Diagnosis and Treatment of Pulmonary Coccidioidomycosis and Paracoccidioidomycosis
Abstract
1. Introduction
2. Coccidioidomycosis
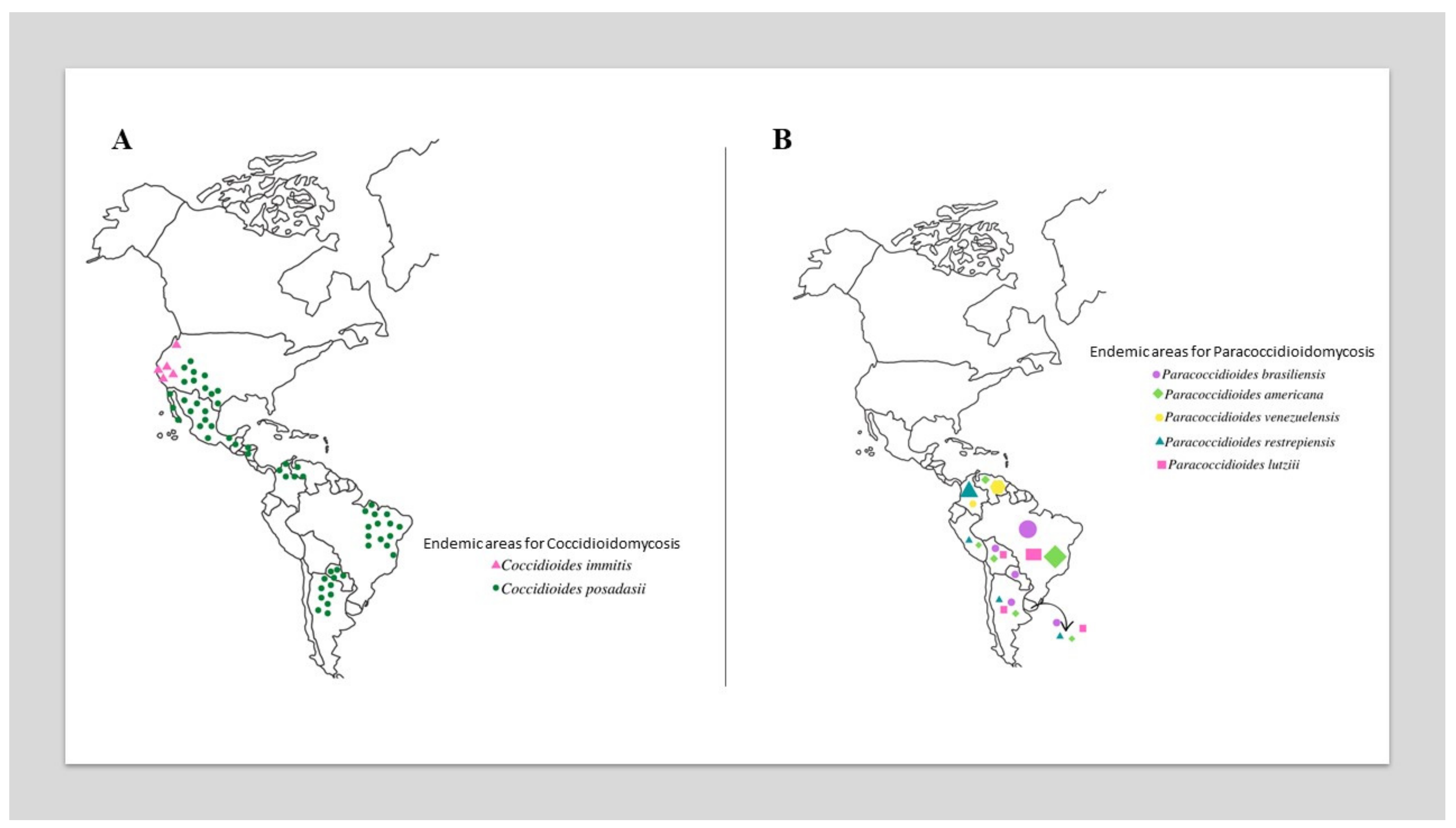
2.1. Ecoepidemiology
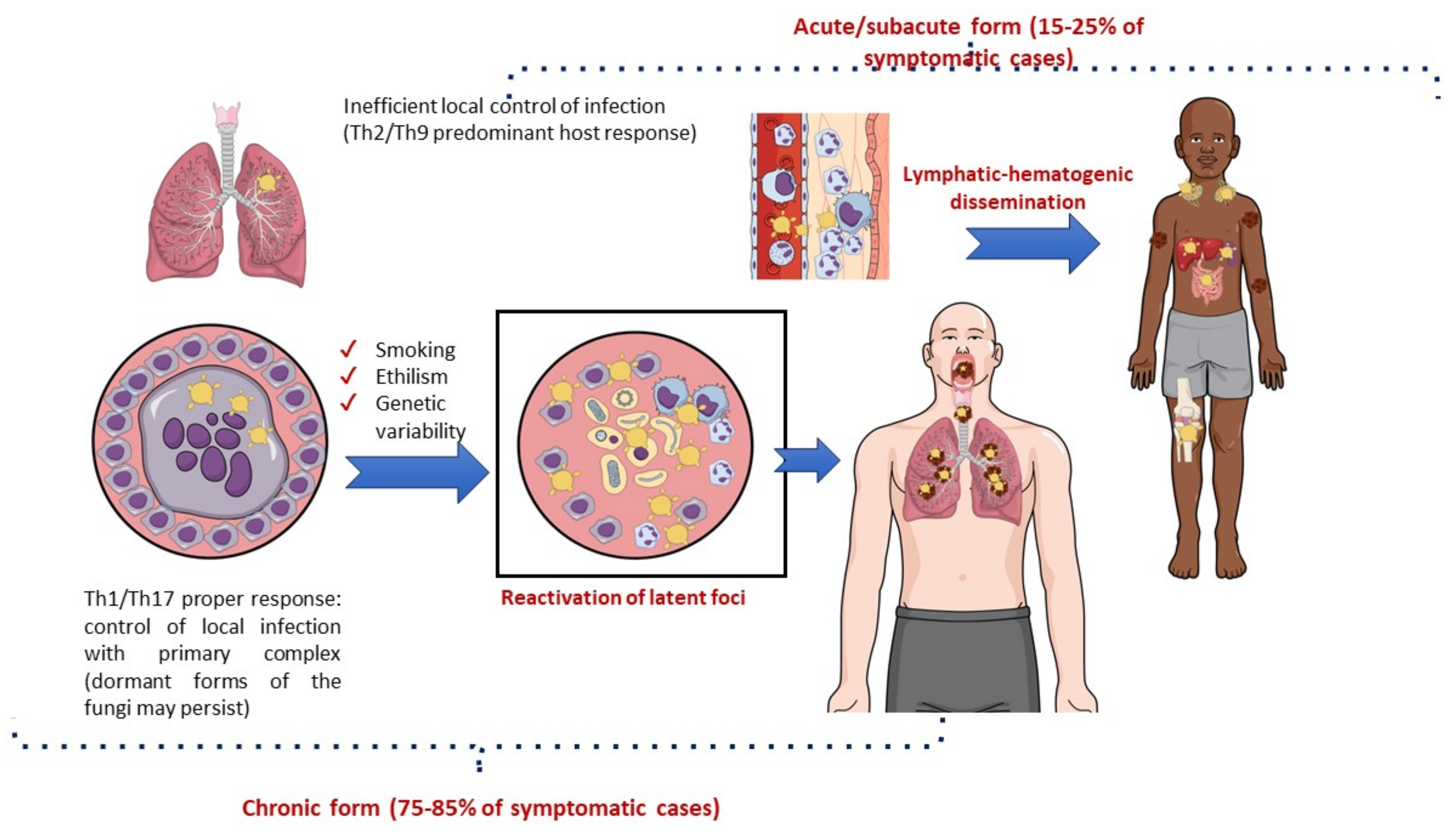
2.2. Pathogenesis
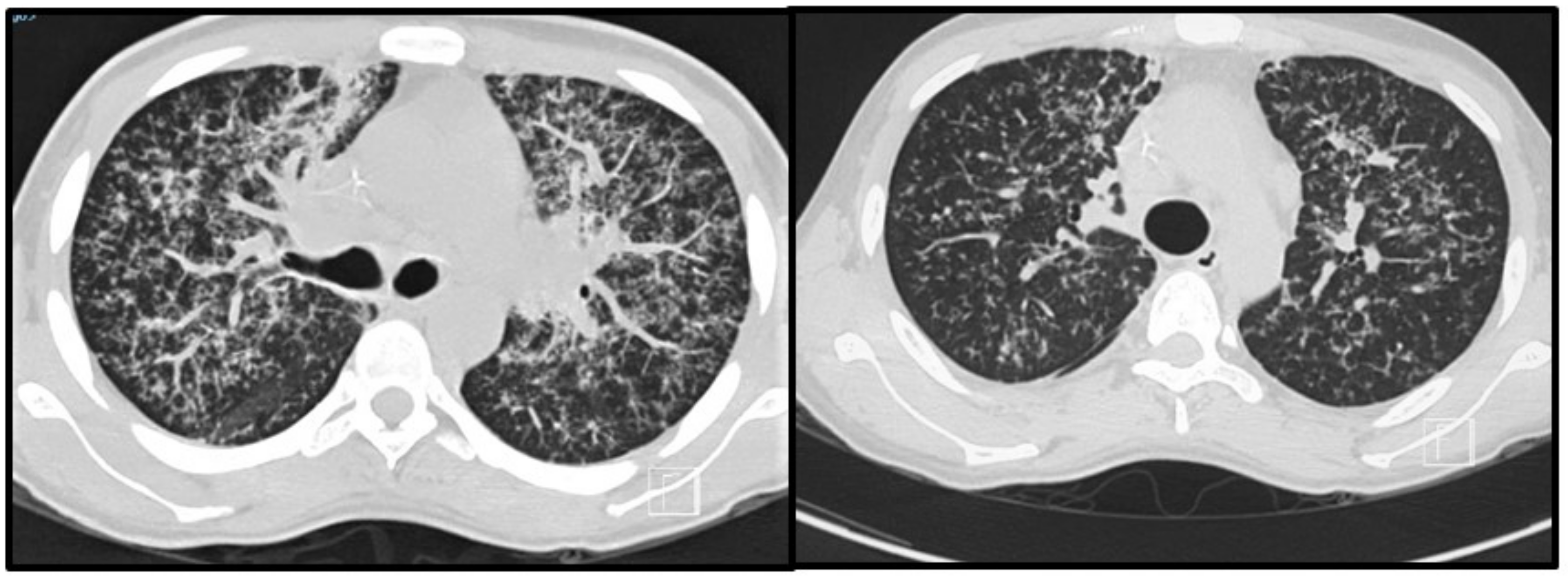
2.3. Clinical Aspects
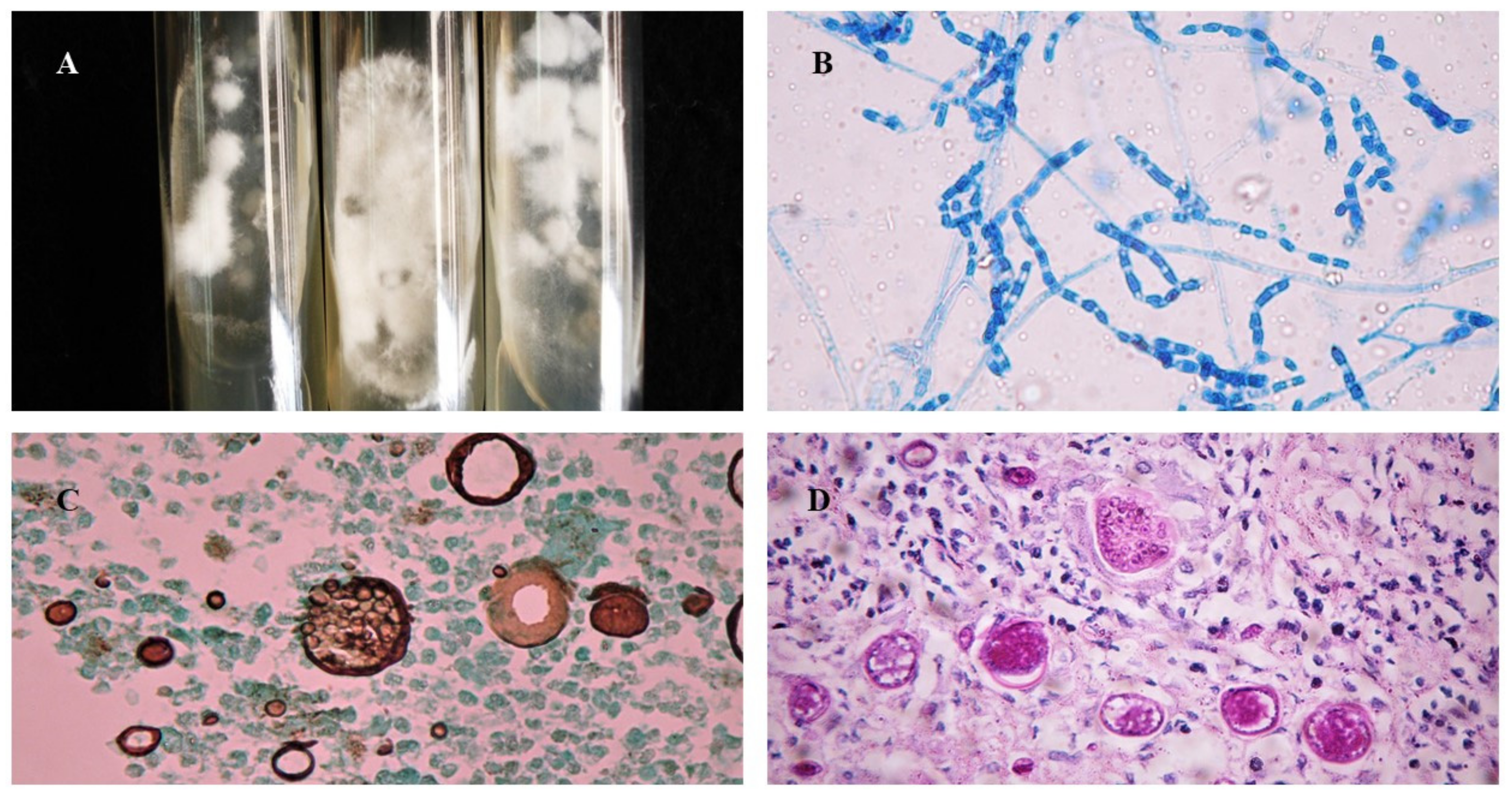
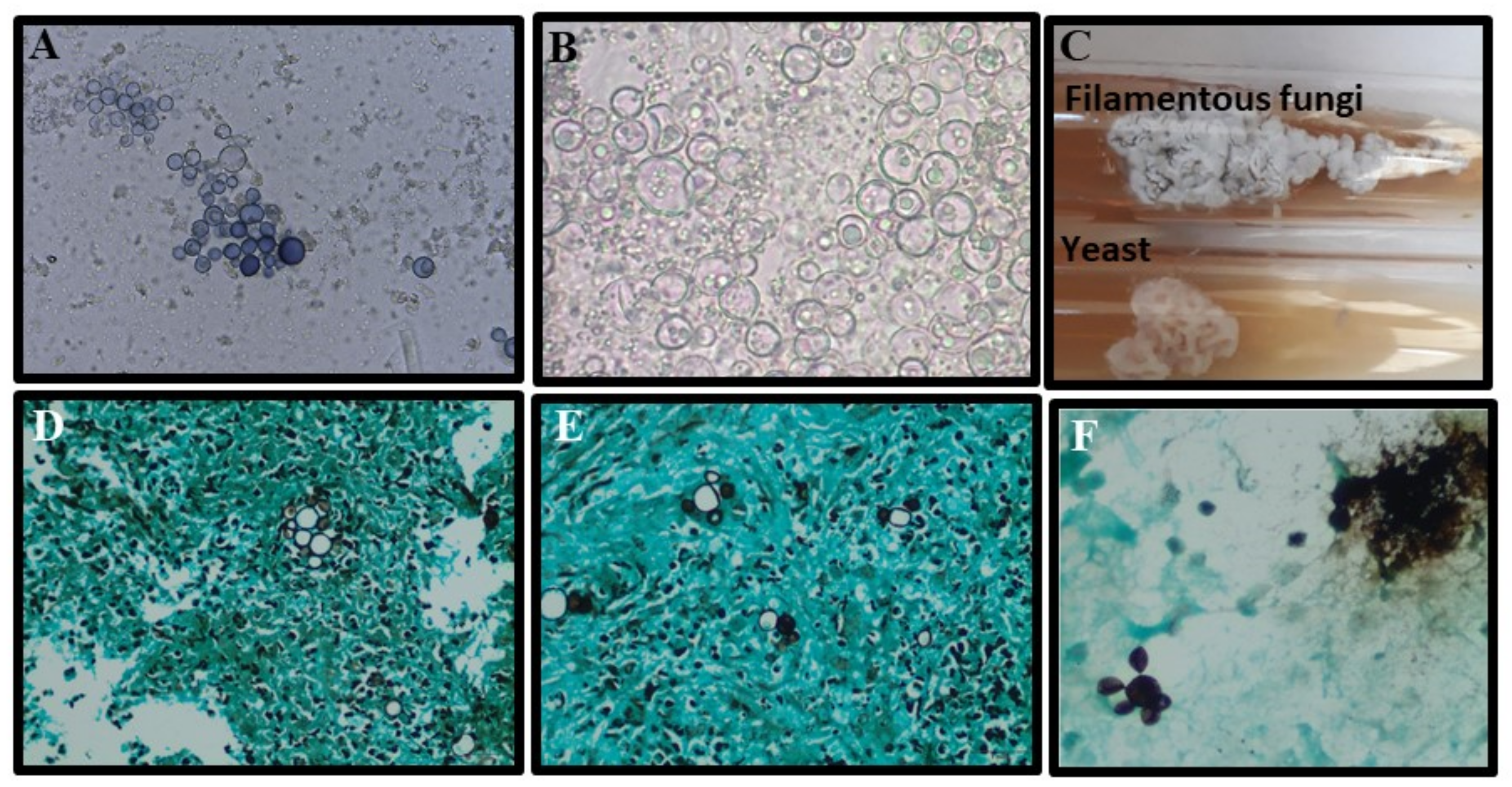
2.4. Laboratory Diagnosis
| Diagnostic Test | Sensitivity | Specificity | Advantageous | Disadvantageous | References |
|---|---|---|---|---|---|
| Paracoccidioidomycosis | |||||
| Mycological | |||||
| Direct Microscopy | 48–75% | High | Pathognomonic fungal elements may be rapidly identified (mother cell with multiple buds: “Ship’s wheel” or “Mickey Mouse”). It may be performed in exudates of tegumental lesions, sputum, and aspirates of lymph nodes. Simple, fast, and low-cost. | The sensitivity is dependent on the operator’s experience, the quality of the material, and the protocol for processing. Small yeasts without multiple buds can be mistaken for other fungi. | [55,56] |
| Histopathology | 70->5% | High | Pathognomonic fungal elements may be rapidly identified (mother cell with multiple buds: “Ship’s wheel” or “Mickey Mouse”). Tissue response and burden of infection may be evaluated and help to characterize the severity of the disease. Results may take less than 24 or 48 h. | An invasive procedure is required to obtain a biopsy. The sensitivity is dependent on the operator’s experience. The quality of the biopsy sample, and the protocol for processing. Specific fungal stains should be included to improve the detection of fungal elements. | [56,57] |
| Culture | 24->90% | 100% | It may be performed in different biologic samples, with higher sensitivities in bronchoalveolar lavage and tissue biopsies. It provides accurate identification of Paracoccidioides spp. at the species level and can possibly check antifungal susceptibility and virulence. Low cost (higher if molecular tests were used to identify species) | Exudates and sputum samples may be contaminated with bacteria which may mitigate the sensitivity of the test (all media cultures should contain antibiotics). Paracoccidioides spp. are fastidious microorganisms and culture will take an average of 3 to 4 weeks to grow. There are biosafety concerns with the manipulation of Paracoccidioides spp. | [55,56] |
| Immunological | |||||
| Antibody (GP43 and GP70) detection by different assays | 80–95% | 80–100% | They may provide quantitative in addition to qualitative results. The test may help with diagnosis and the laboratory monitoring of response to therapy. Results may be provided in hours or a few days. | Cross-reactions may occur with other fungal infections. Sensitivity is highly dependent on host immunity, clinical form, and methodology of detection. False-negative results may be provided for P. lutzii when using P. brasiliensis complex antigen preparation and vice versa. No commercial tests are available. | [58,59] |
| Specific Antigen detection for PCM | 90–96% | 96–100% | It is highly recommended for testing immunocompromised patients with (higher sensitivity than antibody detection). It is a non-invasive procedure. Results may be provided in less than 24 or 48 h in reference centers. | Cross-reactions may be obtained with heterologous sera. No commercial tests are available, and assays are not available in routine laboratories. | [60,61,62] |
| Molecular | |||||
| PCR based methods | 86–100% | It may detect DNA in samples with a low burden of fungal elements. It facilitates the possibility of species identification. Results may be provided in less than 24 or 48 h. | There is no international standardization of the tests. They are not available for most routine laboratories that are located in endemic regions. There is no commercial kit available. Most tests are designed to identify P. brasiliensis. There is a lack of standardized tests to detect P. lutzii infection in biological samples. | [63,64] | |
| Coccidioidomycosis | |||||
| Mycological | |||||
| Direct Microscopy | 15–75 | High | Pathognomonic fungal elements may be rapidly identified (Cocci spherules with endospores). Testing is low-cost, rapid and easy, and various biological materials can be used. | The sensitivity depends on the operator’s experience, the quality of the material, and the protocol for processing. | [23] |
| Cytopathology/Histopathology | 23–84% | High | Pathognomonic fungal elements may be rapidly identified (Cocci spherules with endospores). Testing is low-cost, rapid and easy, and various biological materials may be tested. The specificity and sensibility of the test may be improved by the inclusion of specific fungal stains. | The sensitivity is dependent on the operator’s experience, the quality of the biopsy material, and the protocol for processing. A biopsy requires invasive procedures. | [23] |
| Culture | Close to 50% | High | It may be performed in different biologic samples, with higher sensitivities in broncho-alveolar lavage and tissue biopsies. It provides accurate identification of Coccidioides spp. at the species level as well as the possibility of checking for antifungal susceptibility and virulence. Testing is low-cost (higher if molecular tests are used to identify species). | Manipulation requires a level 3 biosafety laboratory. Results may require up to 7 days. Contamination of samples with bacteria may mitigate the sensitivity of the test. | [65,66] |
| Immunological | |||||
| Specific Antibody (IgG or IgM) detection | 60–100% | 90–96% | They may provide quantitative addition to qualitative results. The test may help with diagnosis and the laboratory monitoring of response to therapy. Results may be provided in hours or a few days. | Cross-reactions may occur with other fungi. Sensitivity is highly dependent on host immunity, clinical form, and methodology of detection. Serological tests are commercially available. | [23,67] |
| CM Specific Antigen tests | 28–70% | 90–100% | It is highly recommended for immunocompromised hosts where the production of antibodies may be low. Tests are usually performed on urine and serum samples. Results may be provided in hours or a few days. | Serum samples usually present lower sensitivity compared with urine samples. Cross-reaction with Histoplasma spp. and Blastomyces spp. may occur. Serological tests are commercially available. | [68,69] |
| Molecular | |||||
| PCR based | >80% | High | They may detect DNA in samples with low fungal burden, exhibiting better performance than culture. There is a possibility of species identification as well as characterization of antifungal susceptibility and virulence. | There is a lack of international standardization. Only in-house tests are available in a limited number of reference medical centers. | [70] |
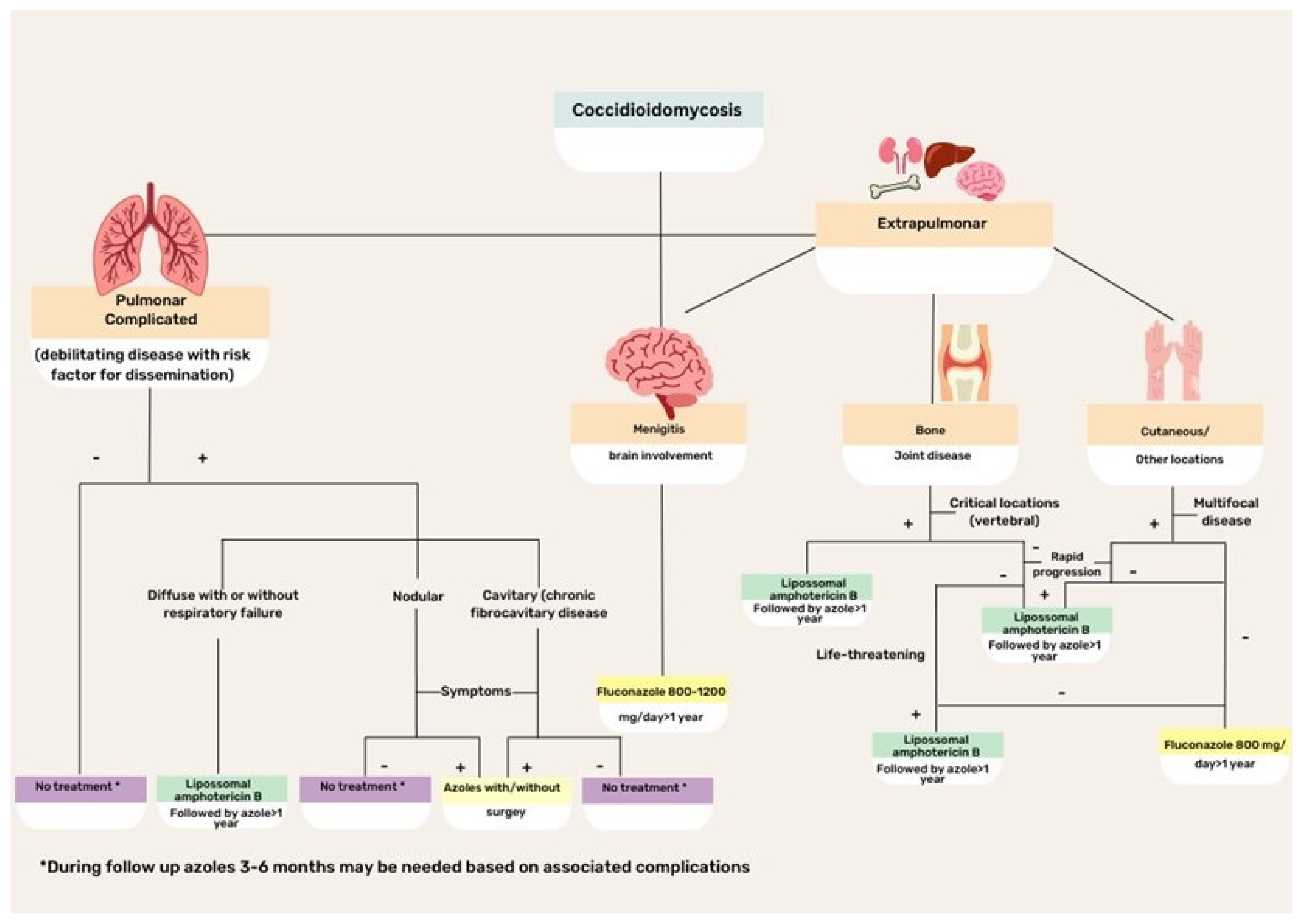
2.5. Treatment
2.6. New Perspectives in the Clinical Management of CM
3. Paracoccidiodomycosis
3.1. Ecoepidemiology
3.2. Pathogenesis
3.3. Clinical Aspects
3.4. Imaging Studies
3.5. Laboratorial Diagnosis
3.6. Treatment
3.7. New Perspectives in the Clinical Management
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colombo, A.L.; Tobón, A.; Restrepo, A.; Queiroz-Telles, F.; Nucci, M. Epidemiology of Endemic Systemic Fungal Infections in Latin America. Med. Mycol. 2011, 49, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, N.; Kubat, R.C.; Poplin, V.; Adenis, A.A.; Denning, D.W.; Wright, L.; McCotter, O.; Schwartz, I.S.; Jackson, B.R.; Chiller, T.; et al. Re-Drawing the Maps for Endemic Mycoses. Mycopathologia 2020, 185, 843–865. [Google Scholar] [CrossRef] [PubMed]
- Tirado-Sánchez, A.; González, G.M.; Bonifaz, A. Endemic Mycoses: Epidemiology and Diagnostic Strategies. Expert Rev. Anti Infect. Ther. 2020, 18, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.H.; Sharma, R.; Kuran, R.; Fong, I.; Heidari, A. Coccidioidomycosis: A Review. J. Investig. Med. 2021, 69, 316–323. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Theodoro, R.C.; de Carvalho, M.J.A.; Fernandes, L.; Paes, H.C.; Hahn, R.C.; Mendoza, L.; Bagagli, E.; San-Blas, G.; Felipe, M.S.S. Phylogenetic Analysis Reveals a High Level of Speciation in the Paracoccidioides Genus. Mol. Phylogenet. Evol. 2009, 52, 273–283. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Cattana, M.E.; Matute, D.R.; Muñoz, J.F.; Arechavala, A.; Isbell, K.; Schipper, R.; Santiso, G.; Tracogna, F.; de los Ángeles Sosa, M.; et al. Genomic Diversity of the Human Pathogen Paracoccidioides across the South American Continent. Fungal Genet. Biol. 2020, 140, 103395. [Google Scholar] [CrossRef]
- Hrycyk, M.F.; Garces, H.G.; Bosco, S.D.M.G.; de Oliveira, S.L.; Marques, S.A.; Bagagli, E. Ecology of Paracoccidioides brasiliensis, P. lutzii and Related Species: Infection in Armadillos, Soil Occurrence and Mycological Aspects. Med. Mycol. 2018, 56, 950–962. [Google Scholar] [CrossRef]
- Turissini, D.A.; Gomez, O.M.; Teixeira, M.M.; McEwen, J.G.; Matute, D.R. Species Boundaries in the Human Pathogen Paracoccidioides. Fungal Genet. Biol. 2017, 106, 9–25. [Google Scholar] [CrossRef]
- Vio de Queiroz-Telles, F.; Pietrobom, P.M.P.; Júnior, M.R.; Baptista, R.D.M.; Peçanha, P.M. New Insights on Pulmonary Paracoccidioidomycosis. Semin. Respir. Crit. Care Med. 2020, 41, 53–68. [Google Scholar] [CrossRef]
- Ekeng, B.E.; Davies, A.A.; Osaigbovo, I.I.; Warris, A.; Oladele, R.O.; Denning, D.W. Pulmonary and Extrapulmonary Manifestations of Fungal Infections Misdiagnosed as Tuberculosis: The Need for Prompt Diagnosis and Management. J. Fungi 2022, 8, 460. [Google Scholar] [CrossRef]
- Matsuda, J.D.S.; Wanke, B.; Balieiro, A.A.D.S.; da Silva Santos, C.S.; Cavalcante, R.C.D.S.; de Medeiros Muniz, M.; Torres, D.R.; Pinheiro, S.B.; Frickmann, H.; Souza, J.V.B.; et al. Prevalence of Pulmonary Mycoses in Smear-Negative Patients with Suspected Tuberculosis in the Brazilian Amazon. Rev. Iberoam Micol. 2021, 38, 111–118. [Google Scholar] [CrossRef]
- Quagliato Júnior, R.; Grangeia, T.D.A.G.; Massucio, R.A.D.C.; de Capitani, E.M.; Rezende, S.M.; Balthazar, A.B. Associação Entre Paracoccidioidomicose e Tuberculose: Realidade e Erro Diagnóstico. J. Brasil. Pneum. 2007, 33, 295–300. [Google Scholar] [CrossRef]
- Griffiths, J.; Colombo, A.L.; Denning, D.W. The Case for Paracoccidioidomycosis to Be Accepted as a Neglected Tropical (Fungal) Disease. PLoS Negl. Trop. Dis. 2019, 13, e0007195. [Google Scholar] [CrossRef]
- Queiroz-Telles, F.; Fahal, A.H.; Falci, D.R.; Caceres, D.H.; Chiller, T.; Pasqualotto, A.C. Neglected Endemic Mycoses. Lancet Infect. Dis. 2017, 17, e367–e377. [Google Scholar] [CrossRef]
- WHO. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Bays, D.J.; Thompson, G.R. Coccidioidomycosis. Infect. Dis. Clin. N. Am. 2021, 35, 453–469. [Google Scholar] [CrossRef]
- Boro, R.; Iyer, P.C.; Walczak, M.A. Current Landscape of Coccidioidomycosis. J. Fungi 2022, 8, 413. [Google Scholar] [CrossRef]
- Ampel, N.M. Coccidioidomycosis: A Review of Recent Advances. Clin. Chest Med. 2009, 30, 241–251. [Google Scholar] [CrossRef]
- Welsh, O.; Vera-Cabrera, L.; Rendon, A.; Gonzalez, G.; Bonifaz, A. Coccidioidomycosis. Clin. Dermatol. 2012, 30, 573–591. [Google Scholar] [CrossRef]
- Dickson, E.C. “Valley Fever” of the San Joaquin Valley and Fungus Coccidioides. Cal. West Med. 1937, 47, 151–155. [Google Scholar]
- Crum, N.F. Coccidioidomycosis: A Contemporary Review. Infect Dis. Ther. 2022, 11, 713–742. [Google Scholar] [CrossRef]
- Chow, N.A.; Kangiser, D.; Gade, L.; McCotter, O.Z.; Hurst, S.; Salamone, A.; Wohrle, R.; Clifford, W.; Kim, S.; Salah, Z.; et al. Factors Influencing Distribution of Coccidioides immitis in Soil, Washington State, 2016. mSphere 2021, 6, 00598-21. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.L.; Chiller, T. Update on the Epidemiology, Diagnosis, and Treatment of Coccidioidomycosis. J. Fungi 2022, 8, 666. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, F.A.; Ellingson, K.D.; Canales, R.A.; Bedrick, E.J.; Galgiani, J.N.; Donovan, F.M. Cross-Sectional Study of Clinical Predictors of Coccidioidomycosis, Arizona, USA. Emerg. Infect. Dis. 2022, 28, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.; Gorris, M.E.; Chiller, T.; Jackson, B.; Beadles, W.; Webb, B.J. Epidemiology, Clinical Features, and Outcomes of Coccidioidomycosis, Utah, 2006–2015. Emerg. Infect. Dis. 2021, 27, 2269–2277. [Google Scholar] [CrossRef]
- McCotter, O.Z.; Benedict, K.; Engelthaler, D.M.; Komatsu, K.; Lucas, K.D.; Mohle-Boetani, J.C.; Oltean, H.; Vugia, D.; Chiller, T.M.; Cooksey, G.L.S.; et al. Update on the Epidemiology of Coccidioidomycosis in the United States. Med. Mycol. 2019, 57, S30–S40. [Google Scholar] [CrossRef]
- Ampel, N.M. What’s Behind the Increasing Rates of Coccidioidomycosis in Arizona and California? Curr. Infect. Dis. Rep. 2010, 12, 211–216. [Google Scholar] [CrossRef]
- Baptista Rosas, R.C.; Riquelme, M. Epidemiología de La Coccidioidomicosis En México. Rev Iberoam Micol 2007, 24, 100–105. [Google Scholar] [CrossRef]
- Laniado-Laborín, R.; Arathoon, E.G.; Canteros, C.; Muñiz-Salazar, R.; Rendon, A. Coccidioidomycosis in Latin America. Med. Mycol. 2019, 57, S46–S55. [Google Scholar] [CrossRef]
- Canteros, C.E.; Toranzo, A.; Ibarra-Camou, B.; David, V.; Carrizo, S.G.; Santillán-Iturres, A.; Serrano, J.; Fernández, N.; Capece, P.; Gorostiaga, J.; et al. Coccidioidomycosis in Argentina, 1892-2009. Rev. Argent Microbiol. 2010, 42, 261–268. [Google Scholar] [CrossRef]
- Cordeiro, R.; Moura, S.; Castelo-Branco, D.; Rocha, M.F.; Lima-Neto, R.; Sidrim, J.J. Coccidioidomycosis in Brazil: Historical Challenges of a Neglected Disease. J. Fungi 2021, 7, 85. [Google Scholar] [CrossRef]
- Diaz, J.H. Travel-Related Risk Factors for Coccidioidomycosis. J. Travel Med. 2018, 25, tay027. [Google Scholar] [CrossRef]
- Carpenter, J.B.; Feldman, J.S.; Leyva, W.H.; DiCaudo, D.J. Clinical and Pathologic Characteristics of Disseminated Cutaneous Coccidioidomycosis. J. Am. Acad. Dermatol. 2010, 62, 831–837. [Google Scholar] [CrossRef]
- Grizzle, A.J.; Wilson, L.; Nix, D.E.; Galgiani, J.N. Clinical and Economic Burden of Valley Fever in Arizona: An Incidence-Based Cost-of-Illness Analysis. Open Forum Infect. Dis. 2021, 8, ofaa623. [Google Scholar] [CrossRef]
- Del Rocío Reyes-Montes, M.; Pérez-Huitrón, M.A.; Ocaña-Monroy, J.L.; Frías-De-León, M.G.; Martínez-Herrera, E.; Arenas, R.; Duarte-Escalante, E. The Habitat of Coccidioides spp. and the Role of Animals as Reservoirs and Disseminators in Nature. BMC Infect. Dis. 2016, 16, 550. [Google Scholar] [CrossRef]
- Kirkland, T.N.; Fierer, J. Coccidioides immitis and posadasii; A Review of Their Biology, Genomics, Pathogenesis, and Host Immunity. Virulence 2018, 9, 1426–1435. [Google Scholar] [CrossRef]
- Huppert, M.; Sun, S.H.; Harrison, J.L. Morphogenesis throughout Saprobic and Parasitic Cycles of Coccidioides immitis. Mycopathologia 1982, 78, 107–122. [Google Scholar] [CrossRef]
- Blair, J.E.; Chang, Y.-H.H.; Cheng, M.-R.; Vaszar, L.T.; Vikram, H.R.; Orenstein, R.; Kusne, S.; Ho, S.; Seville, M.T.; Parish, J.M. Characteristics of Patients with Mild to Moderate Primary Pulmonary Coccidioidomycosis. Emerg. Infect. Dis. 2014, 20, 983. [Google Scholar] [CrossRef]
- Spinello, I.; Munoz, A.; Johnson, R. Pulmonary Coccidioidomycosis. Semin. Respir. Crit. Care Med. 2008, 29, 166–173. [Google Scholar] [CrossRef]
- Stockamp, N.W.; Thompson, G.R. Coccidioidomycosis. Infect. Dis. Clin. N. Am. 2016, 30, 229–246. [Google Scholar] [CrossRef]
- Nguyen, C.; Barker, B.M.; Hoover, S.; Nix, D.E.; Ampel, N.M.; Frelinger, J.A.; Orbach, M.J.; Galgiani, J.N. Recent Advances in Our Understanding of the Environmental, Epidemiological, Immunological, and Clinical Dimensions of Coccidioidomycosis. Clin. Microbiol. Rev. 2013, 26, 505–525. [Google Scholar] [CrossRef]
- Cadena, J.; Hartzler, A.; Hsue, G.; Longfield, R.N. Coccidioidomycosis and Tuberculosis Coinfection at a Tuberculosis Hospital. Medicine 2009, 88, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Galgiani, J.N.; Ampel, N.M.; Blair, J.E.; Catanzaro, A.; Geertsma, F.; Hoover, S.E.; Johnson, R.H.; Kusne, S.; Lisse, J.; MacDonald, J.D.; et al. 2016 Infectious Diseases Society of America (IDSA) Clinical Practice Guideline for the Treatment of Coccidioidomycosis. Clin. Infect. Dis. 2016, 63, e112–e146. [Google Scholar] [CrossRef] [PubMed]
- Garcia Garcia, S.C.; Salas Alanis, J.C.; Flores, M.G.; Gonzalez Gonzalez, S.E.; Vera Cabrera, L.; Ocampo Candiani, J. Coccidioidomycosis and the Skin: A Comprehensive Review. An. Bras. Dermatol. 2015, 90, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.R.; Blair, J.E.; Ampel, N.M. Central Nervous System Infections Due to Coccidioidomycosis. J. Fungi 2019, 5, 54. [Google Scholar] [CrossRef]
- Heaney, A.K.; Head, J.R.; Broen, K.; Click, K.; Taylor, J.; Balmes, J.R.; Zelner, J.; Remais, J.V. Coccidioidomycosis and COVID-19 Co-Infection, United States, 2020. Emerg. Infect. Dis. 2021, 27, 1266–1273. [Google Scholar] [CrossRef]
- Huff, D.; Ampel, N.M.; Blair, J.E. Coccidioidomycosis and COVID-19 Infection. An Analysis from a Single Medical Center Within the Coccidioidal Endemic Area. Mycopathologia 2022, 187, 199–204. [Google Scholar] [CrossRef]
- Aly, F.Z.; Millius, R.; Sobonya, R.; Aboul-Nasr, K.; Klein, R. Cytologic Diagnosis of Coccidioidomycosis: Spectrum of Findings in Southern Arizona Patients over a 10 Year Period. Diagn Cytopathol 2016, 44, 195–200. [Google Scholar] [CrossRef]
- Grosse, A.; Grosse, C. Coccidioidomycosis with Emperipolesis in Fine Needle Aspiration. Cytopathology 2019, 30, 451–452. [Google Scholar] [CrossRef]
- Gastélum-Cano, J.M.; Dautt-Castro, M.; García-Galaz, A.; Felix-Murray, K.; Rascón-Careaga, A.; Cano-Rangel, M.A.; Islas-Osuna, M.A. The Clinical Laboratory Evolution in Coccidioidomycosis Detection: Future Perspectives. J. Med. Mycol. 2021, 31, 101159. [Google Scholar] [CrossRef]
- Gabe, L.M.; Malo, J.; Knox, K.S. Diagnosis and Management of Coccidioidomycosis. Clin. Chest Med. 2017, 38, 417–433. [Google Scholar] [CrossRef]
- Malo, J.; Luraschi-Monjagatta, C.; Wolk, D.M.; Thompson, R.; Hage, C.A.; Knox, K.S. Update on the Diagnosis of Pulmonary Coccidioidomycosis. Ann. Am. Thorac. Soc. 2014, 11, 243–253. [Google Scholar] [CrossRef]
- Binnicker, M.J.; Buckwalter, S.P.; Eisberner, J.J.; Stewart, R.A.; McCullough, A.E.; Wohlfiel, S.L.; Wengenack, N.L. Detection of Coccidioides Species in Clinical Specimens by Real-Time PCR. J. Clin. Microbiol. 2007, 45, 173–178. [Google Scholar] [CrossRef]
- Umeyama, T.; Sano, A.; Kamei, K.; Niimi, M.; Nishimura, K.; Uehara, Y. Novel Approach to Designing Primers for Identification and Distinction of the Human Pathogenic Fungi Coccidioides immitis and Coccidioides posadasii by PCR Amplification. J. Clin. Microbiol. 2006, 44, 1859–1862. [Google Scholar] [CrossRef]
- Hahn, R.C.; Hagen, F.; Mendes, R.P.; Burger, E.; Nery, A.F.; Siqueira, N.P.; Guevara, A.; Rodrigues, A.M.; de Camargo, Z.P. Paracoccidioidomycosis: Current Status and Future Trends. Clin. Microbiol. Rev. 2022. [Google Scholar] [CrossRef]
- Shikanai-Yasuda, M.A.; Mendes, R.P.; Colombo, A.L.; de Queiroz-Telles, F.; Kono, A.S.G.; Paniago, A.M.M.; Nathan, A.; do Valle, A.C.F.; Bagagli, E.; Benard, G.; et al. Brazilian Guidelines for the Clinical Management of Paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 2017, 50, 715–740. [Google Scholar] [CrossRef]
- Dutra, L.M.; Silva, T.H.M.; Falqueto, A.; Peçanha, P.M.; Souza, L.R.M.; Gonçalves, S.S.; Velloso, T.R.G. Oral Paracoccidioidomycosis in a Single-Center Retrospective Analysis from a Brazilian Southeastern Population. J. Infect. Public Health 2018, 11, 530–533. [Google Scholar] [CrossRef]
- de Camargo, Z.P. Serology of Paracoccidioidomycosis. Mycopathologia 2008, 165, 289–302. [Google Scholar] [CrossRef]
- Benard, G. An Overview of the Immunopathology of Human Paracoccidioidomycosis. Mycopathologia 2008, 165, 209–221. [Google Scholar] [CrossRef]
- Da Silva, S.H.M.; Colombo, A.L.; Blotta, M.H.S.L.; Lopes, J.D.; Queiroz-Telles, F.; de Camargo, Z.P. Detection of Circulating Gp43 Antigen in Serum, Cerebrospinal Fluid, and Bronchoalveolar Lavage Fluid of Patients with Paracoccidioidomycosis. J. Clin. Microbiol. 2003, 41, 3675–3680. [Google Scholar] [CrossRef]
- De Mattos Grosso, D.; de Almeida, S.R.; Mariano, M.; Lopes, J.D. Characterization of Gp70 and Anti-Gp70 Monoclonal Antibodies in Paracoccidioides brasiliensis Pathogenesis. Infect. Immun. 2003, 71, 6534–6542. [Google Scholar] [CrossRef]
- Gegembauer, G.; Araujo, L.M.; Pereira, E.F.; Rodrigues, A.M.; Paniago, A.M.M.; Hahn, R.C.; Camargo, Z.P.D. Serology of Paracoccidioidomycosis Due to Paracoccidioides lutzii. PLoS Negl. Trop. Dis. 2014, 8, e2986. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, B.G.; Hahn, R.C.; Camargo, Z.P.D.; Rodrigues, A.M. Molecular Tools for Detection and Identification of Paracoccidioides Species: Current Status and Future Perspectives. J. Fungi 2020, 6, 293. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Silva, F.; Gomes, L.I.; Gracielle-Melo, C.; Goes, A.M.; Caligiorne, R.B. Real Time Polymerase Chain Reaction (Rt-PCR): A New Patent to Diagnostic Purposes for Paracoccidioidomycosis. Recent Pat. Endocr. Metab. Immune Drug Discov. 2017, 10, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Ampel, N.M. The Diagnosis of Coccidioidomycosis. F1000 Med. Rep. 2010, 2. [Google Scholar] [CrossRef]
- Vucicevic, D.; Blair, J.E.; Binnicker, M.J.; McCullough, A.E.; Kusne, S.; Vikram, H.R.; Parish, J.M.; Wengenack, N.L. The Utility of Coccidioides Polymerase Chain Reaction Testing in the Clinical Setting. Mycopathologia 2010, 170, 345–351. [Google Scholar] [CrossRef]
- Kaufman, L.; Sekhon, A.S.; Moledina, N.; Jalbert, M.; Pappagianis, D. Comparative Evaluation of Commercial Premier EIA and Microimmunodiffusion and Complement Fixation Tests for Coccidioides immitis Antibodies. J. Clin. Microbiol. 1995, 33, 618. [Google Scholar] [CrossRef]
- Durkin, M.; Connolly, P.; Kuberski, T.; Myers, R.; Kubak, B.M.; Bruckner, D.; Pegues, D.; Wheat, L.J. Diagnosis of Coccidioidomycosis with Use of the Coccidioides Antigen Enzyme Immunoassay. Clin. Infect. Dis. 2008, 47, e69–e73. [Google Scholar] [CrossRef]
- Thompson, G.R.; Boulware, D.R.; Bahr, N.C.; Clancy, C.J.; Harrison, T.S.; Kauffman, C.A.; Le, T.; Miceli, M.H.; Mylonakis, E.; Nguyen, M.H.; et al. Noninvasive Testing and Surrogate Markers in Invasive Fungal Diseases. Open Forum Infect. Dis. 2022, 9, ofaac112. [Google Scholar] [CrossRef]
- Saubolle, M.A. Laboratory Aspects in the Diagnosis of Coccidioidomycosis. Ann. N. Y. Acad. Sci. 2007, 1111, 301–314. [Google Scholar] [CrossRef]
- Kim, M.M.; Vikram, H.R.; Kusne, S.; Seville, M.T.; Blair, J.E. Treatment of Refractory Coccidioidomycosis With Voriconazole or Posaconazole. Clin. Infect. Dis. 2011, 53, 1060–1066. [Google Scholar] [CrossRef]
- Shubitz, L.F.; Roy, M.E.; Nix, D.E.; Galgiani, J.N. Efficacy of Nikkomycin Z for Respiratory Coccidioidomycosis in Naturally Infected Dogs. Med. Mycol. 2013, 51, 747–754. [Google Scholar] [CrossRef]
- Sass, G.; Larwood, D.J.; Martinez, M.; Shrestha, P.; Stevens, D.A. Efficacy of Nikkomycin Z in Murine CNS Coccidioidomycosis: Modelling Sustained-Release Dosing. J. Antimicrob. Chemother. 2021, 76, 2629–2635. [Google Scholar] [CrossRef]
- Larwood, D.J. Nikkomycin Z—Ready to Meet the Promise? J. Fungi 2020, 6, 261. [Google Scholar] [CrossRef]
- Sass, G.; Larwood, D.J.; Martinez, M.; Chatterjee, P.; Xavier, M.O.; Stevens, D.A. Nikkomycin Z against Disseminated Coccidioidomycosis in a Murine Model of Sustained-Release Dosing. Antimicrob. Agents Chemother. 2021, 65. [Google Scholar] [CrossRef]
- Wiederhold, N.P. Review of the Novel Investigational Antifungal Olorofim. J. Fungi 2020, 6, 122. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Najvar, L.K.; Jaramillo, R.; Olivo, M.; Birch, M.; Law, D.; Rex, J.H.; Catano, G.; Patterson, T.F. The Orotomide Olorofim Is Efficacious in an Experimental Model of Central Nervous System Coccidioidomycosis. Antimicrob. Agents Chemother. 2018, 62, e00999-18. [Google Scholar] [CrossRef]
- Pappagianis, D.; Levine, H.B. The Present Status of Vaccination Against Coccidioidomycosis in Man. Am. J. Epidemiol. 1975, 102, 30–41. [Google Scholar] [CrossRef]
- Cole, G.T.; Xue, J.M.; Okeke, C.N.; Tarcha, E.J.; Basrur, V.; Schaller, R.A.; Herr, R.A.; Yu, J.J.; Hung, C.Y. Prospects of Vaccines for Medically Important Fungi. A Vaccine against Coccidioidomycosis Is Justified and Attainable. Med. Mycol. 2004, 42, 189–216. [Google Scholar] [CrossRef]
- Galgiani, J.N.; Shubitz, L.F.; Orbach, M.J.; Mandel, M.A.; Powell, D.A.; Klein, B.S.; Robb, E.J.; Ohkura, M.; Seka, D.J.; Tomasiak, T.M.; et al. Vaccines to Prevent Coccidioidomycosis: A Gene-Deletion Mutant of Coccidioides posadasii as a Viable Candidate for Human Trials. J. Fungi 2022, 8, 838. [Google Scholar] [CrossRef]
- Martinez, R. New Trends in Paracoccidioidomycosis Epidemiology. J. Fungi 2017, 3, 1. [Google Scholar] [CrossRef]
- Mavengere, H.; Mattox, K.; Teixeira, M.M.; Sepúlveda, V.E.; Gomez, O.M.; Hernandez, O.; McEwen, J.; Matute, D.R. Paracoccidioides Genomes Reflect High Levels of Species Divergence and Little Interspecific Gene Flow. mBio 2020, 11, e01999-20. [Google Scholar] [CrossRef] [PubMed]
- Roberto, T.N.; de Carvalho, J.A.; Beale, M.A.; Hagen, F.; Fisher, M.C.; Hahn, R.C.; de Camargo, Z.P.; Rodrigues, A.M. Trends in the Molecular Epidemiology and Population Genetics of Emerging Sporothrix Species. Stud. Mycol. 2021, 100, 100131. [Google Scholar] [CrossRef] [PubMed]
- Bellissimo-Rodrigues, F.; Machado, A.A.; Martinez, R. Paracoccidioidomycosis Epidemiological Features of a 1,000-Cases Series from a Hyperendemic Area on the Southeast of Brazil. Am. J. Trop. Med. Hyg. 2011, 85, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Peçanha, P.M.; Batista Ferreira, M.E.; Massaroni Peçanha, M.A.; Schmidt, E.B.; Lamas de Araújo, M.; Zanotti, R.L.; Potratz, F.F.; Delboni Nunes, N.E.; Gonçalves Ferreira, C.U.; Delmaestro, D.; et al. Paracoccidioidomycosis: Epidemiological and Clinical Aspects in 546 Cases Studied in the State of Espírito Santo, Brazil. Am. J. Trop. Med. Hyg. 2017, 97, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Bellissimo-Rodrigues, F.; Bollela, V.R.; Fonseca, B.A.L.; Martinez, R. Endemic Paracoccidioidomycosis: Relationship between Clinical Presentation and Patients’ Demographic Features. Med. Mycol. 2013, 51, 313–318. [Google Scholar] [CrossRef]
- Coutinho, Z.F.; da Silva, D.; Lazéra, M.; Petri, V.; de Oliveira, R.M.; Sabroza, P.C.; Wanke, B. Paracoccidioidomycosis Mortality in Brazil (1980-1995). Cad Saude Publica 2002, 18, 1441–1454. [Google Scholar] [CrossRef]
- Coutinho, Z.F.; Wanke, B.; Travassos, C.; Oliveira, R.M.; Xavier, D.R.; Coimbra, C.E.A. Hospital Morbidity Due to Paracoccidioidomycosis in Brazil (1998–2006). Trop. Med. Int. Health 2015, 20, 673–680. [Google Scholar] [CrossRef]
- De Suguiura, I.M.S.; Ono, M.A. Compulsory Notification of Paracoccidioidomycosis: A 14-Year Retrospective Study of the Disease in the State of Paraná, Brazil. Mycoses 2022, 65, 354–361. [Google Scholar] [CrossRef]
- Dantas, K.C.; Mauad, T.; de André, C.D.S.; Bierrenbach, A.L.; Saldiva, P.H.N. A Single-Centre, Retrospective Study of the Incidence of Invasive Fungal Infections during 85 Years of Autopsy Service in Brazil. Sci. Rep. 2021, 11, 3943. [Google Scholar] [CrossRef]
- Fabris, L.R.; Andrade, Ú.V.; Santos, A.F.D.; Marques, A.P.D.C.; Oliveira, S.M.D.V.L.D.; Mendes, R.P.; Paniago, A.M.M. Decreasing Prevalence of the Acute/Subacute Clinical Form of Paracoccidioidomycosis in Mato Grosso do Sul State, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 121–125. [Google Scholar] [CrossRef]
- Vieira, G.D.D.; Alves, T.D.C.; Lima, S.M.D.D.; Camargo, L.M.A.; Sousa, C.M.D. Paracoccidioidomycosis in a Western Brazilian Amazon State: Clinical-Epidemiologic Profile and Spatial Distribution of the Disease. Rev. Soc. Bras. Med. Trop. 2014, 47, 63–68. [Google Scholar] [CrossRef]
- Krakhecke-Teixeira, A.G.; Yamauchi, D.H.; Rossi, A.; de Sousa, H.R.; Garces, H.G.; Júnior, J.L.; Júnior, A.O.S.; Felipe, M.S.S.; Bagagli, E.; de Andrade, H.F.; et al. Clinical and Eco-Epidemiological Aspects of a Novel Hyperendemic Area of Paracoccidioidomycosis in the Tocantins-Araguaia Basin (Northern Brazil), Caused by Paracoccidioides sp. J. Fungi 2022, 8, 502. [Google Scholar] [CrossRef]
- Mangiaterra, M.L.; Giusiano, G.E.; Alonso, J.M.; Gorodner, J.O. Paracoccidioides brasiliensis Infection in a Subtropical Region with Important Environmental Changes. Bull. Soc. Pathol. Exot. 1999, 92, 173–176. [Google Scholar]
- Do Valle, A.C.F.; de Macedo, P.M.; Almeida-Paes, R.; Romão, A.R.; Lazéra, M.D.S.; Wanke, B. Paracoccidioidomycosis after Highway Construction, Rio de Janeiro, Brazil. Emerg. Infect. Dis. 2017, 23, 1917–1919. [Google Scholar] [CrossRef]
- Barrozo, L.V.; Benard, G.; Silva, M.E.S.; Bagagli, E.; Marques, S.A.; Mendes, R.P. First Description of a Cluster of Acute/Subacute Paracoccidioidomycosis Cases and Its Association with a Climatic Anomaly. PLoS Negl. Trop. Dis. 2010, 4, e643. [Google Scholar] [CrossRef]
- Giusiano, G.; Aguirre, C.; Vratnica, C.; Rojas, F.; Corallo, T.; Cattana, M.E.; Fernández, M.; Mussin, J.; de los Angeles Sosa, M. Emergence of Acute/Subacute Infant-Juvenile Paracoccidioidomycosis in Northeast Argentina: Effect of Climatic and Anthropogenic Changes? Med. Mycol. 2019, 57, 30–37. [Google Scholar] [CrossRef]
- Wagner, G.; Moertl, D.; Glechner, A.; Mayr, V.; Klerings, I.; Zachariah, C.; den Nest, M.; Gartlehner, G.; Willinger, B. Paracoccidioidomycosis Diagnosed in Europe—A Systematic Literature Review. J. Fungi 2021, 7, 157. [Google Scholar] [CrossRef]
- Rahman, R.; Davies, L.; Mohareb, A.M.; Peçanha-Pietrobom, P.M.; Patel, N.J.; Solomon, I.H.; Meredith, D.M.; Tsai, H.K.; Guenette, J.P.; Bhattacharyya, S.; et al. Delayed Relapse of Paracoccidioidomycosis in the Central Nervous System: A Case Report. Open Forum Infect Dis. 2020, 7, ofaa077. [Google Scholar] [CrossRef]
- Onda, H.; Komine, M.; Murata, S.; Ohtsuki, M. Letter: Imported Paracoccidioidomycosis in Japan. Dermatol. Online J. 2011, 17, 11. [Google Scholar] [CrossRef]
- Linares, G.; Baker, R.D.; Linares, L. Paracoccidioidomycosis in the United States (South American Blastomycosis). Arch. Otolaryngol. -Head Neck Surg. 1971, 93, 514–518. [Google Scholar] [CrossRef]
- Falcão, E.M.; de Macedo, P.M.; Freitas, D.F.S.; Freitas, A.D.A.; Grinsztejn, B.; Veloso, V.G.; Almeida-Paes, R.; do Valle, A.C.F. Paracoccidioidomycosis in People Living with HIV/AIDS: A Historical Retrospective Cohort Study in a National Reference Center for Infectious Diseases, Rio de Janeiro, Brazil. PLoS Negl. Trop. Dis. 2022, 16, e0010529. [Google Scholar] [CrossRef] [PubMed]
- Morejón, K.M.L.; Machado, A.A.; Martinez, R. Paracoccidioidomycosis in Patients Infected with and Not Infected with Human Immunodeficiency Virus: A Case-Control Study. Am. J. Trop. Med. Hyg. 2009, 80, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Messina, F.; Romero, M.; Benchetrit, A.; Marin, E.; Arechavala, A.; Depardo, R.; Negroni, R.; Santiso, G. Clinical and Microbiological Characteristics of Paracoccidioidomycosis in Patients with AIDS in Buenos Aires, Argentina. Med. Mycol. 2020, 58, 22–29. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, J., Jr.; Peçanha-Pietrobom, P.; Colombo, A. Paracoccidioidomycosis in Immunocompromised Patients: A Literature Review. J. Fungi 2018, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Valentim, F.D.O.; Tsutsui, G.M.; Abbade, L.P.F.; Marques, S.A. Disseminated Paracoccidioidomycosis in a Liver Transplant Patient. An. Bras. Dermatol. 2021, 96, 346–348. [Google Scholar] [CrossRef]
- Peçanha-Pietrobom, P.M.; Falqueto, A.; Rodrigues Gandarella, A.D.; Moyzés, J.V.; Rangel, K.A.; Miranda, L.B.; Hemerly, M.C.; Careta, R.S.; Peçanha, P.M. Case Report: Paracoccidioidomycosis in Solid Organ Transplantation: Disseminated Disease in a Liver Recipient and Literature Review. Am. J. Trop. Med. Hyg. 2019, 101, 1100–1106. [Google Scholar] [CrossRef]
- Felipe, C.R.A.; Silva, A.D.; Penido, M.G.M.G. Disseminated Paracoccidioidomycosis in a Kidney Transplant Recipient. Cureus 2021, 13, e19007. [Google Scholar] [CrossRef]
- Pereira, R.M.; Bucaretchi, F.; Barison, E.D.M.; Hessel, G.; Tresoldi, A.T. Paracoccidioidomycosis in Children: Clinical Presentation, Follow-up and Outcome. Rev. Inst. Med. Trop. Sao Paulo 2004, 46, 127–131. [Google Scholar] [CrossRef]
- Benard, G.; Kavakama, J.; Mendes-Giannini, M.J.S.; Kono, A.; Duarte, A.J.S.; Shikanai-Yasuda, M.A. Contribution to the Natural History of Paracoccidioidomycosis: Identification of the Primary Pulmonary Infection in the Severe Acute Form of the Disease–A Case Report. Clin. Infect. Dis. 2005, 40, e1–e4. [Google Scholar] [CrossRef]
- Mendes, R.P.; Cavalcante, R.S.; Marques, S.A.; Marques, M.E.A.; Venturini, J.; Sylvestre, T.F.; Paniago, A.M.M.; Pereira, A.C.; da Silva, J.D.F.; Fabro, A.T.; et al. Paracoccidioidomycosis: Current Perspectives from Brazil. Open Microbiol. J. 2017, 11, 224–282. [Google Scholar] [CrossRef]
- De Macedo, P.M.; Almeida-Paes, R.; Freitas, D.F.S.; Varon, A.G.; Paixão, A.G.; Romão, A.R.; Coutinho, Z.F.; Pizzini, C.V.; Zancopé-Oliveira, R.M.; do Valle, A.C.F. Acute Juvenile Paracoccidioidomycosis: A 9-Year Cohort Study in the Endemic Area of Rio de Janeiro, Brazil. PLoS Negl. Trop. Dis. 2017, 11, e0005500. [Google Scholar] [CrossRef]
- Restrepo, A.; Salazar, M.E.; Cano, L.E.; Stover, E.P.; Feldman, D.; Stevens, D.A. Estrogens Inhibit Mycelium-to-Yeast Transformation in the Fungus Paracoccidioides brasiliensis: Implications for Resistance of Females to Paracoccidioidomycosis. Infect. Immun. 1984, 46, 346–353. [Google Scholar] [CrossRef]
- Carvalho, F.M.C.; Busser, F.D.; Freitas, V.L.T.; Furucho, C.R.; Sadahiro, A.; Kono, A.S.G.; Criado, P.R.; Moretti, M.L.; Sato, P.K.; Shikanai-Yasuda, M.A. Polymorphisms on IFNG, IL12B and IL12RB1 Genes and Paracoccidioidomycosis in the Brazilian Population. Infect. Genet. Evol. 2016, 43, 245–251. [Google Scholar] [CrossRef]
- Dos Santos, W.A.; da Silva, B.M.; Passos, E.D.; Zandonade, E.; Falqueto, A. Associação Entre Tabagismo e Paracoccidioidomicose: Um Estudo de Caso-Controle No Estado Do Espírito Santo, Brasil. Cad. Saude Publica 2003, 19, 245–253. [Google Scholar] [CrossRef]
- De Messias, I.J.T.; Reis, A.; Brenden, M.; Queiroz-Telles, F.; Mauff, G. Association of Major Histocompatibility Complex Class III Complement Components C2, BF, and C4 with Brazilian Paracoccidioidomycosis. Complement. Inflamm. 1991, 8, 288–293. [Google Scholar] [CrossRef]
- Buccheri, R.; Duarte-Neto, A.N.; Silva, F.L.B.; Haddad, G.C.; da Silva, L.B.R.; Netto, R.A.; Ledesma, F.L.; Taborda, C.P.; Benard, G. Chronic Exposure to Cigarette Smoke Transiently Worsens the Disease Course in a Mouse Model of Pulmonary Paracoccidioidomycosis. Rev. Inst. Med. Trop. Sao Paulo 2022, 64. [Google Scholar] [CrossRef]
- Tobon, A.M.; Agudelo, C.A.; Osorio, M.L.; Alvarez, D.L.; Arango, M.; Cano, L.E.; Restrepo, A. Residual Pulmonary Abnormalities in Adult Patients with Chronic Paracoccidioidomycosis: Prolonged Follow-Up after Itraconazole Therapy. Clin. Infect. Dis. 2003, 37, 898–904. [Google Scholar] [CrossRef]
- Batah, S.S.; Alda, M.A.; Machado-Rugulo, J.R.; Felix, R.G.; Nascimento, E.; Martinez, R.; Pádua, A.I.; Bagagli, E.; Hrycyk, M.F.; Salgado, H.C.; et al. Pulmonary Paracoccidioidomycosis-induced Pulmonary Hypertension. Clin. Transl. Med. 2020, 10, 213. [Google Scholar] [CrossRef]
- Colombo, A.L.; Faiçal, S.; Kater, C.E. Systematic Evaluation of the Adrenocortical Function in Patients with Paracoccidioidomycosis. Mycopathologia 1994, 127, 89–93. [Google Scholar] [CrossRef]
- Francesconi, F.; da Silva, M.T.T.; Costa, R.L.B.; Francesconi, V.A.; Carregal, E.; Talhari, S.; do Valle, A.C.F. Long-Term Outcome of Neuroparacoccidioidomycosis Treatment. Rev. Soc. Bras. Med. Trop. 2011, 44, 22–25. [Google Scholar] [CrossRef]
- Alvarez, M.; Pina, D.R.; de Oliveira, M.; Ribeiro, S.M.; Mendes, R.P.; Duarte, S.B.; Miranda, J.R.A. Objective CT-Based Quantification of Lung Sequelae in Treated Patients With Paracoccidioidomycosis. Medicine 2014, 93, e167. [Google Scholar] [CrossRef] [PubMed]
- Tobon, A.M.; Agudelo, C.A.; Restrepo, C.A.; Villa, C.A.; Quiceno, W.; Estrada, S.; Restrepo, A. Adrenal Function Status in Patients with Paracoccidioidomycosis After Prolonged Post-Therapy Follow Up. Am. Trop. Med. Hyg. 2010, 83, 111–114. [Google Scholar] [CrossRef]
- Costa, A.N.; Benard, G.; Albuquerque, A.L.P.; Fujita, C.L.; Magri, A.S.K.; Salge, J.M.; Shikanai-Yasuda, M.A.; Carvalho, C.R.R. The Lung in Paracoccidioidomycosis: New Insights into Old Problems. Clinics 2013, 68, 441–448. [Google Scholar] [CrossRef]
- Prado, M.; da Silva, M.B.; Laurenti, R.; Travassos, L.R.; Taborda, C.P. Mortality Due to Systemic Mycoses as a Primary Cause of Death or in Association with AIDS in Brazil: A Review from 1996 to 2006. Mem. Inst. Oswaldo Cruz. 2009, 104, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.P.; Padovani, C.R.; Cataneo, A.M. Paracoccidioidomycosis: Radiologic and Pulmonary Study in 58 Cases. Rev. Inst. Med. Trop. Sao Paulo 1991, 33, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.P.; Cataneo, A.J.M. Função Pulmonar Na Evolução de 35 Doentes Com Paracoccidioidomicose. Rev. Inst. Med. Trop. Sao Paulo 1986, 28, 330–336. [Google Scholar] [CrossRef]
- Marques, S.A.; Cortez, D.B.; Lastória, J.C.; Camargo, R.M.P.D.; Marques, M.E.A. Paracoccidioidomicose: Frequência, Morfologia e Patogênese de Lesões Tegumentares. An. Bras. Dermatol. 2007, 82, 411–417. [Google Scholar] [CrossRef]
- Peçanha, P.M.; Peçanha-Pietrobom, P.M.; Grão-Velloso, T.R.; Rosa Júnior, M.; Falqueto, A.; Gonçalves, S.S. Paracoccidioidomycosis: What We Know and What Is New in Epidemiology, Diagnosis, and Treatment. J. Fungi 2022, 8, 1098. [Google Scholar] [CrossRef]
- Rosa Júnior, M.; Baldon, I.V.; Amorim, A.F.C.; Fonseca, A.P.A.; Volpato, R.; Lourenço, R.B.; Baptista, R.M.; de Mello, R.A.F.; Peçanha, P.; Falqueto, A. Imaging Paracoccidioidomycosis: A Pictorial Review from Head to Toe. Eur. J. Radiol. 2018, 103, 147–162. [Google Scholar] [CrossRef]
- Marchiori, E.; Valiante, P.M.; Mano, C.M.; Zanetti, G.; Escuissato, D.L.; Souza, A.S.; Capone, D. Paracoccidioidomycosis: High-Resolution Computed Tomography–Pathologic Correlation. Eur. J. Radiol. 2011, 77, 80–84. [Google Scholar] [CrossRef]
- Muniz, M.; Marchiori, E.; Magnago, M.; Moreira, L. Paracoccidioidomicose Pulmonar: Aspectos Na Tomografia Computadorizada de Alta Resolução. Radiol. Bras. 2002, 35, 147–154. [Google Scholar] [CrossRef]
- Souza, A.S.; Gasparetto, E.L.; Davaus, T.; Escuissato, D.L.; Marchiori, E. High-Resolution CT Findings of 77 Patients with Untreated Pulmonary Paracoccidioidomycosis. Am. J. Roentg. 2006, 187, 1248–1252. [Google Scholar] [CrossRef]
- De Pina, D.R.; Alvarez, M.; Giacomini, G.; Pavan, A.L.M.; Guedes, C.I.A.; de Souza Cavalcante, R.; Mendes, R.P.; Paniago, A.M.M. Paracoccidioidomycosis: Level of Pulmonary Sequelae in High Resolution Computed Tomography Images from Patients of Two Endemic Regions of Brazil. Quant. Imaging Med. Surg. 2017, 7, 318–325. [Google Scholar] [CrossRef]
- Restrepo, A.; Benard, G.; de Castro, C.; Agudelo, C.; Tobón, A. Pulmonary Paracoccidioidomycosis. Semin. Respir. Crit. Care Med. 2008, 29, 182–197. [Google Scholar] [CrossRef]
- Queiroz-Telles, F.; Escuissato, D. Pulmonary Paracoccidioidomycosis. Semin. Respir. Crit. Care Med. 2011, 32, 764–774. [Google Scholar] [CrossRef]
- Talhari, C.; de Souza, J.V.B.; Parreira, V.J.; Reinel, D.; Talhari, S. Oral Exfoliative Cytology as a Rapid Diagnostic Tool for Paracoccidioidomycosis. Mycoses 2008, 51, 177–178. [Google Scholar] [CrossRef]
- Gaviria, M.; Rivera, V.; Muñoz-Cadavid, C.; Cano, L.E.; Naranjo, T.W. Validation and Clinical Application of a Nested PCR for Paracoccidioidomycosis Diagnosis in Clinical Samples from Colombian Patients. Braz. J. Infec. Dis. 2015, 19, 376–383. [Google Scholar] [CrossRef]
- Niño-Vega, G.A.; Calcagno, A.M.; San-Blas, G.; San-Blas, F.; Gooday, G.W.; Gow, N.A.R. RFLP Analysis Reveals Marked Geographical Isolation between Strains of Paracoccidioides brasiliensis. Med. Mycol. 2000, 38, 437–441. [Google Scholar] [CrossRef]
- Nobrega de Almeida, J.; del Negro, G.M.B.; Grenfell, R.C.; Vidal, M.S.M.; Thomaz, D.Y.; de Figueiredo, D.S.Y.; Bagagli, E.; Juliano, L.; Benard, G. Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry for Differentiation of the Dimorphic Fungal Species Paracoccidioides brasiliensis and Paracoccidioides lutzii. J. Clin. Microbiol 2015, 53, 1383–1386. [Google Scholar] [CrossRef]
- Camargo, Z.P.; Berzaghi, R.; Amaral, C.C.; Silva, S.H.M. Simplified Method for Producing Paracoccidioides brasiliensis Exoantigens for Use in Immunodiffusion Tests. Med. Mycol. 2003, 41, 539–542. [Google Scholar] [CrossRef]
- Restrepo, A.; Moncada, L.H. A Slide Latex Test for the Diagnosis of Paracoccidiodomycosis. Bol. Oficina Sanit Panam 1978, 84, 520–532. [Google Scholar] [PubMed]
- Silveira-Gomes, F.; Sarmento, D.N.; Pinto, T.M.; Pimentel, R.F.; Nepomuceno, L.B.; Santo, E.P.T.E.; Mesquita-da-Costa, M.; Camargo, Z.P.; Marques-da-Silva, S.H. Development and Evaluation of a Latex Agglutination Test for the Serodiagnosis of Paracoccidioidomycosis. Clin. Vaccine Immunol. 2011, 18, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Teles, F.R.R.; Martins, M.L. Laboratorial Diagnosis of Paracoccidioidomycosis and New Insights for the Future of Fungal Diagnosis. Talanta 2011, 85, 2254–2264. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Giannini, M.J.; Bueno, J.P.; Shikanai-Yasuda, M.A.; Ferreira, A.W.; Masuda, A. Detection of the 43,000-Molecular-Weight Glycoprotein in Sera of Patients with Paracoccidioidomycosis. J. Clin. Microbiol. 1989, 27, 2842–2845. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.S.M.; Negro, G.M.B.; Vicentini, A.P.; Svidzinski, T.I.E.; Mendes-Giannini, M.J.; Almeida, A.M.F.; Martinez, R.; de Camargo, Z.P.; Taborda, C.P.; Benard, G. Serological Diagnosis of Paracoccidioidomycosis: High Rate of Inter-Laboratorial Variability among Medical Mycology Reference Centers. PLoS Negl. Trop. Dis. 2014, 8, e3174. [Google Scholar] [CrossRef]
- Maricato, J.T.; Batista, W.L.; Kioshima, É.S.; Feitosa, L.S.; e Brito, R.R.N.; Goldman, G.H.; Mariano, M.; Puccia, R.; Lopes, J.D. The Paracoccidioides brasiliensis Gp70 Antigen Is Encoded by a Putative Member of the Flavoproteins Monooxygenase Family. Fungal Genet. Biol. 2010, 47, 179–189. [Google Scholar] [CrossRef]
- De Brito, E.C.A.; Franca, T.; Canassa, T.; Weber, S.S.; Paniago, A.M.M.; Cena, C. Paracoccidioidomycosis Screening Diagnosis by FTIR Spectroscopy and Multivariate Analysis. Photodiagnosis. Photodyn. Ther. 2022, 39, 102921. [Google Scholar] [CrossRef]
- Xavier, M.O.; Pasqualotto, A.C.; Cardoso, I.C.E.; Severo, L.C. Cross-Reactivity of Paracoccidioides brasiliensis, Histoplasma capsulatum, and Cryptococcus Species in the Commercial Platelia Aspergillus Enzyme Immunoassay. Clin. Vaccine Immunol. 2009, 16, 132–133. [Google Scholar] [CrossRef]
- Melo, A.S.D.A.; Santos, D.W.D.C.L.; Lima, S.L.; Rodrigues, A.M.; Camargo, Z.P.; Finkelman, M.; Colombo, A.L. Evaluation of (1 → 3)-β-D-glucan Assay for Diagnosing Paracoccidioidomycosis. Mycoses 2020, 63, 38–42. [Google Scholar] [CrossRef]
- Koishi, A.C.; Vituri, D.F.; Filho, P.S.R.D.; Sasaki, A.A.; Felipe, M.S.S.; Venancio, E.J. A Semi-Nested PCR Assay for Molecular Detection of Paracoccidioides brasiliensis in Tissue Samples. Rev. Soc. Bras. Med. Trop. 2010, 43, 728–730. [Google Scholar] [CrossRef]
- Imai, T.; Sano, A.; Mikami, Y.; Watanabe, K.; Aoki, F.H.; Branchini, M.L.M.; Negroni, R.; Nishimura, K.; Miyaji, M. A New PCR Primer for the Identification of Paracoccidioides Brasiliensis Based on RRNA Sequences Coding the Internal Transcribed Spacers (ITS) and 5·8S Regions. Med. Mycol. 2000, 38, 323–326. [Google Scholar] [CrossRef]
- Tabassum, S.; Zia, M.; Khoja, A.A.; David, J.; Iqbal, M.; Junaid, M. Lepra Reactions: A Study of 130 Cases from Pakistan. J. Pak. Med. Assoc. 2021, 71, 2317–2320. [Google Scholar] [CrossRef]
- Koehler, A.; Scroferneker, M.L.; Pereira, B.A.S.; de Souza, N.M.P.; Cavalcante, R.D.S.; Mendes, R.P.; Corbellini, V.A. Using Infrared Spectroscopy of Serum and Chemometrics for Diagnosis of Paracoccidioidomycosis. J. Pharm. Biomed. Anal. 2022, 221, 115021. [Google Scholar] [CrossRef]
- Thompson, G.R.; Le, T.; Chindamporn, A.; Kauffman, C.A.; Alastruey-Izquierdo, A.; Ampel, N.M.; Andes, D.R.; Armstrong-James, D.; Ayanlowo, O.; Baddley, J.W.; et al. Global Guideline for the Diagnosis and Management of the Endemic Mycoses: An Initiative of the European Confederation of Medical Mycology in Cooperation with the International Society for Human and Animal Mycology. Lancet Infect. Dis. 2021, 21, e364–e374. [Google Scholar] [CrossRef]
- Naranjo, M.S.; Trujillo, M.; Munera, M.I.; Restrepo, P.; Gomez, I.; Restrepo, A. Treatment of Paracoccidioidomycosis with Itraconazole. Med. Mycol. 1990, 28, 67–76. [Google Scholar] [CrossRef]
- Caceres, D.H.; Echeverri Tirado, L.C.; Bonifaz, A.; Adenis, A.; Gomez, B.L.; Flores, C.L.B.; Canteros, C.E.; Santos, D.W.; Arathoon, E.; Soto, E.R.; et al. Current Situation of Endemic Mycosis in the Americas and the Caribbean: Proceedings of the First International Meeting on Endemic Mycoses of the Americas (IMEMA). Mycoses 2022, 65, 1179–1187. [Google Scholar] [CrossRef]
- Domínguez-Gil Hurlé, A.; Sánchez Navarro, A.; García Sánchez, M.J. Therapeutic Drug Monitoring of Itraconazole and the Relevance of Pharmacokinetic Interactions. Clin. Microbiol. Infect. 2006, 12, 97–106. [Google Scholar] [CrossRef]
- Rauseo, A.M.; Mazi, P.; Lewis, P.; Burnett, B.; Mudge, S.; Spec, A. Bioavailability of Single-Dose SUBA-Itraconazole Compared to Conventional Itraconazole under Fasted and Fed Conditions. Antimicrob. Agents Chemother. 2021, 65, e0013421. [Google Scholar] [CrossRef]
- Pappas, P.G.; Spec, A.; Miceli, M.; McGwin, G.; McMullen, R.; Thompson, G.R.R., III. An Open-Label Comparative Trial of SUBA-Itraconazole (SUBA) versus Conventional Itraconazole (c-Itra) for Treatment of Proven and Probable Endemic Mycoses (MSG-15): A Pharmacokinetic (PK) and Adverse Event (AE) Analysis. Open Forum Infect. Dis. 2021, 8, S72. [Google Scholar] [CrossRef]
- Cavalcante, R.D.S.; Sylvestre, T.F.; Levorato, A.D.; de Carvalho, L.R.; Mendes, R.P. Comparison between Itraconazole and Cotrimoxazole in the Treatment of Paracoccidiodomycosis. PLoS Negl. Trop. Dis. 2014, 8, e2793. [Google Scholar] [CrossRef]
- Borges, S.R.C.; da Silva, G.M.S.; da Costa Chambela, M.; Oliveira, R.D.V.D.; Costa, R.L.B.; Wanke, B.; do Valle, A.C.F. Itraconazole vs. Trimethoprim–Sulfamethoxazole: A Comparative Cohort Study of 200 Patients with Paracoccidioidomycosis. Med. Mycol. 2014, 52, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Queiroz-Telles, F.; Goldani, L.Z.; Schlamm, H.T.; Goodrich, J.M.; Espinel-Ingroff, A.; Shikanai-Yasuda, M.A. An Open-Label Comparative Pilot Study of Oral Voriconazole and Itraconazole for Long-Term Treatment of Paracoccidioidomycosis. Clin. Infect. Dis. 2007, 45, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.R.; Rendon, A.; Ribeiro dos Santos, R.; Queiroz-Telles, F.; Ostrosky-Zeichner, L.; Azie, N.; Maher, R.; Lee, M.; Kovanda, L.; Engelhardt, M.; et al. Isavuconazole Treatment of Cryptococcosis and Dimorphic Mycoses. Clin. Infect. Dis. 2016, 63, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Seki Kioshima, E.; Mendonça, P.D.S.B.D.; de Melo Teixeira, M.; Grenier Capoci, I.R.; Amaral, A.; Vilugron Rodrigues-Vendramini, F.A.; Lauton Simões, B.; Rodrigues Abadio, A.K.; Fernandes Matos, L.; Soares Felipe, M.S. One Century of Study: What We Learned about Paracoccidioides and How This Pathogen Contributed to Advances in Antifungal Therapy. J. Fungi 2021, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Sylvestre, T.F.; Franciscone Silva, L.R.; Cavalcante, R.D.S.; Moris, D.V.; Venturini, J.; Vicentini, A.P.; de Carvalho, L.R.; Mendes, R.P. Prevalence and Serological Diagnosis of Relapse in Paracoccidioidomycosis Patients. PLoS Negl. Trop. Dis. 2014, 8, e2834. [Google Scholar] [CrossRef]
- Martins, R.; Marques, S.; Alves, M.; Fecchio, D.; Franco, M.F. de Serological Follow-up of Patients with Paracoccidioidomycosis Treated with Itraconazole Using Dot-Blot, ELISA and Western-Blot. Rev. Inst. Med. Trop. Sao Paulo 1997, 39, 261–270. [Google Scholar] [CrossRef]
- Dias, L.D.S.; Silva, L.B.R.; Nosanchuk, J.D.; Taborda, C.P. Neutrophil Cells Are Essential for The Efficacy of a Therapeutic Vaccine against Paracoccidioidomycosis. J. Fungi 2021, 7, 416. [Google Scholar] [CrossRef]
- Muñoz, J.E.; Luft, V.D.; Amorim, J.; Magalhães, A.; Thomaz, L.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Immunization with P10 Peptide Increases Specific Immunity and Protects Immunosuppressed BALB/c Mice Infected with Virulent Yeasts of Paracoccidioides brasiliensis. Mycopathologia 2014, 178, 177–188. [Google Scholar] [CrossRef]
- Magalhães, A.; Ferreira, K.S.; Almeida, S.R.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Prophylactic and Therapeutic Vaccination Using Dendritic Cells Primed with Peptide 10 Derived from the 43-Kilodalton Glycoprotein of Paracoccidioides brasiliensis. Clin. Vaccine Immunol. 2012, 19, 23–29. [Google Scholar] [CrossRef]
- Taborda, C.P.; Urán, M.E.; Nosanchuk, J.D.; Travassos, L.R. Paracoccidioidomycosis: Challenges in the Development of a Vaccine Against an Endemic Mycosis in the Americas. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 21–24. [Google Scholar] [CrossRef]
- Morais, E.A.; Martins, E.M.D.N.; Boelone, J.N.; Gomes, D.A.; Goes, A.M. Immunization with Recombinant Pb27 Protein Reduces the Levels of Pulmonary Fibrosis Caused by the Inflammatory Response Against Paracoccidioides brasiliensis. Mycopathologia 2015, 179, 31–43. [Google Scholar] [CrossRef]
- González, Á. The Therapy of Pulmonary Fibrosis in Paracoccidioidomycosis: What Are the New Experimental Approaches? J. Fungi 2020, 6, 217. [Google Scholar] [CrossRef]
- Finato, A.C.; Almeida, D.F.; dos Santos, A.R.; Nascimento, D.C.; Cavalcante, R.S.; Mendes, R.P.; Soares, C.T.; Paniago, A.M.M.; Venturini, J. Evaluation of Antifibrotic and Antifungal Combined Therapies in Experimental Pulmonary Paracoccidioidomycosis. Med. Mycol. 2020, 58, 667–678. [Google Scholar] [CrossRef]
- Puerta-Arias, J.D.; Pino-Tamayo, P.A.; Arango, J.C.; Salazar-Peláez, L.M.; González, A. Itraconazole in Combination with Neutrophil Depletion Reduces the Expression of Genes Related to Pulmonary Fibrosis in an Experimental Model of Paracoccidioidomycosis. Med. Mycol. 2018, 56, 579–590. [Google Scholar] [CrossRef]
- Puerta-Arias, J.D.; Pino-Tamayo, P.A.; Arango, J.C.; González, Á. Depletion of Neutrophils Promotes the Resolution of Pulmonary Inflammation and Fibrosis in Mice Infected with Paracoccidioides brasiliensis. PLoS ONE 2016, 11, e0163985. [Google Scholar] [CrossRef]
- Boniche, C.; Rossi, S.A.; Kischkel, B.; Barbalho, F.V.; Moura, Á.N.D.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Immunotherapy against Systemic Fungal Infections Based on Monoclonal Antibodies. J. Fungi 2020, 6, 31. [Google Scholar] [CrossRef]
- Boniche-Alfaro, C.; Kischkel, B.; Thomaz, L.; Carvalho-Gomes, M.M.; Lopes-Bezerra, L.M.; Nosanchuk, J.D.; Taborda, C.P. Antibody- Based Immunotherapy Combined With Antimycotic Drug TMP- SMX to Treat Infection With Paracoccidioides brasiliensis. Front. Immunol. 2021, 12, 725882. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peçanha-Pietrobom, P.M.; Tirado-Sánchez, A.; Gonçalves, S.S.; Bonifaz, A.; Colombo, A.L. Diagnosis and Treatment of Pulmonary Coccidioidomycosis and Paracoccidioidomycosis. J. Fungi 2023, 9, 218. https://doi.org/10.3390/jof9020218
Peçanha-Pietrobom PM, Tirado-Sánchez A, Gonçalves SS, Bonifaz A, Colombo AL. Diagnosis and Treatment of Pulmonary Coccidioidomycosis and Paracoccidioidomycosis. Journal of Fungi. 2023; 9(2):218. https://doi.org/10.3390/jof9020218
Chicago/Turabian StylePeçanha-Pietrobom, Paula Massaroni, Andrés Tirado-Sánchez, Sarah Santos Gonçalves, Alexandro Bonifaz, and Arnaldo Lopes Colombo. 2023. "Diagnosis and Treatment of Pulmonary Coccidioidomycosis and Paracoccidioidomycosis" Journal of Fungi 9, no. 2: 218. https://doi.org/10.3390/jof9020218
APA StylePeçanha-Pietrobom, P. M., Tirado-Sánchez, A., Gonçalves, S. S., Bonifaz, A., & Colombo, A. L. (2023). Diagnosis and Treatment of Pulmonary Coccidioidomycosis and Paracoccidioidomycosis. Journal of Fungi, 9(2), 218. https://doi.org/10.3390/jof9020218







