Vaccination of Elms against Dutch Elm Disease—Are the Associated Epiphytes and Endophytes Affected?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study 1—Epiphytes (Stockholm)
2.2. Study 2—Endophytes (Gotland)
2.3. Data Analysis
3. Results
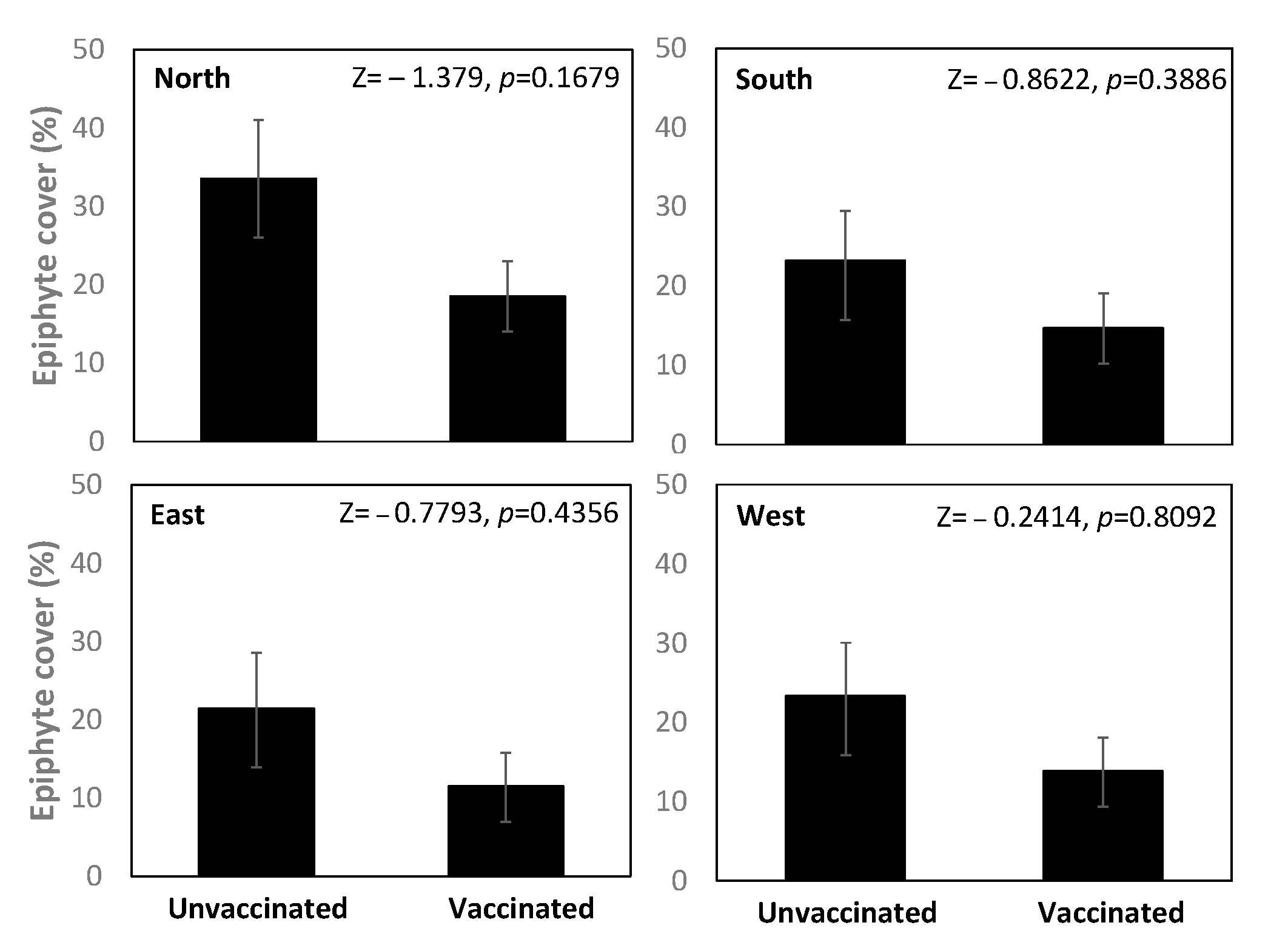
3.1. Study 1—Epiphytes (Stockholm)
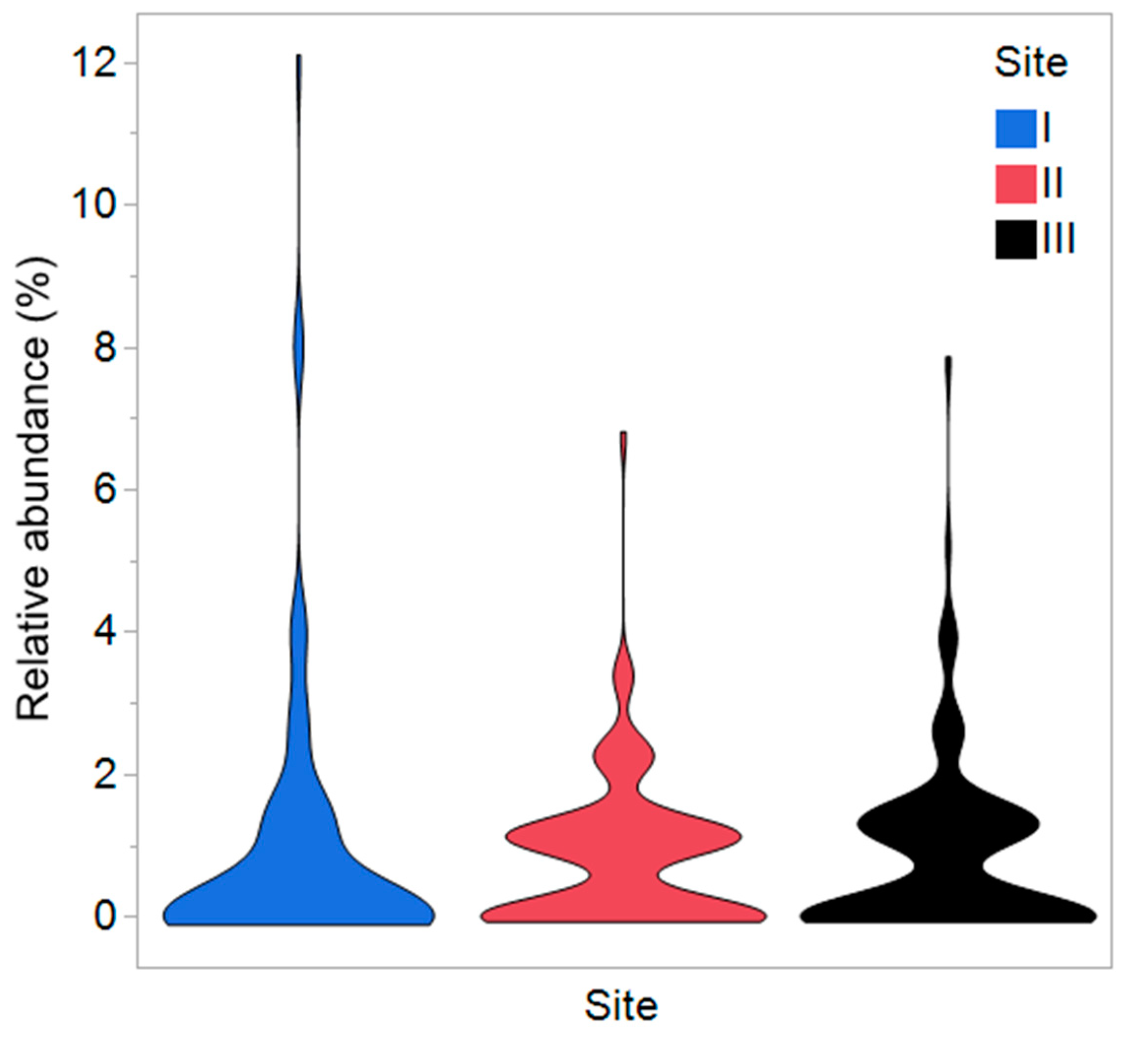
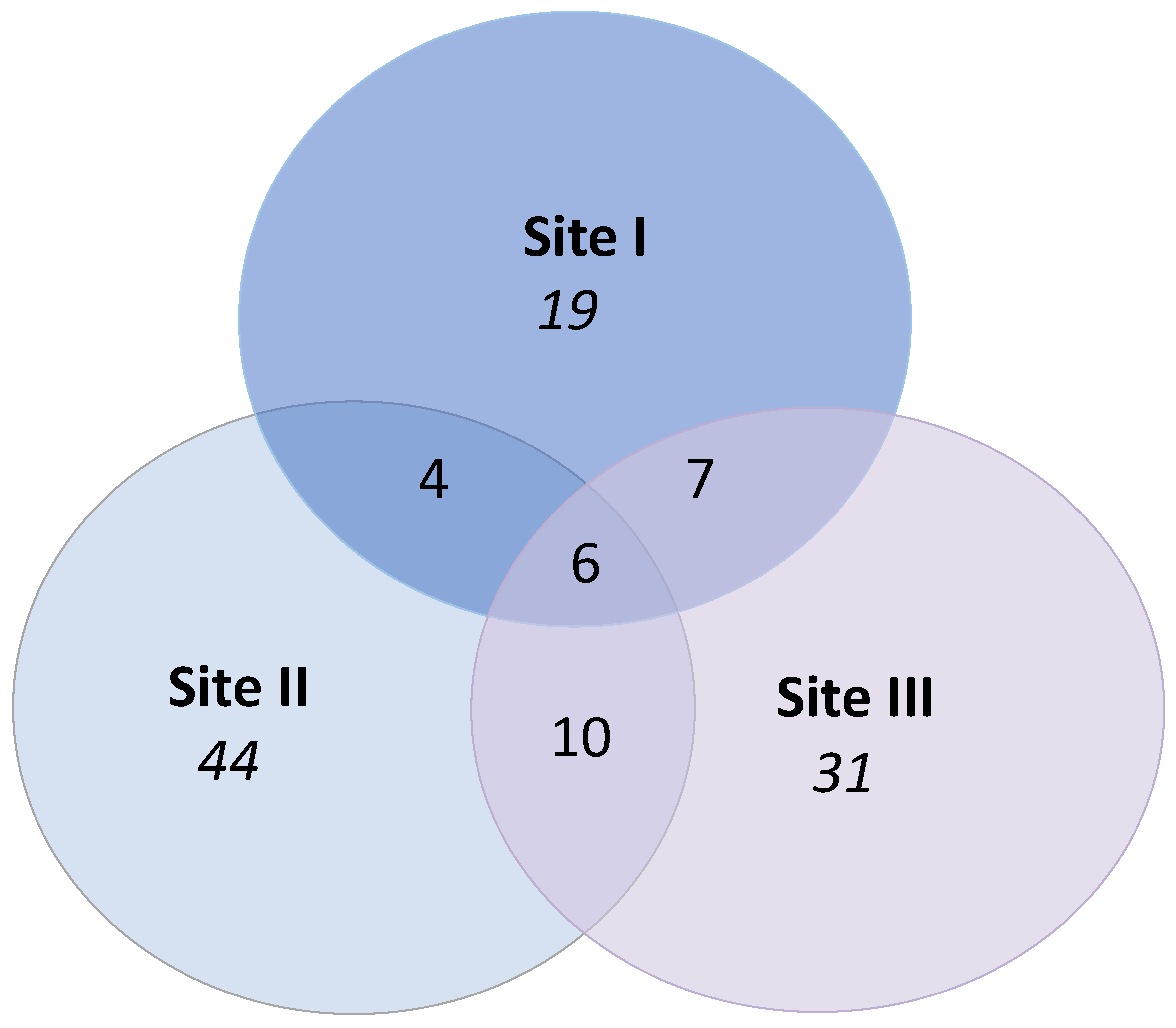
3.2. Study 2—Endophytes (Gotland)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caudullo, G.; de Rigo, D. threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, Luxembourg, 2016; pp. 166–188. [Google Scholar]
- Copeland, C.A.; Harper, R.W.; Brazee, N.J.; Bowlick, F.J. A review of Dutch elm disease and new prospects for Ulmus americana in the urban environment. Arboric. J. 2022, 1–27. [Google Scholar] [CrossRef]
- Whittemore, A.T.; Olsen, R.T. Ulmus americana Ulmaceae is a polyploid complex. Am. J. Bot. 2011, 98, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Thor, G.; Johansson, P.; Jönsson, M.T. Lichen diversity and red-listed lichen species relationships with tree species and diameter in wooded meadows. Biodivers. Conserv. 2010, 19, 2307–2328. [Google Scholar] [CrossRef]
- Hiemstra, J.A.; Buiteveld, J. New perspectives for the use of elms as street trees. Acta Hortic. 2010, 881, 257–261. [Google Scholar] [CrossRef]
- Brasier, C.M. Dual origin of recent Dutch elm disease outbreaks in Europe. Nature 1979, 281, 78–80. [Google Scholar] [CrossRef]
- Brasier, C.M. Ophiostoma novo-ulmi sp. nov., causative agent of the current Dutch elm disease pandemics. Mycopathologia 1991, 115, 151–161. [Google Scholar] [CrossRef]
- Brasier, C.M.; Webber, J.F. Is there evidence for post-epidemic attenuation in the Dutch elm disease pathogen Ophiostoma novo-ulmi? Plant Pathol. 2019, 68, 921–929. [Google Scholar] [CrossRef]
- Pita, P.; Rodríguez-Calcerrada, J.; Medel, D.; Gil, L. Further insights into the components of resistance to Ophiostoma novo-ulmi in Ulmus minor: Hydraulic conductance, stomatal sensitivity and bark dehydration. Tree Physiol. 2018, 38, 252–262. [Google Scholar] [CrossRef]
- Santini, A.; Faccoli, M. Dutch elm disease and elm bark beetles: A century of association. iForest 2015, 8, 126–134. [Google Scholar] [CrossRef]
- Anderbrant, O.; Yuvaraj, J.K.; Martin, J.A.; Gil, L.; Witzell, J. Feeding by bark beetles to test for differently susceptible elm varieties. J. Appl. Entomol. 2017, 141, 417–420. [Google Scholar] [CrossRef]
- Brasier, C.M. Intercontinental spread and continuing evolution of the Dutch elm disease pathogens. In The Elms; Dunn, C.P., Ed.; Springer: Boston, MA, USA, 2000; pp. 61–72. [Google Scholar] [CrossRef]
- Scheffer, R.J.; Voeten, J.G.W.F.; Guries, R.P. Biological control of Dutch elm disease. Plant Dis. 2008, 92, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Postma, J.; Goossen-van de Geijn, H. Twenty-four years of Dutch Trig® application to control Dutch elm disease. BioControl 2016, 61, 305–312. [Google Scholar] [CrossRef]
- Martín, J.A.; Macaya-Sanz, D.; Witzell, J.; Blumenstein, K.; Gil, L. Seven Ulmus minor clones tolerant to Ophiostoma novo-ulmi registered as forest reproductive material in Spain. iForest 2015, 8, 172–180. [Google Scholar] [CrossRef]
- Martín, J.A.; Sobrino-Plata, J.; Rodríguez-Calcerrada, J.; Collada, C.; Gil, L. Breeding and scientific advances in the fight against Dutch elm disease: Will they allow the use of elms in forest restoration? New For. 2019, 50, 183–215. [Google Scholar] [CrossRef]
- Elgersma, D.M.; Overeem, J.C. The relation of mansonones to resistance against dutch elm disease and their accumulation, as induced by several agents. Neth. J. Plant Pathol. 1971, 77, 168–174. [Google Scholar] [CrossRef]
- De Rafael, M.A.; Valle, T.; Babiano, M.J.; Corchete, P. Correlation of resistance and H2O2 production in Ulmus pumila and Ulmus campestris cell suspension cultures inoculated with Ophiostoma novo-ulmi. Physiol. Plant. 2001, 111, 512–518. [Google Scholar] [CrossRef]
- Durkovič, J.; Kačík, F.; Olčák, D.; Kučerová, V.; Krajňáková, J. Host responses and metabolic profiles of wood components in Dutch elm hybrids with a contrasting tolerance to Dutch elm disease. Ann. Bot. 2014, 114, 47–59. [Google Scholar] [CrossRef]
- Beier, G.L.; Blanchette, R.A. Defence responses in the xylem of Ulmus americana cultivars after inoculation with Ophiostoma novo-ulmi. For. Path 2018, 48, e12453. [Google Scholar] [CrossRef]
- Busby, P.E.; Ridout, M.; Newcombe, G. Fungal endophytes: Modifiers of plant disease. Plant Mol. Biol. 2016, 90, 645–655. [Google Scholar]
- Witzell, J.; Martín, J.A. Endophytes and forest health. In Endophytes of Forest Trees; Pirttilä, A.M., Frank, A.C., Eds.; Forestry Sciences 86; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 261–282. [Google Scholar]
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Martín, J.A.; Witzell, J.; Blumenstein, K.; Rozpedowska, E.; Helander, M.; Sieber, T.; Gil, L. Resistance to Dutch elm disease reduces xylem endophytic fungi presence in elms Ulmus spp. PLoS ONE 2013, 82, e56987. [Google Scholar] [CrossRef]
- Flores-Palacios, A.; García-Franco, J.G. The relationship between tree size and epiphyte species richness: Testing four different hypotheses. J. Biogeogr. 2006, 33, 323–330. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949; 144p. [Google Scholar]
- Hutcheson, K. A test for comparing diversities based on the Shannon formula. J. Theor. Biol. 1970, 29, 151–154. [Google Scholar] [CrossRef]
- Eyles, A.; Bonello, P.; Ganley, R.; Mohammed, C. Induced resistance to pests and pathogens in trees. New Phytol. 2010, 185, 893–908. [Google Scholar] [CrossRef]
- Karasov, T.L.; Chae, E.; Herman, J.J.; Bergelson, J. Mechanisms to mitigate the trade-off between growth and defense. Plant Cell 2017, 29, 666–680. [Google Scholar] [CrossRef] [PubMed]
- Gauslaa, Y.; Goward, T.; Asplund, J. Canopy throughfall links canopy epiphytes to terrestrial vegetation in pristine conifer forests. Fungal Ecol. 2021, 52, 101075. [Google Scholar] [CrossRef]
- Jüriado, I.; Liira, J.; Paal, J.; Suija, A. Tree and stand level variables influencing diversity of lichens on temperate broad-leaved trees in boreo-nemoral floodplain forests. Biodivers. Conserv. 2009, 18, 105–125. [Google Scholar] [CrossRef]
- Lamit, L.J.; Lau, M.K.; Schultz, C.M.; Wooley, S.C.; Whitham, T.G.; Gehring, C.A. Tree genotype and genetically based growth traits structure twig endophyte communities. Am. J. Bot. 2014, 101, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Albrectsen, B.R.; Siddique, A.B.; Decker, V.H.; Unterseher, M.; Robinson, K.M. Both plant genotype and herbivory shape aspen endophyte communities. Oecologia 2018, 187, 535–545. [Google Scholar] [CrossRef]
- Romeralo, C.; Martín-García, J.; Martínez-Álvarez, P.; Muñoz-Adalia, E.J.; Reis Gonçalves, D.; Torres, E.; Witzell, J.; Diez Casero, J.J. Pine species determine fungal microbiome composition in a common garden experiment. Fungal Ecol. 2022, 56, 101137. [Google Scholar] [CrossRef]
- Paoli, L.; Pisani, T.; Munzi, S.; Gaggi, C.; Loppi, S. Influence of sun irradiance and water availability on lichen photosynthetic pigments during a Mediterranean summer. Biologia 2010, 65, 776–783. [Google Scholar] [CrossRef]
- Cung, K.; Galvan, L.; Osborne, H.; Spiegel, S. The effects of sunlight and slope on the lichen community of the Sweeney Granite Mountains reserve. CEC Res. 2021, 52, 1–7. [Google Scholar] [CrossRef]
- Coote, L.; Smith, G.F.; Kelly, D.L.; O’Donoghue, S.; Dowding, P.; Iremonger, S.; Mitchell, F.J.G. Epiphytes of Sitka spruce Picea sitchensis plantations in Ireland and the effects of open spaces. Biodivers. Conserv. 2007, 16, 4009–4024. [Google Scholar] [CrossRef]
- Lie, H.; Arup, U.; Grytnes, J.A.; Ohlson, M. The importance of host tree age, size and growth rate as determinants of epiphytic lichen diversity in boreal spruce forests. Biodivers. Conserv. 2009, 18, 3579–3596. [Google Scholar] [CrossRef]
- Lacap, D.C.; Hyde, K.D.; Liew, E.C.Y. An evaluation of the fungal ‘morphotype’ concept based on ribosomal DNA sequences. Fungal Div. 2003, 12, 53–66. [Google Scholar]
- dos Reis, J.B.A.; Lorenzi, A.S.; do Vale, H.M.M. Methods used for the study of endophytic fungi: A review on methodologies and challenges, and associated tips. Arch. Microbiol. 2022, 204, 675. [Google Scholar] [CrossRef] [PubMed]
- Łakomy, P.; Kwaśna, H.; Kuźmiński, R.; Napierała-Filipiak, A.; Filipiak, M.; Behnke, K.; Behnke-Borowczyk, J. Investigation of Ophiostoma population infected elms in Poland. Dendrobiology 2016, 76, 137–144. [Google Scholar] [CrossRef]
- Marčiulynas, A.; Marčiulynienė, D.; Lynikienė, J.; Bakys, R.; Menkis, A. Fungal communities in leaves and roots of healthy-looking and diseased Ulmus glabra. Microorganisms 2022, 10, 2228. [Google Scholar] [CrossRef]
- Albrectsen, B.R.; Björkén, L.; Varad, A.; Hagner, Å.; Wedin, M.; Karlsson, J.; Jansson, S. Endophytic fungi in European aspen Populus tremula leaves—Diversity, detection, and a suggested correlation with herbivory resistance. Fungal Div. 2010, 41, 17–28. [Google Scholar] [CrossRef]
- Doty, S.L.; Joubert, P.M.; Firrincieli, A.; Sher, A.W.; Tournay, R.; Kill, C.; Parikh, S.S.; Okubara, P. Potential biocontrol activities of Populus endophytes against several plant pathogens using different inhibitory mechanisms. Pathogens 2023, 12, 13. [Google Scholar] [CrossRef]
- Witzell, J.; Decker, V.H.G.; Agostinelli, M.; Romeralo, C.; Cleary, M.; Albrectsen, B.R. Aspen leaves as a “chemical landscape” for fungal endophyte diversity—Effects of nitrogen addition. Front. Microbiol. 2022, 13, 846208. [Google Scholar] [CrossRef]
- Knapp, D.G.; Lázár, A.; Molnár, A.; Vajna, B.; Karácsony, Z.; Váczy, K.Z.; Kovács, G.M. Above-ground parts of white grapevine Vitis vinifera cv. Furmint share core members of the fungal microbiome. Environ. Microbiol. Rep. 2021, 13, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Di Foggia, M.; Corbetta, M.; Baldo, D.; Ratti, C.; Baraldi, E. Biocontrol activity and plant growth promotion exerted by Aureobasidium pullulans strains. J. Plant Growth Regul. 2021, 40, 1233–1244. [Google Scholar] [CrossRef]
- Blumenstein, K.; Albrectsen, B.R.; Martín, J.A.; Hultberg, M.; Sieber, T.N.; Helander, M.; Witzell, J. Nutritional niche overlap potentiates the use of endophytes in biocontrol of a tree disease. BioControl 2015, 60, 655–667. [Google Scholar] [CrossRef]
- Shade, A.; Handelsman, J. Beyond the Venn diagram: The hunt for a core microbiome. Environ. Microbiol. 2012, 14, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Toju, H.; Yamamoto, S.; Sato, H.; Tanabe, A.S. Sharing of diverse mycorrhizal and root-endophytic fungi among plant species in an oak-dominated cool–temperate forest. PLoS ONE 2013, 8, e78248. [Google Scholar] [CrossRef] [PubMed]
- Neu, A.T.; Allen, E.E.; Roy, K. Defining and quantifying the core microbiome: Challenges and prospects. Proc. Natl. Acad. Sci. USA 2021, 118, e2104429118. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.V.; Kjøller, R.; Raaijmakers, J.M.; Riber, L.; Christensen, S.; Rasmussen, S.; Christensen, J.H.; Bjorholm Dahl, A.; Westergaard, J.C.; Nielsen, M.; et al. Extension of plant phenotypes by the foliar microbiome. Annu. Rev. Plant Biol. 2021, 72, 823–846. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef]
- Cregger, M.A.; Veach, A.M.; Yang, Z.K.; Crouch, M.J.; Vilgalys, R.; Tuskan, G.A.; Schadt, C.W. The Populus holobiont: Dissecting the effects of plant niches and genotype on the microbiome. Microbiome 2018, 6, 31. [Google Scholar] [CrossRef]
- Oksanen, I. Ecological and biotechnological aspects of lichens. Appl. Microbiol. Biotechnol. 2006, 73, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Albrectsen, B.; Witzell, J. Disentangling functions of fungal endophytes in forest trees. In Fungi: Types, Environmental Impact and Role in Disease; Paz Silva, A., Sol, M., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2012; pp. 235–246. [Google Scholar]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Company, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Asplund, J.; Wardle, D.A. How lichens impact on terrestrial community and ecosystem properties. Biol. Rev. 2017, 92, 1720–1738. [Google Scholar] [CrossRef] [PubMed]
- Mallen-Cooper, M.; Cornwell, W.K. A systematic review of transplant experiments in lichens and bryophytes. Bryologist 2020, 123, 444–454. [Google Scholar] [CrossRef]

| Site I | Site II | Site III | |
|---|---|---|---|
| Endophyte frequency | |||
| Nr of segments plated | 60 | 60 | 60 |
| Nr of segments yielding EF | 60 | 60 | 10 |
| Colonization frequency (CF, %) | 100 | 100 | 100 |
| Total nr of EF isolates (N) | 75 | 89 | 77 |
| Isolation rate (IR) | 1.25 a | 1.48 b | 1.28 ab |
| Nr of isolates belonging to “core community” morphotypes | 26 | 10 | 15 |
| Endophyte diversity | |||
| Nr of MTs (Richness, S) | 36 | 64 | 54 |
| Nr of common * MTs | 24 | 29 | 26 |
| Nr of singleton ** MTs | 12 | 35 | 28 |
| Shannon diversity index (H) | 3.32 a | 4.01 b | 3.85 a |
| Shannon equitability (H/lnS) | 0.92 | 0.96 | 0.96 |
| Growth rate of colonies | |||
| Slow | 13 | 57 | 34 |
| Medium | 36 | 12 | 12 |
| Fast | 51 | 31 | 54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witzell, J.; Sunnerstam, C.; Hansson, T. Vaccination of Elms against Dutch Elm Disease—Are the Associated Epiphytes and Endophytes Affected? J. Fungi 2023, 9, 297. https://doi.org/10.3390/jof9030297
Witzell J, Sunnerstam C, Hansson T. Vaccination of Elms against Dutch Elm Disease—Are the Associated Epiphytes and Endophytes Affected? Journal of Fungi. 2023; 9(3):297. https://doi.org/10.3390/jof9030297
Chicago/Turabian StyleWitzell, Johanna, Caroline Sunnerstam, and Tobias Hansson. 2023. "Vaccination of Elms against Dutch Elm Disease—Are the Associated Epiphytes and Endophytes Affected?" Journal of Fungi 9, no. 3: 297. https://doi.org/10.3390/jof9030297
APA StyleWitzell, J., Sunnerstam, C., & Hansson, T. (2023). Vaccination of Elms against Dutch Elm Disease—Are the Associated Epiphytes and Endophytes Affected? Journal of Fungi, 9(3), 297. https://doi.org/10.3390/jof9030297







