Antifungal Activity of Perillaldehyde on Fusarium solani and Its Control Effect on Postharvest Decay of Sweet Potatoes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Strain and Plant Materials
2.2. Determination of Antifungal Activity
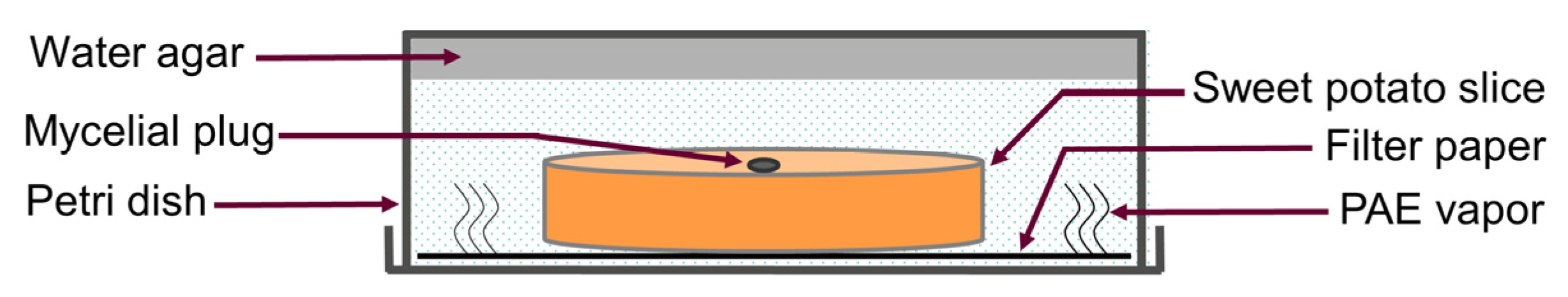
2.3. Determination of the Effect of PAE on Sweet Potato Preservation
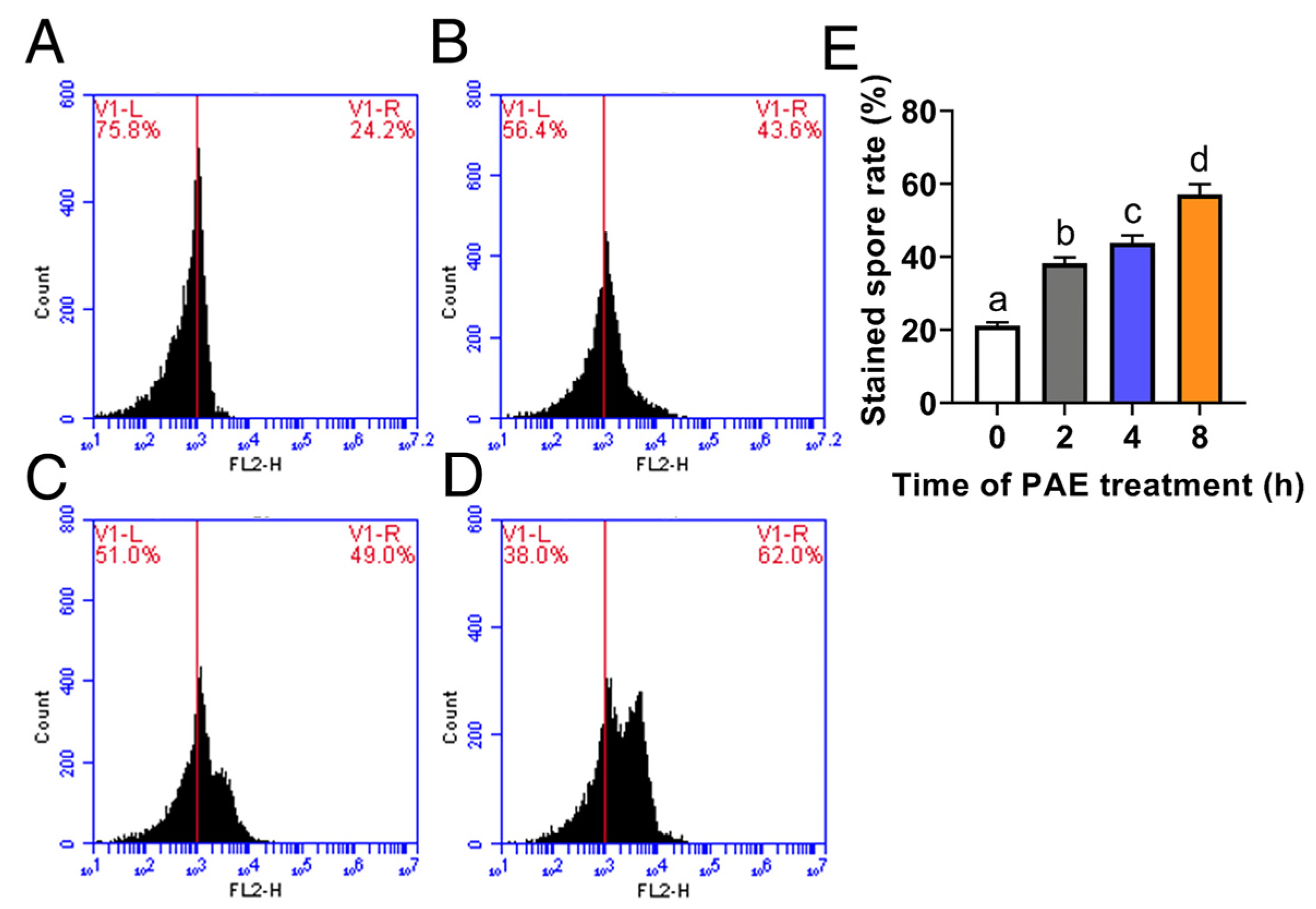
2.4. Measurement of Cell Membrane Integrity
2.5. Determination of MMP
2.6. Determination of ROS Level
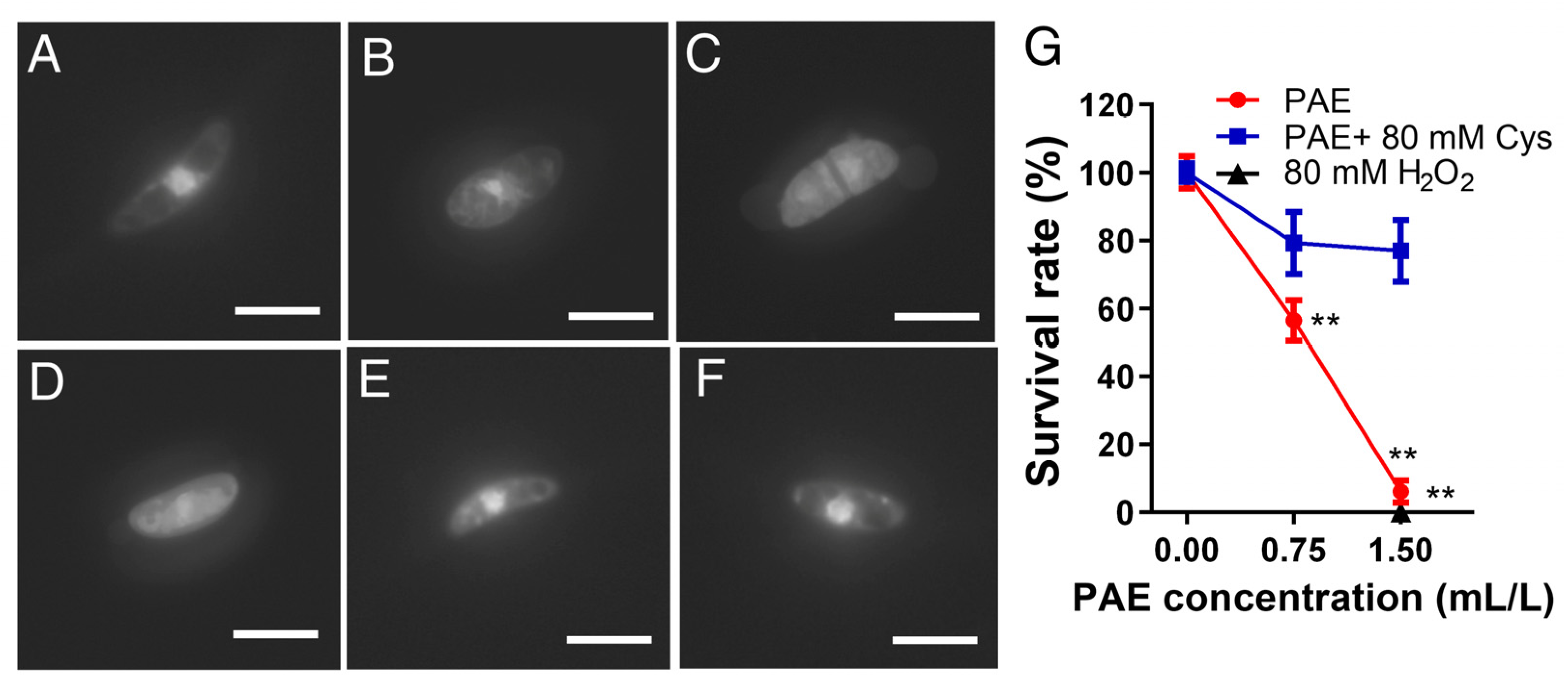
2.7. Determination of Nuclear Morphology
2.8. Statistical Analysis
3. Results
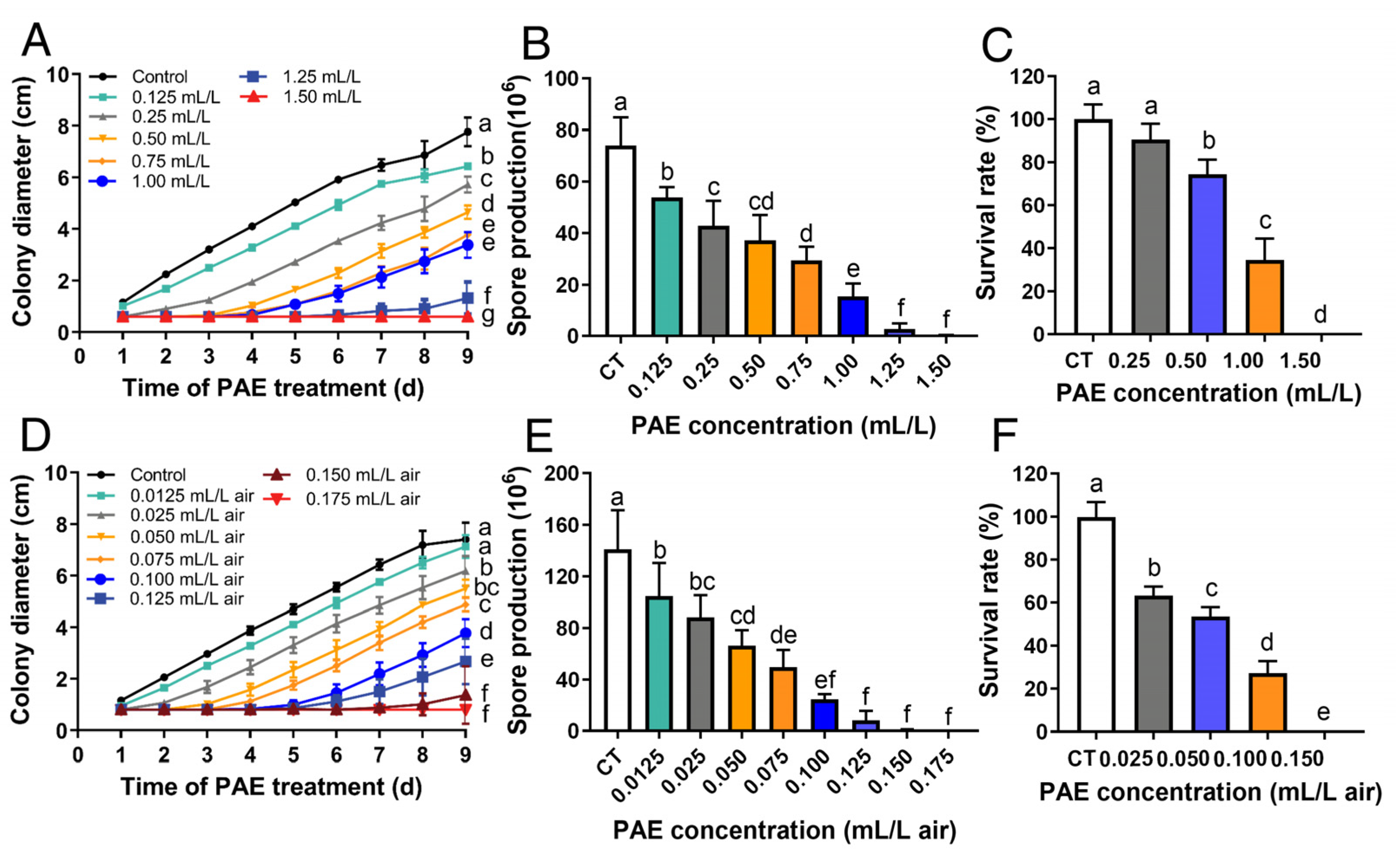
3.1. Antifungal Activity of PAE against F. solani
3.2. Effect of PAE on Sweet Potato Preservation
3.3. Effect of PAE on Cell Membrane Integrity
3.4. Effect of PAE on MMP
3.5. Effect of PAE on ROS Accumulation
3.6. Effect of PAE on Nuclear Morphometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, M.; Cheng, J.; Chen, P.; Zheng, G.; Wang, D.; Hu, Y. Efficient production of succinic acid in engineered Escherichia coli strains controlled by anaerobically-induced nirB promoter using sweet potato waste hydrolysate. J. Environ. Manag. 2019, 237, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Oke, M.O.; Workneh, T.S. A review on sweet potato postharvest processing and preservation technology. Afr. J. Agric. Res. 2013, 8, 4990–5003. [Google Scholar] [CrossRef]
- Xie, S.Y.; Ma, T.; Zhao, N.; Zhang, X.; Fang, B.; Huang, L. Whole-genome sequencing and comparative genome analysis of Fusarium solani-melongenae causing Fusarium root and stem rot in sweetpotatoes. Microbiol. Spectr. 2022, 10, e0068322. [Google Scholar] [CrossRef] [PubMed]
- El Sheikha, A.F.; Ray, R.C. Potential impacts of bioprocessing of sweet potato: Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Scruggs, A.C.; Quesada-Ocampo, L.M. Etiology and epidemiological conditions promoting Fusarium root rot in sweetpotato. Phytopathology 2016, 106, 909–919. [Google Scholar] [CrossRef]
- Ray, R.C.; Ravi, V. Post harvest spoilage of sweetpotato in tropics and control measures. Crit. Rev. Food Sci. Nutr. 2005, 45, 623–644. [Google Scholar] [CrossRef]
- Coleman, J.J.; Rounsley, S.D.; Rodriguez-Carres, M.; Kuo, A.; Wasmann, C.C.; Grimwood, J.; Schmutz, J.; Taga, M.; White, G.J.; Zhou, S.; et al. The genome of Nectria haematococca: Contribution of supernumerary chromosomes to gene expansion. PLoS Genet. 2009, 5, e1000618. [Google Scholar] [CrossRef]
- Zhang, N.; O’Donnell, K.; Sutton, D.A.; Nalim, F.A.; Summerbell, R.C.; Padhye, A.A.; Geiser, D.M. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J. Clin. Microbiol. 2006, 44, 2186–2190. [Google Scholar] [CrossRef]
- Coleman, J.J. The Fusarium solani species complex: Ubiquitous pathogens of agricultural importance. Mol. Plant Pathol. 2016, 17, 146–158. [Google Scholar] [CrossRef]
- Yang, J.-W.; Nam, S.-S.; Lee, H.-U.; Choi, K.-H.; Hwang, S.-G.; Paul, N.C. Fusarium root rot caused by Fusarium solani on sweet potato (Ipomoea batatas) in South Korea. Can. J. Plant Pathol. 2017, 40, 90–95. [Google Scholar] [CrossRef]
- Xu, X.M.; Chen, J.Y.; Li, B.R.; Tang, L.J. Carbendazim residues in vegetables in China between 2014 and 2016 and a chronic carbendazim exposure risk assessment. Food Control 2018, 91, 20–25. [Google Scholar] [CrossRef]
- Fan, R.; Zhang, W.; Li, L.; Jia, L.; Zhao, J.; Zhao, Z.; Peng, S.; Yuan, X.; Chen, Y. Individual and synergistic toxic effects of carbendazim and chlorpyrifos on zebrafish embryonic development. Chemosphere 2021, 280, 130769. [Google Scholar] [CrossRef]
- Yang, K.; Geng, Q.; Luo, Y.; Xie, R.; Sun, T.; Wang, Z.; Qin, L.; Zhao, W.; Liu, M.; Li, Y.; et al. Dysfunction of FadA-cAMP signalling decreases Aspergillus flavus resistance to antimicrobial natural preservative perillaldehyde and AFB1 biosynthesis. Environ. Microbiol. 2022, 86, 326. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zeng, X.B.; Zhang, S.; Wang, Y.Z.; Zhang, P.; Lu, A.J.; Peng, X. Regional variation in components and antioxidant and antifungal activities of Perilla frutescens essential oils in China. Ind. Crops Prod. 2014, 59, 69–79. [Google Scholar] [CrossRef]
- Hobbs, C.A.; Taylor, S.V.; Beevers, C.; Lloyd, M.; Bowen, R.; Lillford, L.; Maronpot, R.; Hayashi, S.M. Genotoxicity assessment of the flavouring agent, perillaldehyde. Food Chem. Toxicol. 2016, 97, 232–242. [Google Scholar] [CrossRef]
- Erhunmwunsee, F.; Pan, C.; Yang, K.; Li, Y.; Liu, M.; Tian, J. Recent development in biological activities and safety concerns of perillaldehyde from Perilla plants: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6328–6340. [Google Scholar] [CrossRef] [PubMed]
- Mcgeady, P.; Wansley, D.L.; Logan, D.A. Carvone and perillaldehyde interfere with the serum-induced formation of filamentous structures in Candida albicans at substantially lower concentrations than those causing significant inhibition of growth. J. Nat. Prod. 2002, 65, 953–955. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Pan, C.; Zhang, M.; Gan, Y.Y.; Pan, S.Y.; Liu, M.; Li, Y.X.; Zeng, X.B. Induced cell death in Ceratocystis fimbriata by pro-apoptotic activity of a natural organic compound, perillaldehyde, through Ca2+ overload and accumulation of reactive oxygen species. Plant Pathol. 2019, 68, 344–357. [Google Scholar] [CrossRef]
- Li, Y.X.; Erhunmwunsee, F.; Liu, M.; Yang, K.; Zheng, W.; Tian, J. Antimicrobial mechanisms of spice essential oils and application in food industry. Food Chem. 2022, 382, 132312. [Google Scholar] [CrossRef]
- Helmerhorst, E.J.; Troxler, R.F.; Oppenheim, F.G. The human salivary peptide histatin 5 exerts its antifungal activity through the formation of reactive oxygen species. Proc. Natl. Acad. Sci. USA 2001, 98, 14637–14642. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.; Lu, Z.; Sun, C.; Zhang, M.; Zhu, A.; Peng, X. Perillaldehyde, a promising antifungal agent used in food preservation, triggers apoptosis through a metacaspase-dependent pathway in Aspergillus flavus. J. Agric. Food Chem. 2016, 64, 7404–7413. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Li, Y.X.; Yang, K.; Famous, E.; Ma, Y.; He, X.; Geng, Q.; Liu, M.; Tian, J. The molecular mechanism of perillaldehyde inducing cell death in Aspergillus flavus by inhibiting energy metabolism revealed by transcriptome sequencing. Int. J. Mol. Sci. 2020, 21, 1518. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.; Stasch, S.; Kaerger, K.; Hamprecht, A.; Roth, M.; Cornely, O.A.; Geerling, G.; Mackenzie, C.R.; Kurzai, O.; von Lilienfeld-Toal, M. Fusarium keratitis in Germany. J. Clin. Microbiol. 2017, 55, 2983–2995. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Micozzi, A.; Gentile, G.; Polonelli, L.; Morace, G.; Bianco, P.; Avvisati, G.; Papa, G.; Martino, P. Invasive Fusarium solani infections in patients with acute leukemia. Rev. Infect. Dis. 1988, 10, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Gao, B.; Li, X.H.; Ma, J.; Chen, S.L. First report of Fusarium solani causing Fusarium root rot and stem canker on storage roots of sweet potato in China. Plant Dis. 2014, 98, 160–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, X.; Zhou, Z.; Xing, K.; Tessema, A.; Zeng, H.; Tian, J. Inhibitory effect of nerol against Aspergillus niger on grapes through a membrane lesion mechanism. Food Control 2015, 55, 54–61. [Google Scholar] [CrossRef]
- Gulluce, M.; Sokmen, M.; Daferera, D.; Agar, G.; Ozkan, H.; Kartal, N.; Polissiou, M.; Sokmen, A.; Sahin, F. In vitro antibacterial, antifungal, and antioxidant activities of the essential oil and methanol extracts of herbal parts and callus cultures of Satureja hortensis L. J. Agric. Food Chem. 2003, 51, 3958–3965. [Google Scholar] [CrossRef]
- Gong, Q.W.; Zhang, C.; Lu, F.X.; Zhao, H.Z.; Bie, X.M.; Lu, Z.X. Identification of bacillomycin D from Bacillus subtilis fmbJ and its inhibition effects against Aspergillus flavus. Food Control 2014, 36, 8–14. [Google Scholar] [CrossRef]
- da Silva, W.L.; Clark, C.A. Infection of sweetpotato by Fusarium solani and Macrophomina phaseolina prior to harvest. Plant Dis. 2013, 97, 1636–1644. [Google Scholar] [CrossRef]
- Pan, C.; Yang, K.; Erhunmwunsee, F.; Li, Y.-X.; Liu, M.; Pan, S.; Yang, D.; Lu, G.; Ma, D.; Tian, J. Inhibitory effect of cinnamaldehyde on Fusarium solani and its application in postharvest preservation of sweet potato. Food Chem. 2023, 408, 135213. [Google Scholar] [CrossRef]
- Li, X.; Liu, M.; Huang, T.; Yang, K.; Zhou, S.; Li, Y.; Tian, J. Antifungal effect of nerol via transcriptome analysis and cell growth repression in sweet potato spoilage fungi Ceratocystis fimbriata. Postharvest. Biol. Technol. 2021, 171, 111343. [Google Scholar] [CrossRef]
- Perreault, S.D.; Jeffay, S.; Poss, P.; Laskey, J.W. Use of the fungicide carbendazim as a model compound to determine the impact of acute chemical exposure during oocyte maturation and fertilization on pregnancy outcome in the hamster. Toxicol. Appl. Pharmacol. 1992, 114, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Krist, S.; Buchbauer, G. Antimicrobial effect of trans-cinnamaldehyde, (–)-perillaldehyde, (–)-citronellal, citral, eugenol and carvacrol on airborne microbes using an airwasher. Biol. Pharm. Bull. 2006, 29, 2292–2294. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods–a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wang, Y.; Zeng, H.; Li, Z.; Zhang, P.; Tessema, A.; Peng, X. Efficacy and possible mechanisms of perillaldehyde in control of Aspergillus niger causing grape decay. Int. J. Food Microbiol. 2015, 202, 27–34. [Google Scholar] [CrossRef]
- Druille, M.; Cabello, M.N.; Omacini, M.; Golluscio, R.A. Glyphosate reduces spore viability and root colonization of arbuscular mycorrhizal fungi. Appl. Soil. Ecol. 2013, 64, 99–103. [Google Scholar] [CrossRef]
- Clark, C.A. End rot, surface rot, and stem lesions caused on sweet potato by Fusarium solani. Phytopathology 1980, 70, 109–112. [Google Scholar] [CrossRef]
- Ravi, V.; Chakrabarti, S.; Makeshkumar, T.; Saravanan, R. Molecular regulation of storage root formation and development in sweet potato. Hortic. Rev. 2014, 42, 157–208. [Google Scholar] [CrossRef]
- Xing, K.; Li, T.J.; Liu, Y.F.; Zhang, J.; Zhang, Y.; Shen, X.Q.; Li, X.Y.; Miao, X.M.; Feng, Z.Z.; Peng, X.; et al. Antifungal and eliciting properties of chitosan against Ceratocystis fimbriata in sweet potato. Food Chem. 2018, 268, 188–195. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Betts, G.; Hoskins, N.; Edwards, M.; Ercolini, D.; Mauriello, G. Membrane toxicity of antimicrobial compounds from essential oils. J. Agric. Food Chem. 2007, 55, 4863–4870. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, Y.; Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Quek, S.Y.; Yao, W. Antifungal effects of thymol and salicylic acid on cell membrane and mitochondria of Rhizopus stolonifer and their application in postharvest preservation of tomatoes. Food Chem. 2019, 285, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cai, Y.; Wang, R.; Li, B.; Weng, Q. Effect of Ethylenediaminetetraacetic acid (EDTA) on perillaldehyde-mediated regulation of postharvest Aspergillus flavus growth on peanuts. LWT Food Sci. Technol. 2022, 154, 112826. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, C.; Yang, K.; Erhunmwunsee, F.; Wang, B.; Yang, D.; Lu, G.; Liu, M.; Li, Y.; Tian, J. Antifungal Activity of Perillaldehyde on Fusarium solani and Its Control Effect on Postharvest Decay of Sweet Potatoes. J. Fungi 2023, 9, 257. https://doi.org/10.3390/jof9020257
Pan C, Yang K, Erhunmwunsee F, Wang B, Yang D, Lu G, Liu M, Li Y, Tian J. Antifungal Activity of Perillaldehyde on Fusarium solani and Its Control Effect on Postharvest Decay of Sweet Potatoes. Journal of Fungi. 2023; 9(2):257. https://doi.org/10.3390/jof9020257
Chicago/Turabian StylePan, Chao, Kunlong Yang, Famous Erhunmwunsee, Bo Wang, Dongjing Yang, Guoquan Lu, Man Liu, Yongxin Li, and Jun Tian. 2023. "Antifungal Activity of Perillaldehyde on Fusarium solani and Its Control Effect on Postharvest Decay of Sweet Potatoes" Journal of Fungi 9, no. 2: 257. https://doi.org/10.3390/jof9020257
APA StylePan, C., Yang, K., Erhunmwunsee, F., Wang, B., Yang, D., Lu, G., Liu, M., Li, Y., & Tian, J. (2023). Antifungal Activity of Perillaldehyde on Fusarium solani and Its Control Effect on Postharvest Decay of Sweet Potatoes. Journal of Fungi, 9(2), 257. https://doi.org/10.3390/jof9020257






