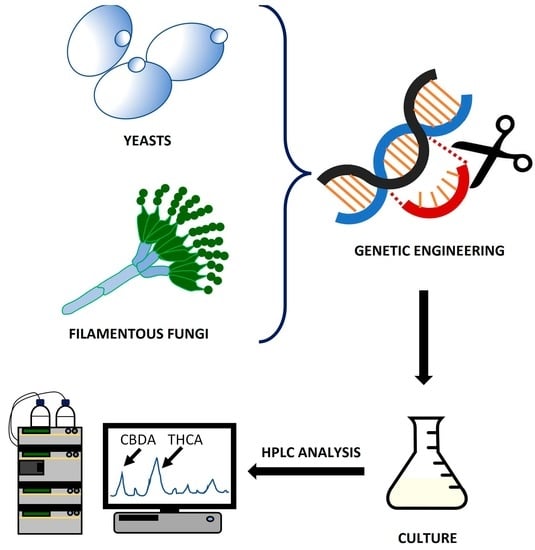
Biotechnological Fungal Platforms for the Production of Biosynthetic Cannabinoids
Abstract
:1. Introduction
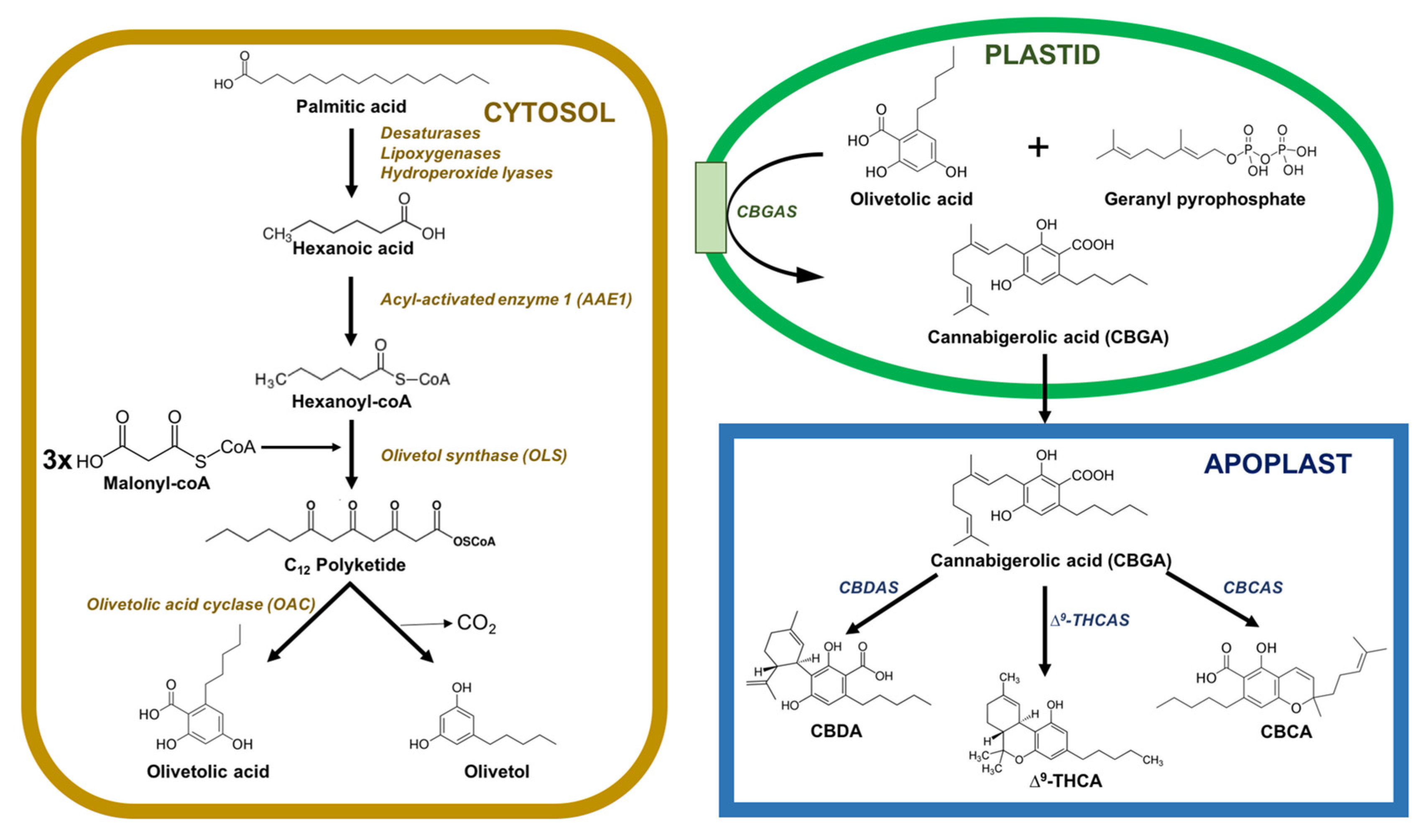
2. Chemical Structure of Cannabinoids, Types, and Metabolic Routes for Biosynthesis
3. Importance, Applications, and Impact of Cannabinoids
4. Development of Biotechnological Fungal Platforms for the Biosynthesis of Cannabinoids
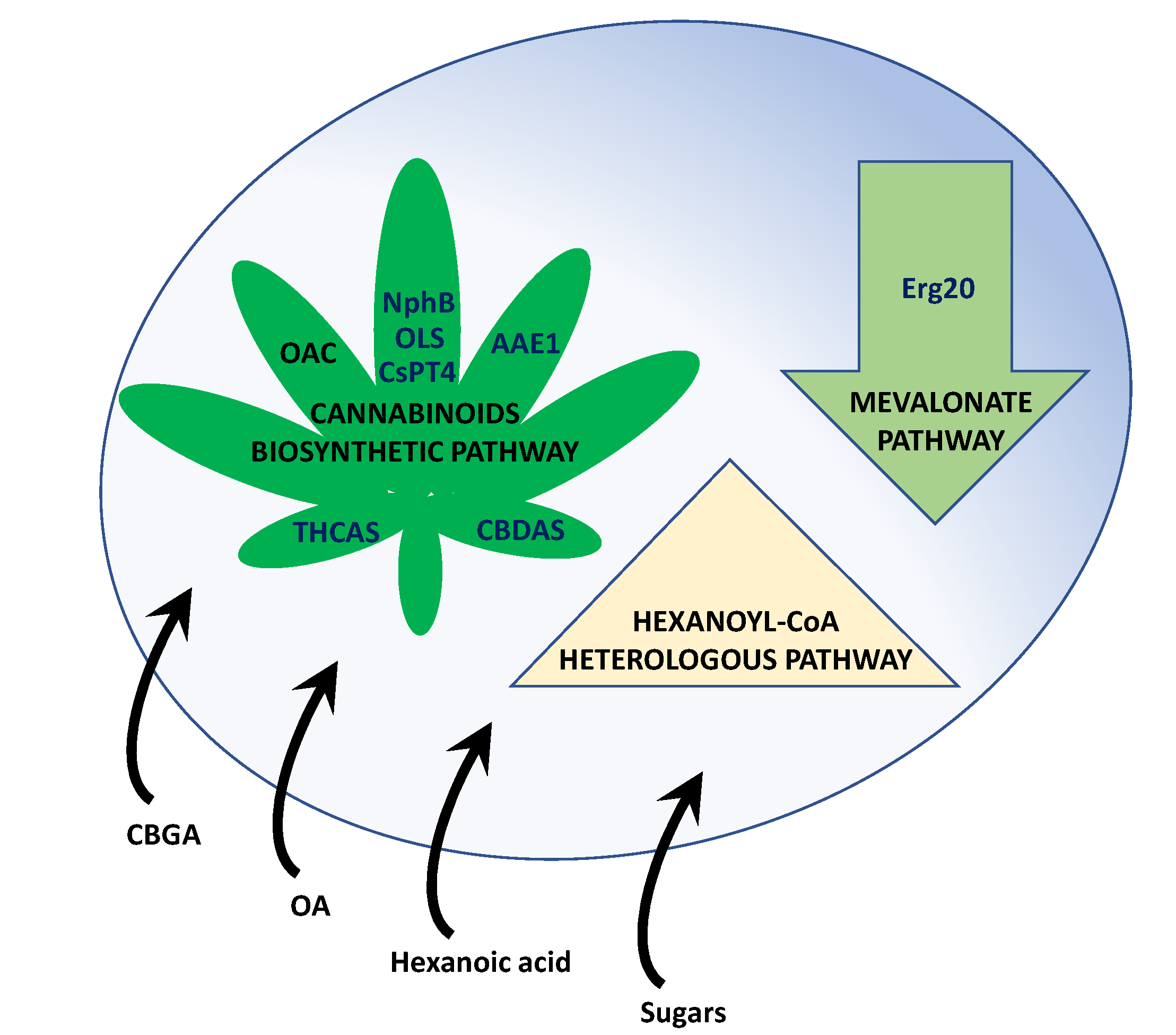
4.1. Biosynthesis of Cannabinoids in Yeasts
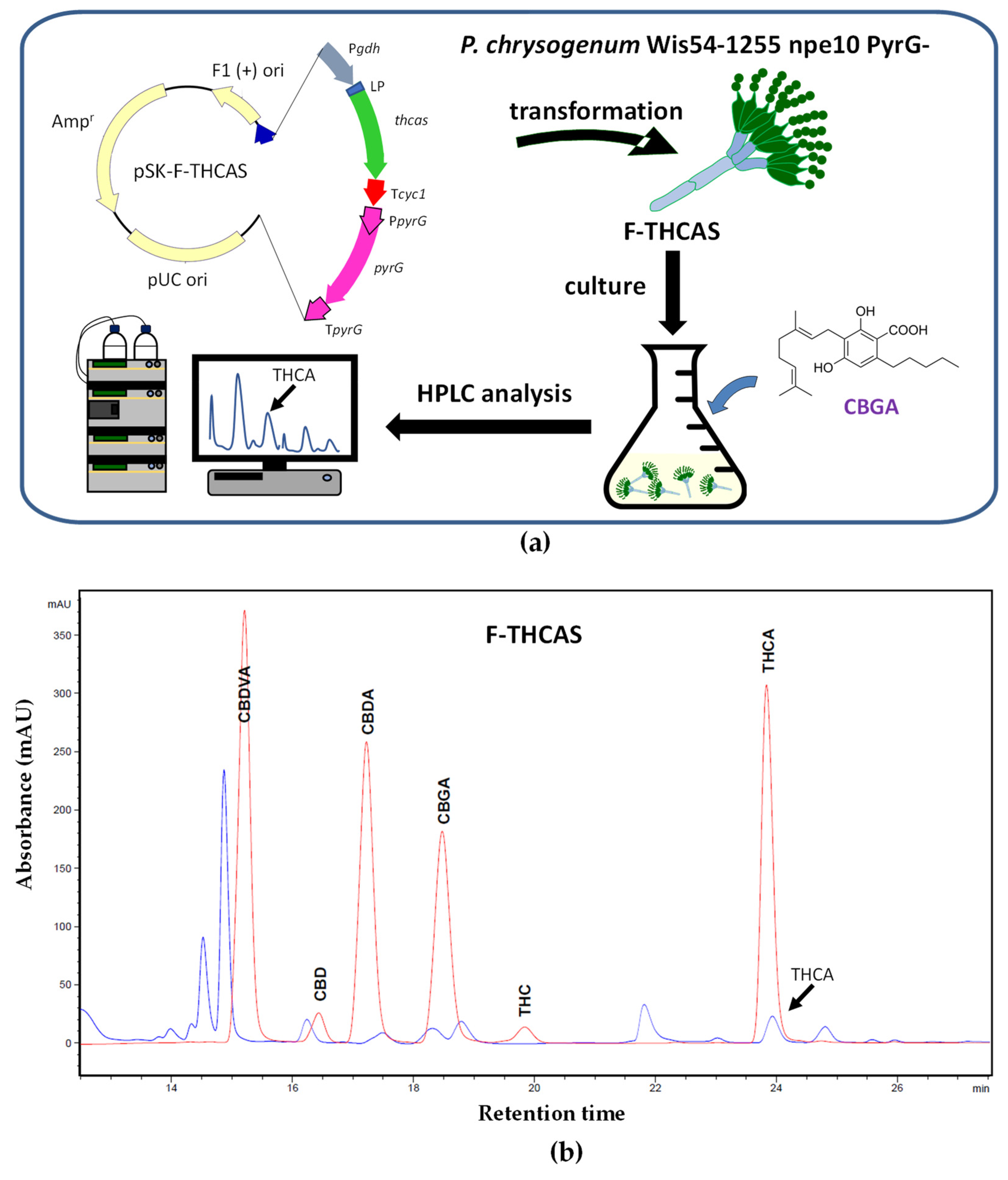
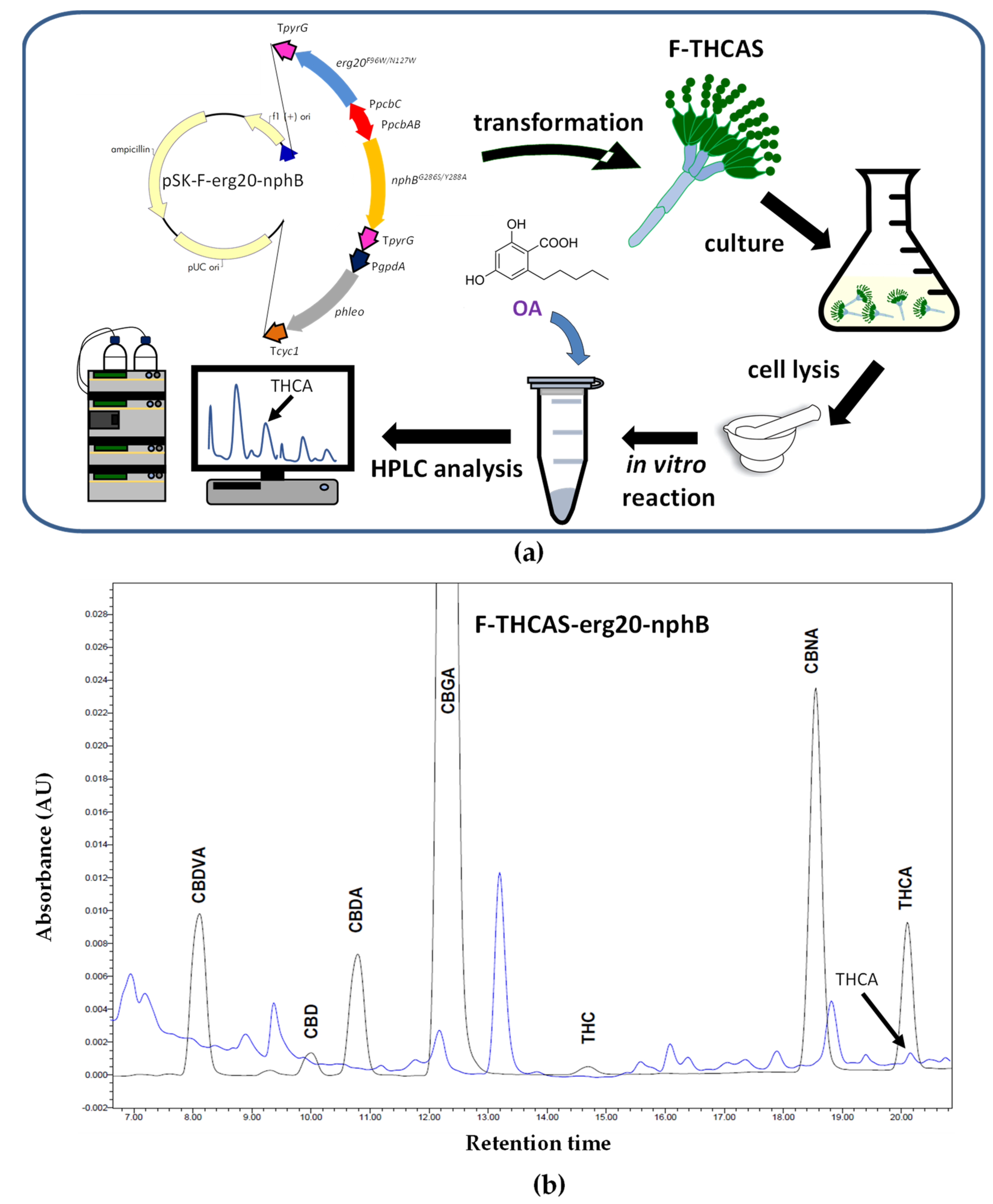
4.2. Biosynthesis of Cannabinoids in Filamentous Fungi: The Paradigm of P. chrysogenum
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atakan, Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254. [Google Scholar] [CrossRef]
- Thomas, B.F.; ElSohly, M.A. The Botany of Cannabis sativa L. In The Analytical Chemistry of Cannabis; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–26. [Google Scholar] [CrossRef]
- Hartsel, J.A.; Eades, J.; Hickory, B.; Makriyannis, A. Cannabis sativa and Hemp. In Nutraceuticals: Efficacy, Safety and Toxicity; Academic Press: Princeton, NJ, USA, 2016; pp. 735–754. [Google Scholar] [CrossRef]
- Warf, B. High points: An historical geography of cannabis. Geogr. Rev. 2014, 104, 414–438. [Google Scholar] [CrossRef]
- Li, H.-L. An archaeological and historical account of cannabis in China. Econ. Bot. 1974, 28, 437–448. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, X.; Li, Y.; Ridout, K.; Serrano-Serrano, M.L.; Yang, Y.; Liu, A.; Ravikanth, G.; Nawaz, M.A.; Mumtaz, A.S.; et al. Large-scale whole-genome resequencing unravels the domestication history of Cannabis sativa. Sci. Adv. 2021, 7, eabg2286. [Google Scholar] [CrossRef] [PubMed]
- Small, E.; Cronquist, A. A practical and natural taxonomy for cannabis. Taxon 1976, 25, 405–435. [Google Scholar] [CrossRef]
- McPartland, J.M. Cannabis systematics at the levels of Family, Genus, and Species. Cannabis Cannabinoid Res. 2018, 3, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Pollio, A. The name of Cannabis: A short guide for nonbotanists. Cannabis Cannabinoid Res. 2016, 1, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Schilling, S.; Dowling, C.A.; Shi, J.; Ryan, L.; Hunt, D.J.L.; O’Reilly, E.; Perry, A.S.; Kinnane, O.; McCabe, P.F.; Melzer, R. The cream of the crop: Biology, breeding, and applications of Cannabis sativa. Annu. Plant Rev. Online 2021, 4, 471–528. [Google Scholar] [CrossRef]
- Iftikhar, A.; Zafar, U.; Ahmed, W.; Shabbir, M.A.; Sameen, A.; Sahar, A.; Bhat, Z.F.; Kowalczewski, P.Ł.; Jarzębski, M.; Aadil, R.M. Applications of Cannabis sativa L. in Food and Its Therapeutic Potential: From a Prohibited Drug to a Nutritional Supplement. Molecules 2021, 26, 7699. [Google Scholar] [CrossRef] [PubMed]
- Britch, S.C.; Babalonis, S.; Walsh, S.L. Cannabidiol: Pharmacology and therapeutic targets. Psychopharmacology 2021, 238, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A Unified Critical Inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [PubMed]
- Chanda, D.; Neumann, D.; Glatz, J.F.C. The endocannabinoid system: Overview of an emerging multi-faceted therapeutic target. Prostaglandins. Leukot. Essent. Fatty Acids 2019, 140, 51–56. [Google Scholar] [CrossRef]
- Dei Cas, M.; Casagni, E.; Casiraghi, A.; Minghetti, P.; Fornasari, D.M.M.; Ferri, F.; Arnoldi, S.; Gambaro, V.; Roda, G. Phytocannabinoids profile in medicinal cannabis oils: The impact of plant varieties and preparation methods. Front. Pharmacol. 2020, 11, 570616. [Google Scholar] [CrossRef]
- Taura, F.; Sirikantaramas, S.; Shoyama, Y.; Shoyama, Y.; Morimoto, S. Phytocannabinoids in Cannabis sativa: Recent studies on biosynthetic enzymes. Chem. Biodivers. 2007, 4, 1649–1663. [Google Scholar] [CrossRef] [PubMed]
- Zirpel, B. Recombinant Expression and Functional Characterization of Cannabinoid Producing Enzymes in Komagataella phaffii. Ph.D. Thesis, Technische Universität Dortmund, Dortmund, Germany, 2018. [Google Scholar]
- Kinghorn, A.D.; Falk, H.; Gibbons, S.; Kobayashi, J. Phytocannabinoids. Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa; Progress in the Chemistry of Organic Natural Products; Springer International Publishing: Cham, Switzerland, 2017; Volume 103, ISBN 978-3-319-45539-6. [Google Scholar]
- Elsohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary metabolism in cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Happyana, N.; Agnolet, S.; Muntendam, R.; Van Dam, A.; Schneider, B.; Kayser, O. Analysis of cannabinoids in laser-microdissected trichomes of medicinal Cannabis sativa using LCMS and cryogenic NMR. Phytochemistry 2013, 87, 51–59. [Google Scholar] [CrossRef]
- Luo, X.; Reiter, M.A.; D’Espaux, L.; Wong, J.; Denby, C.M.; Lechner, A.; Zhang, Y.; Grzybowski, A.T.; Harth, S.; Lin, W.; et al. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 2019, 567, 123–126. [Google Scholar] [CrossRef]
- Gagne, S.J.; Stout, J.M.; Liu, E.; Boubakir, Z.; Clark, S.M.; Page, J.E. Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides. Proc. Natl. Acad. Sci. USA 2012, 109, 12811–12816. [Google Scholar] [CrossRef]
- Raharjo, T.J.; Chang, W.-T.; Choi, Y.H.; Peltenburg-Looman, A.M.; Verpoorte, R. Olivetol as product of a polyketide synthase in Cannabis sativa L. Plant Sci. 2004, 166, 381–385. [Google Scholar] [CrossRef]
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and biosynthesis. Trends Plant Sci. 2020, 25, 985–1004. [Google Scholar] [CrossRef] [PubMed]
- Geissler, M.; Volk, J.; Stehle, F.; Kayser, O.; Warzecha, H. Subcellular localization defines modification and production of Δ9-tetrahydrocannabinolic acid synthase in transiently transformed Nicotiana benthamiana. Biotechnol. Lett. 2018, 40, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Pollastro, F.; Caprioglio, D.; Del Prete, D.; Rogati, F.; Minassi, A.; Taglialatela-Scafati, O.; Munoz, E.; Appendino, G. Cannabichromene. Nat. Prod. Commun. 2018, 13, 1934578X1801300. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Ali, E.M.M.; Almagboul, A.Z.I.; Khogali, S.M.E.; Gergeir, U.M.A. Antimicrobial activity of Cannabis sativa L. Chin. Med. 2012, 3, 61–64. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Van Klingeren, B.; Ten Ham, M. Antibacterial activity of delta9-tetrahydrocannabinol and cannabidiol. Antonie Van Leeuwenhoek 1976, 42, 9–12. [Google Scholar] [CrossRef]
- Pellechia, T. Legal Cannabis Industry Poised for Big Growth, in North America and around the World. Forbes. 2018. Available online: https://www.forbes.com/sites/thomaspellechia/2018/03/01/double-digit-billions-puts-north-america-in-the-worldwide-cannabis-market-lead/?sh=573f10bc6510 (accessed on 1 February 2023).
- Kendall, D.A.; Yudowski, G.A. Cannabinoid receptors in the central nervous system: Their signaling and roles in disease. Front. Cell. Neurosci. 2016, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Peters, M.; Murillo-Rodriguez, E.; Hanus, L.O. Cannabidiol—Recent advances. Chem. Biodivers. 2007, 4, 1678–1692. [Google Scholar] [CrossRef]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol adverse effects and toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) Estimating the Size of the Main Illicit Drug Markets in Europe: An Update; EMCDDA: Lisbon, Portugal, 2019; ISBN 9789294974440.
- Blatt-Janmaat, K.; Qu, Y. The biochemistry of phytocannabinoids and metabolic engineering of their production in heterologous systems. Int. J. Mol. Sci. 2021, 22, 2454. [Google Scholar] [CrossRef]
- Barreiro, C.; García-Estrada, C. Recent developments in genome design and assembly tools. In New Frontiers and Applications of Synthetic Biology; Singh, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 45–65. ISBN 9780128244692. [Google Scholar]
- Wang, J.; Yang, Y.; Yan, Y. Bioproduction of resveratrol. In Biotechnology of Natural Products; Springer International Publishing: Cham, Switzerland, 2018; pp. 61–79. [Google Scholar]
- Wang, G.; Huang, M.; Nielsen, J. Exploring the potential of Saccharomyces cerevisiae for biopharmaceutical protein production. Curr. Opin. Biotechnol. 2017, 48, 77–84. [Google Scholar] [CrossRef]
- Martínez, J.L.; Liu, L.; Petranovic, D.; Nielsen, J. Pharmaceutical protein production by yeast: Towards production of human blood proteins by microbial fermentation. Curr. Opin. Biotechnol. 2012, 23, 965–971. [Google Scholar] [CrossRef]
- Madzak, C. Yarrowia lipolytica: Recent achievements in heterologous protein expression and pathway engineering. Appl. Microbiol. Biotechnol. 2015, 99, 4559–4577. [Google Scholar] [CrossRef] [PubMed]
- Madzak, C. Engineering Yarrowia lipolytica for Use in Biotechnological Applications: A Review of Major Achievements and Recent Innovations. Mol. Biotechnol. 2018, 60, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Naumov, G.I.; Naumova, E.S.; Tyurin, O.V.; Kozlov, D.G. Komagataella kurtzmanii sp. nov., a new sibling species of Komagataella (Pichia) pastoris based on multigene sequence analysis. Antonie Van Leeuwenhoek 2013, 104, 339–347. [Google Scholar] [CrossRef]
- Kurtzman, C.P. Description of Komagataella phaffii sp. nov. and the transfer of Pichia pseudopastoris to the methylotrophic yeast genus Komagataella. Int. J. Syst. Evol. Microbiol. 2005, 55, 973–976. [Google Scholar] [CrossRef]
- Gao, L.; Cai, M.; Shen, W.; Xiao, S.; Zhou, X.; Zhang, Y. Engineered fungal polyketide biosynthesis in Pichia pastoris: A potential excellent host for polyketide production. Microb. Cell Fact. 2013, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gong, J.-S.; Su, C.; Li, H.; Li, H.; Rao, Z.-M.; Xu, Z.-H.; Shi, J.-S. Pathway engineering facilitates efficient protein expression in Pichia pastoris. Appl. Microbiol. Biotechnol. 2022, 106, 5893–5912. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Meyer, V. Genetic engineering of filamentous fungi—Progress, obstacles and future trends. Biotechnol. Adv. 2008, 26, 177–185. [Google Scholar] [CrossRef]
- Sakekar, A.A.; Gaikwad, S.R.; Punekar, N.S. Protein expression and secretion by filamentous fungi. J. Biosci. 2021, 46, 5. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, M.A.; Albang, R.; Albermann, K.; Badger, J.H.; Daran, J.M.; Driessen, A.J.M.; Garcia-Estrada, C.; Fedorova, N.D.; Harris, D.M.; Heijne, W.H.M.; et al. Genome sequencing and analysis of the filamentous fungus Penicillium chrysogenum. Nat. Biotechnol. 2008, 26, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, C.; García-Estrada, C. Proteomics and Penicillium chrysogenum: Unveiling the secrets behind penicillin production. J. Proteom. 2019, 198, 119–131. [Google Scholar] [CrossRef]
- García-Estrada, C.; Martín, J.F.; Cueto, L.; Barreiro, C. Omics approaches applied to Penicillium chrysogenum and penicillin production: Revealing the secrets of improved productivity. Genes 2020, 11, 712. [Google Scholar] [CrossRef]
- García-Calvo, L.; Rodríguez-Castro, R.; Ullán, R.V.; Albillos, S.M.; Fernández-Aguado, M.; Vicente, C.M.; Degnes, K.F.; Sletta, H.; Barreiro, C. Penicillium chrysogenum as a fungal factory for feruloyl esterases. Appl. Microbiol. Biotechnol. 2023, 107, 691–717. [Google Scholar] [CrossRef]
- Fierro, F.; Vaca, I.; Castillo, N.I.; García-Rico, R.O.; Chávez, R. Penicillium chrysogenum, a Vintage Model with a Cutting-Edge Profile in Biotechnology. Microorganisms 2022, 10, 573. [Google Scholar] [CrossRef]
- Van den Berg, M.A. Impact of the Penicillium chrysogenum genome on industrial production of metabolites. Appl. Microbiol. Biotechnol. 2011, 92, 45–53. [Google Scholar] [CrossRef]
- Clevenger, K.D.; Bok, J.W.; Ye, R.; Miley, G.P.; Verdan, M.H.; Velk, T.; Chen, C.; Yang, K.; Robey, M.T.; Gao, P.; et al. A scalable platform to identify fungal secondary metabolites and their gene clusters. Nat. Chem. Biol. 2017, 13, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Chávez, F.; Zwahlen, R.D.; Bovenberg, R.A.L.; Driessen, A.J.M. Engineering of the Filamentous Fungus Penicillium chrysogenum as Cell Factory for Natural Products. Front. Microbiol. 2018, 9, 2768. [Google Scholar] [CrossRef] [PubMed]
- Arnau, J.; Yaver, D.; Hjort, C.M. Strategies and challenges for the development of industrial enzymes using fungal cell factories. In Grand Challenges in Fungal Biotechnology; Springer: Cham, Switzerland, 2020; pp. 179–210. [Google Scholar]
- Punt, P.J.; van Biezen, N.; Conesa, A.; Albers, A.; Mangnus, J.; van den Hondel, C. Filamentous fungi as cell factories for heterologous protein production. Trends Biotechnol. 2002, 20, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Adrio, J.-L.; Demain, A.L. Recombinant organisms for production of industrial products. Bioeng. Bugs 2010, 1, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-M.; Oakley, C.E.; Ahuja, M.; Entwistle, R.; Schultz, A.; Chang, S.-L.; Sung, C.T.; Wang, C.C.C.; Oakley, B.R. An Efficient System for Heterologous Expression of Secondary Metabolite Genes in Aspergillus nidulans. J. Am. Chem. Soc. 2013, 135, 7720–7731. [Google Scholar] [CrossRef]
- Anyaogu, D.C.; Mortensen, U.H. Heterologous production of fungal secondary metabolites in Aspergilli. Front. Microbiol. 2015, 6, 77. [Google Scholar] [CrossRef]
- Wang, Q.; Zhong, C.; Xiao, H. Genetic Engineering of Filamentous Fungi for Efficient Protein Expression and Secretion. Front. Bioeng. Biotechnol. 2020, 8, 293. [Google Scholar] [CrossRef]
- Li, W.; Cui, L.; Mai, J.; Shi, T.-Q.; Lin, L.; Zhang, Z.-G.; Ledesma-Amaro, R.; Dong, W.; Ji, X.-J. Advances in Metabolic Engineering Paving the Way for the Efficient Biosynthesis of Terpenes in Yeasts. J. Agric. Food Chem. 2022, 70, 9246–9261. [Google Scholar] [CrossRef]
- Taura, F.; Tanaka, S.; Taguchi, C.; Fukamizu, T.; Tanaka, H.; Shoyama, Y.; Morimoto, S. Characterization of olivetol synthase, a polyketide synthase putatively involved in cannabinoid biosynthetic pathway. FEBS Lett. 2009, 583, 2061–2066. [Google Scholar] [CrossRef]
- Taura, F.; Morimoto, S.; Shoyama, Y. Purification and characterization of cannabidiolic-acid synthase from Cannabis sativa L. Biochemical analysis of a novel enzyme that catalyzes the oxidocyclization of cannabigerolic acid to cannabidiolic acid. J. Biol. Chem. 1996, 271, 17411–17416. [Google Scholar] [CrossRef]
- Fellermeier, M.; Zenk, M.H. Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol. FEBS Lett. 1998, 427, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Sirikantaramas, S.; Morimoto, S.; Shoyama, Y.; Ishikawa, Y.; Wada, Y.; Shoyama, Y.; Taura, F. The gene controlling marijuana psychoactivity: Molecular cloning and heterologous expression of Delta1-tetrahydrocannabinolic acid synthase from Cannabis sativa L. J. Biol. Chem. 2004, 279, 39767–39774. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.M.; Boubakir, Z.; Ambrose, S.J.; Purves, R.W.; Page, J.E. The hexanoyl-CoA precursor for cannabinoid biosynthesis is formed by an acyl-activating enzyme in Cannabis sativa trichomes. Plant J. 2012, 71, 353–365. [Google Scholar] [CrossRef]
- Taura, F.; Dono, E.; Sirikantaramas, S.; Yoshimura, K.; Shoyama, Y.; Morimoto, S. Production of Delta(1)-tetrahydrocannabinolic acid by the biosynthetic enzyme secreted from transgenic Pichia pastoris. Biochem. Biophys. Res. Commun. 2007, 361, 675–680. [Google Scholar] [CrossRef]
- Lange, K.; Schmid, A.; Julsing, M.K. Δ(9)-Tetrahydrocannabinolic acid synthase production in Pichia pastoris enables chemical synthesis of cannabinoids. J. Biotechnol. 2015, 211, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zirpel, B.; Stehle, F.; Kayser, O. Production of Δ9-tetrahydrocannabinolic acid from cannabigerolic acid by whole cells of Pichia (Komagataella) pastoris expressing Δ9-tetrahydrocannabinolic acid synthase from Cannabis sativa L. Biotechnol. Lett. 2015, 37, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Zirpel, B.; Degenhardt, F.; Martin, C.; Kayser, O.; Stehle, F. Engineering yeasts as platform organisms for cannabinoid biosynthesis. J. Biotechnol. 2017, 259, 204–212. [Google Scholar] [CrossRef]
- Zirpel, B.; Degenhardt, F.; Zammarelli, C.; Wibberg, D.; Kalinowski, J.; Stehle, F.; Kayser, O. Optimization of Δ9-tetrahydrocannabinolic acid synthase production in Komagataella phaffii via post-translational bottleneck identification. J. Biotechnol. 2018, 272–273, 40–47. [Google Scholar] [CrossRef]
- Zirpel, B.; Kayser, O.; Stehle, F. Elucidation of structure-function relationship of THCA and CBDA synthase from Cannabis sativa L. J. Biotechnol. 2018, 284, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Schmidt, C.; Kayser, O. Bioengineering studies and pathway modeling of the heterologous biosynthesis of tetrahydrocannabinolic acid in yeast. Appl. Microbiol. Biotechnol. 2020, 104, 9551–9563. [Google Scholar] [CrossRef]
- Gülck, T.; Booth, J.K.; Carvalho, Â.; Khakimov, B.; Crocoll, C.; Motawia, M.S.; Møller, B.L.; Bohlmann, J.; Gallage, N.J. Synthetic Biology of Cannabinoids and Cannabinoid Glucosides in Nicotiana benthamiana and Saccharomyces cerevisiae. J. Nat. Prod. 2020, 83, 2877–2893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zeng, W.; Xu, S.; Zhou, J. Metabolism and strategies for enhanced supply of acetyl-CoA in Saccharomyces cerevisiae. Bioresour. Technol. 2021, 342, 125978. [Google Scholar] [CrossRef] [PubMed]
- Dusséaux, S.; Wajn, W.T.; Liu, Y.; Ignea, C.; Kampranis, S.C. Transforming yeast peroxisomes into microfactories for the efficient production of high-value isoprenoids. Proc. Natl. Acad. Sci. USA 2020, 117, 31789–31799. [Google Scholar] [CrossRef] [PubMed]
- Poulos, J.L.; Farnia, A.N. Production of Cannabinoids in Yeast 2016. WO2016010827A1. Librede Inc, USA. Available online: https://patents.google.com/patent/WO2016010827A1/en (accessed on 1 February 2023).
- Szamecz, B.K.; Varszegi, S.; Nemeth, A.; Szabo, L. Microorganisms and Methods for the Fermentation of Cannabinoids 2020. WO2019071000A1. INTREXON CORPORATION, USA. Available online: https://patents.google.com/patent/WO2019071000A1/en (accessed on 1 February 2023).
- Sayre, R.T.; Carvalho-Gonçalves, E.; Zidenga, T. Generation of Water-Soluble Cannabinoid Compounds in Yeast and Plant Cell Suspension Cultures and Compositions of Matter 2019. US20190078168A1. Trait Biosciences Inc., USA. Available online: https://patents.google.com/patent/US20190078168A1/en (accessed on 1 February 2023).
- Mookerjee, S.; Campbell, A.J.; Wiltshire, Z.D.; Chen, K.J.; Hyasynth Biologicals Inc. Method and Cell Line for Production of Phytocannabinoids and Phytocannabinoid Analogues in Yeast. WO2018148848A1. Hyasynth Biologicals Inc., Canada. 2020. Available online: https://patents.google.com/patent/WO2018148848A1/en (accessed on 1 February 2023).
- Keasling, J.D.; D’espaux, L.; Wong, J.; Luo, X.; Reiter, M.; Denby, C.; Lechner, A. Recombinant Microorganisms and Methods for Producing Cannabinoids and Cannabinoid Derivatives 2020. US10563211B2. The Regents of the University of California, USA. Available online: https://patents.google.com/patent/US10563211B2/en (accessed on 1 February 2023).
- Kayser, O.; Stehle, F.-O. Biotechnological Production of Cannabinoids 2020. WO2020016287A1. Technische Universität Dortmund, Germany. Available online: https://patents.google.com/patent/WO2020016287A1/en (accessed on 1 February 2023).
- Horwitz, A.; D’espaux, L.; Wong, J.; Bector, R.; Hjelmeland, A.K.; Platt, D.; Ubersax, J. Optimized Expression Systems for Producing Cannabinoid Synthase Polypeptides, Cannabinoids, and Cannabinoid Derivatives 2020. WO2020069214A3. Demetrix Inc., USA. Available online: https://patents.google.com/patent/WO2020069214A3/en (accessed on 1 February 2023).
- Bowie, J.U.; Korman, T.P.; Valliere, M. Biosynthetic Platform for the Production of Cannabinoids and other Prenylated Compounds 2021. WO2020028722A1. The Regents of the University of California, USA. Available online: https://patents.google.com/patent/WO2020028722A1/en (accessed on 1 February 2023).
- Beardslee, T.A. Biosynthetic Cannabinoid Production in Engineered Microorganisms 2020. WO2020198679A1. Rynetech Bio Inc., USA. Available online: https://patents.google.com/patent/WO2020198679A1/en (accessed on 1 February 2023).
- Ma, J.; Gu, Y.; Xu, P. Biosynthesis of cannabinoid precursor olivetolic acid in genetically engineered Yarrowia lipolytica. Commun. Biol. 2022, 5, 1239. [Google Scholar] [CrossRef]
- Okorafor, I.C.; Chen, M.; Tang, Y. High-Titer Production of Olivetolic Acid and Analogs in Engineered Fungal Host Using a Nonplant Biosynthetic Pathway. ACS Synth. Biol. 2021, 10, 2159–2166. [Google Scholar] [CrossRef]
- Elander, R.P. Industrial production of beta-lactam antibiotics. Appl. Microbiol. Biotechnol. 2003, 61, 385–392. [Google Scholar] [CrossRef]
- Cantoral, J.M.; Gutiérrez, S.; Fierro, F.; Gil-Espinosa, S.; van Liempt, H.; Martín, J.F. Biochemical characterization and molecular genetics of nine mutants of Penicillium chrysogenum impaired in penicillin biosynthesis. J. Biol. Chem. 1993, 268, 737–744. [Google Scholar] [CrossRef]
- Fierro, F.; Montenegro, E.; Gutiérrez, S.; Martín, J.F. Mutants blocked in penicillin biosynthesis show a deletion of the entire penicillin gene cluster at a specific site within a conserved hexanucleotide sequence. Appl. Microbiol. Biotechnol. 1996, 44, 597–604. [Google Scholar] [CrossRef]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Jami, M.S.; García-Estrada, C.; Barreiro, C.; Cuadrado, A.A.; Salehi-Najafabadi, Z.; Martín, J.F. The Penicillium chrysogenum extracellular proteome. Conversion from a food-rotting strain to a versatile cell factory for white biotechnology. Mol. Cell. Proteom. 2010, 9, 2729–2744. [Google Scholar] [CrossRef] [PubMed]
- Jami, M.S.; Barreiro, C.; García-Estrada, C.; Martín, J.F. Proteome analysis of the penicillin producer Penicillium chrysogenum: Characterization of protein changes during the industrial strain improvement. Mol. Cell. Proteom. 2010, 9, 1182–1198. [Google Scholar] [CrossRef]
- De Backer, B.; Debrus, B.; Lebrun, P.; Theunis, L.; Dubois, N.; Decock, L.; Verstraete, A.; Hubert, P.; Charlier, C. Innovative development and validation of an HPLC/DAD method for the qualitative and quantitative determination of major cannabinoids in cannabis plant material. J. Chromatogr. B 2009, 877, 4115–4124. [Google Scholar] [CrossRef] [PubMed]
- Valliere, M.A.; Korman, T.P.; Arbing, M.A.; Bowie, J.U. A bio-inspired cell-free system for cannabinoid production from inexpensive inputs. Nat. Chem. Biol. 2020, 16, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosalková, K.; Barreiro, C.; Sánchez-Orejas, I.-C.; Cueto, L.; García-Estrada, C. Biotechnological Fungal Platforms for the Production of Biosynthetic Cannabinoids. J. Fungi 2023, 9, 234. https://doi.org/10.3390/jof9020234
Kosalková K, Barreiro C, Sánchez-Orejas I-C, Cueto L, García-Estrada C. Biotechnological Fungal Platforms for the Production of Biosynthetic Cannabinoids. Journal of Fungi. 2023; 9(2):234. https://doi.org/10.3390/jof9020234
Chicago/Turabian StyleKosalková, Katarina, Carlos Barreiro, Isabel-Clara Sánchez-Orejas, Laura Cueto, and Carlos García-Estrada. 2023. "Biotechnological Fungal Platforms for the Production of Biosynthetic Cannabinoids" Journal of Fungi 9, no. 2: 234. https://doi.org/10.3390/jof9020234
APA StyleKosalková, K., Barreiro, C., Sánchez-Orejas, I.-C., Cueto, L., & García-Estrada, C. (2023). Biotechnological Fungal Platforms for the Production of Biosynthetic Cannabinoids. Journal of Fungi, 9(2), 234. https://doi.org/10.3390/jof9020234