Molecular Regulatory Mechanism of the Iron-Ion-Promoted Asexual Sporulation of Antrodia cinnamomea in Submerged Fermentation Revealed by Comparative Transcriptomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain
2.2. Submerged Fermentation of A. cinnamomea
2.3. Effects of Different Concentrations of Fe2+ on the Sporulation and Biomass of A. cinnamomea
2.4. Sample Preparation for RNA-Seq
2.5. RNA-Seq and Bioinformatics Analysis
2.6. RT-qPCR Analysis
2.7. Statistical Analysis of Data
3. Results and Discussion
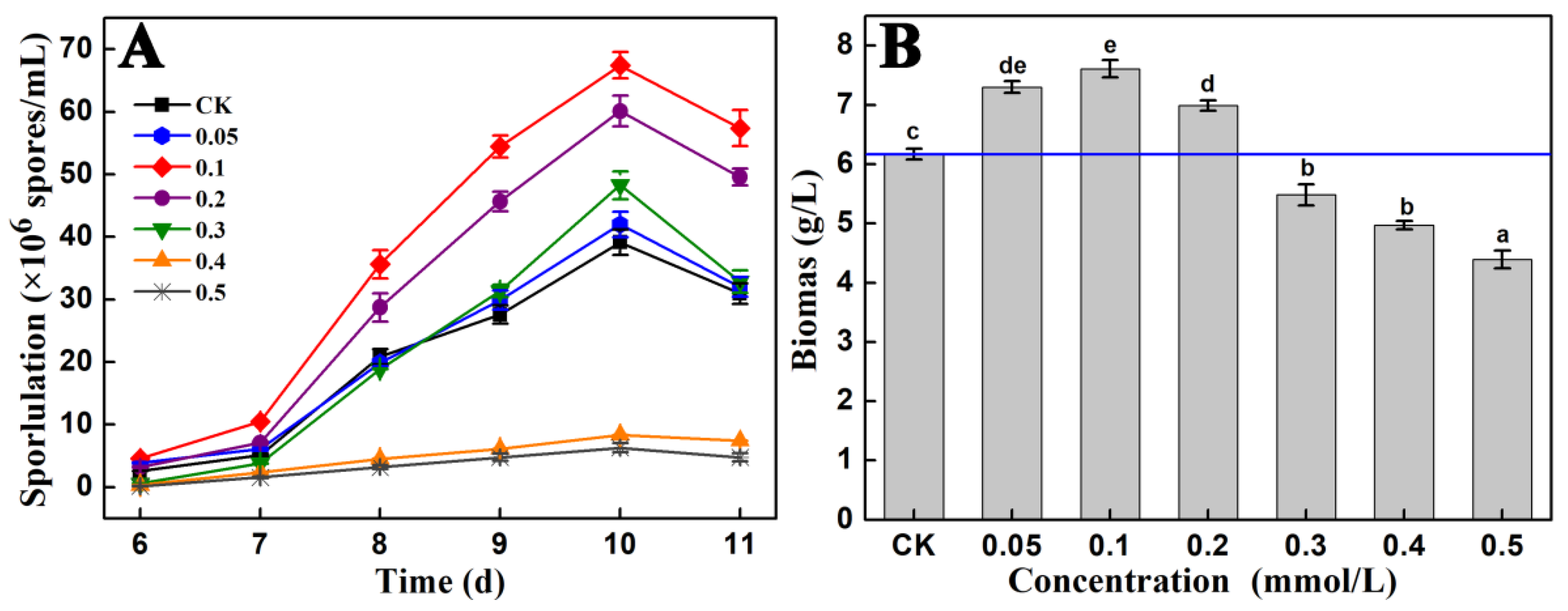
3.1. Effects of Fe2+ on the Sporulation Capacity of A. cinnamomea
3.2. RNA-Seq and Statistical Analysis
3.2.1. Preparation of Sequencing Samples
3.2.2. Statistical Analysis of Sample Repeatability and DEGs
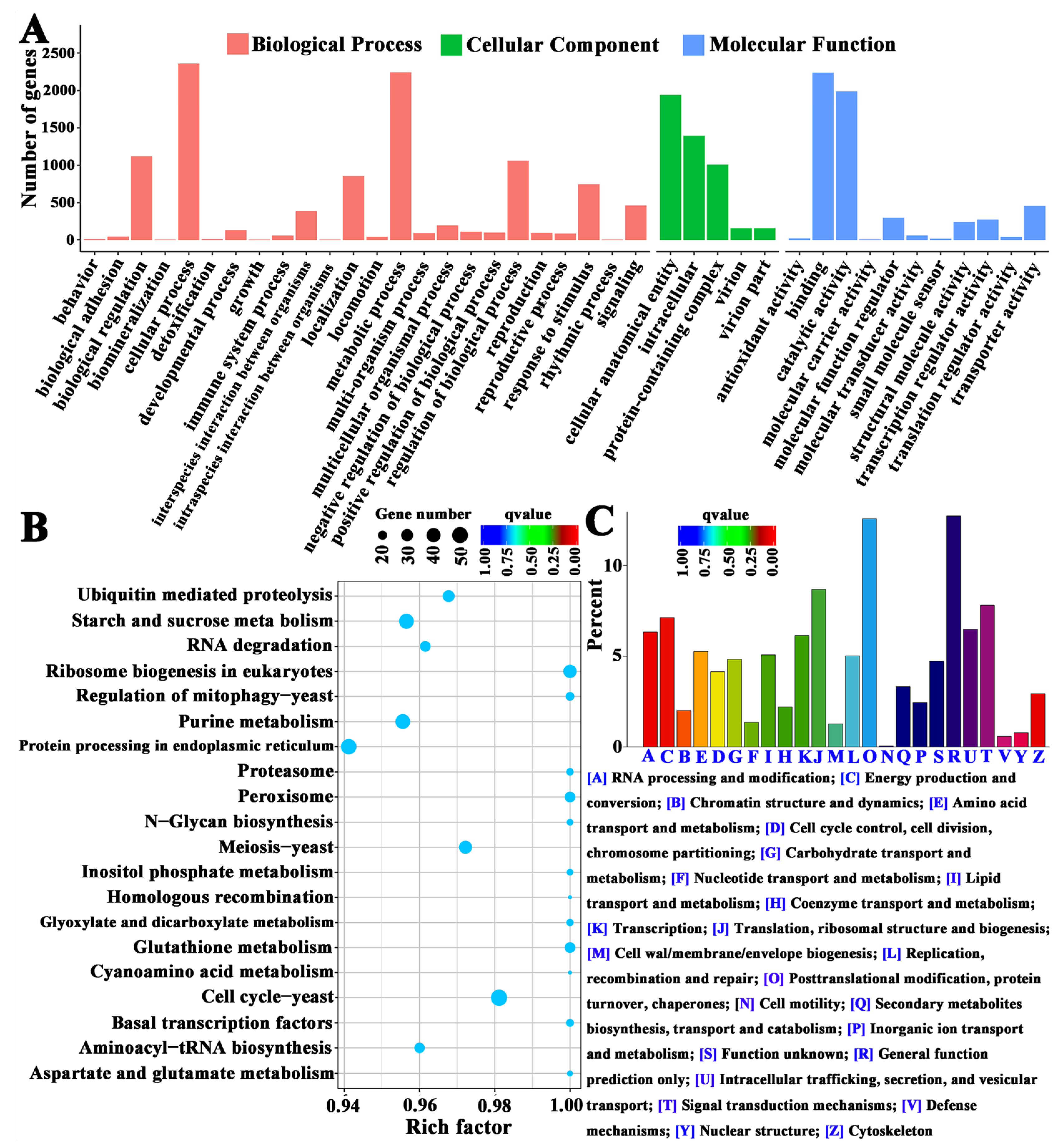
3.2.3. Enrichment Analysis of DEGs
3.3. Bioinformatic Analysis
3.4. RT-qPCR Analysis
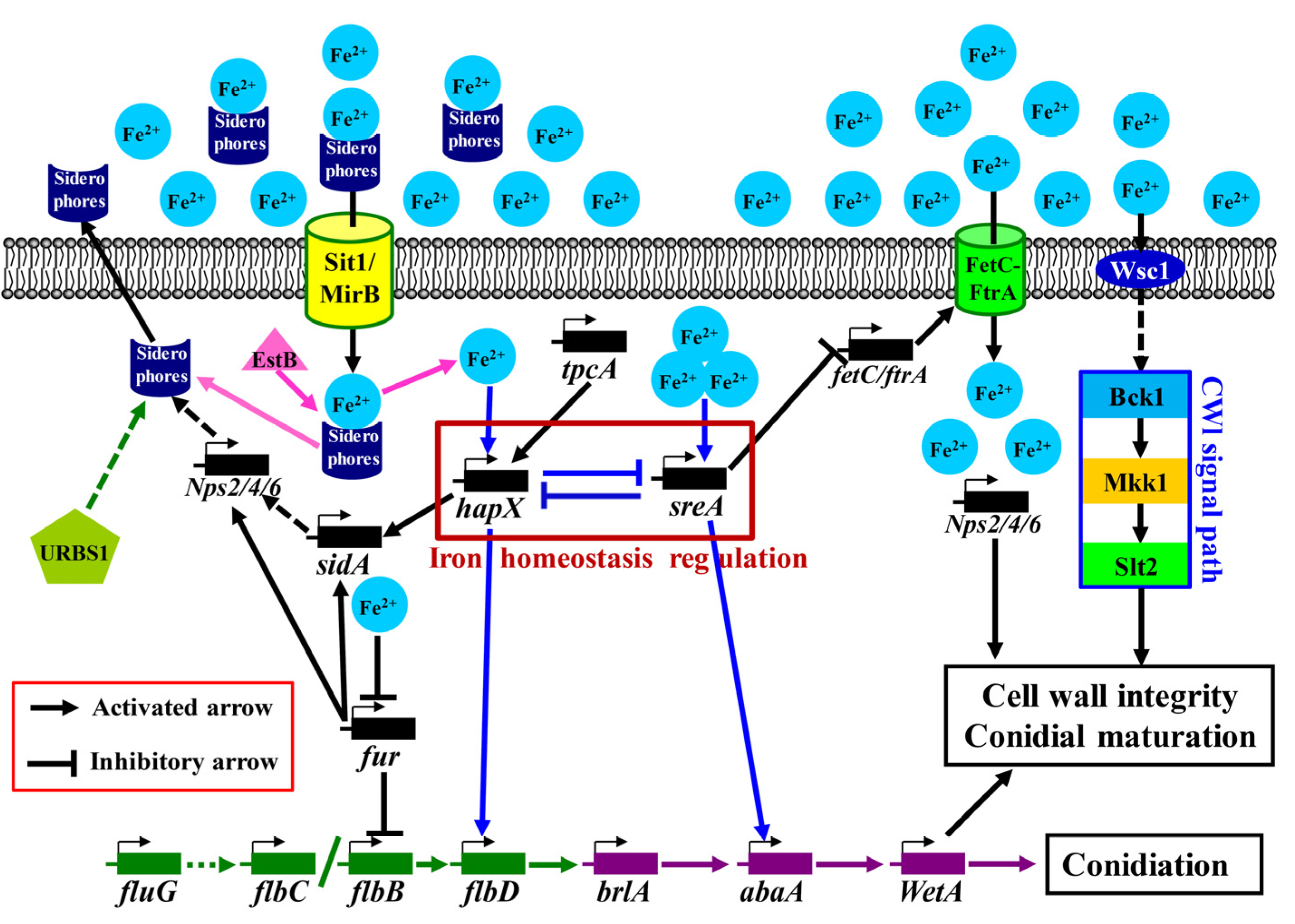
3.5. Model of the Signaling Pathway of the Iron-Ion-Promoted Asexual Sporulation of A. cinnamomea
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.L.; Li, W.C.; Chuang, Y.C.; Liu, H.C.; Huang, C.H.; Lo, K.Y.; Chen, C.Y.; Chang, F.M.; Chang, G.A.; Lin, Y.L.; et al. Sexual crossing, chromosome-level genome sequences, and comparative genomic analyses for the medicinal mushroom Taiwanofungus camphoratus (Syn. Antrodia Cinnamomea, Antrodia Camphorata). Microbiol. Spectr. 2022, 10, e02032-21. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Geng, Y.; Xu, H.X.; Ren, Y.; Liu, D.Y.; Mao, Y. Antrodia camphorata-derived antrodin C inhibits liver fibrosis by blocking TGF-Beta and PDGF signaling pathways. Front. Mol. Biosci. 2022, 9, 835508. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.F.; Wang, S.W.; Lai, Y.W.; Liu, S.C.; Chen, Y.J.; Hsueh, T.Y.; Lin, C.C.; Lin, C.H.; Chung, C.H. 4-Acetylantroquinonol B suppresses prostate cancer growth and angiogenesis via a VEGF/PI3K/ERK/mTOR-dependent signaling pathway in subcutaneous xenograft and in vivo angiogenesis Models. Int. J. Mol. Sci. 2022, 23, 1446. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, N.; Baskaran, R.; Velmurugan, B.K.; Thanh, N.C. Antrodia cinnamomea—An updated minireview of its bioactive components and biological activity. J. Food Biochem. 2019, 43, e12936. [Google Scholar] [CrossRef]
- Lu, C.L.; Li, H.X.; Zhu, X.Y.; Luo, Z.S.; Rao, S.Q.; Yang, Z.Q. Regulatory effect of intracellular polysaccharides from Antrodia cinnamomea on the intestinal microbiota of mice with antibiotic-associated diarrhea. Qual. Assur. Saf. Crops Foods 2022, 14, 124–134. [Google Scholar] [CrossRef]
- Li, H.X.; Wang, J.J.; Lu, C.L.; Gao, Y.J.; Gao, L.; Yang, Z.Q. Review of bioactivity, isolation, and identification of active compounds from Antrodia cinnamomea. Bioengineering 2022, 9, 494. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W.; Wong, J.H.; Ng, T.; Ye, X. Diversity of potentially exploitable pharmacological activities of the highly prized edible medicinal fungus Antrodia camphorata. Appl. Microbiol. Biotechnol. 2019, 103, 7843–7867. [Google Scholar] [CrossRef]
- Lu, M.C.; El-Shazly, M.; Wu, T.Y.; Du, Y.C.; Chang, T.T.; Chen, C.F.; Hsu, Y.M.; Lai, K.H.; Chiu, C.P.; Chang, F.R.; et al. Recent research and development of Antrodia cinnamomea. Pharmacol. Therapeut. 2013, 139, 124–156. [Google Scholar] [CrossRef]
- Liu, X.; Yu, S.; Zhang, Y.; Zhang, W.; Zhong, H.; Lu, X.; Guan, R. A review on the protective effect of active components in Antrodia camphorata against alcoholic liver injury. J. Ethnopharmacol. 2023, 300, 115740. [Google Scholar] [CrossRef]
- Lu, Z.M.; He, Z.; Li, H.X.; Gong, J.S.; Geng, Y.; Xu, H.Y.; Xu, G.H.; Shi, J.S.; Xu, Z.H. Modified arthroconidial inoculation method for the efficient fermentation of Antrodia camphorata ATCC 200183. Biochem. Eng. J. 2014, 87, 41–49. [Google Scholar] [CrossRef]
- Li, H.X.; Lu, Z.M.; Geng, Y.; Gong, J.S.; Zhang, X.J.; Shi, J.S.; Xu, Z.H.; Ma, Y.H. Efficient production of bioactive metabolites from Antrodia camphorata ATCC 200183 by asexual reproduction-based repeated batch fermentation. Bioresour. Technol. 2015, 194, 334–343. [Google Scholar] [CrossRef]
- Zhang, B.B.; Guan, Y.Y.; Hu, P.F.; Chen, L.; Xu, G.R.; Liu, L.; Cheung, P.C.K. Production of bioactive metabolites by submerged fermentation of the medicinal mushroom Antrodia cinnamomea: Recent advances and future development. Crit. Rev. Biotechnol. 2019, 39, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.S.; Wang, C.C.; Sung, S.C. Cultivating conditions influence lipase production by the edible basidiomycete Antrodia cinnamomea in submerged culture. Enzym. Microb. Technol. 2006, 39, 98–102. [Google Scholar] [CrossRef]
- Klingen, I.; Holthe, M.P.; Westrum, K.; Suthaparan, A.; Torp, T. Effect of light quality and light-dark cycle on sporulation patterns of the mite pathogenic fungus Neozygites floridana (Neozygitales: Entomophthoromycota), a natural enemy of Tetranychus urticae. J. Invertebr. Pathol. 2016, 137, 43–48. [Google Scholar] [CrossRef]
- Zhao, X.; Fan, Y.; Xiang, M.; Kang, S.; Wang, S.; Liu, X. DdaCrz1, a C2H2-type transcription factor, regulates growth, conidiation, and stress resistance in the nematode-trapping fungus Drechslerella dactyloides. J. Fungi 2022, 8, 750. [Google Scholar] [CrossRef]
- Wang, F.; Lu, Y.Y.; Liu, M.M.; Xiao, S.Q.; Gao, Y.B.; Yuan, M.Y.; Xue, C.S. Effects of iron on the asexual reproduction and major virulence factors of Curvularia lunata. Eur. J. Plant Pathol. 2020, 157, 497–507. [Google Scholar] [CrossRef]
- Da Silva Hellwig, A.H.; Pagani, D.M.; Rios, I.d.S.; Ribeiro, A.C.; Zanette, R.A.; Scroferneker, M.L. Influence of iron on growth and on susceptibility to itraconazole in Sporothrix spp. Med. Mycol. 2021, 59, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Punja, Z.K. Influence of iron on cylindrocarpon root rot development on ginseng. Phytopathology 2006, 96, 1179–1187. [Google Scholar] [CrossRef]
- Canessa, P.; Munoz-Guzman, F.; Vicuna, R.; Larrondo, L.F. Characterization of PIR1, a GATA family transcription factor involved in iron responses in the white-rot fungus Phanerochaete chrysosporium. Fungal Genet. Biol. 2012, 49, 626–634. [Google Scholar] [CrossRef]
- Jirakkakul, J.; Wichienchote, N.; Likhitrattanapisal, S.; Ingsriswang, S.; Yoocha, T.; Tangphatsornruang, S.; Wasuwan, R.; Cheevadhanarak, S.; Tanticharoen, M.; Amnuaykanjanasin, A. Iron homeostasis in the absence of ferricrocin and its consequences in fungal development and insect virulence in Beauveria bassiana. Sci. Rep. 2021, 11, 19624. [Google Scholar] [CrossRef]
- Li, H.X.; Lu, Z.M.; Zhu, Q.; Gong, J.S.; Geng, Y.; Shi, J.S.; Xu, Z.H.; Ma, Y.H. Comparative transcriptomic and proteomic analyses reveal a flug-mediated signaling pathway relating to asexual sporulation of Antrodia camphorata. Proteomics 2017, 17, 1700256. [Google Scholar] [CrossRef]
- Li, H.X.; Ji, D.; Luo, Z.S.; Ren, Y.L.; Lu, Z.M.; Yang, Z.Q.; Xu, Z.H. Comparative transcriptomic analyses reveal the regulatory mechanism of nutrient limitation-induced sporulation of Antrodia cinnamomea in submerged fermentation. Foods 2022, 11, 2715. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, J.; Ying, S.H.; Feng, M.G. Three mitogen-activated protein kinases required for cell wall integrity contribute greatly to biocontrol potential of a fungal entomopathogen. PLoS ONE 2014, 9, e87948. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Davidson, N.M.; Oshlack, A. Corset: Enabling differential gene expression analysis for de novo assembled transcriptomes. Genome Biol. 2014, 15, 410. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, J.; Ying, S.H.; Feng, M.G. The GPI-anchored protein Ecm33 is vital for conidiation, cell wall integrity, and multi-stress tolerance of two filamentous entomopathogens but not for virulence. Appl. Microbiol. Biotechnol. 2014, 98, 5517–5529. [Google Scholar] [CrossRef] [PubMed]
- Haas, H. Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat. Prod. Rep. 2014, 31, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Eisendle, M.; Oberegger, H.; Zadra, I.; Haas, H. The siderophore system is essential for viability of Aspergillus nidulans: Functional analysis of two genes encoding l-ornithine N 5-monooxygenase (sidA) and a non-ribosomal peptide synthetase (sidC). Mol. Microbiol. 2003, 49, 359–375. [Google Scholar] [CrossRef]
- Kim, K.-H.; Cho, Y.; La Rota, M.; Cramer, R.A., Jr.; Lawrence, C.B. Functional analysis of the Alternaria brassicicola non-ribosomal peptide synthetase gene AbNPS2 reveals a role in conidial cell wall construction. Mol. Plant Pathol. 2007, 8, 23–39. [Google Scholar] [CrossRef]
- Kragl, C.; Schrettl, M.; Abt, B.; Sarg, B.; Lindner, H.H.; Haas, H. EstB-mediated hydrolysis of the siderophore triacetylfusarinine C optimizes iron uptake of Aspergillus fumigatus. Eukaryot. Cell 2007, 6, 1278–1285. [Google Scholar] [CrossRef]
- Raymond-Bouchard, I.; Carroll, C.S.; Nesbitt, J.R.; Henry, K.A.; Pinto, L.J.; Moinzadeh, M.; Scott, J.K.; Moore, M.M. Structural requirements for the activity of the MirB ferrisiderophore transporter of Aspergillus fumigatus. Eukaryot. Cell 2012, 11, 1333–1344. [Google Scholar] [CrossRef]
- Schrettl, M.; Beckmann, N.; Varga, J.; Heinekamp, T.; Jacobsen, I.D.; Jochl, C.; Moussa, T.A.; Wang, S.; Gsaller, F.; Blatzer, M.; et al. HapX-mediated adaption to iron starvation is crucial for virulence of Aspergillus fumigatus. PLoS Pathog. 2010, 6, e1001124. [Google Scholar] [CrossRef]
- Schrettl, M.; Kim, H.S.; Eisendle, M.; Kragl, C.; Nierman, W.C.; Heinekamp, T.; Werner, E.R.; Jacobsen, I.; Illmer, P.; Yi, H.; et al. SreA-mediated iron regulation in Aspergillus fumigatus. Mol. Microbiol. 2008, 70, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.H.; Seth, E.; Gilmore, S.A.; Sil, A. SRE1 regulates iron-dependent and -independent pathways in the fungal pathogen Histoplasma capsulatum. Eukaryot. Cell 2012, 11, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Bom, V.L.; de Castro, P.A.; Winkelstroter, L.K.; Marine, M.; Hori, J.I.; Ramalho, L.N.; dos Reis, T.F.; Goldman, M.H.; Brown, N.A.; Rajendran, R.; et al. The Aspergillus fumigatus sitA phosphatase homologue is important for adhesion, cell wall integrity, biofilm formation, and virulence. Eukaryot. Cell 2015, 14, 728–744. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, P.; Aubert-Frambourg, A.; Guillot, A.; Auger, S. The MarR-like protein PchR (YvmB) regulates expression of genes involved in pulcherriminic acid biosynthesis and in the initiation of sporulation in Bacillus subtilis. BMC Microbiol. 2016, 16, 190. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, Y.; Jia, J.; Xie, J.; Cheng, J.; Liu, H.; Jiang, D.; Fu, Y. Uninterrupted expression of CmSIT1 in a sclerotial parasite Coniothyrium minitans leads to reduced growth and enhanced antifungal ability. Front. Microbiol. 2017, 8, 2208. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; He, Y.; Xu, J.; Xiao, X.; Wang, F.P. The regulatory role of ferric uptake regulator (Fur) during anaerobic respiration of Shewanella piezotolerans WP3. PLoS ONE 2013, 8, e75588. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.J.; Hou, J.; Zhang, H.; Lei, J.H.; Lin, H.Y.; Ding, J.L.; Feng, M.G.; Ying, S.H. Systematic contributions of CFEM domain-containing proteins to iron acquisition are essential for interspecies interaction of the filamentous pathogenic fungus Beauveria bassiana. Environ. Microbiol. 2022, 24, 3693–3704. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.N.; Kroken, S.; Chou, D.Y.; Robbertse, B.; Yoder, O.C.; Turgeon, B.G. Functional analysis of all nonribosomal peptide synthetases in Cochliobolus heterostrophus reveals a factor, NPS6, involved in virulence and resistance to oxidative stress. Eukaryot. Cell 2005, 4, 545–555. [Google Scholar] [CrossRef]
- An, Z.; Zhao, Q.; McEvoy, J.; Yuan, W.M.; Markley, J.L.; Leong, S.A. The second finger of Urbs1 is required for iron-mediated repression of sid1 in Ustilago maydis. Proc. Natl. Acad. Sci. USA 1997, 94, 5882–5887. [Google Scholar] [CrossRef]
- Schrettl, M.; Haas, H. Iron homeostasis-Achilles’ heel of Aspergillus fumigatus? Curr. Opin. Microbiol. 2011, 14, 400–405. [Google Scholar] [CrossRef]
- Haas, H. Iron—A key nexus in the virulence of Aspergillus fumigatus. Front. Microbiol. 2012, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Stanford, F.A.; Matthies, N.; Cseresnyes, Z.; Figge, M.T.; Hassan, M.I.A.; Voigt, K. Expression patterns in reductive iron assimilation and functional consequences during phagocytosis of Lichtheimia corymbifera, an emerging cause of mucormycosis. J. Fungi 2021, 7, 272. [Google Scholar] [CrossRef]
- Peng, Y.J.; Wang, J.J.; Lin, H.Y.; Ding, J.L.; Feng, M.G.; Ying, S.H. HapX, an indispensable bZIP transcription factor for iron acquisition, regulates infection Iinitiation by orchestrating conidial oleic acid homeostasis and cytomembrane functionality in mycopathogen Beauveria bassiana. mSystems 2020, 5, e00695-20. [Google Scholar] [CrossRef]
- Hortschansky, P.; Eisendle, M.; Al-Abdallah, Q.; Schmidt, A.D.; Bergmann, S.; Thon, M.; Kniemeyer, O.; Abt, B.; Seeber, B.; Werner, E.R.; et al. Interaction of HapX with the CCAAT-binding complex—A novel mechanism of gene regulation by iron. EMBO J. 2007, 26, 3157–3168. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Berges, M.S.; Capilla, J.; Turra, D.; Schafferer, L.; Matthijs, S.; Jochl, C.; Cornelis, P.; Guarro, J.; Haas, H.; Di Pietro, A. HapX-mediated iron homeostasis is essential for rhizosphere competence and virulence of the soilborne pathogen Fusarium oxysporum. Plant Cell 2012, 24, 3805–3822. [Google Scholar] [CrossRef] [PubMed]
- Emri, T.; Sumegi-Gyori, V.M.; Pall, K.; Gila, B.C.; Pocsi, I. Effect of the combinatorial iron-chelation and oxidative stress on the growth of Aspergillus species. Res. Microbiol. 2022, 173, 103969. [Google Scholar] [CrossRef]
- Oberegger, H.; Schoeser, M.; Zadra, I.; Abt, B.; Haas, H. SREA is involved in regulation of siderophore biosynthesis, utilization and uptake in Aspergillus nidulans. Mol. Microbiol. 2001, 41, 1077–1089. [Google Scholar] [CrossRef]
- Nazik, H.; Sass, G.; Ansari, S.R.; Ertekin, R.; Haas, H.; Deziel, E.; Stevens, D.A. Novel intermicrobial molecular interaction: Pseudomonas aeruginosa quinolone signal (PQS) modulates Aspergillus fumigatus response to iron. Microbiology 2020, 166, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, J.; Hu, Y.; Ying, S.H.; Feng, M.G. Roles of six Hsp70 genes in virulence, cell wall integrity, antioxidant activity and multiple stress tolerance of Beauveria bassiana. Fungal Genet. Biol. 2020, 144, 103437. [Google Scholar] [CrossRef] [PubMed]
- Valiante, V.; Macheleidt, J.; Foge, M.; Brakhage, A.A. The Aspergillus fumigatus cell wall integrity signaling pathway: Drug target, compensatory pathways, and virulence. Front. Microbiol. 2015, 6, 325. [Google Scholar] [CrossRef]
- Eisendle, M.; Schrettl, M.; Kragl, C.; Muller, D.; Illmer, P.; Haas, H. The intracellular siderophore ferricrocin is involved in iron storage, oxidative-stress resistance, germination, and sexual development in Aspergillus nidulans. Eukaryot. Cell 2006, 5, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.M. The crucial role of iron uptake in Aspergillus fumigatus virulence. Curr. Opin. Microbiol. 2013, 16, 692–699. [Google Scholar] [CrossRef]
- Albarouki, E.; Deising, H.B. Infection structure-specific reductive iron assimilation is required for cell wall integrity and full virulence of the maize pathogen Colletotrichum graminicola. Mol. Plant-Microbe Interact. 2013, 26, 695–708. [Google Scholar] [CrossRef]
- Rouault, T.; Klausner, R. Regulation of Iron Metabolism in Eukaryotes. Curr. Top. Cell. Regul. 1997, 35, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Voss, B.; Kirschhofer, F.; Brenner-Weiss, G.; Fischer, R. Alternaria alternata uses two siderophore systems for iron acquisition. Sci. Rep. 2020, 10, 3587. [Google Scholar] [CrossRef]
- Blachowicz, A.; Chiang, A.J.; Romsdahl, J.; Kalkum, M.; Wang, C.C.C.; Venkateswaran, K. Proteomic characterization of Aspergillus fumigatus isolated from air and surfaces of the international space station. Fungal Genet. Biol. 2019, 124, 39–46. [Google Scholar] [CrossRef]


| Gene Name | Upstream Primer (5′→3′) | Downstream Primer (5′→3′) | Product (bp) |
|---|---|---|---|
| flbD | AATGTCTGAAGGTCGTGATGCC | GCCGTATCGTTAGCCGTATGG | 126 |
| abaA | TGTGCGAGTGCGGAGACC | GTAGACGACGGACAGGAGGAC | 116 |
| bck1 | GTCAACAGTATAGATATGC | GTCAACAGTATAGATATGC | 127 |
| mkk1 | CATAAAGGTCTTCGCTAT | CATAAAGGTCTTCGCTAT | 165 |
| slt2 | ATCTCCTTTAGAAGACATC | ATCTCCTTTAGAAGACATC | 103 |
| 18S rRNA | GCTGGTCGCTGGCTTCTTAG | CGCTGGCTCTGTCAGTGTAG | 123 |
| Unigene ID | Genome ID | Gene Name | Accession Number | E Value | Score |
|---|---|---|---|---|---|
| Cluster-140.3091 | ACg001255 | mirB | NC_007196.1 | 1 × 10−19 | 198 |
| Cluster-140.1965 | ACg005881 | ftrA | NC_007198.1 | 5 × 10−11 | 82 |
| Cluster-140.3564 | ACg006970 | hapX | NC_007198.1 | 4 × 10−18 | 183 |
| Cluster-140.2153 | ACg000929 | sreA | NC_007198.1 | 6 × 10−12 | 89 |
| Cluster-140.3137 | ACg005708 | fetC | NC_032094.1 | 2 × 10−16 | 138 |
| Cluster-140.2788 | ACg000854 | bck1 | NW_007930838.1 | 8 × 10−16 | 156 |
| Cluster-140.3618 | ACg001175 | uvt | KJ158162.1 | 2 × 10−17 | 164 |
| Cluster-140.2669 | ACg007032 | urbs1 | NC_026479.1 | 7 × 10−16 | 154 |
| Cluster-140.3500 | ACg005433 | sit | MF447899.1 | 6 × 10−14 | 102 |
| Cluster-140.2357 | ACg001741 | fre | NC_007197.1 | 4 × 10−14 | 100 |
| Cluster-140.4088 | ACg006852 | slt2 | AEU60018.1 | 2 × 10−90 | 326 |
| Cluster-140.2081 | ACg007003 | ssiG 06045 | XP_001593123.1 | 6 × 10−18 | 189 |
| Cluster-140.1623 | ACg002353 | feoB | NC_000913.3 | 3 × 10−16 | 145 |
| Cluster-140.3137 | ACg005708 | tpcA | NW_020939752.1 | 1 × 10−18 | 175 |
| Cluster-140.3451 | ACg003216 | nrps | KIM81356.1 | 0 | 2771 |
| - | ACg008442 | nps2 | NC_031953.1 | 7 × 10−11 | 84 |
| Cluster-140.3206 | ACg002074 | nps4 | KY471559.1 | 1 × 10−25 | 268 |
| Cluster-140.4587 | ACg007029 | clpP | NC_000964.3 | 3 × 10−15 | 126 |
| Cluster-140.4385 | ACg003470 | mkk1 | NW_007930837.1 | 2 × 10−10 | 79 |
| - | ACg000676 | sidA | NC_007194.1 | 8 × 10−19 | 202 |
| Cluster-140.3564 | ACg006969 | yvmB | NC_020507.1 | 2 × 10−18 | 179 |
| Cluster-140.132 | ACg001175 | fur | NC_016845.1 | 3 × 10−14 | 97 |
| Cluster-140.2003 | ACg007734 | estB | NC_007196.1 | 5 × 10−16 | 149 |
| Cluster-140.2389 | ACg007303 | wsc1 | NC_007198.1 | 3 × 10−16 | 146 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Dai, J.; Shi, Y.; Zhu, X.; Jia, L.; Yang, Z. Molecular Regulatory Mechanism of the Iron-Ion-Promoted Asexual Sporulation of Antrodia cinnamomea in Submerged Fermentation Revealed by Comparative Transcriptomics. J. Fungi 2023, 9, 235. https://doi.org/10.3390/jof9020235
Li H, Dai J, Shi Y, Zhu X, Jia L, Yang Z. Molecular Regulatory Mechanism of the Iron-Ion-Promoted Asexual Sporulation of Antrodia cinnamomea in Submerged Fermentation Revealed by Comparative Transcriptomics. Journal of Fungi. 2023; 9(2):235. https://doi.org/10.3390/jof9020235
Chicago/Turabian StyleLi, Huaxiang, Jianing Dai, Yu Shi, Xiaoyan Zhu, Luqiang Jia, and Zhenquan Yang. 2023. "Molecular Regulatory Mechanism of the Iron-Ion-Promoted Asexual Sporulation of Antrodia cinnamomea in Submerged Fermentation Revealed by Comparative Transcriptomics" Journal of Fungi 9, no. 2: 235. https://doi.org/10.3390/jof9020235
APA StyleLi, H., Dai, J., Shi, Y., Zhu, X., Jia, L., & Yang, Z. (2023). Molecular Regulatory Mechanism of the Iron-Ion-Promoted Asexual Sporulation of Antrodia cinnamomea in Submerged Fermentation Revealed by Comparative Transcriptomics. Journal of Fungi, 9(2), 235. https://doi.org/10.3390/jof9020235







