Three New Species of Gongronella (Cunninghamellaceae, Mucorales) from Soil in Hainan, China Based on Morphology and Molecular Phylogeny
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Morphological Observation
2.2. DNA Extraction and Amplification
2.3. Phylogenetic Analyses
3. Results
3.1. Phylogenetic Analyses
3.2. Taxonomy
3.2.1. Gongronella multiramosa Yi Xin Wang, H. Zhao & X.Y. Liu, sp. nov., Figure 2

3.2.2. Gongronella qichaensis Yi Xin Wang, H. Zhao & X.Y. Liu, sp. nov., Figure 3
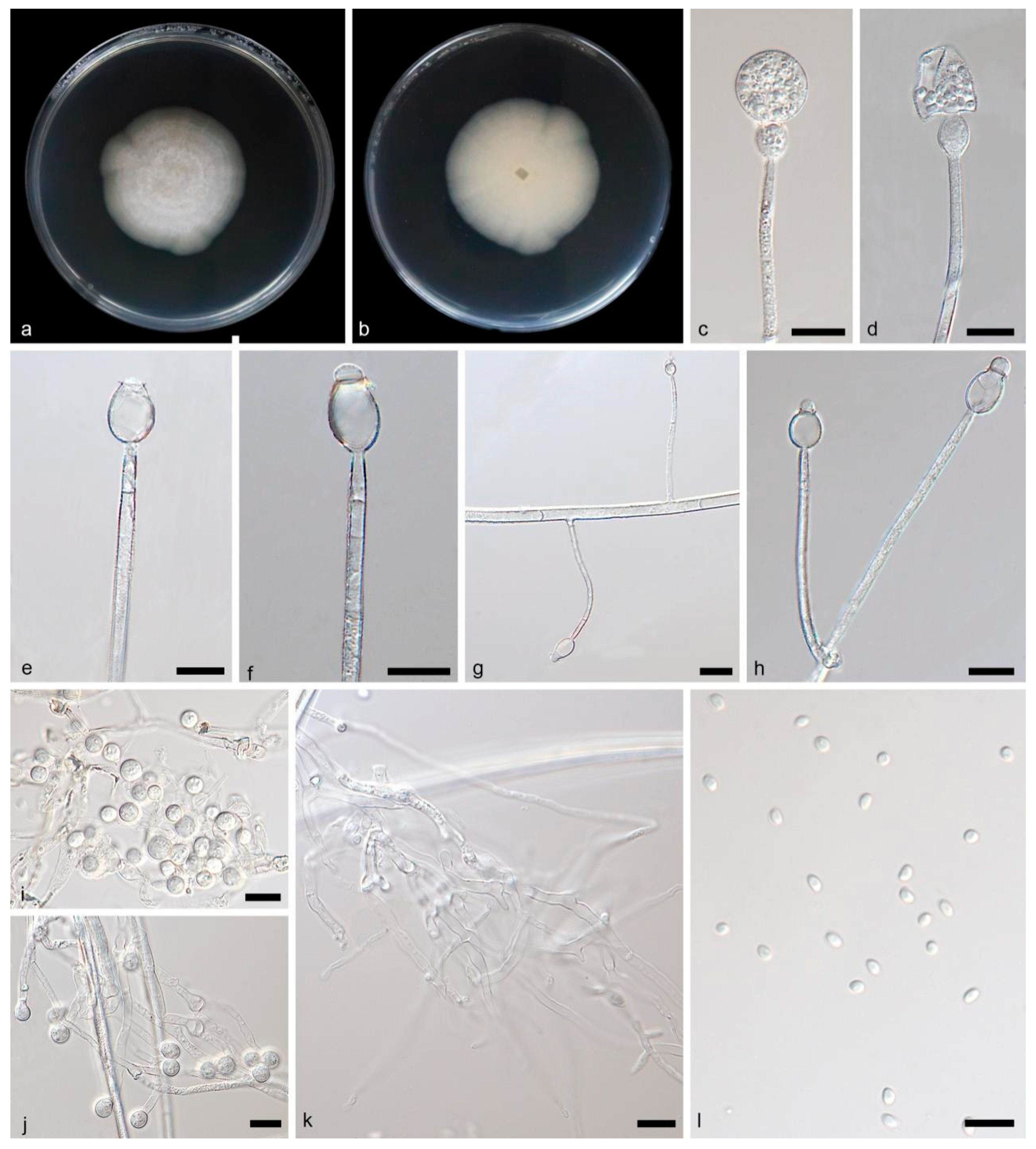
3.2.3. Gongronella oleae Yi Xin Wang, H. Zhao & X.Y. Liu, sp. nov., Figure 4

4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, S.C.; Tan, T.K.; Wong, S.M.; Khor, E. The chitosan yield of zygomycetes at their optimum harvesting time. Carbohydr. Polym. 1996, 30, 239–242. [Google Scholar] [CrossRef]
- Babu, A.G.; Kim, S.W.; Adhikari, M.; Yadav, D.R.; Um, Y.H.; Kim, C.; Lee, H.B.; Lee, Y.S. A New Record of Gongronella butleri Isolated in Korea. Mycobiology 2015, 43, 166–169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.; Zhou, W.; Yuan, H.; Wang, Y. Characterization of a novel fungal chitosanase Csn2 from Gongronella sp. JG. Carbohydr. Res. 2008, 343, 2583–2588. [Google Scholar] [CrossRef]
- Zhou, W.; Yuan, H.; Wang, J.; Yao, J. Production, purification and characterization of chitosanase produced by Gongronella sp. JG. Lett. Appl. Microbiol. 2008, 46, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Doilom, M.; Guo, J.-W.; Phookamsak, R.; Mortimer, P.E.; Karunarathna, S.C.; Dong, W.; Liao, C.-F.; Yan, K.; Pem, D.; Suwannarach, N.; et al. Screening of Phosphate-Solubilizing Fungi From Air and Soil in Yunnan, China: Four Novel Species in Aspergillus, Gongronella, Penicillium, and Talaromyces. Front. Microbiol. 2020, 11, 585215. [Google Scholar] [CrossRef]
- Martins, M.R.; Santos, C.; Soares, C.; Santos, C.; Lima, N. Gongronella eborensis sp. nov., from vineyard soil of Alentejo (Portugal). Int. J. Syst. Evol. Microbiol. 2020, 70, 3475–3482. [Google Scholar] [CrossRef]
- Dong, Y.; Sun, Q.; Zhang, Y.; Wang, X.; Liu, P.; Xiao, Y.; Fang, Z. Complete genome of Gongronella sp. w5 provides insight into its relationship with plant. J. Biotechnol. 2018, 286, 1–4. [Google Scholar] [CrossRef]
- Wei, F.; Hong, Y.; Liu, J.; Yuan, J.; Fang, W.; Peng, H.; Xiao, Y. Gongronella sp. induces overproduction of laccase Inpanus rudis. J. Basic Microbiol. 2010, 50, 98–103. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Y.; Xu, Y.; Sun, Q.; Liu, J.; Fang, W.; Xiao, Y.; Kües, U.; Fang, Z. Gongronella sp. w5 elevates Coprinopsis cinerea laccase production by carbon source syntrophism and secondary metabolite induction. Appl. Microbiol. Biotechnol. 2019, 103, 411–425. [Google Scholar] [CrossRef]
- Tedersoo, L.; Sánchez-Ramírez, S.; Kõljalg, U.; Bahram, M.; Döring, M.; Schigel, D.; May, T.; Ryberg, M.; Abarenkov, K. High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Divers. 2018, 90, 135–159. [Google Scholar] [CrossRef]
- Ribaldi, M. Sopra un interessante zigomicete terricolo: Gongronella urceolifera n. gen. et n. sp. Riv. Biol. 1952, 44, 157–166. [Google Scholar] [PubMed]
- Upadhyay, H.P. Soil fungi from North-East and North Brazil—VIII. Persoonia Mol. Phylogeny Evol. Fungi 1970, 6, 111–117. [Google Scholar]
- Zhang, Z.-Y.; Han, Y.-F.; Chen, W.-H.; Liang, Z.-Q. Gongronella sichuanensis (Cunninghamellaceae, Mucorales), a new species isolated from soil in China. Phytotaxa 2019, 416, 167–174. [Google Scholar] [CrossRef]
- Nie, Y.; Cai, Y.; Gao, Y.; Yu, D.-S.; Wang, Z.-M.; Liu, X.-Y.; Huang, B. Three new species of Conidiobolus sensu stricto from plant debris in eastern China. MycoKeys 2020, 73, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Qin, L.; Yu, D.-S.; Liu, X.-Y.; Huang, B. Two new species of Conidiobolus occurring in Anhui, China. Mycol. Prog. 2018, 17, 1203–1211. [Google Scholar] [CrossRef]
- Peyronel, B.; Vesco, G.D. Ricerche sulla microflora di un terreno agrario presso Torino. Allionia 1955, 2, 357–417. [Google Scholar]
- Corry, J.E.L. Rose bengal chloramphenicol (RBC) agar. In Progress in Industrial Microbiology; Elsevier: Amsterdam, The Netherlands, 1995; pp. 431–433. [Google Scholar]
- Zhang, Z.; Liu, R.; Liu, S.; Mu, T.; Zhang, X.; Xia, J. Morphological and phylogenetic analyses reveal two new species of Sporocadaceae from Hainan, China. MycoKeys 2022, 88, 171–192. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L.; Brown, A.H.D. Chloroplast DNA Phylogenetic Affinities of Newly Described Species in Glycine (Leguminosae: Phaseoleae). Syst. Bot. 1990, 15, 466. [Google Scholar] [CrossRef]
- Guo, L.D.; Hyde, K.D.; Liew, E.C.Y. Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytol. 2000, 147, 617–630. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W.; Karami, A.; Movaghar, A.F.; Mercier, S.; Ferre, L.; Seligmann, H.; Proença, D.; et al. A Greedy Algorithm for Aligning DNA Sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. The CIPRES science gateway: Enabling high-impact science for phylogenetics researchers with limited resources. In Proceedings of the 1st Conference of the Extreme Science and Engineering Discovery Environment: Bridging from the Extreme to the Campus and Beyond; Association for Computing Machinery: Chicago, IL, USA, 2012. [Google Scholar]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J. MrModeltest V2: Program distributed by the author. Bioinformatics 2004, 24, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Waren, L. Gongronella pederalhadensis, a new specification of Mucorales (Mucoromycota) isolated from the Brazilian Atlantic forest, with an identification key for the genus. Sydowia 2020, 73, 61–68. [Google Scholar]
- Zhao, H.; Nie, Y.; Zong, T.-K.; Wang, K.; Lv, M.-L.; Cui, Y.-J.; Tohtirjap, A.; Chen, J.-J.; Zhao, C.-L.; Wu, F.; et al. Species diversity, updated classification and divergence times of the phylum Mucoromycota. Fungal Divers. 2023, 1–109. [Google Scholar] [CrossRef]
- Adamčík, S.; Cai, L.; Chakraborty, D.; Chen, X.-H.; Cotter, H.V.T.; Dai, D.-Q.; Dai, Y.-C.; Das, K.; Deng, C.; Ghobad-Nejhad, M.; et al. Fungal Biodiversity Profiles 1–10. Cryptogam. Mycol. 2015, 36, 121–166. [Google Scholar] [CrossRef]
- Tibpromma, S.; Hyde, K.D.; Jeewon, R.; Maharachchikumbura, S.S.N.; Liu, J.-K.; Bhat, D.J.; Jones, E.B.G.; McKenzie, E.H.C.; Camporesi, E.; Bulgakov, T.S.; et al. Fungal diversity notes 491–602: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2017, 83, 1–261. [Google Scholar] [CrossRef]
- Li, G.J.; Hyde, K.D.; Zhao, R.L.; Hongsanan, S.; Abdel-Aziz, F.A.; Abdel-Wahab, M.A.; Alvarado, P.; Alves-Silva, G.; Ammirati, J.F.; Ariyawansa, H.A.; et al. Fungal diversity notes 253–366: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016, 78, 1–237. [Google Scholar] [CrossRef]

| Species | Strains | Substrates | Countries | GenBank Accession Numbers | |
|---|---|---|---|---|---|
| ITS | LSU | ||||
| G. banzhaoae | BRIP 75171a * | Soil | Australia | OR271908 | OR259049 |
| G. brasiliensis | URM 7487 * | Soil | Brazil | NR_155148 | KY114932 |
| URM 7488 | Soil | Brazil | KY114931 | KY114933 | |
| G. butleri | CBS 415.67 | Soil | Brazil | JN206288 | MH870714 |
| CBS 216.58 * | Soil | UK | JN206285 | MH869292 | |
| G. chlamydospora | CGMCC 3.16118 | Soil | China | OL678157.1 | n.a. |
| G. eborensis | MUM 10.262 *=CCMI 1100 * | Soil | Portugal | KT809408 | MN947301 |
| MUM 10.263=CCMI 1101 | Soil | Portugal | GU244500 | MN947302 | |
| G. guangdongensis | CGMCC 2.15212 * | Soil | China | NR_158464 | MN947303 |
| CGMCC 2.15213 | Soil | China | KC462740 | MN947304 | |
| G. hydei | KUMCC 18.0198 | Soil | China | NR_171964 | MT907273 |
| G. koreana | EML-TS2Bp * | Soil | Korea | KP636529 | KP636530 |
| EML-TS2Bp-2 | Soil | Korea | KP835545 | KP835542 | |
| G. lacrispora | ATCC 24412 * | Soil | Brazil | GU244498 | JN206609 |
| G. multiramosa | CGMCC 3.26216 * | Soil | China | OR733546 | OR733611 |
| SAUCC 4056-4 | Soil | China | OR733545 | OR733610 | |
| G. multispora | CGMCC 3.16119 | Soil | China | OL678158.1 | n.a. |
| G. namwonensis | CNUFC WW2-12 | Soil | Korea | NR_175640 | MN658482 |
| G. oleae | CGMCC 3.26217 * | Soil | China | OR742078 | OR733608 |
| SAUCC 4164-2 | Soil | China | OR742079 | OR733609 | |
| G. orasabula | EML-QF12-1 * | Soil | Korea | NR_148087 | KT936263 |
| EML-QF12-2 | Soil | Korea | KT936270 | KT936264 | |
| G. pamphilae | BRIP 74936a | Soil | Australia | n.a. | n.a. |
| G. qichaensis | CGMCC 3.26218 * | Soil | China | OR733544 | OR733607 |
| SAUCC 4137-3 | Soil | China | OR733543 | OR733606 | |
| G. pedratalhadensis | URM 8182 | Soil | Brazil | MN912512 | MN912508 |
| G. sichuanensis | CGMCC 3.19651 * | Soil | China | MK813373 | MK813855 |
| CGMCC 3.19652 | Soil | China | MK813374 | MK813856 | |
| CGMCC 3.19653 | Soil | China | MK813375 | MK813857 | |
| G. zunyiensis | CGMCC 3.19899 * | Soil | China | MN453856 | MN453853 |
| CGMCC 3.19900 | Soil | China | MN453857 | MN453854 | |
| CGMCC 3.19901 | Soil | China | MN453858 | MN453855 | |
| Cunninghamella echinulata | CBS 156.28 | n.a. | n.a. | JN205895 | MH877699 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-X.; Zhao, H.; Ding, Z.-Y.; Ji, X.-Y.; Zhang, Z.-X.; Wang, S.; Zhang, X.-G.; Liu, X.-Y. Three New Species of Gongronella (Cunninghamellaceae, Mucorales) from Soil in Hainan, China Based on Morphology and Molecular Phylogeny. J. Fungi 2023, 9, 1182. https://doi.org/10.3390/jof9121182
Wang Y-X, Zhao H, Ding Z-Y, Ji X-Y, Zhang Z-X, Wang S, Zhang X-G, Liu X-Y. Three New Species of Gongronella (Cunninghamellaceae, Mucorales) from Soil in Hainan, China Based on Morphology and Molecular Phylogeny. Journal of Fungi. 2023; 9(12):1182. https://doi.org/10.3390/jof9121182
Chicago/Turabian StyleWang, Yi-Xin, Heng Zhao, Zi-Ying Ding, Xin-Yu Ji, Zhao-Xue Zhang, Shi Wang, Xiu-Guo Zhang, and Xiao-Yong Liu. 2023. "Three New Species of Gongronella (Cunninghamellaceae, Mucorales) from Soil in Hainan, China Based on Morphology and Molecular Phylogeny" Journal of Fungi 9, no. 12: 1182. https://doi.org/10.3390/jof9121182
APA StyleWang, Y.-X., Zhao, H., Ding, Z.-Y., Ji, X.-Y., Zhang, Z.-X., Wang, S., Zhang, X.-G., & Liu, X.-Y. (2023). Three New Species of Gongronella (Cunninghamellaceae, Mucorales) from Soil in Hainan, China Based on Morphology and Molecular Phylogeny. Journal of Fungi, 9(12), 1182. https://doi.org/10.3390/jof9121182







