cell Biol. 2017, 89, 136–146. [Google Scholar] [CrossRef]
- Navarro-Mendoza, M.I.; Pérez-Arques, C.; Murcia, L.; Martínez-García, P.; Lax, C.; Sanchis, M.; Capilla, J.; Nicolás, F.E.; Garre, V. Components of a new gene family of ferroxidases involved in virulence are functionally specialized in fungal dimorphism. Sci. Rep. 2018, 8, 7660. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Gebremariam, T.; Lin, L.; Luo, G.; Husseiny, M.I.; Skory, C.D.; Fu, Y.; French, S.W.; Edwards, J.E., Jr.; Spellberg, B. The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis. Mol. Microbiol. 2010, 77, 587–604. [Google Scholar] [CrossRef]
- Schwartze, V.U.; Winter, S.; Shelest, E.; Marcet-Houben, M.; Horn, F.; Wehner, S.; Linde, J.; Valiante, V.; Sammeth, M.; Riege, K.; et al. Gene Expansion Shapes Genome Architecture in the Human Pathogen Lichtheimia corymbifera: An Evolutionary Genomics Analysis in the Ancient Terrestrial Mucorales (Mucoromycotina). PLoS Genet. 2014, 10, e1004496. [Google Scholar] [CrossRef]
- Shirazi, F.; Kontoyiannis, D.P.; Ibrahim, A.S. Iron starvation induces apoptosis in Rhizopus oryzae in vitro. Virulence 2015, 6, 121–126. [Google Scholar] [CrossRef]
- Caramalho, R.; Tyndall, J.D.A.; Monk, B.C.; Larentis, T.; Lass-Flörl, C.; Lackner, M. Intrinsic short-tailed azole resistance in mucormycetes is due to an evolutionary conserved aminoacid substitution of the lanosterol 14α-demethylase. Sci. Rep. 2017, 7, 15898. [Google Scholar] [CrossRef]
- Szebenyi, C.; Gu, Y.; Gebremariam, T.; Kocsubé, S.; Kiss-Vetráb, S.; Jáger, O.; Patai, R.; Spisák, K.; Sinka, R.; Binder, U.; et al. cotH Genes Are Necessary for Normal Spore Formation and Virulence in Mucor lusitanicus. MBio 2023, 14, e03386-22. [Google Scholar] [CrossRef]
- Gebremariam, T.; Alkhazraji, S.; Soliman, S.S.M.; Gu, Y.; Jeon, H.H.; Zhang, L.; French, S.W.; Stevens, D.A.; Edwards, J.E.; Filler, S.G.; et al. Anti-CotH3 antibodies protect mice from mucormycosis by prevention of invasion and augmenting opsonophagocytosis. Sci. Adv. 2019, 5, eaaw1327. [Google Scholar] [CrossRef]
- López-Fernández, L.; Sanchis, M.; Navarro-Rodríguez, P.; Nicolás, F.E.; Silva-Franco, F.; Guarro, J.; Garre, V.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; Capilla, J. Understanding Mucor circinelloides pathogenesis by comparative genomics and phenotypical studies. Virulence 2018, 9, 707–720. [Google Scholar] [CrossRef]
- Pérez-Arques, C.; Navarro-Mendoza, M.I.; Murcia, L.; Navarro, E.; Garre, V.; Nicolás, F.E. The RNAi Mechanism Regulates a New Exonuclease Gene Involved in the Virulence of Mucorales. Int. J. Mol. Sci. 2021, 22, 2282. [Google Scholar] [CrossRef]
- Pérez-Arques, C.; Navarro-Mendoza, M.I.; Murcia, L.; Lax, C.; Martínez-García, P.; Heitman, J.; Nicolás, F.E.; Garre, V. Mucor circinelloides Thrives inside the Phagosome through an Atf-Mediated Germination Pathway. Mbio 2019, 10, e02765-18. [Google Scholar] [CrossRef]
- Trieu, T.A.; Navarro-Mendoza, M.I.; Perez-Arques, C.; Sanchis, M.; Capilla, J.; Navarro-Rodriguez, P.; Lopez-Fernandez, L.; Torres-Martinez, S.; Garre, V.; Ruiz-Vazquez, R.M.; et al. RNAi-Based Functional Genomics Identifies New Virulence Determinants in Mucormycosis. PLoS Pathog. 2017, 13, e1006150. [Google Scholar] [CrossRef]
- Lee, S.C.; Li, A.; Calo, S.; Heitman, J. Calcineurin plays key roles in the dimorphic transition and virulence of the human pathogenic zygomycete Mucor circinelloides. PLoS Pathog. 2013, 9, e1003625. [Google Scholar] [CrossRef]
- Lee, S.C.; Li, A.; Calo, S.; Inoue, M.; Tonthat, N.K.; Bain, J.M.; Louw, J.; Shinohara, M.L.; Erwig, L.P.; Schumacher, M.A.; et al. Calcineurin orchestrates dimorphic transitions, antifungal drug responses and host-pathogen interactions of the pathogenic mucoralean fungus Mucor circinelloides. Mol. Microbiol. 2015, 97, 844–865. [Google Scholar] [CrossRef]
- Ocampo, J.; Nuñez, L.F.; Silva, F.; Pereyra, E.; Moreno, S.; Garre, V.; Rossi, S. A subunit of protein kinase a regulates growth and differentiation in the fungus mucor circinelloides. Eukaryot. Cell 2009, 8, 933–944. [Google Scholar] [CrossRef]
- Valle-Maldonado, M.I.; Patiño-Medina, J.A.; Pérez-Arques, C.; Reyes-Mares, N.Y.; Jácome-Galarza, I.E.; Ortíz-Alvarado, R.; Vellanki, S.; Ramírez-Díaz, M.I.; Lee, S.C.; Garre, V.; et al. The heterotrimeric G-protein beta subunit Gpb1 controls hyphal growth under low oxygen conditions through the protein kinase A pathway and is essential for virulence in the fungus Mucor circinelloides. Cell Microbiol. 2020, 22, e13236. [Google Scholar] [CrossRef]
- Pérez-Arques, C.; Navarro-Mendoza, M.I.; Murcia, L.; Navarro, E.; Garre, V.; Nicolás, F.E. A non-canonical RNAi pathway controls virulence and genome stability in Mucorales. PLoS Genet. 2020, 16, e1008611. [Google Scholar] [CrossRef]
- Thieken, A.; Winkelmann, G. Rhizoferrin: A complexone type siderophore of the Mucorales and entomophthorales (Zygomycetes). FEMS Microbiol. Lett. 1992, 73, 37–41. [Google Scholar] [CrossRef]
- Haas, H. Molecular genetics of fungal siderophore biosynthesis and uptake: The role of siderophores in iron uptake and storage. Appl. Microbiol. Biotechnol. 2003, 62, 316–330. [Google Scholar] [CrossRef]
- Liu, M.; Lin, L.; Gebremariam, T.; Luo, G.; Skory, C.D.; French, S.W.; Chou, T.F.; Edwards, J.E.; Ibrahim, A.S.; Edwards, J.E., Jr.; et al. Fob1 and Fob2 Proteins Are Virulence Determinants of Rhizopus oryzae via Facilitating Iron Uptake from Ferrioxamine. PLoS Pathog. 2015, 11, e1004842. [Google Scholar] [CrossRef]
- Groll, A.H.; Gea-Banacloche, J.C.; Glasmacher, A.; Just-Nuebling, G.; Maschmeyer, G.; Walsh, T.J. Clinical pharmacology of antifungal compounds. Infect. Dis. Clin. N. Am. 2003, 17, 159–191. [Google Scholar] [CrossRef]
- Lass-Flörl, C. Triazole antifungal agents in invasive fungal infections: A comparative review. Drugs 2011, 71, 2405–2419. [Google Scholar] [CrossRef]
- Watson, P.F.; Rose, M.E.; Ellis, S.W.; England, H.; Kelly, S.L. Defective sterol C5-6 desaturation and azole resistance: A new hypothesis for the mode of action of azole antifungals. Biochem. Biophys. Res. Commun. 1989, 164, 1170–1175. [Google Scholar] [CrossRef]
- Chayakulkeeree, M.; Ghannoum, M.A.; Perfect, J.R. Zygomycosis: The re-emerging fungal infection. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 215–229. [Google Scholar] [CrossRef]
- Nishimoto, A.T.; Sharma, C.; Rogers, P.D. Molecular and genetic basis of azole antifungal resistance in the opportunistic pathogenic fungus Candida albicans. J. Antimicrob. Chemother. 2019, 75, 257–270. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Hagen, F.; Meis, J.F. Exploring azole antifungal drug resistance in Aspergillus fumigatus with special reference to resistance mechanisms. Future Microbiol. 2014, 9, 697–711. [Google Scholar] [CrossRef]
- Vitale, R.G.; De Hoog, G.S.; Schwarz, P.; Dannaoui, E.; Deng, S.; Machouart, M.; Voigt, K.; Van De Sande, W.W.J.; Dolatabadi, S.; Meis, J.F.; et al. Antifungal susceptibility and phylogeny of opportunistic members of the order Mucorales. J. Clin. Microbiol. 2012, 50, 66–75. [Google Scholar] [CrossRef]
- Valle-Maldonado, M.I.; Jácome-Galarza, I.E.; Díaz-Pérez, A.L.; Martínez-Cadena, G.; Campos-García, J.; Ramírez-Díaz, M.I.; Reyes-De la Cruz, H.; Riveros-Rosas, H.; Díaz-Pérez, C.; Meza-Carmen, V. Phylogenetic analysis of fungal heterotrimeric G protein-encoding genes and their expression during dimorphism in Mucor circinelloides. Fungal. Biol. 2015, 119, 1179–1193. [Google Scholar] [CrossRef]
- Luo, G.; Gebremariam, T.; Lee, H.; French, S.W.; Wiederhold, N.P.; Patterson, T.F.; Filler, S.G.; Ibrahim, A.S. Efficacy of liposomal amphotericin B and posaconazole in intratracheal models of murine mucormycosis. Antimicrob. Agents Chemother. 2013, 57, 3340–3347. [Google Scholar] [CrossRef] [PubMed]
- Maurer, E.; Binder, U.; Sparber, M.; Lackner, M.; Caramalho, R.; Lass-Flörl, C. Susceptibility profiles of amphotericin B and posaconazole against clinically relevant Mucorales species under hypoxic conditions. Antimicrob. Agents Chemother. 2015, 59, 1344–1346. [Google Scholar] [CrossRef] [PubMed]
- Leonardelli, F.; Macedo, D.; Dudiuk, C.; Cabeza, M.S.; Gamarra, S.; Garcia-Effron, G. Aspergillus fumigatus Intrinsic Fluconazole Resistance Is Due to the Naturally Occurring T301I Substitution in Cyp51Ap. Antimicrob. Agents Chemother. 2016, 60, 5420–5426. [Google Scholar] [CrossRef] [PubMed]
- Snelders, E.; Karawajczyk, A.; Schaftenaar, G.; Verweij, P.E.; Melchers, W.J.G. Azole resistance profile of amino acid changes in Aspergillus fumigatus CYP51A based on protein homology modeling. Antimicrob. Agents Chemother. 2010, 54, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Guerra, T.M.; Mellado, E.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. A point mutation in the 14α-sterol demethylase gene cyp51a contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2003, 47, 1120–1224. [Google Scholar] [CrossRef] [PubMed]
- Abdolrasouli, A.; Rhodes, J.; Beale, M.A.; Hagen, F.; Rogers, T.R.; Chowdhary, A.; Meis, J.F.; Armstrong-James, D.; Fisher, M.C. Genomic context of azole resistance mutations in Aspergillus fumigatus determined using whole-genome sequencing. MBio 2015, 6, e00536-15. [Google Scholar] [CrossRef]
- Hagiwara, D.; Watanabe, A.; Kamei, K.; Goldman, G.H. Epidemiological and Genomic Landscape of Azole Resistance Mechanisms in Aspergillus Fungi. Front. Microbiol. 2016, 7, 1382. [Google Scholar] [CrossRef] [PubMed]
- Sagatova, A.A.; Keniya, M.V.; Wilson, R.K.; Sabherwal, M.; Tyndall, J.D.A.; Monk, B.C. Triazole resistance mediated by mutations of a conserved active site tyrosine in fungal lanosterol 14α-demethylase. Sci. Rep. 2016, 6, 26213. [Google Scholar] [CrossRef]
- McKenney, P.T.; Driks, A.; Eichenberger, P. The Bacillus subtilis endospore: Assembly and functions of the multilayered coat. Nat. Rev. Microbiol. 2013, 11, 33–44. [Google Scholar] [CrossRef]
- Nguyen, K.B.; Sreelatha, A.; Durrant, E.S.; Lopez-Garrido, J.; Muszewska, A.; Dudkiewicz, M.; Grynberg, M.; Yee, S.; Pogliano, K.; Tomchick, D.R.; et al. Phosphorylation of spore coat proteins by a family of atypical protein kinases. Proc. Natl. Acad. Sci. USA 2016, 113, E3482–E3491. [Google Scholar] [CrossRef] [PubMed]
- Saggese, A.; Scamardella, V.; Sirec, T.; Cangiano, G.; Isticato, R.; Pane, F.; Amoresano, A.; Ricca, E.; Baccigalupi, L. Antagonistic role of CotG and CotH on spore germination and coat formation in Bacillus subtilis. PLoS ONE 2014, 9, e104900. [Google Scholar] [CrossRef]
- Chibucos, M.C.; Soliman, S.; Gebremariam, T.; Lee, H.; Daugherty, S.; Orvis, J.; Shetty, A.C.; Crabtree, J.; Hazen, T.H.; Etienne, K.A.; et al. An integrated genomic and transcriptomic survey of mucormycosis-causing fungi. Nat. Commun. 2016, 7, 12218. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, T.; Liu, M.; Luo, G.; Bruno, V.; Phan, Q.T.; Waring, A.J.; Edwards, J.E.; Filler, S.G.; Yeaman, M.R.; Ibrahim, A.S. CotH3 mediates fungal invasion of host cells during mucormycosis. J. Clin. Investig. 2014, 124, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Alqarihi, A.; Gebremariam, T.; Gu, Y.; Swidergall, M.; Alkhazraji, S.; Soliman, S.S.M.; Bruno, V.M.; Edwards, J.E.; Filler, S.G.; Uppuluri, P.; et al. GRP78 and Integrins Play Different Roles in Host Cell Invasion during Mucormycosis. MBio 2020, 11, e01087-20. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, A.; Meslet-Cladière, L.; Morin-Sardin, S.; Coton, E.; Jany, J.L.; Barbier, G.; Corre, E. Comparative analysis of five Mucor species transcriptomes. Genomics 2019, 111, 1306–1314. [Google Scholar] [CrossRef]
- Baldin, C.; Soliman, S.S.M.; Jeon, H.H.; Alkhazraji, S.; Gebremariam, T.; Gu, Y.; Bruno, V.M.; Cornely, O.A.; Leather, H.L.; Sugrue, M.W.; et al. PCR-based approach targeting Mucorales-specific gene family for diagnosis of mucormycosis. J. Clin. Microbiol. 2018, 56, e00746-18. [Google Scholar] [CrossRef]
- Boyce, K.J.; Andrianopoulos, A. Fungal dimorphism: The switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol. Rev. 2015, 39, 797–811. [Google Scholar] [CrossRef]
- Jacobsen, I.D.; Wilson, D.; Wächtler, B.; Brunke, S.; Naglik, J.R.; Hube, B. Candida albicans dimorphism as a therapeutic target. Expert Rev. Anti-Infect. Ther. 2014, 10, 85–93. [Google Scholar] [CrossRef]
- Wolff, A.M.; Appel, K.F.; Petersen, J.B.; Poulsen, U.; Arnau, J. Identification and analysis of genes involved in the control of dimorphism in Mucor circinelloides (syn. racemosus). FEMS Yeast Res. 2002, 2, 203–213. [Google Scholar]
- Orlowski, M. Mucor dimorphism. Microbiol. Rev. 1991, 55, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Billmyre, R.B.; Lee, S.C.; Heitman, J. Broad antifungal resistance mediated by RNAi-dependent epimutation in the basal human fungal pathogen Mucor circinelloides. PLoS Genet. 2019, 15, e1007957. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Vázquez, R.M.; Nicolás, F.E.; Torres-Martínez, S.; Garre, V. Distinct RNAi Pathways in the Regulation of Physiology and Development in the Fungus Mucor circinelloides. Adv. Genet. 2015, 91, 55–102. [Google Scholar]
- Calo, S.; Nicolás, F.E.; Vila, A.; Torres-Martínez, S.; Ruiz-Vázquez, R.M.; Nicolas, F.E.; Vila, A.; Torres-Martinez, S.; Ruiz-Vazquez, R.M. Two distinct RNA-dependent RNA polymerases are required for initiation and amplification of RNA silencing in the basal fungus Mucor circinelloides. Mol. Microbiol. 2012, 83, 379–394. [Google Scholar] [CrossRef]
- de Haro, J.P.; Calo, S.; Cervantes, M.; Nicolás, F.E.; Torres-Martínez, S.; Ruiz-Vázquez, R.M. A Single dicer Gene Is Required for Efficient Gene Silencing Associated with Two Classes of Small Antisense RNAs in Mucor circinelloides. Eukaryot. Cell 2009, 8, 1486–1497. [Google Scholar] [CrossRef]
- Cervantes, M.; Vila, A.; Nicolás, F.E.; Moxon, S.; de Haro, J.P.; Dalmay, T.; Torres-Martínez, S.; Ruiz-Vázquez, R.M. A Single Argonaute Gene Participates in Exogenous and Endogenous RNAi and Controls Cellular Functions in the Basal Fungus Mucor circinelloides. PLoS ONE 2013, 8, e69283. [Google Scholar] [CrossRef]
- Nicolas, F.E.; Moxon, S.; de Haro, J.P.; Calo, S.; Grigoriev, I.V.; Torres-MartÍnez, S.; Moulton, V.; Ruiz-Vázquez, R.M.; Dalmay, T. Endogenous short RNAs generated by Dicer 2 and RNA-dependent RNA polymerase 1 regulate mRNAs in the basal fungus Mucor circinelloides. Nucleic Acids Res. 2010, 38, 5535–5541. [Google Scholar] [CrossRef]
- Nicolás, F.E.; Vila, A.; Moxon, S.; Cascales, M.D.; Torres-Martínez, S.; Ruiz-Vázquez, R.M.; Garre, V. The RNAi machinery controls distinct responses to environmental signals in the basal fungus Mucor circinelloides. BMC Genom. 2015, 16, 237. [Google Scholar] [CrossRef] [PubMed]
- Calo, S.; Nicolás, F.E.; Lee, S.C.; Vila, A.; Cervantes, M.; Torres-Martinez, S.; Ruiz-Vazquez, R.M.; Cardenas, M.E.; Heitman, J. A non-canonical RNA degradation pathway suppresses RNAi-dependent epimutations in the human fungal pathogen Mucor circinelloides. PLoS Genet. 2017, 13, e1006686. [Google Scholar] [CrossRef]
- Trieu, T.A.; Calo, S.; Nicolás, F.E.; Vila, A.; Moxon, S.; Dalmay, T.; Torres-Martínez, S.; Garre, V.; Ruiz-Vázquez, R.M. A Non-canonical RNA Silencing Pathway Promotes mRNA Degradation in Basal Fungi. PLoS Genet. 2015, 11, e1005168. [Google Scholar] [CrossRef]
- Chang, Z.; Heitman, J. Drug-resistant epimutants exhibit organ-specific stability and induction during murine infections caused by the human fungal pathogen Mucor circinelloides. MBio 2019, 10, e02579-19. [Google Scholar] [CrossRef] [PubMed]
- Cánovas-Márquez, J.T.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; Lax, C.; Tahiri, G.; Pérez-Ruiz, J.A.; Lorenzo-Gutiérrez, D.; Calo, S.; López-García, S.; Navarro, E.; et al. Role of the non-canonical rnai pathway in the antifungal resistance and virulence of Mucorales. Genes 2021, 12, 586. [Google Scholar] [CrossRef]
- Nicolás, F.E.; Murcia, L.; Navarro, E.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; Garre, V. Mucorales species and macrophages. J. Fungi 2020, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Cánovas-Márquez, J.T.; Falk, S.; Nicolás, F.E.; Padmanabhan, S.; Zapata-Pérez, R.; Sánchez-Ferrer, Á.; Navarro, E.; Garre, V. A ribonuclease III involved in virulence of Mucorales fungi has evolved to cut exclusively single-stranded RNA. Nucleic Acids Res. 2021, 49, 5294–5307. [Google Scholar] [CrossRef]
- Court, D.L.; Gan, J.; Liang, Y.-H.; Shaw, G.X.; Tropea, J.E.; Costantino, N.; Waugh, D.S.; Ji, X. RNase III: Genetics and Function; Structure and Mechanism. Annu. Rev. Genet. 2013, 47, 405–431. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.C.; Nguyen, T.A.; Choi, Y.G.; Jo, M.H.; Hohng, S.; Kim, V.N.; Woo, J.S. Structure of Human DROSHA. Cell 2016, 164, 81–90. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Cheng, H.; Ke, X.; Sun, L.; Zhang, Q.C.; Wang, H.W. Cryo-EM Structure of Human Dicer and Its Complexes with a Pre-miRNA Substrate. Cell 2018, 173, 1191–1203. [Google Scholar] [CrossRef]
- Gan, J.; Shaw, G.; Tropea, J.E.; Waugh, D.S.; Court, D.L.; Ji, X. A stepwise model for double-stranded RNA processing by ribonuclease III. Mol. Microbiol. 2008, 67, 143–154. [Google Scholar] [CrossRef]
- Challa, S. Mucormycosis: Pathogenesis and Pathology. Curr. Fungal Infect. Rep. 2019, 13, 11–20. [Google Scholar] [CrossRef]
- Harpf, V.; Rambach, G.; Parth, N.; Neurauter, M.; Fleischer, V.; Lackner, M.; Lass-Flörl, C.; Würzner, R.; Speth, C. Complement, but Not Platelets, Plays a Pivotal Role in the Outcome of Mucormycosis In Vivo. J. Fungi 2023, 9, 162. [Google Scholar] [CrossRef]
- Voelz, K.; Gratacap, R.L.; Wheeler, R.T. A zebrafish larval model reveals early tissue-specific innate immune responses to Mucor circinelloides. Dis. Model. Mech. 2015, 8, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, J. The model organism diaspora. Hered 2019, 123, 14–17. [Google Scholar] [CrossRef] [PubMed]
- van Heeswijck, R.; Roncero, M.I.G. High frequency transformation of Mucor with recombinant plasmid DNA. Carlsberg Res. Commun. 1984, 49, 691–702. [Google Scholar] [CrossRef]
- Navarro, E.; Lorca-Pascual, J.; Quiles-Rosillo, M.; Nicolás, F.; Garre, V.; Torres-Martínez, S.; Ruiz-Vázquez, R. A negative regulator of light-inducible carotenogenesis in Mucor circinelloides. Mol. Genet. Genom. 2001, 266, 463–470. [Google Scholar] [CrossRef]
- Nicolas, F.E.; Torres-Martínez, S.; Ruiz-Vázquez, R.M. Two classes of small antisense RNAs in fungal RNA silencing triggered by non-integrative transgenes. EMBO J. 2003, 22, 3983–3991. [Google Scholar] [CrossRef]
- Navarro-Mendoza, M.I.; Pérez-Arques, C.; Panchal, S.; Nicolás, F.E.; Mondo, S.J.; Ganguly, P.; Pangilinan, J.; Grigoriev, I.V.; Heitman, J.; Sanyal, K.; et al. Early Diverging Fungus Mucor circinelloides Lacks Centromeric Histone CENP-A and Displays a Mosaic of Point and Regional Centromeres. Curr. Biol. 2019, 29, 3791–3802.e6. [Google Scholar] [CrossRef]
- Roncero, M.I.G. Enrichment method for the isolation of auxotrophic mutants of Mucor using the polyene antibiotic N-glycosyl-polifungin. Carlsberg Res. Commun. 1984, 49, 685–690. [Google Scholar] [CrossRef]
- Anaya, N.; Roncero, M.I.G. Transformation of a methionine auxotrophic mutant of Mucor circinelloides by direct cloning of the corresponding wild type gene. Mol. Genom. 1991, 230, 449–455. [Google Scholar] [CrossRef]
- Nicolás, F.E.; de Haro, J.P.; Torres-Martínez, S.; Ruiz-Vázquez, R.M.; Nicolas, F.E.; de Haro, J.P.; Torres-Martinez, S.; Ruiz-Vazquez, R.M. Mutants defective in a Mucor circinelloides dicer-like gene are not compromised in siRNA silencing but display developmental defects. Fungal Genet. Biol. 2007, 44, 504–516. [Google Scholar] [CrossRef]
- Nicolas, F.E.; Calo, S.; Murcia-Flores, L.; Garre, V.; Ruiz-Vazquez, R.M.; Torres-Martinez, S. A RING-finger photocarotenogenic repressor involved in asexual sporulation in Mucor circinelloides. FEMS Microbiol. Lett. 2008, 280, 81–88. [Google Scholar] [CrossRef]
- Nicolás-Molina, F.E.; Navarro, E.; Ruiz-Vázquez, R.M. Lycopene over-accumulation by disruption of the negative regulator gene crgA in Mucor circinelloides. Appl. Microbiol. Biotechnol. 2008, 78, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Arques, C.; Navarro-Mendoza, M.I.; Murcia, L.; Lax, C.; Sanchis, M.; Capilla, J.; Navarro, E.; Garre, V.; Nicolás, F.E. A Mucoralean White Collar-1 Photoreceptor Controls Virulence by Regulating an Intricate Gene Network during Host Interactions. Microorganisms 2021, 9, 459. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.F.; Torres-Martínez, S.; Garre, V.; Torres-Martinez, S.; Garre, V. Distinct white collar-1 genes control specific light responses in Mucor circinelloides. Mol. Microbiol. 2006, 61, 1023–1037. [Google Scholar] [CrossRef]
- Navarro, E.; Peñaranda, A.; Hansberg, W.; Torres-Martínez, S.; Garre, V. A White Collar 1-like protein mediates opposite regulatory functions in Mucor circinelloides. Fungal Genet. Biol. 2013, 52, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Navarro, E.; Peñaranda, A.; Murcia-Flores, L.; Torres-Martínez, S.; Garre, V. A RING-finger protein regulates carotenogenesis via proteolysis-independent ubiquitylation of a White Collar-1-like activator. Mol. Microbiol. 2008, 70, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Nicolás, F.; Ruiz-Vázquez, R. Functional Diversity of RNAi-Associated sRNAs in Fungi. Int. J. Mol. Sci. 2013, 14, 15348–15360. [Google Scholar] [CrossRef] [PubMed]
- Nicolás, F.E.; Torres-Martínez, S.; Ruiz-Vázquez, R.M. Transcriptional activation increases RNA silencing efficiency and stability in the fungus Mucor circinelloides. J. Biotechnol. 2009, 142, 123–126. [Google Scholar] [CrossRef]
- Nicolás, F.E.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; López-García, S.; Navarro, E.; Torres-Martínez, S.; Garre, V. Molecular tools for carotenogenesis analysis in the mucoral Mucor circinelloides. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2018; Volume 1852, pp. 221–237. [Google Scholar]
- Prakash, H.; Chakrabarti, A. Global epidemiology of mucormycosis. J. Fungi 2019, 5, 26. [Google Scholar] [CrossRef]
- Partida-Martinez, L.P.; Hertweck, C. Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature 2005, 437, 884–888. [Google Scholar] [CrossRef]
- Mondo, S.J.; Lastovetsky, O.A.; Gaspar, M.L.; Schwardt, N.H.; Barber, C.C.; Riley, R.; Sun, H.; Grigoriev, I.V.; Pawlowska, T.E. Bacterial endosymbionts influence host sexuality and reveal reproductive genes of early divergent fungi. Nat. Commun. 2017, 8, 1843. [Google Scholar] [CrossRef]
- Itabangi, H.; Sephton-Clark, P.C.S.; Tamayo, D.P.; Zhou, X.; Starling, G.P.; Mahamoud, Z.; Insua, I.; Probert, M.; Correia, J.; Moynihan, P.J.; et al. A bacterial endosymbiont of the fungus Rhizopus microsporus drives phagocyte evasion and opportunistic virulence. Curr. Biol. 2022, 32, 1115–1130.e6. [Google Scholar] [CrossRef] [PubMed]
- Lax, C.; Navarro-Mendoza, M.I.; Pérez-Arques, C.; Navarro, E.; Nicolás, F.E.; Garre, V. Transformation and CRISPR-Cas9-Mediated homologous recombination in the fungus Rhizopus microsporus. Star. Protoc. 2022, 3, 101237. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Ibrahim, A.S.; Skory, C.; Grabherr, M.G.; Burger, G.; Butler, M.; Elias, M.; Idnurm, A.; Lang, B.F.; Sone, T.; et al. Genomic analysis of the basal lineage fungus Rhizopus oryzae reveals a whole-genome duplication. PLoS Genet. 2009, 5, e1000549. [Google Scholar] [CrossRef]
- Lax, C.; Pérez-arques, C.; Navarro-mendoza, M.I.; Cánovas-márquez, J.T.; Tahiri, G.; Pérez-ruiz, J.A.; Osorio-concepción, M.; Murcia-flores, L.; Navarro, E.; Garre, V.; et al. Genes, pathways, and mechanisms involved in the virulence of Mucorales. Genes 2020, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Katragkou, A.; Walsh, T.J.; Roilides, E. Why is mucormycosis more difficult to cure than more common mycoses? Clin. Microbiol. Infect. 2014, 20, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Corrochano, L.M.; Kuo, A.; Marcet-Houben, M.; Polaino, S.; Salamov, A.; Villalobos-Escobedo, J.M.; Grimwood, J.; Álvarez, M.I.; Avalos, J.; Bauer, D.; et al. Expansion of Signal Transduction Pathways in Fungi by Extensive Genome Duplication. Curr. Biol. 2016, 26, 1577–1584. [Google Scholar] [CrossRef]
- Binder, U.; Navarro-Mendoza, M.I.; Naschberger, V.; Bauer, I.; Nicolas, F.E.; Pallua, J.D.; Lass-Flörl, C.; Garre, V. Generation of a mucor circinelloides reporter strain—A promising new tool to study antifungal drug efficacy and mucormycosis. Genes 2018, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- López-Muñoz, A.; Nicolás, F.E.; García-Moreno, D.; Pérez-Oliva, A.B.; Navarro-Mendoza, M.I.; Hernández-Oñate, M.A.; Herrera-Estrella, A.; Torres-Martínez, S.; Ruiz-Vázquez, R.M.; Garre, V.; et al. An Adult Zebrafish Model Reveals that Mucormycosis Induces Apoptosis of Infected Macrophages. Sci. Rep. 2018, 8, 12802. [Google Scholar] [CrossRef]
- Lax, C.; Cánovas-Márquez, J.T.; Tahiri, G.; Navarro, E.; Garre, V.; Nicolás, F.E. Genetic Manipulation in Mucorales and New Developments to Study Mucormycosis. Int. J. Mol Sci. 2022, 23, 3454. [Google Scholar] [CrossRef] [PubMed]
- Vellanki, S.; Navarro-Mendoza, M.I.; Garcia, A.; Murcia, L.; Perez-Arques, C.; Garre, V.; Nicolas, F.E.; Lee, S.C. Mucor circinelloides: Growth, Maintenance, and Genetic Manipulation. Curr. Protoc. Microbiol. 2018, 49, e53. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Concepción, M.; Lax, C.; Navarro, E.; Nicolás, F.E.; Garre, V. DNA Methylation on N6-Adenine Regulates the Hyphal Development during Dimorphism in the Early-Diverging Fungus Mucor lusitanicus. J. Fungi 2021, 7, 738. [Google Scholar] [CrossRef] [PubMed]
| Study Model | Gene | Function | Reference |
|---|---|---|---|
| M. lusitanicus | fet3a | Iron uptake | [15] |
| M. lusitanicus | fet3b | Iron uptake | [15] |
| M. lusitanicus | fet3c | Iron uptake | [15] |
| R. delemar | ftr1 | Iron uptake | [16] |
| Several | cyp51 f1 | Ergosterol synthesis | [19] |
| Several | cyp51 f5 | Ergosterol synthesis | [19] |
| M. lusitanicus and R. delemar | cotH family | Cell wall antigen | [20,21] |
| M. lusitanicus | ID112092 | Secreted, unknown | [22] |
| M. lusitanicus | wex1 | Exonuclease, unknown | [23] |
| M. lusitanicus | atf1 and atf2 | Transcription factors | [24] |
| M. lusitanicus | mcplD | Signaling | [25] |
| M. lusitanicus | mcmyo5 | Intracellular transport | [25] |
| M. lusitanicus | cnaA, cnaB, cnaC, and cnaR | Calcineurin, pleiotropic | [26,27] |
| M. lusitanicus | pkar1, pkar2, and pkar4 | Dimorphism | [28] |
| M. lusitanicus | gpb1 | Dimorphism | [29] |
| M. lusitanicus | r3b2 | RNAi | [30] |
| Rhizopus microsporus | pyrF | Uracile synthesis | [9] |
| Study Model | Genetic Tool | Year of Development | Reference |
|---|---|---|---|
| M. lusitanicus | Plasmid transformation | 1984 | [7,83] |
| Stable homologous recombination | 2001 | [84] | |
| Gene complementation | 2001 | [84] | |
| RNAi | 2003 | [85] | |
| Amino acid substitution | 2015 | [70] | |
| Genomic RNAi libraries | 2017 | [70] | |
| Fluorescent protein tagging | 2019 | [86] | |
| R. delemar | Plasmid transformation | 2010 | [16] |
| RNAi | 2010 | [16] | |
| Unstable homologous recombination (Heterokaryons) | 2010 | [16] | |
| R. microsporus | Plasmid transformation | 2021 | [9] |
| Stable homologous recombination | 2021 | [9] | |
| Gene complementation | 2021 | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahiri, G.; Lax, C.; Cánovas-Márquez, J.T.; Carrillo-Marín, P.; Sanchis, M.; Navarro, E.; Garre, V.; Nicolás, F.E. Mucorales and Mucormycosis: Recent Insights and Future Prospects. J. Fungi 2023, 9, 335. https://doi.org/10.3390/jof9030335
Tahiri G, Lax C, Cánovas-Márquez JT, Carrillo-Marín P, Sanchis M, Navarro E, Garre V, Nicolás FE. Mucorales and Mucormycosis: Recent Insights and Future Prospects. Journal of Fungi. 2023; 9(3):335. https://doi.org/10.3390/jof9030335
Chicago/Turabian StyleTahiri, Ghizlane, Carlos Lax, José Tomás Cánovas-Márquez, Pablo Carrillo-Marín, Marta Sanchis, Eusebio Navarro, Victoriano Garre, and Francisco Esteban Nicolás. 2023. "Mucorales and Mucormycosis: Recent Insights and Future Prospects" Journal of Fungi 9, no. 3: 335. https://doi.org/10.3390/jof9030335
APA StyleTahiri, G., Lax, C., Cánovas-Márquez, J. T., Carrillo-Marín, P., Sanchis, M., Navarro, E., Garre, V., & Nicolás, F. E. (2023). Mucorales and Mucormycosis: Recent Insights and Future Prospects. Journal of Fungi, 9(3), 335. https://doi.org/10.3390/jof9030335









