Pulmonary Histoplasmosis: A Clinical Update
Abstract
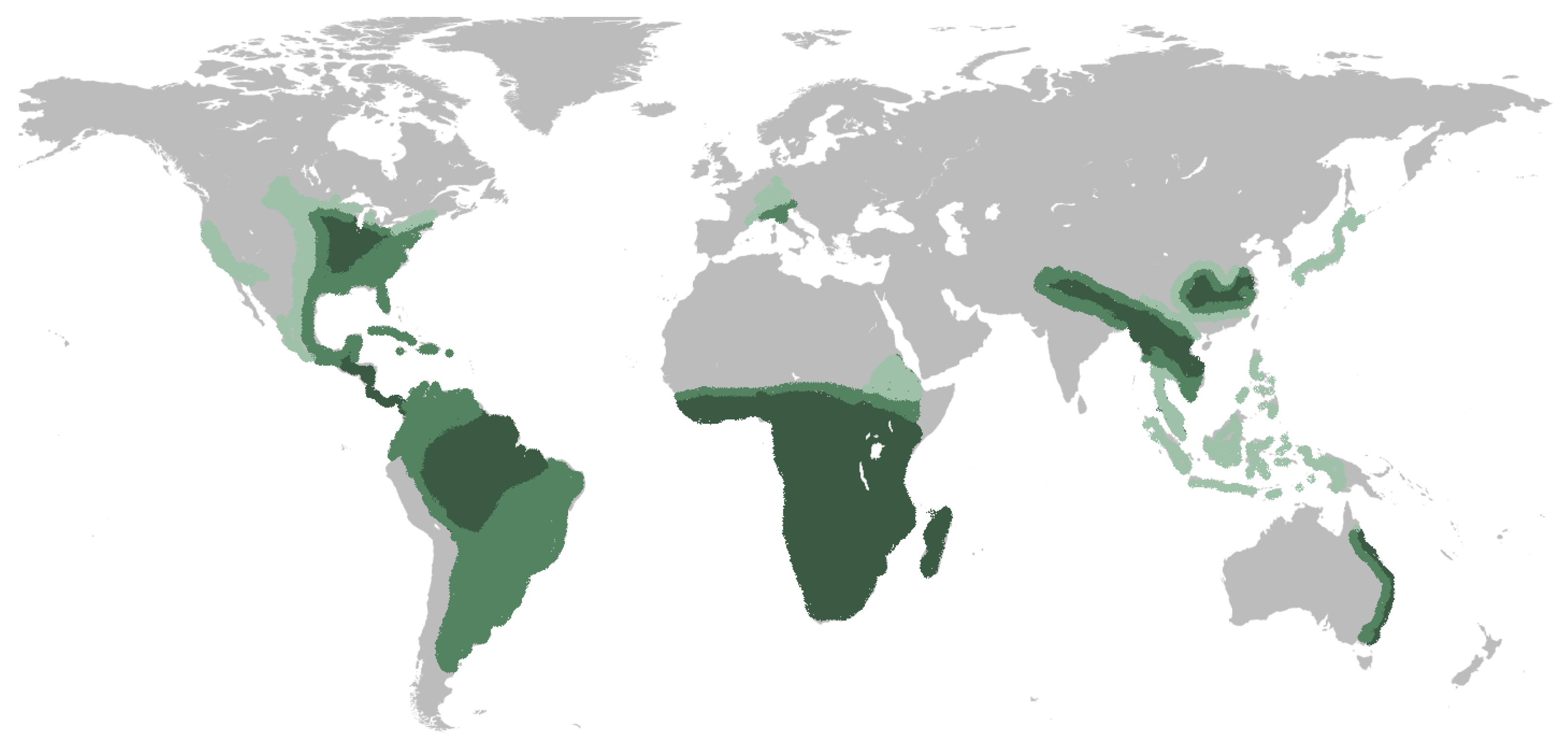
1. Introduction
2. Pathogenesis
3. Diagnosis
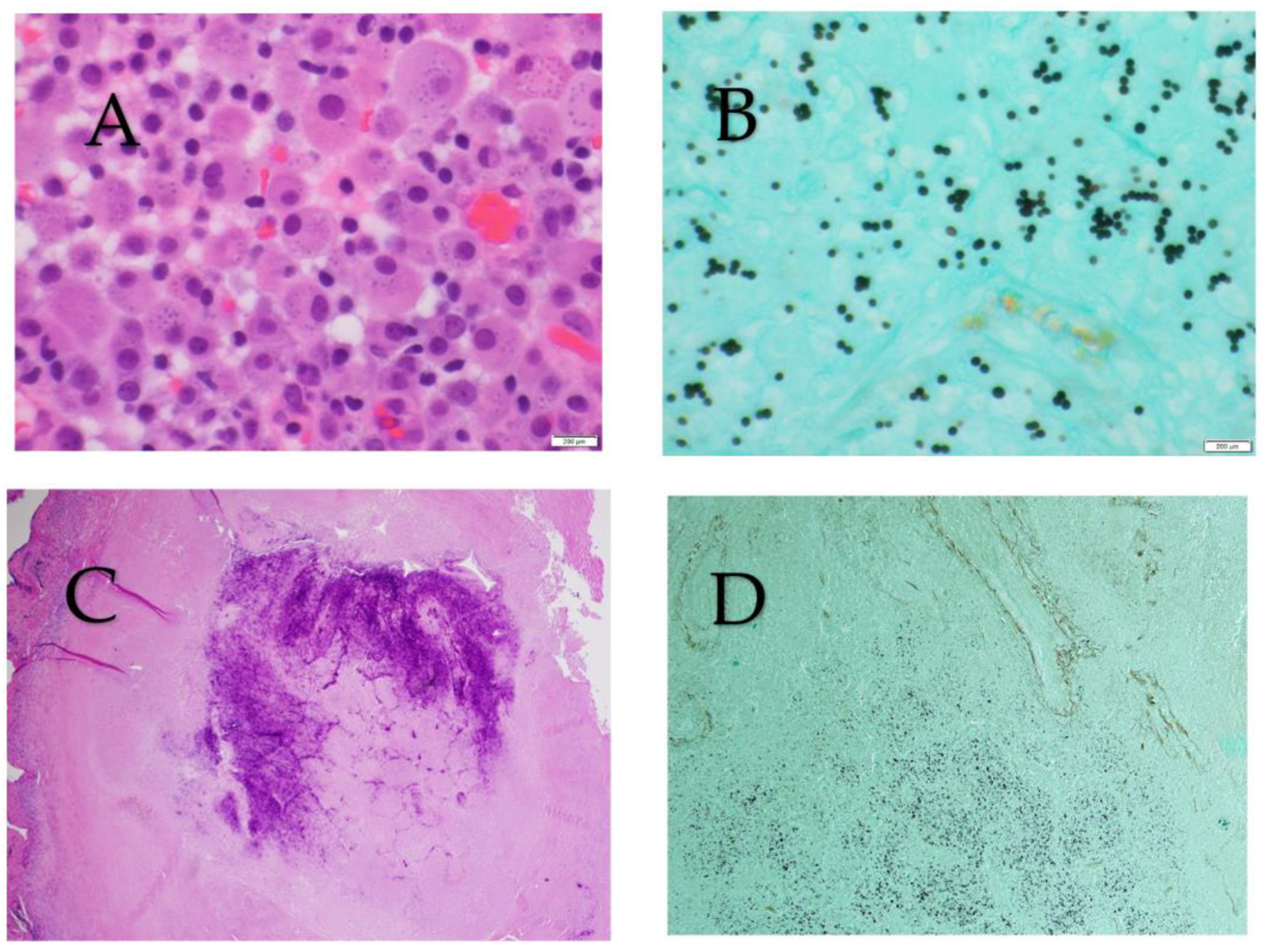
3.1. Culture, Histopathology, and Cytopathology
| Pulmonary | Mediastinal | ||||||
|---|---|---|---|---|---|---|---|
| Method | Acute | Subacute | Chronic Cavitary | Adenitis * | Granuloma * | Fibrosis * | Progressive Disseminated |
| Antigen | 83% | 30% | 88% | May be positive | Usually negative | Negative | 92% |
| Antibody | 64% | 95% | 83% | Usually positive | Usually positive | Usually positive | 75% |
| Pathology | 20% | 42% | 75% | May be positive | May be positive | Uncommonly positive | 76% |
| Culture | 42% | 54% | 67% | May be positive | Uncommonly positive | Negative | 74% |
3.2. Antigen Culture, Histopathology, and Cytopathology
3.3. Serology
3.4. Molencular-Based Diagnostics
4. Clinical Presentation and Management
4.1. Pulmonary Histoplasmosis
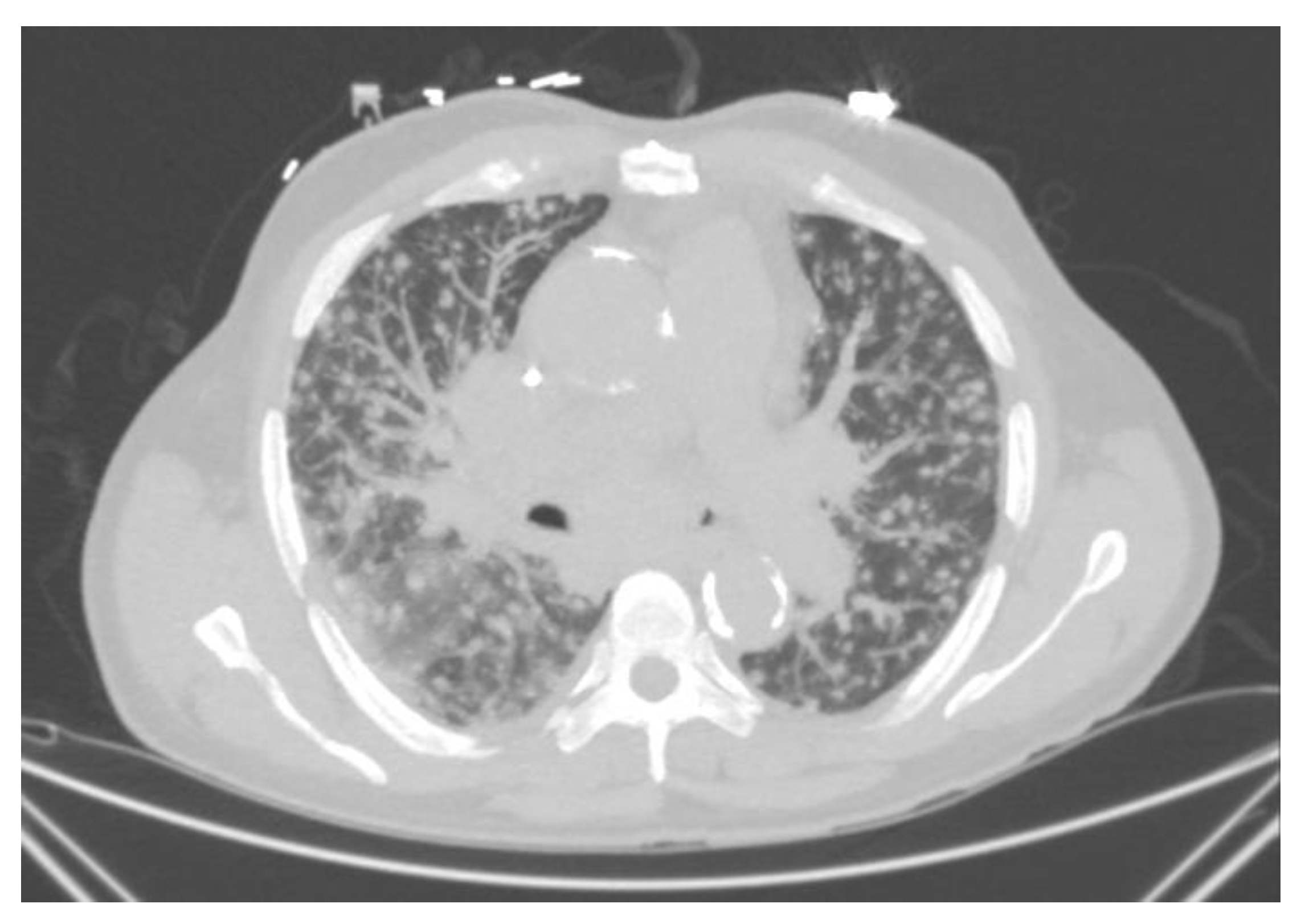
4.1.1. Acute and Subacute Pulmonary Histoplasmosis
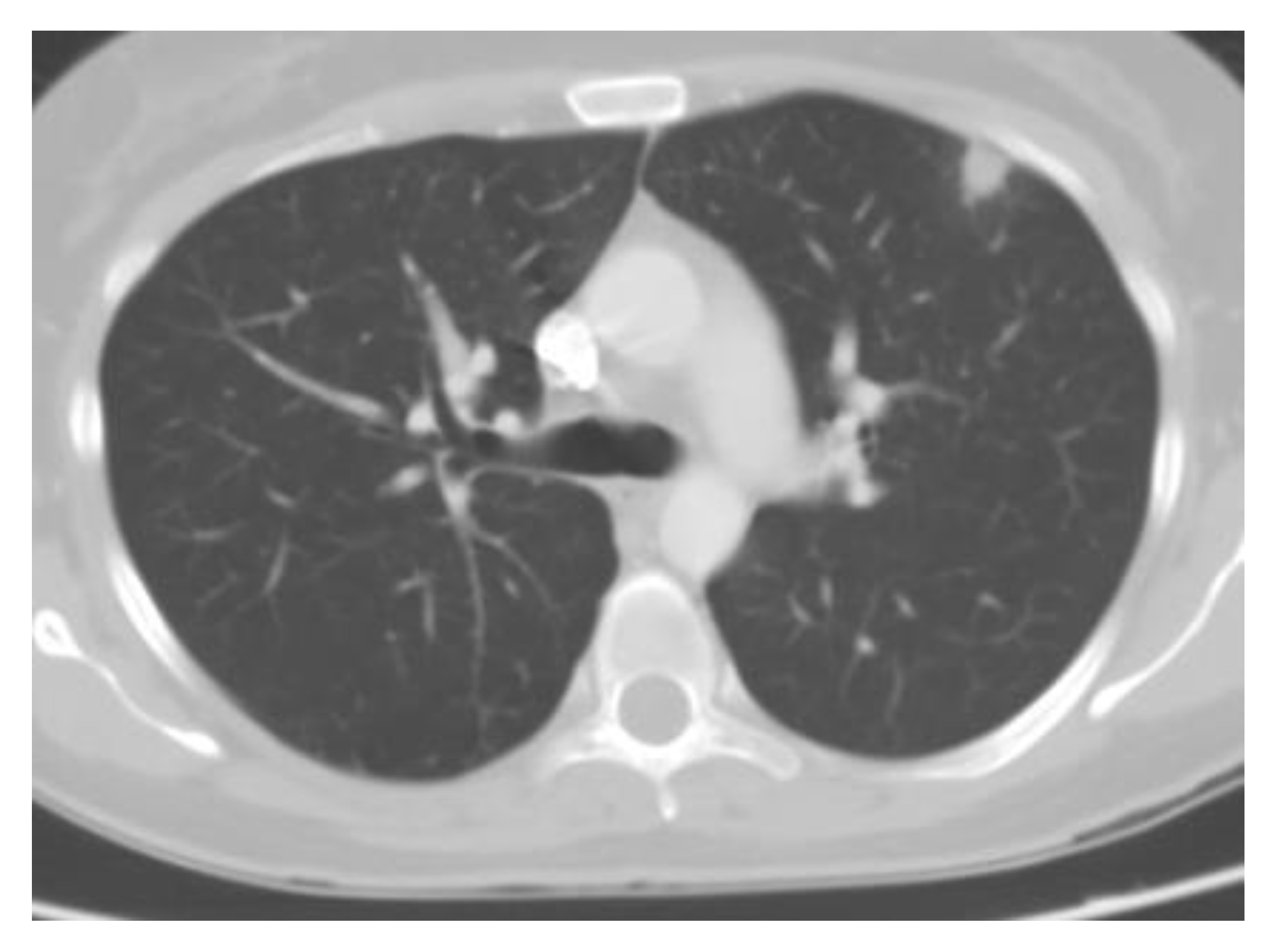
4.1.2. Pulmonary Nodules
4.1.3. Chronic Cavitary Pulmonary Histoplasmosis
5. Mediastinal Histoplasmosis
5.1. Mediastinal Lymphadenopathy (or Mediastinal Adenitis)
5.2. Mediastinal Granuloma
5.3. Fibrosing Mediastinitis
6. Progressive Disseminated Histoplasmosis
7. Other Considerations: Therapeutic Drug Monitoring
8. Special Populations
8.1. Solid Organ Transplant Recipients
8.2. Hematological Malignancies and Stem Cell Transplant Recipients
8.3. Human Immunodeficiency
8.4. Biological and Small Molecule Targeted Immunomodulatory Therapies
9. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wheat, L.J.; Azar, M.M.; Bahr, N.C.; Spec, A.; Relich, R.F.; Hage, C. Histoplasmosis. Infect. Dis. Clin. N. Am. 2016, 30, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Baddley, J.W.; Winthrop, K.L.; Patkar, N.M.; Delzell, E.; Beukelman, T.; Xie, F.; Chen, L.; Curtis, J.R. Geographic distribution of endemic fungal infections among older persons, United States. Emerg. Infect. Dis. 2011, 17, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.B.; Acquaviva, F.A.; Livesay, V.T.; Cross, F.W.; Palmer, C.E. An atlas of sensitivity to tuberculin, PPD-B, and histoplasmin in the United States. Am. Rev. Respir. Dis. 1969, 99 (Suppl. 1), 132. [Google Scholar]
- Bahr, N.C.; Antinori, S.; Wheat, L.J.; Sarosi, G.A. Histoplasmosis infections worldwide: Thinking outside of the Ohio River valley. Curr. Trop. Med. Rep. 2015, 2, 70–80. [Google Scholar] [CrossRef]
- Mazi, P.B.; Sahrmann, J.M.; Olsen, M.A.; Coler-Reilly, A.; Rauseo, A.M.; Pullen, M.; Zuniga-Moya, J.C.; Powderly, W.G.; Spec, A. The Geographic Distribution of Dimorphic Mycoses in the United States for the Modern Era. Clin. Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Adenis, A.A.; Valdes, A.; Cropet, C.; McCotter, O.Z.; Derado, G.; Couppie, P.; Chiller, T.; Nacher, M. Burden of HIV-associated histoplasmosis compared with tuberculosis in Latin America: A modelling study. Lancet Infect. Dis. 2018, 18, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, N.; Kubat, R.C.; Poplin, V.; Adenis, A.A.; Denning, D.W.; Wright, L.; McCotter, O.; Schwartz, I.S.; Jackson, B.R.; Chiller, T.; et al. Re-drawing the Maps for Endemic Mycoses. Mycopathologia 2020, 185, 843–865. [Google Scholar] [CrossRef]
- Myint, T.; Leedy, N.; Villacorta Cari, E.; Wheat, L.J. HIV-Associated Histoplasmosis: Current Perspectives. HIV AIDS 2020, 12, 113–125. [Google Scholar] [CrossRef]
- Colombo, A.L.; Tobon, A.; Restrepo, A.; Queiroz-Telles, F.; Nucci, M. Epidemiology of endemic systemic fungal infections in Latin America. Med. Mycol. 2011, 49, 785–798. [Google Scholar] [CrossRef]
- Mittal, J.; Ponce, M.G.; Gendlina, I.; Nosanchuk, J.D. Histoplasma capsulatum: Mechanisms for Pathogenesis. Curr. Top. Microbiol. Immunol. 2019, 422, 157–191. [Google Scholar] [CrossRef]
- Valdez, A.F.; Miranda, D.Z.; Guimaraes, A.J.; Nimrichter, L.; Nosanchuk, J.D. Pathogenicity & virulence of Histoplasma capsulatum—A multifaceted organism adapted to intracellular environments. Virulence 2022, 13, 1900–1919. [Google Scholar] [CrossRef]
- Fregonezi, N.F.; Oliveira, L.T.; Singulani, J.L.; Marcos, C.M.; Dos Santos, C.T.; Taylor, M.L.; Mendes-Giannini, M.J.S.; de Oliveira, H.C.; Fusco-Almeida, A.M. Heat Shock Protein 60, Insights to Its Importance in Histoplasma capsulatum: From Biofilm Formation to Host-Interaction. Front. Cell Infect. Microbiol. 2020, 10, 591950. [Google Scholar] [CrossRef] [PubMed]
- Nosanchuk, J.D.; Gacser, A. Histoplasma capsulatum at the host-pathogen interface. Microbes Infect. 2008, 10, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Gildea, L.A.; Morris, R.E.; Newman, S.L. Histoplasma capsulatum yeasts are phagocytosed via very late antigen-5, killed, and processed for antigen presentation by human dendritic cells. J. Immunol. 2001, 166, 1049–1056. [Google Scholar] [CrossRef]
- McDermott, A.J.; Klein, B.S. Helper T-cell responses and pulmonary fungal infections. Immunology 2018, 155, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Arias, J.D.; Mejia, S.P.; Gonzalez, A. The Role of the Interleukin-17 Axis and Neutrophils in the Pathogenesis of Endemic and Systemic Mycoses. Front. Cell Infect. Microbiol. 2020, 10, 595301. [Google Scholar] [CrossRef] [PubMed]
- Leopold Wager, C.M.; Wormley, F.L., Jr. Classical versus alternative macrophage activation: The Ying and the Yang in host defense against pulmonary fungal infections. Mucosal Immunol. 2014, 7, 1023–1035. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef]
- Azar, M.M.; Hage, C.A. Laboratory Diagnostics for Histoplasmosis. J. Clin. Microbiol. 2017, 55, 1612–1620. [Google Scholar] [CrossRef]
- Stockman, L.; Clark, K.A.; Hunt, J.M.; Roberts, G.D. Evaluation of commercially available acridinium ester-labeled chemiluminescent DNA probes for culture identification of Blastomyces dermatitidis, Coccidioides immitis, Cryptococcus neoformans, and Histoplasma capsulatum. J. Clin. Microbiol. 1993, 31, 845–850. [Google Scholar] [CrossRef]
- Valero, C.; Buitrago, M.J.; Gago, S.; Quiles-Melero, I.; Garcia-Rodriguez, J. A matrix-assisted laser desorption/ionization time of flight mass spectrometry reference database for the identification of Histoplasma capsulatum. Med. Mycol. 2018, 56, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.A.; Ribes, J.A.; Wengenack, N.L.; Baddour, L.M.; Assi, M.; McKinsey, D.S.; Hammoud, K.; Alapat, D.; Babady, N.E.; Parker, M.; et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin. Infect. Dis. 2011, 53, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Caceres, D.H.; Knuth, M.; Derado, G.; Lindsley, M.D. Diagnosis of Progressive Disseminated Histoplasmosis in Advanced HIV: A Meta-Analysis of Assay Analytical Performance. J. Fungi 2019, 5, 76. [Google Scholar] [CrossRef]
- Hage, C.A.; Azar, M.M.; Bahr, N.; Loyd, J.; Wheat, L.J. Histoplasmosis: Up-to-Date Evidence-Based Approach to Diagnosis and Management. Semin. Respir. Crit. Care Med. 2015, 36, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, C.A. Histoplasmosis: A clinical and laboratory update. Clin. Microbiol. Rev. 2007, 20, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.A.; Davis, T.E.; Fuller, D.; Egan, L.; Witt, J.R., 3rd; Wheat, L.J.; Knox, K.S. Diagnosis of histoplasmosis by antigen detection in BAL fluid. Chest 2010, 137, 623–628. [Google Scholar] [CrossRef]
- Martinez-Gamboa, A.; Niembro-Ortega, M.D.; Torres-Gonzalez, P.; Santiago-Cruz, J.; Velazquez-Zavala, N.G.; Rangel-Cordero, A.; Crabtree-Ramirez, B.; Gamboa-Dominguez, A.; Reyes-Gutierrez, E.; Reyes-Teran, G.; et al. Diagnostic accuracy of antigen detection in urine and molecular assays testing in different clinical samples for the diagnosis of progressive disseminated histoplasmosis in patients living with HIV/AIDS: A prospective multicenter study in Mexico. PLoS Negl. Trop. Dis. 2021, 15, e0009215. [Google Scholar] [CrossRef]
- Wheat, L.J.; Conces, D.; Allen, S.D.; Blue-Hnidy, D.; Loyd, J. Pulmonary histoplasmosis syndromes: Recognition, diagnosis, and management. Semin. Respir. Crit. Care Med. 2004, 25, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Swartzentruber, S.; Rhodes, L.; Kurkjian, K.; Zahn, M.; Brandt, M.E.; Connolly, P.; Wheat, L.J. Diagnosis of acute pulmonary histoplasmosis by antigen detection. Clin. Infect. Dis. 2009, 49, 1878–1882. [Google Scholar] [CrossRef]
- Bloch, K.C.; Myint, T.; Raymond-Guillen, L.; Hage, C.A.; Davis, T.E.; Wright, P.W.; Chow, F.C.; Woc-Colburn, L.; Khairy, R.N.; Street, A.C.; et al. Improvement in Diagnosis of Histoplasma Meningitis by Combined Testing for Histoplasma Antigen and Immunoglobulin G and Immunoglobulin M Anti-Histoplasma Antibody in Cerebrospinal Fluid. Clin. Infect. Dis. 2018, 66, 89–94. [Google Scholar] [CrossRef]
- Myint, T.; Anderson, A.M.; Sanchez, A.; Farabi, A.; Hage, C.; Baddley, J.W.; Jhaveri, M.; Greenberg, R.N.; Bamberger, D.M.; Rodgers, M.; et al. Histoplasmosis in patients with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS): Multicenter study of outcomes and factors associated with relapse. Medicine 2014, 93, 11–18. [Google Scholar] [CrossRef]
- Segal, B.H.; Herbrecht, R.; Stevens, D.A.; Ostrosky-Zeichner, L.; Sobel, J.; Viscoli, C.; Walsh, T.J.; Maertens, J.; Patterson, T.F.; Perfect, J.R.; et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin. Infect. Dis. 2008, 47, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Wheat, L.J.; Connolly, P.; Haddad, N.; Le Monte, A.; Brizendine, E.; Hafner, R. Antigen clearance during treatment of disseminated histoplasmosis with itraconazole versus fluconazole in patients with AIDS. Antimicrob. Agents Chemother. 2002, 46, 248–250. [Google Scholar] [CrossRef]
- Ghorra, N.; Goushchi, A.; Konopnicki, D.; Libois, A.; Lagrou, K.; Wind, A.; Montesinos, I.; Hallin, M.; Deyi, V.Y.M. Disseminated histoplasmosis diagnosed by cross-reactivity with the Aspergillus galactomannan antigen in an HIV-positive patient. J. Mycol. Med. 2022, 32, 101244. [Google Scholar] [CrossRef]
- Wheat, J.; Wheat, H.; Connolly, P.; Kleiman, M.; Supparatpinyo, K.; Nelson, K.; Bradsher, R.; Restrepo, A. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin. Infect. Dis. 1997, 24, 1169–1171. [Google Scholar] [CrossRef]
- Kuberski, T.; Myers, R.; Wheat, L.J.; Durkin, M.; Connolly, P.; Kubak, B.M.; Bruckner, D.; Pegues, D. Diagnosis of coccidioidomycosis by antigen detection using cross-reaction with a Histoplasma antigen. Clin. Infect. Dis. 2007, 44, e50–e54. [Google Scholar] [CrossRef]
- Assi, M.; Lakkis, I.E.; Wheat, L.J. Cross-reactivity in the Histoplasma antigen enzyme immunoassay caused by sporotrichosis. Clin. Vaccine Immunol. 2011, 18, 1781–1782. [Google Scholar] [CrossRef]
- Wheat, J.; French, M.L.; Kohler, R.B.; Zimmerman, S.E.; Smith, W.R.; Norton, J.A.; Eitzen, H.E.; Smith, C.D.; Slama, T.G. The diagnostic laboratory tests for histoplasmosis: Analysis of experience in a large urban outbreak. Ann. Intern. Med. 1982, 97, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Richer, S.M.; Smedema, M.L.; Durkin, M.M.; Herman, K.M.; Hage, C.A.; Fuller, D.; Wheat, L.J. Improved Diagnosis of Acute Pulmonary Histoplasmosis by Combining Antigen and Antibody Detection. Clin. Infect. Dis. 2016, 62, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Veron, V.; Boukhari, R.; Blanchet, D.; Aznar, C. Detection of Histoplasma capsulatum DNA in human samples by real-time polymerase chain reaction. Diagn. Microbiol. Infect. Dis. 2010, 66, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.W.; Li, H.; Durkin, M.M.; Sefers, S.E.; Meng, S.; Connolly, P.A.; Stratton, C.W.; Wheat, L.J. Urine polymerase chain reaction is not as sensitive as urine antigen for the diagnosis of disseminated histoplasmosis. Diagn. Microbiol. Infect. Dis. 2006, 54, 283–287. [Google Scholar] [CrossRef]
- Gomez, C.A.; Budvytiene, I.; Zemek, A.J.; Banaei, N. Performance of Targeted Fungal Sequencing for Culture-Independent Diagnosis of Invasive Fungal Disease. Clin. Infect. Dis. 2017, 65, 2035–2041. [Google Scholar] [CrossRef]
- Tsang, C.C.; Teng, J.L.L.; Lau, S.K.P.; Woo, P.C.Y. Rapid Genomic Diagnosis of Fungal Infections in the Age of Next-Generation Sequencing. J. Fungi 2021, 7, 636. [Google Scholar] [CrossRef]
- Chang, B.; Saleh, T.; Wales, C.; Kuklinski, L.; Malla, P.; Yang, S.; Fuller, D.; Nielsen-Saines, K. Case report: Disseminated histoplasmosis in a renal transplant recipient from a non-endemic region. Front. Pediatr. 2022, 10, 985475. [Google Scholar] [CrossRef]
- Wheat, L.J.; Wass, J.; Norton, J.; Kohler, R.B.; French, M.L. Cavitary histoplasmosis occurring during two large urban outbreaks. Analysis of clinical, epidemiologic, roentgenographic, and laboratory features. Medicine 1984, 63, 201–209. [Google Scholar] [CrossRef]
- Wheat, L.J.; Slama, T.G.; Eitzen, H.E.; Kohler, R.B.; French, M.L.; Biesecker, J.L. A large urban outbreak of histoplasmosis: Clinical features. Ann. Intern. Med. 1981, 94, 331–337. [Google Scholar] [CrossRef]
- Sepulveda, V.E.; Williams, C.L.; Goldman, W.E. Comparison of phylogenetically distinct Histoplasma strains reveals evolutionarily divergent virulence strategies. mBio 2014, 5, e01376-14. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.A.; Knox, K.S.; Wheat, L.J. Endemic mycoses: Overlooked causes of community acquired pneumonia. Respir. Med. 2012, 106, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.C.; Arakkal, A.T.; Koeneman, S.H.; Cavanaugh, J.E.; Thompson, G.R.; Baddley, J.W.; Polgreen, P.M. Frequency and Duration of, and Risk Factors for, Diagnostic Delays Associated with Histoplasmosis. J. Fungi 2022, 8, 438. [Google Scholar] [CrossRef]
- Sizemore, T.C. Rheumatologic manifestations of histoplasmosis: A review. Rheumatol. Int. 2013, 33, 2963–2965. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Marchiori, E.; Youssef, A.; Mohammed, T.L.; Patel, P.; Irion, K.; Pasini, R.; Mancano, A.; Souza, A.; Pasqualotto, A.C.; et al. Chest Imaging in Systemic Endemic Mycoses. J. Fungi 2022, 8, 1132. [Google Scholar] [CrossRef]
- Kunin, J.R.; Flors, L.; Hamid, A.; Fuss, C.; Sauer, D.; Walker, C.M. Thoracic Endemic Fungi in the United States: Importance of Patient Location. Radiographics 2021, 41, 380–398. [Google Scholar] [CrossRef]
- Wheat, L.J.; Freifeld, A.G.; Kleiman, M.B.; Baddley, J.W.; McKinsey, D.S.; Loyd, J.E.; Kauffman, C.A.; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007, 45, 807–825. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.N.; Lawrence, D.S.; Meya, D.B.; Kagimu, E.; Kasibante, J.; Mpoza, E.; Rutakingirwa, M.K.; Ssebambulidde, K.; Tugume, L.; Rhein, J.; et al. Single-Dose Liposomal Amphotericin B Treatment for Cryptococcal Meningitis. N. Engl. J. Med. 2022, 386, 1109–1120. [Google Scholar] [CrossRef]
- Reed, R.M.; Amoroso, A.; Hashmi, S.; Kligerman, S.; Shuldiner, A.R.; Mitchell, B.D.; Netzer, G. Calcified granulomatous disease: Occupational associations and lack of familial aggregation. Lung 2014, 192, 841–847. [Google Scholar] [CrossRef]
- Mashburn, J.D.; Dawson, D.F.; Young, J.M. Pulmonary calcifications and histoplasmosis. Am. Rev. Respir. Dis. 1961, 84, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Straub, M.; Schwarz, J. The healed primary complex in histoplasmosis. Am. J. Clin. Pathol. 1955, 25, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Wilcox, B.E.; Myers, J.L.; Bryant, S.C.; Buckwalter, S.P.; Wengenack, N.L.; Yi, E.S.; Aughenbaugh, G.L.; Specks, U.; Aubry, M.C. Pulmonary necrotizing granulomas of unknown cause: Clinical and pathologic analysis of 131 patients with completely resected nodules. Chest 2013, 144, 813–824. [Google Scholar] [CrossRef]
- Azour, L.; Ko, J.P.; Washer, S.L.; Lanier, A.; Brusca-Augello, G.; Alpert, J.B.; Moore, W.H. Incidental Lung Nodules on Cross-sectional Imaging: Current Reporting and Management. Radiol. Clin. N. Am. 2021, 59, 535–549. [Google Scholar] [CrossRef]
- Earp, E.; Gordon, P.M.; Tan, A.; Page, I.; Thum, C.K.; Mackenzie, A.I.; Johnson, E.; Biswas, A. Disseminated Mucocutaneous Histoplasmosis Diagnosed in the United Kingdom, Presumably as a Result of Recrudescence Decades after Primary Infection Following Immunosuppressive Treatment of Its Mimic, Sarcoidosis: A Multidisciplinary Cautionary Tale. Am. J. Derm. 2022, 44, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Croft, D.R.; Trapp, J.; Kernstine, K.; Kirchner, P.; Mullan, B.; Galvin, J.; Peterson, M.W.; Gross, T.; McLennan, G.; Kern, J.A. FDG-PET imaging and the diagnosis of non-small cell lung cancer in a region of high histoplasmosis prevalence. Lung Cancer 2002, 36, 297–301. [Google Scholar] [CrossRef]
- Kadaria, D.; Archie, D.S.; SultanAli, I.; Weiman, D.S.; Freire, A.X.; Zaman, M.K. Dual time point positron emission tomography/computed tomography scan in evaluation of intrathoracic lesions in an area endemic for histoplasmosis and with high prevalence of sarcoidosis. Am. J. Med. Sci. 2013, 346, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Deppen, S.A.; Massion, P.P.; Blume, J.; Walker, R.C.; Antic, S.; Chen, H.; Durkin, M.M.; Wheat, L.J.; Grogan, E.L. Accuracy of a Novel Histoplasmosis Enzyme Immunoassay to Evaluate Suspicious Lung Nodules. Cancer Epidemiol. Biomark. Prev. 2019, 28, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Nasim, F.; Ost, D.E. Management of the solitary pulmonary nodule. Curr. Opin. Pulm. Med. 2019, 25, 344–353. [Google Scholar] [CrossRef]
- Georgiadou, S.P.; Sampsonas, F.L.; Rice, D.; Granger, J.M.; Swisher, S.; Kontoyiannis, D.P. Open-lung biopsy in patients with undiagnosed lung lesions referred at a tertiary cancer center is safe and reveals noncancerous, noninfectious entities as the most common diagnoses. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Connell, J.V.; Muhm, J.R. Radiographic manifestations of pulmonary histoplasmosis: A 10-year review. Radiology 1976, 121, 281–285. [Google Scholar] [CrossRef]
- Kennedy, C.C.; Limper, A.H. Redefining the clinical spectrum of chronic pulmonary histoplasmosis: A retrospective case series of 46 patients. Medicine 2007, 86, 252–258. [Google Scholar] [CrossRef]
- Furcolow, M.L.; Brasher, C.A. Chronic progressive (cavitary) histoplasmosis as a problem in tuberculosis sanatoriums. Am. Rev. Tuberc. 1956, 73, 609–619. [Google Scholar] [CrossRef]
- Goodwin, R.A., Jr.; Owens, F.T.; Snell, J.D.; Hubbard, W.W.; Buchanan, R.D.; Terry, R.T.; Des Prez, R.M. Chronic pulmonary histoplasmosis. Medicine 1976, 55, 413–452. [Google Scholar] [CrossRef]
- Baker, J.; Kosmidis, C.; Rozaliyani, A.; Wahyuningsih, R.; Denning, D.W. Chronic Pulmonary Histoplasmosis—A Scoping Literature Review. Open Forum Infect. Dis. 2020, 7, ofaa119. [Google Scholar] [CrossRef]
- Furcolow, M.L. Comparison of Treated and Untreated Severe Histoplasmosis: A Communicable Disease Center Cooperative Mycoses Study. JAMA 1963, 183, 823–829. [Google Scholar] [CrossRef]
- Parish, J.M.; Rosenow, E.C., 3rd. Mediastinal granuloma and mediastinal fibrosis. Semin. Respir. Crit. Care Med. 2002, 23, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Loyd, J.E.; Tillman, B.F.; Atkinson, J.B.; Des Prez, R.M. Mediastinal fibrosis complicating histoplasmosis. Medicine 1988, 67, 295–310. [Google Scholar] [CrossRef]
- Peebles, R.S.; Carpenter, C.T.; Dupont, W.D.; Loyd, J.E. Mediastinal fibrosis is associated with human leukocyte antigen-A2. Chest 2000, 117, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Strock, S.B.; Gaudieri, S.; Mallal, S.; Yu, C.; Mitchell, D.; Cogan, J.; Mason, W.; Crowe, D.; Loyd, J.E. Fibrosing mediastinitis complicating prior histoplasmosis is associated with human leukocyte antigen DQB1*04:02—A case control study. BMC Infect. Dis. 2015, 15, 206. [Google Scholar] [CrossRef]
- Goodwin, R.A.; Nickell, J.A.; Des Prez, R.M. Mediastinal fibrosis complicating healed primary histoplasmosis and tuberculosis. Medicine 1972, 51, 227–246. [Google Scholar] [CrossRef]
- Peikert, T.; Colby, T.V.; Midthun, D.E.; Pairolero, P.C.; Edell, E.S.; Schroeder, D.R.; Specks, U. Fibrosing mediastinitis: Clinical presentation, therapeutic outcomes, and adaptive immune response. Medicine 2011, 90, 412–423. [Google Scholar] [CrossRef]
- Westerly, B.D.; Johnson, G.B.; Maldonado, F.; Utz, J.P.; Specks, U.; Peikert, T. Targeting B lymphocytes in progressive fibrosing mediastinitis. Am. J. Respir. Crit. Care Med. 2014, 190, 1069–1071. [Google Scholar] [CrossRef]
- Koene, R.J.; Catanese, J.; Sarosi, G.A. Adrenal hypofunction from histoplasmosis: A literature review from 1971 to 2012. Infection 2013, 41, 757–759. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Ivancic, S.; Eckardt, P.A.; Lemos-Ramirez, J.C.; Niu, J. A Case of Pulmonary Histoplasmosis Presenting with Hypercalcemia and Altered Mental Status in a Patient Following Allogeneic Hematopoietic Stem Cell Transplantation. Am. J. Case Rep. 2020, 21, e919724. [Google Scholar] [CrossRef]
- Johnson, P.C.; Wheat, L.J.; Cloud, G.A.; Goldman, M.; Lancaster, D.; Bamberger, D.M.; Powderly, W.G.; Hafner, R.; Kauffman, C.A.; Dismukes, W.E.; et al. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann. Intern. Med. 2002, 137, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Huprikar, S.; Burdette, S.D.; Morris, M.I.; Blair, J.E.; Wheat, L.J.; American Society of Transplantation, Infectious Diseases Community of Practice, Donor-Derived Fungal Infection Working Group. Donor-derived fungal infections in organ transplant recipients: Guidelines of the American Society of Transplantation, infectious diseases community of practice. Am. J. Transpl. 2012, 12, 2414–2428. [Google Scholar] [CrossRef]
- Gupta, A.O.; Singh, N. Immune reconstitution syndrome and fungal infections. Curr. Opin. Infect. Dis. 2011, 24, 527–533. [Google Scholar] [CrossRef]
- Andes, D.; Pascual, A.; Marchetti, O. Antifungal therapeutic drug monitoring: Established and emerging indications. Antimicrob Agents Chemother. 2009, 53, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Laverdiere, M.; Bow, E.J.; Rotstein, C.; Autmizguine, J.; Broady, R.; Garber, G.; Haider, S.; Hussaini, T.; Husain, S.; Ovetchkine, P.; et al. Therapeutic drug monitoring for triazoles: A needs assessment review and recommendations from a Canadian perspective. Can. J. Infect. Dis. Med. Microbiol. 2014, 25, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Ashbee, H.R.; Barnes, R.A.; Johnson, E.M.; Richardson, M.D.; Gorton, R.; Hope, W.W. Therapeutic drug monitoring (TDM) of antifungal agents: Guidelines from the British Society for Medical Mycology. J. Antimicrob. Chemother. 2014, 69, 1162–1176. [Google Scholar] [CrossRef]
- Park, W.B.; Kim, N.H.; Kim, K.H.; Lee, S.H.; Nam, W.S.; Yoon, S.H.; Song, K.H.; Choe, P.G.; Kim, N.J.; Jang, I.J.; et al. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: A randomized controlled trial. Clin. Infect. Dis. 2012, 55, 1080–1087. [Google Scholar] [CrossRef]
- Pascual, A.; Calandra, T.; Bolay, S.; Buclin, T.; Bille, J.; Marchetti, O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 2008, 46, 201–211. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Hoenigl, M.; Sprute, R.; Egger, M.; Arastehfar, A.; Cornely, O.A.; Krause, R.; Lass-Florl, C.; Prattes, J.; Spec, A.; Thompson, G.R., 3rd; et al. The Antifungal Pipeline: Fosmanogepix, Ibrexafungerp, Olorofim, Opelconazole, and Rezafungin. Drugs 2021, 81, 1703–1729. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef]
- Neofytos, D.; Fishman, J.A.; Horn, D.; Anaissie, E.; Chang, C.H.; Olyaei, A.; Pfaller, M.; Steinbach, W.J.; Webster, K.M.; Marr, K.A. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl. Infect. Dis. 2010, 12, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Rodriguez, J.; Avery, R.K.; Lard, M.; Budev, M.; Gordon, S.M.; Shrestha, N.K.; van Duin, D.; Oethinger, M.; Mawhorter, S.D. Histoplasmosis in solid organ transplant recipients: 10 years of experience at a large transplant center in an endemic area. Clin. Infect. Dis. 2009, 49, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Saullo, J.L.; Miller, R.A. Updates on Histoplasmosis in Solid Organ Transplantation. Curr. Fungal Infect. Rep. 2022, 16, 165–178. [Google Scholar] [CrossRef]
- Assi, M.; Martin, S.; Wheat, L.J.; Hage, C.; Freifeld, A.; Avery, R.; Baddley, J.W.; Vergidis, P.; Miller, R.; Andes, D.; et al. Histoplasmosis after solid organ transplant. Clin. Infect. Dis. 2013, 57, 1542–1549. [Google Scholar] [CrossRef]
- Vail, G.M.; Young, R.S.; Wheat, L.J.; Filo, R.S.; Cornetta, K.; Goldman, M. Incidence of histoplasmosis following allogeneic bone marrow transplant or solid organ transplant in a hyperendemic area. Transpl. Infect. Dis. 2002, 4, 148–151. [Google Scholar] [CrossRef]
- Miller, R.; Assi, M.; AST Infectious Diseases Community of Practice. Endemic fungal infections in solid organ transplant recipients-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transpl. 2019, 33, e13553. [Google Scholar] [CrossRef]
- Gajurel, K.; Dhakal, R.; Deresinski, S. Histoplasmosis in transplant recipients. Clin. Transpl. 2017, 31, e13087. [Google Scholar] [CrossRef]
- Natarajan, M.; Swierzbinski, M.J.; Maxwell, S.; Zelazny, A.M.; Fahle, G.A.; Quezado, M.; Barrett, J.; Battiwalla, M.; Lionakis, M.S. Pulmonary Histoplasma Infection After Allogeneic Hematopoietic Stem Cell Transplantation: Case Report and Review of the Literature. Open Forum Infect. Dis. 2017, 4, ofx041. [Google Scholar] [CrossRef]
- Shahani, L. Disseminated histoplasmosis mimicking relapsed chronic lymphocytic leukaemia. BMJ Case Rep. 2018, 2018, 224498. [Google Scholar] [CrossRef]
- Limper, A.H.; Adenis, A.; Le, T.; Harrison, T.S. Fungal infections in HIV/AIDS. Lancet Infect. Dis. 2017, 17, e334–e343. [Google Scholar] [CrossRef] [PubMed]
- McKinsey, D.S.; Spiegel, R.A.; Hutwagner, L.; Stanford, J.; Driks, M.R.; Brewer, J.; Gupta, M.R.; Smith, D.L.; O’Connor, M.C.; Dall, L. Prospective study of histoplasmosis in patients infected with human immunodeficiency virus: Incidence, risk factors, and pathophysiology. Clin. Infect. Dis. 1997, 24, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Baddley, J.W. Guideline for Diagnosing and Managing Disseminated Histoplasmosis among People Living with HIV; WHO: Washington, DC, USA, 2020. [CrossRef]
- Allendoerfer, R.; Deepe, G.S., Jr. Blockade of endogenous TNF-alpha exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J. Immunol. 1998, 160, 6072–6082. [Google Scholar] [CrossRef]
- Smith, J.A.; Kauffman, C.A. Endemic fungal infections in patients receiving tumour necrosis factor-alpha inhibitor therapy. Drugs 2009, 69, 1403–1415. [Google Scholar] [CrossRef]
- Vergidis, P.; Avery, R.K.; Wheat, L.J.; Dotson, J.L.; Assi, M.A.; Antoun, S.A.; Hamoud, K.A.; Burdette, S.D.; Freifeld, A.G.; McKinsey, D.S.; et al. Histoplasmosis complicating tumor necrosis factor-alpha blocker therapy: A retrospective analysis of 98 cases. Clin. Infect. Dis. 2015, 61, 409–417. [Google Scholar] [CrossRef]
- Davis, J.S.; Ferreira, D.; Paige, E.; Gedye, C.; Boyle, M. Infectious Complications of Biological and Small Molecule Targeted Immunomodulatory Therapies. Clin. Microbiol. Rev. 2020, 33, e00035-19. [Google Scholar] [CrossRef] [PubMed]




| Clinical Form | ||||
|---|---|---|---|---|
| Characteristic | Acute | Subacute | Nodular | Chronic Cavitary |
| Age | Any | Any | Any | >50-year-old with structural lung disease |
| Clinical manifestation | Fever, headache, dry cough, chills, chest pain, malaise, myalgias and arthritis | Same as acute but symptoms are milder | Usually asymptomatic | Fever, productive cough, dyspnea, weight loss, hemoptysis, night sweats, chest pain |
| Symptom duration | 1–2 weeks | Weeks to months | - | Months to years |
| Mimicked disease | Community acquired pneumonia | Community acquired pneumonia | Neoplasm | Tuberculosis, Sarcoidosis |
| Pathology | Granuloma with acute lung injury | Well-formed granulomas | Well-formed granulomas | Cavities with granulomas, tissue destruction |
| Radiologic findings | Diffuse bilateral patchy opacities | Focal or patchy opacities | Nodules | Cavitation, fibrosis, volume loss, pleural thickening. Right upper lobe is most commonly affected |
| Hilar and Mediastinal lymph nodes | Enlarged | Enlarged | Not enlarged | Not enlarged. Occasionally calcified |
| Calcifications | None | None | Present | Present |
| Indications for treatment | Severe disease | Symptoms over 1 month | None | Yes |
| Clinical Form | Treatment Recommendation |
|---|---|
| Pulmonary | |
| Acute—Mild to moderate | |
| Immunocompetent host | |
| <4 weeks | Usually unnecessary |
| >4 weeks | Itraconazole for 6–12 weeks |
| Immunocompromised host | |
| Regardless of duration | Itraconazole for 12 months |
| Acute—Moderately severe or severe | |
| Immunocompetent host | Lipid Amphotericin B for 1–2 weeks followed by Itraconazole for 12 weeks |
| Immunocompromised host | Lipid Amphotericin B for 1–2 weeks followed by Itraconazole for at least 12 |
| months and negative or low antigen (<2 ng/mL) | |
| Methylprednisolone 0.5–1 mg/Kg during the first 1–2 weeks if the patient | |
| develops ARDS | |
| Subacute | Itraconazole for 6–12 weeks |
| Nodular | None |
| Chronic cavitary | Itraconazole for at least 12 months |
| Mediastinal | |
| Adenitis | As acute pulmonary |
| Granuloma | |
| Asymptomatic | None |
| Symptomatic | Itraconazole for 6–12 weeks |
| Fibrosis | Symptomatic management (e.g., stents) |
| Antifungal therapy not recommended | |
| Can consider Rituximab in certain cases | |
| Progressive disseminated | |
| Mild to moderate | |
| Immunocompetent host | Itraconazole for 6–12 weeks |
| Immunocompromised host | Itraconazole for 12 months |
| Moderately severe or severe | |
| Immunocompetent host | Lipid Amphotericin B for 1–2 weeks followed by Itraconazole for 12 weeks |
| Immunocompromised host | Lipid Amphotericin B for 1–2 weeks followed by Itraconazole for at least 12 |
| months and negative or low antigen (<2 ng/mL) | |
| Methylprednisolone 0.5–1 mg/Kg during the first 1–2 weeks if the patient | |
| develops ARDS |
| Clinical Form | |||
|---|---|---|---|
| Characteristic | Adenitis | Granulomatous | Fibrosing |
| Age | Usually < 20 y | All ages > 2 y | Typically 20–30 y |
| Clinical manifestation | Usually detected during diagnosis of acute pulmonary histoplasmosis. Can have mild obstructive symptoms | Obstructive syndromes (e.g., SVC, dysphagia and chest pain can occur) | Obstructive syndromes (e.g., SVC, pulmonary artery veins, dysphagia, dyspnea) |
| Pathology | Granulomas | Granulomas with extensive necrosis | Extensive fibrosis with or without granulomas |
| Radiologic findings | Large nodes, not calcified | Large mass, subcapsular or diffuse calcifications | Proliferative calcified mediastinal nodes with obstruction |
| Calcifications | None | Usually | Extensive |
| Indication for treatment | As acute pulmonary | Obstruction or pain | Recurrent hemoptysis, obstruction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, N.; Wheat, J.L.; Hage, C. Pulmonary Histoplasmosis: A Clinical Update. J. Fungi 2023, 9, 236. https://doi.org/10.3390/jof9020236
Barros N, Wheat JL, Hage C. Pulmonary Histoplasmosis: A Clinical Update. Journal of Fungi. 2023; 9(2):236. https://doi.org/10.3390/jof9020236
Chicago/Turabian StyleBarros, Nicolas, Joseph L. Wheat, and Chadi Hage. 2023. "Pulmonary Histoplasmosis: A Clinical Update" Journal of Fungi 9, no. 2: 236. https://doi.org/10.3390/jof9020236
APA StyleBarros, N., Wheat, J. L., & Hage, C. (2023). Pulmonary Histoplasmosis: A Clinical Update. Journal of Fungi, 9(2), 236. https://doi.org/10.3390/jof9020236





